Abstract
Autoantibodies, a hallmark of both autoimmunity and cancer, represent an easily accessible surrogate for measuring adaptive immune responses to cancer. Sera can now be assayed for reactivity against thousands of proteins using microarrays, but there is no agreed-upon standard to analyze results. We developed a set of tailored quality control and normalization procedures based on ELISA validation to allow patient comparisons and determination of individual cutoffs for specificity and sensitivity. Sera from 60 patients with pancreatic cancer, 51 patients with ovarian cancer, and 53 age-matched healthy donors were used to assess the binding of IgG antibodies against a panel of >8000 human antigens using protein microarrays and fluorescence detection. The resulting data interpretation led to the definition and ranking of proteins with preferred recognition by the sera from cancer patients in comparison with healthy donors, both by frequency and strength of signal. We found that 202 proteins were preferentially immunogenic in ovarian cancer sera compared to 29 in pancreatic cancer, with few overlaps. Correlates of autoantibody signatures with known tumor expression of corresponding antigens, functional pathways, clinical stage, and outcome were examined. Serological analysis of arrays displaying the complete human proteome (seromics) represents a new era in cancer immunology, opening the way to defining the repertoire of the humoral immune response to cancer.
Keywords: serum antibody, biomarkers, protein microarrays, serology, autoantigen
Protein microarrays allow for the detection of specific serum antibodies against a very large number of targets simultaneously. Arrays can be used with high throughput to determine patterns of antigens recognized by autoantibodies during the course of diseases, such as autoimmunity or cancer (1–9), as well as a way to characterize the repertoire of serological responses in healthy individuals (10). Defined protein collections assembled from phage expression systems or purified from recombinant sources were used to detect antibody responses in the serum of ovarian (11, 12), breast (13), colorectal (2), pancreatic (14), or lung (15, 16) cancer patients, and some of these responses appear to have diagnostic significance. In addition, protein array tools are also useful to measure changes in antibody responses to vaccination or immunotherapies, although it has yet to be applied to the monitoring of cancer treatments (17, 18). So far, studies have often concentrated either on small numbers of defined antigenic targets tested across large serum datasets, or on large numbers of proteins probed by a limited number of patient sera, likely because of prohibitive cost constraints and arduous handling of large data samples. Still, the opportunity to define the cancer “serome,” that is, the repertoire of antigens recognized by the humoral immune system, is now within reach, provided adequate analyses of the vast amount of data generated by these microarrays can be properly interpreted.
We have recently validated the use of such arrays by comparing serological results obtained with pedigreed sera in classic methods such as ELISA (19), and defined a set of normalization and calculation conditions for stringent data analysis tailored to the definition of protein targets of autoantibodies (20). When analyzing a series of lung cancer and healthy control sera on a small array (329 proteins) for antigen reactivity using this antibody profiling method, referred to here as “seromics,” we were able to detect known antigens with sensitivity and specificity comparable to ELISA, as well as new antigens that are now under further investigation. Contrary to gene microarrays where changes in the pattern of gene expression are detected in clusters, antibody responses to antigens on protein arrays typically fit a pattern of discrete responses in individual cancer patients. By applying the set of analyses determined from our initial validation study (interquartile and quantile normalization, individual cutoffs for each antigen based on interquartile differences, scoring by strength and frequency of positives), reactivities that could be otherwise dismissed as outliers in genomic analyses can be now measured, ranked, and assessed for cancer specificity (20).
Using commercially available microarrays containing over 8200 proteins translated from genes randomly selected throughout the human genome (21), we now asked whether we could detect autoantibody responses to previously described or unknown antigens to define: (i) autoantibody targets and their frequency in patients with ovarian and pancreatic cancers; (ii) potential differences in the immunogenicity of these two cancer types; and (iii) autoantibody signatures representative of cancer with potential clinical value.
For this purpose, sera were selected from patients with resectable cancer at time of surgery to be representative of the following categories: 60 pancreatic cancer patients, mostly stage IIB and IV, chosen to include long-term survivors with localized disease as well as short-term survivors with either localized disease or distant metastatic disease; 51 ovarian cancer patients, mostly stage IIIC, including long-term and short-term survivors; and 53 healthy donors taken from the blood bank and matched for age with both cancer cohorts.
We describe here the validation and normalization procedures and analysis of the data that allowed us to define and rank top immunogenic antigens in ovarian and pancreatic cancer patients.
Results
Measuring Autoantibodies to Known Tumor Antigens by ELISA.
