Abstract
Compared with notable successes in the genetics of basic sensory transduction, progress on the genetics of higher level perception and cognition has been limited. We propose that investigating specific cognitive abilities with well-defined neural substrates, such as face recognition, may yield additional insights. In a twin study of face recognition, we found that the correlation of scores between monozygotic twins (0.70) was more than double the dizygotic twin correlation (0.29), evidence for a high genetic contribution to face recognition ability. Low correlations between face recognition scores and visual and verbal recognition scores indicate that both face recognition ability itself and its genetic basis are largely attributable to face-specific mechanisms. The present results therefore identify an unusual phenomenon: a highly specific cognitive ability that is highly heritable. Our results establish a clear genetic basis for face recognition, opening this intensively studied and socially advantageous cognitive trait to genetic investigation.
Keywords: behavioral genetic, face recognition, individual differences, specialist gene, generalist gene
General intelligence, or g, has received great attention in both quantitative genetic and molecular genetic studies of cognition (1). Strong heritability is shown by g, and neurobiological correlates of g have been identified (1–4). However, molecular studies searching for genetic correlates of g have only found chromosomal regions with very small effects (5), and studies identifying candidate genes often fail to replicate (6). On the other hand, recent studies investigating reading and spoken language, abilities that rely on more specific cognitive and neural mechanisms, have identified major contributing genes (7, 8). Thus, at least some specific abilities appear relatively tractable to genetic investigation, and it is plausible that abilities involving fewer cognitive and neural mechanisms may generally depend on fewer genes. However, heritabilities of cognitive traits typically decrease as their relation to g decreases, and few highly heritable specific abilities have been identified (1, 9). We present here an exception to this trend by demonstrating both high heritability and face specificity of face recognition ability, whose well-defined neural basis and established animal models provide promising tools for investigating its genetic basis at the neural level (10).
Face recognition is a paradigmatic example of a cognitively and neurally dissociable trait. Psychophysical studies suggest that the cognitive representation of faces relies on different computational processes than other stimuli (11), and neuroimaging has identified occipitotemporal areas in humans and macaques that respond much more strongly to faces than to other stimuli (12). Single-unit recording shows that macaque face patches consist of cells that respond exclusively to faces (13). Consistent with these findings, studies with patients and transcranial magnetic stimulation have demonstrated selective impairments or selective sparing of face recognition (14–16).
The heritability of face recognition ability has not been previously investigated, but several findings suggest that this ability may have a genetic basis. Face recognition deficits with a developmental basis run in families (17, 18), with some familial cases exhibiting normal object recognition (19). In addition, a twin study found that the structure of and functional MRI response within ventral visual cortex, a region containing several face-selective areas, were more similar in monozygotic (MZ) twins than in dizygotic (DZ) twins (10).
We used a classic twin design to determine the relative contributions of genes and environment to face recognition ability. We also used an individual differences-based study to dissociate face recognition ability from other memory abilities.
Results
Our main measure of interest was the Cambridge Face Memory Test (CFMT) (20), a widely used test of face recognition ability requiring study and then recognition of faces in different views and different lighting (Fig. 1A). CFMT isolates face-specific mechanisms by presenting faces without hair or other cues contributing to person recognition (20) (Materials and Methods).
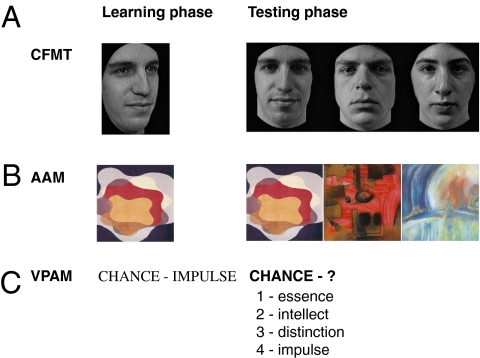
Fig. 1.
Examples of stimuli from the tests used. Each test consisted of a learning phase during which target stimuli were memorized and a testing phase during which participants identified the memorized stimuli among different stimuli. (A) CFMT was our test of primary interest (20). (B) AAM was our visual memory control test. (C) VPAM was our nonvisual memory control test (22).
