Abstract
Adaptive shifts associated with human origins are brought to light as we examine the human fossil record and study our own genome and that of our closest ape relatives. However, the more ancient roots of many human characteristics are revealed through the study of a broader array of living anthropoids and the increasingly dense fossil record of the earliest anthropoid radiations. Genomic data and fossils of early primates in Asia and Africa clarify relationships among the major clades of primates. Progress in comparative anatomy, genomics, and molecular biology point to key changes in sensory ecology and brain organization that ultimately set the stage for the emergence of the human lineage.
Keywords: primate, Haplorhini, Strepsirrhini, human evolution, phylogeny
Human evolution did not begin 6–8 million years ago with the phylogenetic split between the chimpanzee and human lineages. Although the ape–human transition is of tremendous interest to specialists and the general public, many key genetic events and adaptive shifts leading to the origin of humans had already been set in motion long before this split. The seeds of these changes can be identified in the earlier branches of the primate family tree, many millions of years in the past. Changes in sensory ecology, increases in brain size, and reorganization of the brain's components set the stage for the development of language and for many of our other exceptional cognitive abilities. Recent well-publicized descriptions of fossil primates from Africa and Eurasia have reignited interest in the origins of Anthropoidea—monkeys, apes, and humans. Fossil discoveries are expanding our understanding of the diversity and biogeography of this clade. Developments in molecular phylogenetics are establishing a phylogenetic framework of living primates against which hypotheses about relationships of fossils can be tested. Advances in genomics and the study of living primate ecology are enabling us to understand key adaptive changes in anthropoid evolution and have reframed many of our adaptive questions about human origins in the context of deep time. Here we review recent fossil discoveries, developments in the study of the sensory ecology and neurobiology of anthropoids, and implications from genetic research that bear on the study of anthropoid origins.
Phylogenetics of Crown Primates
In the past, separate origins were considered for the two living clades of anthropoid primates: the New World platyrrhines and the Old World catarrhines. Today, Anthropoidea is accepted universally as a monophyletic group. The monophyly of the strepsirrhine primates (lemurs and lorises) is also well supported by morphological and molecular evidence. However, the question of whether tarsiers are more closely related to anthropoids or strepsirrhines has been less clear. Some classifications united tarsiers with strepsirrhines in the suborder Prosimii because they retain primitive features such as grooming claws and an unfused mandibular symphysis. An alternative phylogenetic classification linked tarsiers with anthropoids in the semiorder Haplorhini. Evidence for a haplorhine clade comes from an increasingly long list of derived hard and soft tissue morphological features and from molecular features. Molecular phylo-genetic studies using DNA sequence data generally failed to provide strong support for either Haplorhini or Prosimii (1). However, genetic markers called short interspersed elements (SINEs) offer strong evidence in support of both haplorhine and strepsirrhine monophyly. SINEs are short segments of DNA that insert into the genome at apparently random positions and are excellent phylogenetic markers with an extraor-dinarily low probability of convergent evolution (2). Because there are billions of potential insertion sites in any primate genome, the probability of a SINE inserting precisely in the same locus in two separate evolutionary lineages is “exceedingly minute, and for all practical purposes, can be ignored” (p. 151, ref. 3). Five SINEs support haplorhine mono-phyly to the exclusion of strepsirrhines and eight are shared among strepsirrhines (4). Thus, the haplorhine–strepsirrhine split may now be regarded as fact and must underlie any phylogenetic reconstruction of fossil primates.
Identification of which taxa of early primates are haplorhines and which are strepsirrhines is an ongoing challenge because homoplasy is common, the evolutionary acquisition of diagnostic haplorhine and anthropoid characters occurred in mosaic fashion, and the fossil record remains sparse and incomplete. For decades, anthropoid ancestors were sought in two taxonomically diverse radiations of primarily Eocene taxa: Omomyiformes and Adapiformes (sometimes identified at the family or superfamily level). Both groups are common in North American and European Paleogene faunas and also occur in Africa and Asia (5). Omomyiforms are considered haplorhines, and adapiforms strepsirrhines by most workers (6–8, but see ref. 9), although few characters unambiguously support omomyiforms as haplorhine. The German adapiform Darwinius was described as having features allying it, and by extension, other adapiforms with Haplorhini (10). However, Darwinius and other adapiforms share derived features with extant strepsirrhines and lack derived features shared by extant tarsiers and anthropoids (6, 11–15).