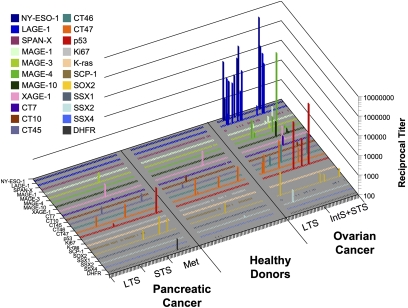
To establish a profile of autoantibody reactivity to tumor antigens, we tested a panel of 22 recombinant proteins, including cancer/testis antigens (NY-ESO-1, LAGE-1, MAGE-A, MAGE-C, SSX, XAGE, CT45, CT46, CT47, SPANX), mutational antigens (TP53, K-ras), and embryonic/stem cell antigens (SOX2), for recognition by ELISA. We found that ovarian cancer appeared more immunogenic overall than pancreatic cancer in terms of frequency of seroreactivity against this antigen panel (Fig. 1). As expected from previous studies, NY-ESO-1 and LAGE-1 were recognized with the highest frequency by ovarian cancer sera (17% and 19%, respectively), but not by pancreatic cancer sera or by healthy donor sera, echoing differences in CT antigen expression in these two tumor types, ovarian cancer being CT antigen-rich (22–24) whereas pancreatic cancer was previously shown to be CT antigen-poor (25, 26). Accordingly, serum reactivity to MAGE antigens, SSX2, CT7, or CT10 was also found primarily in ovarian cancer samples (Fig. 1). Among CT antigens, only CT45, CT47, and XAGE-1 appeared to react with sera from both cancer types, but also with some healthy donor sera. Among non-CT antigens, SOX2 and p53 were the most immunogenic in both tumor types, with higher frequency in ovarian cancer samples. Overall, antibody responses to one or more antigens from the panel were found in 52% of ovarian cancer samples, compared to 15% of healthy donor samples and 20% of pancreatic cancer samples. Except for NY-ESO-1 and LAGE-1, all other antigens tested reacted with less than 10% of sera within each cohort, indicating that detectable spontaneous immunogenicity to these antigens represents an uncommon event. Ovarian cancer sera were more likely to react to multiple antigens simultaneously, and did so with higher average titers compared to sera from healthy donors and pancreatic cancer patients.
Fig. 1.
ELISA with sera from 60 pancreatic cancer patients, 53 healthy donors, and 51 ovarian cancer patients. Results are shown as extrapolated IgG titers against a series of 22 recombinant antigens listed. Three ovarian cancer patient sera were considered not evaluable because of reactivity to more than 50% of antigens tested and were therefore excluded from the graph and from statistics. Results are considered positive if reciprocal titers are >100. Results are representative of at least two repeat assays. LTS, long-term survivor; STS, short-term survivor; IntS, intermediate-term survivor.
Validating the Use of Protein Microarrays.
Having previously established that protein microarrays (including ProtoArrays) were a suitable method for detecting antibody responses from the serum of non-small-cell lung cancer patients (20), we reexamined the concordance between ELISA and seromics for the current investigation. Because only two of the proteins tested in ELISA, MAGE-A4 and TP53, were present on ProtoArrays, we sought to extend the correlation between assays by spotting most of the panel of recombinant proteins used for ELISAs (see above) in a customized fashion on ProtoArrays, along with additional control proteins.
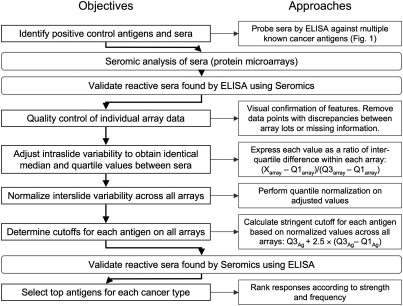
All sera were tested at a dilution of 1:500 as described in Materials and Methods, and antigen-specific IgG responses to each of the proteins present on the array were measured by fluorescence. Reproducibility of results was confirmed using duplicate microarrays for selected sera. After extensive visual quality control of spot alignment and duplicates, a series of normalization steps was applied to allow interslide comparisons, and specificity was determined with a stringent yet adaptable calculation highlighting sera with outlying reactivity in an antigen-specific manner. The analysis strategy is summarized in Fig. 2 and described in more detail in ref. 20.
Fig. 2.
Flowchart of normalization, validation, and antigen selection strategies for seromics. X = any of the values on the array; Q1 = 25th percentile of all values; Q3 = 75th percentile of all values.
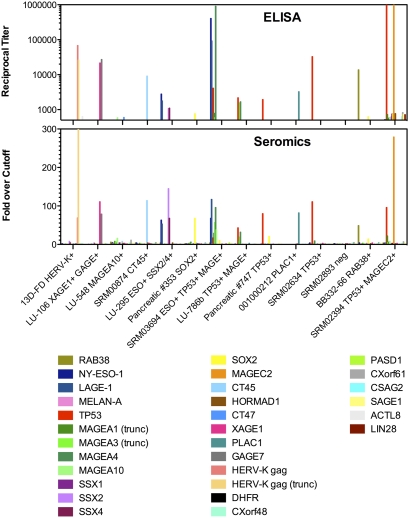
To compare ELISA and seromics results, we used a series of human sera known for their specific reactivity to individual antigens from our ELISA panel and tested them for reactivity with customized arrays (Fig. 3). Results showed a high degree of similarity between the two methods, and all of the reactivities expected from ELISA were also observed in seromics. Overall, there was an excellent correlation (P < 0.0001, Pearson correlation test) between the strength of antibody responses in ELISA as measured by titers, compared to seromics measured by fold-over-cutoff results, suggesting that signal intensity in protein arrays may reflect relative actual titers.
Fig. 3.
Comparison of ELISA and seromics data on panel of antigens using sera with known specificity. Fourteen control sera, plotted along the x axis and known to react with individual antigens shown next to their name, were tested against a series of 30 proteins indicated. In the top panel, reciprocal titers were determined by ELISA from serial dilutions for each serum against each protein, as described in Materials and Methods. Only reciprocal titers greater than 500 are shown in ELISA to allow relevant comparison with seromics data that were generated using a single 1:500 serum dilution. In the lower panel, fold-over-cutoff results of seromics are shown following data transformation and normalization, indicating seroreactivity against the same 30 proteins spotted on ProtoArrays. Trunc, truncated proteins.