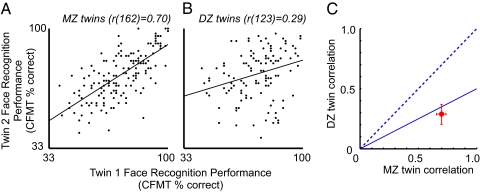
For our twin study, we administered CFMT to 164 MZ and 125 same-gender DZ twin pairs. Because both MZ and DZ twins share family environment but MZ twins share twice as many genes as DZ twins (100% vs. 50%), any greater correlation in MZ than DZ twins supports an effect of genes (1). Fig. 2 shows scatter plots of CFMT scores for MZ and DZ twins, with each dot representing a pair of twins. Because MZ twins share both family environment and all their genes, the MZ correlation represents the sum total of family resemblance for a trait. We found an intraclass correlation between MZ twins of r(162) = 0.70 [Fig. 2A; 95% confidence interval (CI): 0.61–0.77].
Fig. 2.
CFMT twin correlations. The second-born twin’s CFMT score (y axis) plotted against the first-born twin’s score (x axis) for MZ (n = 164) twins (A) and DZ (n = 125) twins (B). The score is the total % correct of 72 items. Chance performance is 24 of 72 items correct (33.3%). MZ intraclass correlation is rMZ(162) = 0.70, and DZ intraclass correlation is rDZ(123) = 0.29. (C) Plot of DZ vs. MZ twin intraclass correlations. Lines represent the extreme cases in which all family resemblance is caused by shared environmental (dotted line, rDZ = rMZ) or additive genetic (solid line, rDZ = 0.5*rMZ) effects, according to the classic twin ACE model (1). Error bars represent 68% (±SE) CIs.
As Fig. 2C shows, the 0.70 MZ correlation we observed was more than double the 0.29 DZ correlation (intraclass r(123) = 0.29, 95% CI: 0.12–0.44; for difference between correlations, P < 0.0001). For the standard “ACE” twin model that includes additive genetic influence (A), shared environmental influence (C), and the combination of measurement error plus nonfamilial environmental influence (E), an MZ correlation at least double the DZ correlation indicates that 100% of familial resemblance (A + C) is genetic (1). As expected, given these MZ and DZ correlations, a maximum likelihood-based fit of the ACE model to our data controlling for age and gender attributed 100% of familial resemblance in CFMT performance to additive genetic effects (95% CI: 87–100%; detailed modeling analyses are described in SI Text S1). These analyses provide evidence that familial resemblance in face recognition ability results primarily from genetic factors.
The difference between measurement reliability and the MZ correlation represents the influence of nonfamilial environment. We calculated several measures of CFMT’s reliability: internal consistency reliabilities (Cronbach’s α, Guttman’s λ2, McDonald’s ωt, and greatest lower bound) (21) ranged from 0.89 to 0.90, test-retest reliability with a mean delay of 6 months was 0.70 (n = 389), and alternate-forms reliability with a mean delay of 2 months was 0.76 (n = 42) (SI Text S2). The 0.20 difference between the MZ correlation and our highest reliability estimate is consistent with a modest but nontrivial nonfamilial environmental contribution to CFMT performance.
To determine whether the observed genetic contribution to CFMT performance is face-specific, we asked whether CFMT performance dissociates from nonface memory ability. We tested large nontwin cohorts (n > 1,500) and a subset of our twins (n = 120) on CFMT and two control nonface memory tests: a verbal paired-associates memory test (VPAM) (22) (Fig. 1C) and a newly developed abstract art memory test (AAM) (Fig. 1B). VPAM served as a nonvisual control recognition test to CFMT. VPAM tests memory for linguistic information (word pairs), minimizing the contribution of nonlinguistic visual processes by using abstract words that are difficult to translate into visual images (Fig. 1C). AAM served as a nonface control visual recognition test to CFMT. Like CFMT, AAM uses hard-to-verbalize stimuli to minimize the contribution of linguistic processes to visual recognition (Fig. 1B).
Indeed, although VPAM and AAM both showed high internal consistency in our large nontwin sample (Cronbach’s α = 0.80 and 0.79, respectively; Table 1), their respective correlations with CFMT were only 0.17 and 0.26 (n = 1,532 and n = 3,004, respectively); we found similar correlations of 0.15 and 0.31 in our twin participants (n = 120; Table 1). These low correlations are similar to those found on the Wechsler Memory Scale III between face memory and both verbal paired associates (r = 0.18–0.22) and nonface visual memory (r = 0.28–0.30) (23).
Table 1.