Several hypotheses about tarsier and anthropoid origins have been proposed. Some researchers argue that anthropoids and tarsiers share a common ancestor within a paraphyletic Omomyiformes whereas others assert that only tarsiers have omomyiform ancestry. An alternative hypothesis that we favor is that the tarsier-anthropoid clade is the sister group of omomyiforms as a whole. This last option is supported by a comprehensive phylo-genetic analysis (11). The hypothetical common ancestor of tarsiers, anthro-poids, and omomyiforms has yet to be identified, but must predate the oldest omomyiform, Teilhardina, from the earliest Eocene of North America, Europe, and Asia (16).
What Is an Anthropoid?
By definition, crown Anthropoidea includes all species, living and fossil, descended from the last common ancestor of extant anthropoids. Stem Anthropoidea includes all fossil taxa that are more closely related to crown anthropoids than they are to tarsiers, but are outside the anthropoid crown group. Crown anthropoids share a number of important features. On the basis of evidence from comprehensive phylogenetic analyses (11, 13, 17, 18), many of these features are considered synapomorphies. Four peculiarities of embryonic implantation have been described (19). Extant anthropoids share 27 Alu-SINE markers (4). Most anthropoids have orbits that are relatively small, forward facing, and convergent (20). A bony lamina posterior to the orbit completely separates the eyes from the chewing muscles in the temporal fossa (21). A number of other soft-tissue features of the visual system (e.g., an all-cone retinal fovea) are anthropoid synapomorphies (22). Anthropoid features of the auditory region include a distinctive configuration of the internal carotid arterial system that supplies the orbit and much of the cerebrum (23). The middle ear cavity of the temporal bone extends forward into an air-filled accessory chamber containing a network of bony trabeculae (24). The tympanic bone that supports the eardrum is fused to the bony sidewall of the middle ear. Early fusion occurs in both the frontal metopic suture and the mandibular symphysis (25). The body of the mandible is relatively deep (26). Other anthropoid dental features include small, vertically implanted and spatulate lower incisors, simplified molar trigonids, and lower third molars with short heels (8).
The bony anatomy of the crown anthropoid foot is distinctive: the facet between the talus and fibula is steep-sided, and the groove for the tendon of the flexor fibularis muscle is in a midtrochlear position (6). The calcaneus is wide with a shortened heel and a distinctive calcaneocuboid joint shape (27). The peroneal tubercle on the first metatarsal that receives the tendon of the peroneus longus muscle is reduced in size (28).
We do not know the order in which most of these crown anthropoid features evolved. Most features probably appeared in a mosaic fashion in stem anthropoids. Some features may be primitive for Anthropoidea or Primates as a whole and others may have evolved in parallel in multiple crown anthropoid clades. We do know that most of the hard tissue features mentioned above are evident in Afro-Arabian fossils of late Eocene age, although several taxa lack some derived features found in living anthropoids, as discussed below. We do not know the character states for many of the presumed Eocene anthropoids from Asia because they are not yet known from adequate cranial materials. Postcranial materials of putative Asian stem anthropoids have been found in isolation, complicating their specific attribution.
Anthropoid Fossils and Revised Dates
Afro-Arabian Anthropoids.
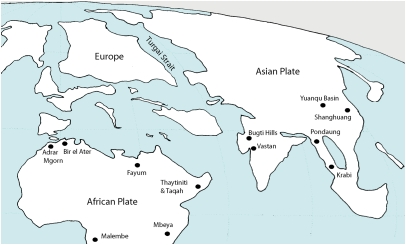
In the past decade, finds of fossil primates have augmented our picture of early anthropoids and broadened the biogeographic focus of studies of anthropoid origins (Fig. 1 and Fig. 2). Early anthropoids are best known from the phylogenetically diverse faunas of Afro-Arabia, especially those of the Fayum Depression in Egypt. The youngest of the Fayum primate-bearing deposits date to the Oligocene, ∼30 Ma, whereas the oldest are late Eocene, ∼37 Ma (29). There are 15 known Fayum anthropoid genera from four clades: parapithecoids, proteopithecids, oligopithecids, and propliopithecids.
Fig. 1.
Map showing localities containing Paleogene anthropoids. Coastlines and continental positions represent roughly the situation in the early Eocene to early Oligocene. Modified from refs. 8 and 119 with a more northerly position of India.
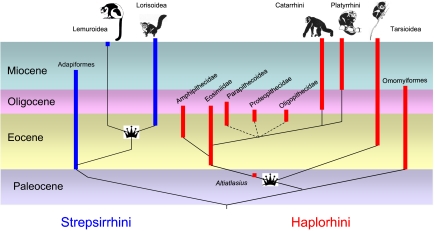
Fig. 2.
A dendrogram of primate phylogeny with Eocene families represented based on cladograms of refs. 11, 13, and 18. Paleocene through Miocene is proportionally scaled; post Miocene time is not to scale.