Discovery of Targets of Autoantibody Responses in Ovarian and Pancreatic Cancer.
Following normalization and validation, individual antigens from microarrays were ranked according to the frequency of cancer sera reacting in comparison with the healthy cohort as well as by the mean strength of signal elicited. Contrary to gene array studies aiming at discovering small but consistent changes between two cohorts, the method used for seromics was designed to identify rare but clear events, corresponding to antigens only occasionally recognized with high titers among sera tested. To be considered of interest, antigen-specific responses had to occur more frequently in cancer patient sera than in healthy donor sera and be found in at least two patients within the cancer cohort, thus possibly representing shared tumor-associated events. Approximately 20% of all antigens failed to specifically react with any sera, and another 30% reacted with a single serum only. Out of all 8277 antigens, ovarian cancer sera reacted on average with 218 antigens (standard deviation = 92), pancreatic cancer with 120 antigens (SD = 118), and healthy sera with 250 antigens (SD = 121). Antigens eliciting responses with similar frequency and strength in patients and healthy donors failed to achieve a sufficient score to qualify as top antigens, and were thus not considered in the present analysis (even though some may represent important targets of autoimmunity or cancer immunosurveillance).
We found 197 distinct proteins with increased immunogenicity in ovarian cancer patients (Table 1 and Table S1). For 5 of these proteins (APEX1, CSNK2A1, GAS7, MAPKAPK5, SUB1), there were redundant sequences present on the array because of transcript variants produced independently, which also reacted with similar sets of sera, thereby confirming antigen specificity and bringing the total of frequently immunogenic gene products in ovarian cancer to 202.
Table 1.
Top 15 antigens out of 202 candidate antigens found immunogenic in ovarian cancer compared with healthy donor sera
| Locus | Symbol | Frequency in healthy, % | Frequency in ovarian, % | Intensity* healthy | Intensity* ovarian | Overall score† |
| NM_152277.1 | UBTD2 | 6 | 24 | 1.82 | 3.93 | 30.2 |
| BC029920.1 | TGIF2LX | 4 | 10 | 1.77 | 27.12 | 24.9 |
| NM_030935.1 | TSC22D4 | 2 | 12 | 1.08 | 8.69 | 22.3 |
| NM_007067.3 | MYST2 | 0 | 14 | 1.00 | 3.08 | 20.0 |
| NM_016360.1 | CCDC44 | 4 | 16 | 1.53 | 3.51 | 19.5 |
| NM_021809.2 | TGIF2 | 4 | 10 | 1.62 | 12.63 | 18.4 |
| NM_022104.1 | C20orf67 | 0 | 10 | 1.00 | 6.42 | 18.2 |
| NM_033201.1 | C16orf45 | 4 | 14 | 7.89 | 6.02 | 17.5 |
| NM_006442.2 | DRAP1 | 0 | 14 | 1.00 | 2.07 | 17.5 |
| NM_006993.1 | NPM3 | 4 | 16 | 1.44 | 2.65 | 17.5 |
| NM_015850.2 | FGFR1 | 4 | 12 | 3.20 | 7.16 | 17.1 |
| BC003596.1 | TP53 | 0 | 10 | 1.00 | 4.82 | 16.6 |
| BC043346.2 | UBL4A | 2 | 8 | 1.07 | 13.11 | 16.6 |
| NM_005246.1 | FER | 0 | 12 | 1.00 | 2.77 | 16.5 |
| BC002859.1 | ZNF434 | 0 | 14 | 1.00 | 1.71 | 16.4 |
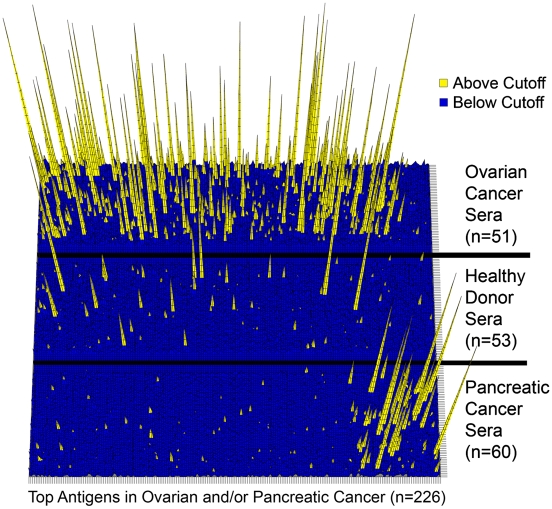
In contrast, we found only 28 proteins with increased immunogenicity in pancreatic cancer patients, with only one additional redundant sequence (MAPK9) recognized (Table 2 and Table S2). Similarly to what had been observed in ELISA, it appears that pancreatic cancer sera had overall less reactivity compared to ovarian cancer sera. Six antigens from the top list for pancreatic cancer were also present in the top list of ovarian cancer antigens (C4orf16, CD79B, GAS2, NY-SAR-48, TMOD1, TMSB10). Fig. 4 shows the distribution and strength of seroreactivity for each cohort against all top antigens, and highlights that, although some occasional reactivity is observed in healthy donors, these antigens react preferentially and more strongly with sera from cancer patients.
Table 2.