Performance on CFMT, AAM, and VPAM
| Test (population, testing method) | N (female) | Age (SD or range) | Mean % correct (SD) | Cronbach’s α |
| CFMT (twin, web) | 578 (540) | 37 (11) | 74.2 (13.7) | 0.89 |
| CFMT (nontwin, web) | 3,004 (1,932) | 28 (12) | 75.6 (13.4) | 0.90 |
| CFMT (nontwin, laboratory) (33) | 153 (80) | 24 (5) | 72.2 (12.8) | 0.86* |
| CFMT (nontwin, laboratory) (30) | 124 (73) | 23 (18–32) | 76.9 (11.8) | 0.88 |
| CFMT (nontwin, laboratory) (20) | 50 (29) | 20 (2) | 80.4 (11.0) | 0.86 |
| AAM (twin, web) | 120 (89) | 38 (11) | 66.0 (12.3) | 0.77 |
| AAM (nontwin, web) | 3,004 (1932) | 28 (12) | 66.2 (12.6) | 0.79 |
| VPAM (twin, web) | 120 (89) | 38 (11) | 41.5 (17.5) | 0.72 |
| VPAM (nontwin, web) | 1,532 (986) | 27 (11) | 50.2 (20.4) | 0.80 |
α estimated by applying Spearman–Brown correction to α’s within CFMT blocks 2 and 3 (33).
The low correlations between VPAM and CFMT performance in both twins and nontwins demonstrate that the heritability we observed for CFMT was not the result of factors such as motivation, attention, computer literacy, g, or general memory. Because AAM is very similar to CFMT but uses abstract art rather than faces, the modest correlations between CFMT and AAM indicate that general visual processes make only limited contributions to CFMT performance. These low correlations suggest that both face recognition ability itself and its genetic basis depend primarily on face-specific mechanisms (SI Text S3).
Discussion
Our twin findings indicate that genetic differences can account for most of the stable variation in face recognition ability in healthy adults. Our investigation of CFMT’s face specificity demonstrates that face recognition ability overlaps little with other visual and memory abilities, suggesting that both face recognition ability itself and its genetic basis are largely face-specific. The present results therefore identify a rare phenomenon: a highly specific cognitive ability that is highly heritable (1, 9).
Recent documentation of families with multiple prosopagnosic members demonstrated that specific face recognition deficits can run in families (20, 22). However, such familial clustering could result from either familial environment or familial genes. The current results demonstrate that familial clustering in face recognition ability has a strong genetic basis. The high heritability of face recognition indicates that linkage and positional cloning studies investigating the genetic basis of face recognition may be successful. Such studies can complement association studies in families with multiple prosopagnosics.
Although indicating that familial resemblance in face recognition ability results primarily from genetic factors, our data are also consistent with a modest nonfamilial environmental contribution to CFMT performance. Indeed, prior literature demonstrates that face processing is sensitive to environmental input. Face perception shows adaptation effects (24), perceptual narrowing occurs with development (25, 26), and recognition of faces from infrequently observed races is notoriously poor (27, 28). Extreme environmental variables, such as visual deprivation in the months after birth, also have an impact on face processing (29).
More generally, the discovery that face recognition is highly heritable indicates that cognitive neuroscience can usefully guide behavioral genetic investigations. Dividing the brain into its component systems is a fundamental task in cognitive neuroscience, and investigation of these systems may be an effective means to link genes and behavior.
Materials and Methods
Our main measure of interest, CFMT, was designed to assess face-specific mechanisms (20). Evidence that CFMT taps face-specific mechanisms comes from three sources. First, CFMT performance in normal controls decreases dramatically (from 80.4% correct to 58.4% correct) when the faces are inverted (20). Second, CFMT performance correlates robustly with tests of both face perception [Cambridge Face Perception Test: r(87) = 0.60, P < 0.0001] (30) and long-term face memory [Before They Were Famous Test: r(27) = 0.70, P < 0.001 (31); Famous Faces Test: r(778) = 0.51, P < 0.0001; SI Text S4]. Third, CFMT powerfully discriminates individuals with specific face processing deficits (prosopagnosia) from normal controls (20), and prosopagnosics with normal object recognition have shown severe impairments on CFMT (14, 19).
For our twin study, we recruited 289 twin pairs aged 18–57 years: 164 MZ twins (mean age = 37.1, 122 female) and 125 same-gender DZ twins (mean age = 37.2, 91 female). Of the 289 twin pairs, 238 pairs (119 MZ, 82 female; 122 DZ, 88 female) participated following direct mailings from the Australian Twin Registry; the rest responded to online advertisements or constituted normal traffic to Test My Brain, our web-based testing environment. Each twin completed CFMT. Because data from registry-recruited and web-recruited twins did not differ significantly in any respect, data were combined. We classified twins as MZ or DZ via latent class analysis with a standard survey instrument, a method that correctly classified 99.6% of individual twins (241 of 242) in a validation study when compared with direct genetic testing (32). Because the single reported misclassification using this method happened for only 1 of the 2 twins in a pair (32), we dropped from our analysis twin pairs in which one twin was classified as MZ and the other DZ (n = 8 pairs). No combination of MZ and DZ classification of these dropped pairs changed our estimated genetic contribution to face recognition by more than a fraction of 1%.