Top 15 antigens out of 29 candidate antigens found immunogenic in pancreatic cancer compared to healthy donor sera
| Locus | Symbol | Frequency in healthy,% | Frequency in pancreatic,% | Intensity* healthy | Intensity* pancreatic | Overall score† |
| BC032539.1 | MAPK9 | 0 | 8 | 1.00 | 2.33 | 11.0 |
| BC041421.1 | NR2E3 | 4 | 13 | 1.94 | 1.55 | 10.7 |
| NM_153344.1 | C6orf141 | 0 | 8 | 1.00 | 1.96 | 10.4 |
| NM_002752.3 | MAPK9 | 0 | 8 | 1.00 | 1.60 | 9.8 |
| NM_004560.2 | ROR2 | 4 | 12 | 1.25 | 1.68 | 9.8 |
| NM_080840.1 | PTPRA | 0 | 7 | 1.00 | 3.10 | 9.7 |
| BC063126.1 | FAM13A1 | 0 | 5 | 1.00 | 6.58 | 9.4 |
| BC037982.1 | LRRC49 | 0 | 7 | 1.00 | 2.78 | 9.4 |
| NM_017886.1 | ULK4 | 0 | 7 | 1.00 | 2.14 | 8.6 |
| BC002660.1 | TMOD1 | 2 | 7 | 1.10 | 3.83 | 8.5 |
| NM_177553.1 | GAS2 | 2 | 8 | 1.11 | 1.65 | 7.9 |
| NM_052958.1 | C8orf34 | 0 | 7 | 1.00 | 1.48 | 7.6 |
| NM_020954.1 | KIAA1618 | 2 | 8 | 2.77 | 1.64 | 7.2 |
| BC028124.1 | C17orf46 | 0 | 5 | 1.00 | 2.88 | 7.1 |
| BC009739.1 | HERPUD1 | 0 | 5 | 1.00 | 2.77 | 7.0 |
Fig. 4.
Three-dimensional heat map representation of seroreactivity against top antigens in sera from ovarian and pancreatic cancer patients and healthy donors, as indicated. Sera are arranged along the y axis, whereas antigens listed in Tables S1 and Tables S2 are arranged along the x axis, with those preferentially immunogenic in ovarian cancer on the left and those preferentially immunogenic in pancreatic cancer on the right, with some overlap. Each peak represents the reactivity of an individual serum to one antigen, expressed as the number of fold-over cutoff, indicating the strength of antibody response. If the ratio to cutoff is greater than 1, the serum is considered to react significantly and peaks appear as yellow. Peaks have graded bars to indicate number of actual folds over cutoff (shown up to 20× over cutoff).
The antigen with the highest score in ovarian cancer was UBTD2, also known as DC-UbP: It was immunogenic in 24% of patients, with an average reactivity of 4× over the cutoff, as compared to 6% of healthy donors reacting against it with less than 2× over the cutoff. Most other top antigens were recognized by less than 14% of patient serum samples, with a median differential frequency of 6%, and therefore represented rare events (Table 1 and Table S1).
The frequency of autoantibody responses in pancreatic cancer patients was even smaller, with a median of 5% of patients responding compared to healthy donors. No top antigen achieved immunogenicity in more than 15% of patients (Table 2 and Table S2).
Specificity Confirmation and Gene Ontology.
A total of 19/197 (10%) antigens immunogenic by seromics in ovarian cancer patients and 2/28 (7%) in pancreatic cancer patients have been previously identified by serological screening of cDNA expression libraries from various other cancer types (SEREX; Table S3), thereby confirming their immunogenic potential. For example, antigens ANXA2 or DNAJB1 were previously found to elicit autoantibodies in non-small-cell lung cancer (Tables S4 and Tables S5). Additionally, one target of ovarian cancer sera (MAPKAPK3) was recently identified as an immunoreactive antigen in colorectal cancer in one of the only other studies that used a similar strategy with ProtoArrays in a smaller sample set (2).
Additionally, several top antigens immunogenic in ovarian cancer have been previously described associated with germ cells, oocyte maturation, or gonadal tumorigenesis (Table S4), thus suggesting that humoral responses detected in seromics had specificity against the tumor type. More generally, a large number of top antigens have been found overexpressed in various cancer tissues, including pancreatic and ovarian, or associated with carcinogenesis. Yet, a majority of other proteins from the top lists as well as previously unknown proteins, only discovered through domain homology or sequencing, have no assigned function or description of cancer association in the literature.
In an attempt to categorize top antigens according to biological pathways, we performed a gene ontology analysis of the top immunogenic candidate antigens in ovarian cancer. We were not able to define a unique functional or structural signature associated with candidate molecules, rather these genes belonged to many different pathways without obvious connection to each other.
Clinical Implications.
Finally, we asked whether any of the top immunogenic antigens had a prognostic value for cancer survival. A clear limitation for such analyses lies in the low frequency of antibody responses observed, as well as in the heterogeneity of prior treatments as well as in stage heterogeneity, making statistical estimations difficult. To address this situation, an exploratory approach was followed. Survival analyses stratified for antibody responses against single antigens showed marked differences (Fig. S1), and individual antigens were found to be more often immunogenic in the serum of ovarian and pancreatic cancer patients with either favorable or bad prognosis (Fig. S1). These observations will need to be confirmed in a larger patient cohort for each tumor entity. Given the low frequency of antibody responses to single antigens, a combined analysis could have a higher predictive power. As an exploratory analysis and as a basis for further studies, potential good or bad prognostic antibody responses were determined using antigens in combination, and Fig. 5 shows examples of markers with potential prognostic value. Because patient cohorts were selected on the basis of clinical outcome, these markers will need to be validated independently in a larger number of sera, once they are available as recombinant antigens for high-throughput screening. So these exploratory observations give a first impression of the possible importance of antibody responses and show that antibody responses could indicate either a positive or negative clinical course.
Fig. 5.
Kaplan–Meier analyses of overall survival of cancer patients according to the presence of antibody response to a set of antigens. Detection of antibody response to any of the antigens listed above each graph was measured in ovarian (A and B) and pancreatic (C and D) cancer patients. Significant associations of autoantibody responses with better (A and C) or worse (B and D) clinical outcome were found by comparing differences between curves with the log-rank method. The accession numbers of genes coding for these antigens are listed in Tables S1 and Tables S2.
Discussion
We describe here a series of antigens recognized by autoantibodies present in the serum of ovarian and/or pancreatic cancer patients, and suggest that they may be useful individually or as signature sets as (i) diagnostic markers, preferentially immunogenic in cancer, (ii) prognostic markers, associated with favorable or unfavorable clinical outcome, and (iii) potential targets of immune responses for the development of new immunotherapeutic reagents. Some of these autoantibody targets overlap with antigens found by SEREX in ovarian or other cancers (27), thereby validating the protein array methodology for antigen discovery, but the overlap is only partial (10%), indicating that a previously untapped repertoire may be detected by seromics. It is possible that technical aspects, such as the spotting of recombinant proteins on nitrocellulose, affects serum recognition when compared to phage display methodologies. More likely, there may be increased sensitivity in the seromics approach due to the large number of probing sera used, in comparison with a single serum from SEREX analyses.
We also stress the importance of the data analysis and dangers in interpretation. From our previous validation study, we derived a set of calculations tailored for autoantibody target discovery (20). In contrast to gene array data analyses, the current evaluation emphasizes individual outlier events on a per-antigen basis, rather than widespread but modest shifts from global thresholds. It is therefore more closely related to the definition of antibody reactivity in ELISA, where discrete titers are extrapolated from negative and positive control sera used for each antigen tested (19). The final antigen panel was selected to be representative of proteins more frequently and strongly recognized by either ovarian or pancreatic cancer sera compared to normal sera. A remarkably small number of proteins fit these characteristics, and represented only 28 and 197 distinct targets for pancreatic and ovarian cancer, respectively, with 6 overlaps. The relative lower immunogenicity of pancreatic cancer may be related to elements in the microenvironment of this tumor type and reflect deeper levels of associated immunosuppressive mechanisms. Yet, many more proteins not present in the top lists were found to be immunogenic, either with equal frequency in cancer patients as in healthy donors, or had reactivity only in individual patients. These latter antigens, although not showing frequent immunogenicity, may still highlight individual mutations/overexpression/translocation events and potential pathways of interest or important mechanisms of tumorigenicity to be further studied.
Other studies have used protein arrays to define individual targets from the sera of ovarian and pancreatic cancer patients (9, 11, 14). Whereas some studies used undefined protein extracts from tumor lysates (11, 14, 28–30), others focused on limited numbers of candidate tumor antigens (24, 25). To our knowledge, only two studies have performed a large-scale profiling of autoantibodies related to cancer using the extensive panel of human antigens present on ProtoArrays, one study probing with 12 colorectal cancer sera and 8 control sera (2), and the other probing with 30 ovarian cancer sera and 30 healthy controls (9). The study in colorectal cancer (2) led to the definition of five antigens, one of which was also found immunogenic in our present study in ovarian cancer (MAPKAPK3). Surprisingly, the study with ovarian cancer sera found 10 immunogenic protein candidates, but none matched our top antigens (9). A possibility for explaining these different findings is that the arrays used in this study from 2007 were of a previous generation, with a third less content, but it also underscores how interpretation and normalization of data may lead to different results. Indeed, based on their use of alternate statistical methods to select significant responses, the authors of the study on ovarian cancer (9) did find additional antigens to be immunogenic, and there was some overlap with our data (ARL8B, MAGEA12, VRK3, along with a series of family members of proteins with possible cross-reactive serological recognition, such as MAGE-B antigens). Even though additional statistical tools may be developed to ensure greater specificity and sensitivity, our data analysis procedure was validated using previously known immunogenic control antigens and reactive sera to guide the determination of serological specificity and sensitivity.
To date, some of the best-studied antigens with expression restricted to cancer and able to induce measurable frequent spontaneous antibody responses in cancer patients only are NY-ESO-1, p53, Her2/neu, survivin, WT1, and XAGE-1 (31–34). The frequency of serological responses to these antigens varies according to tumor type, stage, or grade. For example, antibodies to NY-ESO-1 are found in ≈50% of stage IV melanoma patients expressing NY-ESO-1 in their tumor, but the overall frequency in melanoma is about 8% when considering earlier stages (with less frequent NY-ESO-1 expression) and nonexpressing tumors (31). Similarly, p53 and XAGE-1 are immunogenic in 10–30% of non-small-cell lung cancers, depending on type and stage, but very rarely so in melanoma (35–37). Therefore, in the current study, the frequencies of seroreactivity observed against top antigens in pancreatic and ovarian cancer patients have to be placed in the context of cognate antigen expression frequency, tumor type selected, and stage. Even with 4% of patients responding, some of these antigens could represent important targets for immunotherapy development. In this regard, a validation of antigen expression by immunohistochemistry and/or RT-PCR will be important in cancer tissues from patients showing seroreactivity, as well as in other tissue types, similarly to what has been established for model tumor antigen NY-ESO-1 (31). Our preliminary data indicate that GAS7, for example, appears to have preferred expression in metastatic ovarian cancer from gene microarray analyses. Although limited access to tumor blocks or material may make this task difficult, evidence of organ-specific or tumor-specific expression from the literature, as we showed here, may also help.