For our study of the face specificity of CFMT, we recruited a large nontwin cohort (n = 3,004) from normal traffic to Test My Brain. All 3,004 nontwin participants completed CFMT and AAM, and 1,532 of these participants completed VPAM. CFMT mean performance and internal consistency for our web-recruited participants were similar to those of laboratory-tested participants from prior studies (Table 1), evidence that our web-recruited participants produced data of a quality comparable to that produced by laboratory testing. A subset of our twins (n = 120) also completed AAM and VPAM as well as CFMT.
All tests were administered via the web. Each test consisted of a learning phase and a testing phase (Fig. 1). For CFMT (Fig. 1A), participants memorized target faces and were then tested for recognition of these faces, choosing on each trial which one of three faces they saw previously. CFMT consists of three blocks of increasing difficulty: The first block tests recognition of images identical to those in the study phase, the second block uses different images, and the third block uses different images with added visual noise. For VPAM (Fig. 1C), participants memorized 25 target word pairs and were then tested for recognition of these word pairs, choosing on each trial which one of four words was paired with a given word. For AAM (Fig. 1B), participants memorized 50 target abstract art images and were then tested for recognition of these images, choosing on each trial which one of three images they saw previously. This research was approved by the Harvard University and Macquarie University Institutional Review Boards and conformed to the Declaration of Helsinki.
Supplementary Material
Acknowledgments
This research was facilitated through the Australian Twin Registry, which is supported by an Enabling Grant from the National Health and Medical Research Council administered by the University of Melbourne. Margaret Gerbasi provided research assistance. Funding for this project was provided by the Economic and Social Research Council (to B.D.), National Eye Institute Grant EY-013602 (to K.N.), National Institutes of Health Fellowship F32 EY16933-02 (to J.W.), a National Science Foundation grant (to J. Richard Hackman and Stephen M. Kosslyn) that supported C.F.C.’s contributions, and a Director of Central Intelligence Postdoctoral Fellowship (to C.F.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913053107/DCSupplemental.
See Commentary on page 4795.
References
- 1.Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics. 5th Ed. New York: Worth; 2008. [Google Scholar]
- 2.Lee KH, et al. Neural correlates of superior intelligence: Superior recruitment of posterior parietal cortex. NeuroImage. 2006;29:578–586. doi: 10.1016/j.neuroimage.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 3.Luders E, Narr KL, Thompson PM, Toga AW. Neuroanatomical correlates of intelligence. Intelligence. 2008;37:156–163. doi: 10.1016/j.intell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDaniel MA. A meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 2005;33:337–346. [Google Scholar]
- 5.Butcher LM, Davis OS, Craig IW, Plomin R. Genome-wide quantitative trait locus association scan of general cognitive ability using pooled DNA and 500K single nucleotide polymorphism microarrays. Genes Brain Behav. 2008;7:435–446. doi: 10.1111/j.1601-183X.2007.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deary IJ, Johnson W, Houlihan LM. Genetic foundations of human intelligence. Hum Genet. 2009;126:215–232. doi: 10.1007/s00439-009-0655-4. [DOI] [PubMed] [Google Scholar]
- 7.Fisher SE, Scharff C. FOXP2 as a molecular window into speech and language. Trends Genet. 2009;25:166–177. doi: 10.1016/j.tig.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Galaburda AM, LoTurco J, Ramus F, Fitch RH, Rosen GD. From genes to behavior in developmental dyslexia. Trends Genet. 2006;9:1213–1217. doi: 10.1038/nn1772. [DOI] [PubMed] [Google Scholar]
- 9.Stromswold K. The heritability of language: A review and metaanalysis of twin, adoption, and linkage studies. Language. 2001;77:647–723. [Google Scholar]
- 10.Polk TA, Park J, Smith MR, Park DC. Nature versus nurture in ventral visual cortex: A functional magnetic resonance imaging study of twins. J Neurosci. 2007;27:13921–13925. doi: 10.1523/JNEUROSCI.4001-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKone E, Kanwisher NK, Duchaine BC. Can generic expertise explain special processing for faces? Trends Cogn Sci. 2007;11:8–15. doi: 10.1016/j.tics.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. Proc Natl Acad Sci USA. 2008;105:19514–19519. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duchaine B, Yovel G, Butterworth E, Nakayama K. Prosopagnosia as an impairment to face-specific mechanisms: Elimination of the alternative hypotheses in a developmental case. Cognit Neuropsychol. 2006;23:714–747. doi: 10.1080/02643290500441296. [DOI] [PubMed] [Google Scholar]