Still, there are many potential pitfalls in our analysis that need to be considered: We were not able to match gender for both cancer cohorts, because of the nature of the diseases analyzed and the prohibitive current costs to expand microarray analyses to more samples. The heterogeneity of the population analyzed and the low frequency of antibody responses clearly limit statistical evaluations. These preliminary differences in overall survival and corresponding antibody response have to be confirmed in larger cohorts, but the observed differences in (i) the either good or poor prognostic role of antibody responses to single antigens and (ii) the possibility of generating a prognostic signature clearly incite further studies and give clear directions for antigen or protein selection. In the present study, it should also be noted that many proteins had to be removed from the analysis after quality control because of lack of information or lot discrepancy, thereby potentially missing important targets. Most importantly, seromics should be considered as a discovery tool that will require further validation. Ultimately, our results should be confirmed on a larger scale, to ascertain that each identified target is indeed specifically and frequently immunogenic in larger cohorts of patients. To this end, ELISA is one of the most suitable methodologies, but requires each candidate antigen to be produced in sufficient amounts as recombinant protein, which is a limiting factor. We have started this project, and have already reconfirmed the specificities observed against a few targets, such as GAGE7 and ERG found immunogenic in individual ovarian cancer patients. Because proteins were produced in Escherichia coli as full-length His-tagged recombinant proteins, we could rule out any nonspecific reactivity against the GST tag or against insect cell contaminants from the baculovirus-produced proteins on arrays. Current assessment of UBTD2, TGIF2, TGIF2LX, YWHAB, UBL4A, MYST2, and GAS7 as recombinant bacterial proteins for ELISA confirmation has also shown some partial expected correlation with ELISA, but also points to possible individual differences with seromics results, linked to possible conformational and posttranslational differences between baculovirus- and E. coli-derived proteins that will need further exploration. Accumulating information from seromics analyses will lead to the development of customized arrays containing antigens showing greatest cancer specificity and sensitivity.
Materials and Methods
Study Population.
Patient characteristics are presented in Table S6. Sera were collected from ovarian and pancreatic cancers at time of surgery. Of the 51 ovarian cancer patients examined, 29 (57%) were dead before the end of the observation period. The median duration of follow-up for the entire group was 47.7 months (range, 1.0–167 months). The median age of the study population was 62 years (range, 26–88 years). The majority of patients presented with grade-3 tumors (82%), at stage IIIC (86%), and with serous histology (82%). The median survival for all patients was 48 months [95% confidence interval (C.I.) = 27–infinity months]. The 5-year survival for the entire study population was 45%. The ovarian cancer patients were selected to include 26 long-term survivors (median survival = 74 months), 7 intermediate-term survivors (median survival = 27 months), and 18 short-term survivors (median survival = 8 months). The 60 pancreatic cancer patients, mostly stage IIB and IV (median age = 63), were 21 long-term survivors with localized disease (median survival = 35 months), 19 short-term survivors with localized disease (median survival = 9 months), and 20 short-term survivors with distant metastatic disease (median survival = 11 months). The control sample (n = 53) had a median age of 62 years (range, 38–92 years) and was 49% female. The healthy control serum samples were from the Data Bank and BioRepository of Roswell Park Cancer Institute (37, or 70%) and from the New York Blood Bank (16, or 30%).
ELISA.
Patient plasma or donor serum samples were analyzed by ELISA for seroreactivity to bacterially produced recombinant proteins NY-ESO-1/CTAG1B, LAGE-1/CTAG2, MAGEA1, MAGEA3, MAGEA4, MAGEA10, CT7/MAGEC1, CT10/MAGEC2, CT45/RP13-36C9.1, CT46/HORMAD1, CT47/RP6-166C19.1, Ki-67/MKI67, KRAS, SCP1/SYCP1, SOX2, SPANXA1, SSX1, SSX2, SSX4, p53/TP53, XAGE1B, and DHFR (19). Plasma or serum was diluted serially from 1:100–1:100,000 and added to low-volume 96-well plates (Corning) coated overnight at 4 °C with 1 μg/mL antigen in 25 μl and blocked for 2 h at RT with PBS containing 5% nonfat milk. After overnight incubation, plates were extensively washed with PBS containing 0.2% Tween 20 and rinsed with PBS (BioTek ELx405 automated washer). Plasma IgG (total or subclasses) bound to antigens was detected with specific monoclonal antibodies conjugated to alkaline phosphatase (Southern Biotech). Following addition of ATTOPHOS substrate (Fisher Scientific), absorbance was measured using a Cytofluor Series 4000 fluorescence reader (PerSeptive Biosystems). A reciprocal titer was calculated for each plasma sample as the maximal dilution still significantly reacting to a specific antigen. Specificity was determined by comparing seroreactivity among the various antigens tested. In each assay, sera of patients with known presence or absence of specific reactivity were used as controls. A positive result was defined as extrapolated reciprocal titers > 100.
Seromics: Array Profiling Assay.