- 15.Moscovitch M, Winocur G, Behrmann M. What is special about face recognition? Nineteen experiments on a person with visual object agnosia and dyslexia but normal face recognition. J Cognit Neurosci. 1997;9:555–604. doi: 10.1162/jocn.1997.9.5.555. [DOI] [PubMed] [Google Scholar]
- 16.Pitcher D, Charles L, Devlin J, Walsh V, Duchaine B. Triple dissociation of faces, bodies and objects in extrastriate cortex. Curr Biol. 2009;19:319–324. doi: 10.1016/j.cub.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Duchaine B, Germine L, Nakayama K. Family resemblance: Ten family members with prosopagnosia and within-class object agnosia. Cognit Neuropsychol. 2007;24:419–430. doi: 10.1080/02643290701380491. [DOI] [PubMed] [Google Scholar]
- 18.Schmalzl L, Palermo R, Coltheart M. Cognitive heterogeneity in genetically based prosopagnosia: A family study. J Neuropsychol. 2008;2:99–117. doi: 10.1348/174866407x256554. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y, Duchaine B, Wilson H, Nakayama K. Three cases of developmental prosopagnosia from one family: Detailed neuropsychological and psychophysical investigation of face processing. Cortex. 2009 doi: 10.1016/j.cortex.2009.07.012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Duchaine B, Nakayama K. The Cambridge Face Memory Test: Results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic subjects. Neuropsychologia. 2006;44:576–585. doi: 10.1016/j.neuropsychologia.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Sijtsma K. Reliability beyond theory and into practice. Psychometrika. 2009;74:169–173. doi: 10.1007/s11336-008-9103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woolley AW, Gerbasi ME, Chabris CF, Kosslyn SM, Hackman JR. Bringing in the experts: How team ability composition and collaborative planning jointly shape analytic effectiveness. Small Group Research. 2008;39:352–371. [Google Scholar]
- 23.Wechsler D. Wechsler Memory Scale. 3rd Ed. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 24.Webster MA, Kaping D, Mizokami Y, Duhamel P. Adaptation to natural facial categories. Nature. 2004;428:558–561. doi: 10.1038/nature02420. [DOI] [PubMed] [Google Scholar]
- 25.Kelly DJ, et al. Development of the other-race effect during infancy: Evidence toward universality? J Exp Child Psychol. 2009;104:105–114. doi: 10.1016/j.jecp.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascalis O, et al. Plasticity of face processing in infancy. Proc Natl Acad Sci USA. 2005;102:5297–5300. doi: 10.1073/pnas.0406627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levin DT. Race as a visual feature: Using visual search and perceptual discrimination tasks to understand face categories and the cross-race recognition deficit. J Exp Psychol Gen. 2000;129:559–574. doi: 10.1037//0096-3445.129.4.559. [DOI] [PubMed] [Google Scholar]
- 28.Rhodes G, Hayward WG, Winkler C. Expert face coding: Configural and component coding of own-race and other-race faces. Psychon Bull Rev. 2006;13:499–505. doi: 10.3758/bf03193876. [DOI] [PubMed] [Google Scholar]
- 29.Le Grand R, Mondloch C, Maurer D, Brent HP. Early visual experience and face processing. Nature. 2001;410:890. doi: 10.1038/35073749. [DOI] [PubMed] [Google Scholar]
- 30.Bowles DC, et al. Diagnosing prosopagnosia: Effects of aging, sex, and participant-stimulus ethnic match on the Cambridge Face Memory Test and Cambridge Face Perception Test. Cognit Neuropsychol. 2009 doi: 10.1080/02643290903343149. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Russell R, Duchaine B, Nakayama K. Super-recognizers: People with extraordinary face recognition ability. Psychon Bull Rev. 2009;16:252–257. doi: 10.3758/PBR.16.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heath AC, et al. Zygosity diagnosis in the absence of genotypic data: An approach using latent class analysis. Twin Res. 2003;6:22–26. doi: 10.1375/136905203762687861. [DOI] [PubMed] [Google Scholar]
- 33.Herzmann G, Danthiir V, Schacht A, Sommer W, Wilhelm O. Toward a comprehensive test battery for face cognition: Assessment of the tasks. Behav Res Methods. 2008;40:840–857. doi: 10.3758/brm.40.3.840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.