ProtoArrays microarrays (v4.0; Invitrogen) were purchased and used according to the manufacturer’s instructions. Briefly, after blocking for 1 h at 4 °C and washing, arrays were incubated in Quadriperm dishes (Greiner Bio One) placed on a horizontal shaker (50 rpm) for 90 min at 4 °C with individual sera diluted 1:500 in 5 ml washing buffer [0.1% Tween 20 (vol/vol), 1% BSA (wt/vol) in PBS]. After washes, binding of IgG was detected by incubation with Alexa Fluor 647 goat anti-human IgG (Invitrogen) diluted 1:2000 in assay buffer for 90 min at 4 °C. Arrays were washed again and dried by centrifugation. Arrays were scanned at 10-μm resolution using a microarray scanner (Axon 4200AL with GenePix Pro Software; Molecular Devices) and fluorescence was detected according to the manufacturer’s instructions. Images were saved as 16-bit tif files and analysis was performed using GenePix. The median net intensity in relative fluorescence units (rfu) was reported for each spot.
Calculations and Statistics.
Data from arrays were adjusted and normalized as described before, with minor modifications (20). Rather than averaging values from duplicate spots, the lower median rfu values of duplicates were used for each antigen within each array to minimize potential false positive results caused by artifacts. Additionally, to prevent erroneous interpretation in reactivity due to different antigen content between the two lots of arrays in this study, all antigens with discrepant or missing information for QC data provided by the vendor were removed from the analysis (removed content amounted to 2% of the total number of antigens). All other steps are described in ref. 20, and include a transformation of data as a ratio of interquartile differences per array followed by a quantile normalization across all arrays. Scoring included frequency of responses in the cancer patient cohort in comparison with the healthy donor cohort, and also included relative strength of reactivity observed. A high score (>5) was considered to indicate that most seroreactivity observed was attributable to cancer patients. For ELISA versus seromics comparisons, the Pearson correlation test was used. For clinical correlations of overall survival with the presence of specific antibodies, results were analyzed by the Kaplan–Meier method and assessed for significance using the log-rank method.
Supplementary Material
Acknowledgments
This work was conducted as part of The Atlantic Philanthropies/Ludwig Institute for Cancer Research Program and supported in part by grants from the Cancer Research Institute (Ovarian Cancer Working Group, Seromics, Cancer Vaccine Collaborative). K.O. and C.A. were supported in part by National Cancer Institute Center grant CA16056 (Roswell Park Cancer Institute). N.H. and I.Z. were supported by the postdoctoral program of the University Heidelberg.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914213107/DCSupplemental.
References
- 1.Quintana FJ, et al. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc Natl Acad Sci USA. 2008;105:18889–18894. doi: 10.1073/pnas.0806310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babel I, et al. Identification of tumor-associated autoantigens for the diagnosis of colorectal cancer in serum using high density protein microarrays. Mol Cell Proteomics. 2009;8:2382–2395. doi: 10.1074/mcp.M800596-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belousov PV, et al. Cancer-associated antigens and antigen arrays in serological diagnostics of malignant tumors. Biochemistry (Mosc) 2008;73:562–572. doi: 10.1134/s000629790805009x. [DOI] [PubMed] [Google Scholar]
- 4.Kijanka G, Murphy D. Protein arrays as tools for serum autoantibody marker discovery in cancer. J Proteomics. 2009;72:936–944. doi: 10.1016/j.jprot.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee M, Wojciechowski J, Tainsky MA. Discovery of antibody biomarkers using protein microarrays of tumor antigens cloned in high throughput. Methods Mol Biol. 2009;520:21–38. doi: 10.1007/978-1-60327-811-9_3. [DOI] [PubMed] [Google Scholar]
- 6.Tan EM, Zhang J. Autoantibodies to tumor-associated antigens: Reporters from the immune system. Immunol Rev. 2008;222:328–340. doi: 10.1111/j.1600-065X.2008.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canzler U, et al. Detection of autoantibodies to tumour-associated antigens in sera of patients with systemic autoimmunity using a novel protein microblot array. Scand J Immunol. 2009;69:563–569. doi: 10.1111/j.1365-3083.2009.02257.x. [DOI] [PubMed] [Google Scholar]
- 8.Hueber W, et al. Antigen microarray profiling of autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2005;52:2645–2655. doi: 10.1002/art.21269. [DOI] [PubMed] [Google Scholar]
- 9.Hudson ME, Pozdnyakova I, Haines K, Mor G, Snyder M. Identification of differentially expressed proteins in ovarian cancer using high-density protein microarrays. Proc Natl Acad Sci USA. 2007;104:17494–17499. doi: 10.1073/pnas.0708572104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madi A, et al. Organization of the autoantibody repertoire in healthy newborns and adults revealed by system level informatics of antigen microarray data. Proc Natl Acad Sci USA. 2009;106:14484–14489. doi: 10.1073/pnas.0901528106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor DD, Gercel-Taylor C, Parker LP. Patient-derived tumor-reactive antibodies as diagnostic markers for ovarian cancer. Gynecol Oncol. 2009;115:112–120. doi: 10.1016/j.ygyno.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Chatterjee M, et al. Diagnostic markers of ovarian cancer by high-throughput antigen cloning and detection on arrays. Cancer Res. 2006;66:1181–1190. doi: 10.1158/0008-5472.CAN-04-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson KS, et al. Application of protein microarrays for multiplexed detection of antibodies to tumor antigens in breast cancer. J Proteome Res. 2008;7:1490–1499. doi: 10.1021/pr700804c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong SH, et al. An autoantibody-mediated immune response to calreticulin isoforms in pancreatic cancer. Cancer Res. 2004;64:5504–5510. doi: 10.1158/0008-5472.CAN-04-0077. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, et al. Autoantibody profiles reveal ubiquilin 1 as a humoral immune response target in lung adenocarcinoma. Cancer Res. 2007;67:3461–3467. doi: 10.1158/0008-5472.CAN-06-4475. [DOI] [PubMed] [Google Scholar]
- 16.Albertus DL, et al. AZGP1 autoantibody predicts survival and histone deacetylase inhibitors increase expression in lung adenocarcinoma. J Thorac Oncol. 2008;3:1236–1244. doi: 10.1097/JTO.0b013e318189f5ec. [DOI] [PubMed] [Google Scholar]
- 17.Li B, et al. Protein microarray for profiling antibody responses to Yersinia pestis live vaccine. Infect Immun. 2005;73:3734–3739. doi: 10.1128/IAI.73.6.3734-3739.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies DH, et al. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics. 2007;7:1678–1686. doi: 10.1002/pmic.200600926. [DOI] [PubMed] [Google Scholar]
- 19.Gnjatic S, Old LJ, Chen YT. Autoantibodies against cancer antigens. In: Tainsky MA, editor. Methods in Molecular Biology. Vol 520. Totowa, NJ: Humana; 2009. pp. 11–19. [DOI] [PubMed] [Google Scholar]
- 20.Gnjatic S, et al. Seromic analysis of antibody responses in non-small cell lung cancer patients and healthy donors using conformational protein arrays. J Immunol Methods. 2009;341:50–58. doi: 10.1016/j.jim.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Michaud GA, et al. Analyzing antibody specificity with whole proteome microarrays. Nat Biotechnol. 2003;21:1509–1512. doi: 10.1038/nbt910. [DOI] [PubMed] [Google Scholar]
- 22.Odunsi K, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003;63:6076–6083. [PubMed] [Google Scholar]
- 23.Yakirevich E, et al. Expression of the MAGE-A4 and NY-ESO-1 cancer-testis antigens in serous ovarian neoplasms. Clin Cancer Res. 2003;9:6453–6460. [PubMed] [Google Scholar]
- 24.Tammela J, et al. OY-TES-1 expression and serum immunoreactivity in epithelial ovarian cancer. Int J Oncol. 2006;29:903–910. [PubMed] [Google Scholar]
- 25.Wadle A, et al. Serological immune response to cancer testis antigens in patients with pancreatic cancer. Int J Cancer. 2006;119:117–125. doi: 10.1002/ijc.21744. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz-Winnenthal FH, et al. Potential target antigens for immunotherapy in human pancreatic cancer. Cancer Lett. 2007;252:290–298. doi: 10.1016/j.canlet.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y-T. Cancer Immun. 2004. Identification of human tumor antigens by serological expression cloning: An online review on SEREX. Available at http://www.cancerimmunity.org/SEREX. [Google Scholar]
- 28.Philip R, et al. Shared immunoproteome for ovarian cancer diagnostics and immunotherapy: Potential theranostic approach to cancer. J Proteome Res. 2007;6:2509–2517. doi: 10.1021/pr0606777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patwa TH, et al. The identification of phosphoglycerate kinase-1 and histone H4 autoantibodies in pancreatic cancer patient serum using a natural protein microarray. Electrophoresis. 2009;30:2215–2226. doi: 10.1002/elps.200800857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehmann M, et al. Identification of potential markers for the detection of pancreatic cancer through comparative serum protein expression profiling. Pancreas. 2007;34:205–214. doi: 10.1097/01.mpa.0000250128.57026.b2. [DOI] [PubMed] [Google Scholar]
- 31.Gnjatic S, et al. NY-ESO-1: Review of an immunogenic tumor antigen. Adv Cancer Res. 2006;95:1–30. doi: 10.1016/S0065-230X(06)95001-5. [DOI] [PubMed] [Google Scholar]
- 32.Reuschenbach M, von Knebel Doeberitz M, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58:1535–1544. doi: 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soussi T. p53 antibodies in the sera of patients with various types of cancer: A review. Cancer Res. 2000;60:1777–1788. [PubMed] [Google Scholar]
- 34.Gaiger A, et al. WT1-specific serum antibodies in patients with leukemia. Clin Cancer Res. 2001;7(Suppl 3):761s–765s. [PubMed] [Google Scholar]
- 35.Bergqvist M, et al. The presence of anti-p53 antibodies in sera prior to thoracic surgery in non small cell lung cancer patients: Its implications on tumor volume, nodal involvement, and survival. Neoplasia. 2003;5:283–287. doi: 10.1016/S1476-5586(03)80021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakagawa K, et al. XAGE-1 expression in non-small cell lung cancer and antibody response in patients. Clin Cancer Res. 2005;11:5496–5503. doi: 10.1158/1078-0432.CCR-05-0216. [DOI] [PubMed] [Google Scholar]
- 37.Mitsudomi T, et al. Clinical implications of p53 autoantibodies in the sera of patients with non-small-cell lung cancer. J Natl Cancer Inst. 1998;90:1563–1568. doi: 10.1093/jnci/90.20.1563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.