Parapithecoids (e.g., Simonsius, Apidium, Parapithecus, and Biretia) are thought by some researchers to be stem catarrhines (30), but cranial and postcranial evidence suggests that they are stem anthropoids (13). Proteopithecids (Proteopithecus, and possibly Serapia) have been linked with the oligopithecids Catopithecus and Oligopithecus (8), but postcranial evidence (31–33) does not support this link. Proteopithecids also exhibit some resemblance to platyrrhines (34), but a close phylogenetic relationship between these two groups is supported neither by character distribution (35) nor phylogenetic analyses (36). The most that can be said is that proteopithecids are stem anthropoids sufficiently primitive to have given rise to catarrhines, platyrrhines, both, or neither (13).
Propliopithecids (Aegyptopithecus and Propliopithecus) are widely accepted as the earliest definitive representatives of the catarrhine primates because of their dental formula (two premolars) and details of molar anatomy greatly resembling early Miocene catarrhines from east Africa (37). Oligopithecids may be catarrhines. They retain some primitive characteristics (small brain and unfused mandibular symphysis), yet the dental formula, large ascending process of the premaxilla, and several features of the talus, humerus, and femur all support catarrhine affinities (33).
Other poorly known African Paleogene primates may be anthropoids. Altiatlasius (late Paleocene, Morocco) was described as an omomyiform (38) but may be a stem anthropoid (11, 38, 39) or a stem euprimate (40). Afrotarsius, from the Egyptian Oligocene, resembles stem anthropoids (41), but also tarsiids (13, 42). Algeripithecus (early to middle Eocene, Algeria) was commonly recognized as an anthropoid (39), but recent discoveries of more complete specimens suggest that it is not anthropoid but instead may belong to the Azibiidae (43). Azibiids are argued to be adapiforms, early euprimates, plesiadapiforms (possible stem primates), or even nonprimates (14, 44, 45), and there is no consensus on their phylogenetic position. However, the oblique orientation of the lacrimal canal relative to the infraorbital canal indicates that they are not crown haplorhines (Tarsius and anthropoids have more vertically oriented lacrimal canals) (43). Specimens attributed to another supposed anthropoid from Algeria, Tabelia, are probably specimens of Algeripithecus or Azibius (43). This leaves Altiatlasius as the only potentially anthropoid African taxon from earlier than ∼37 Ma.
Stem Anthropoids of Asia.
Eosimias was described in 1994 on the basis of fragmentary dental remains from the middle Eocene of China (46) and was placed in a previously undescribed anthropoid family, Eosimiidae. To date, as many as 11 species in six genera are attributed to the Eosimiidae. Eosimias and Phenacopithecus are known from the middle Eocene of China (e.g., 46, 47). Outside of China, Bahinia and another eosimiid have been recovered from the late middle Eocene of Burma (48, 49), and Phileosimias is known from the Oligocene of Pakistan (50).
The fossil record for eosimiids was extended to the Indian subcontinent by the discovery of fragmentary dental remains of Anthrasimias (11) from the early Eocene of India. Some researchers have argued that Anthrasimias is an adapiform (12, 51), although a calcaneus from the same locality is eosimiid-like in its proportions and morphology (12).
Southeast Asian Amphipithecidae were first described from fragments of upper and lower jaws (Amphipithecus, Pondaungia) from the late middle Eocene of Burma. In the past decade, much amphipithecid material has been recovered from Burma (Myanmarpithecus, Ganlea) (52, 53), the late Eocene of Thailand (Siamopithecus) (54), and the early Oligocene of Pakistan (Bugtipithecus) (50).
Relationships of North African Anthropoids, Eosimiids, and Amphipithecids.
The anthropoid status of the late Eocene to early Oligocene Afro-Arabian taxa is undisputed because they are known from crania that possess the above-mentioned derived anthropoid features of the orbit and ear region, mandible, and teeth. Some taxa (Catopithecus and Proteopithecus) lack a few features found in extant anthropoids (e.g., fused mandibular symphysis), but have the other crown anthropoid hallmarks (23, 24, 55).
The phylogenetic position of Asian eosimiids is more controversial. Comprehensive phylogenetic analyses place eosimiids within the anthropoid radiation (11–13), and the majority of specialists agree that eosimiids are stem anthropoids. Nevertheless, some researchers continue to question the anthropoid status of some or all eosimiids (42, 51, 56, 57). Although eosimiids retain many primitive dental features (e.g., paraconid on lower molars, an unfused mandibular symphysis), they also demonstrate key anthropoid synapomorphies, including tarsal features (midtrochlear position of the flexor fibularis, a wide calcaneus with a shortened heel, and a reduced peroneal tubercle of the first metatarsal), a deep mandible, various dental features (absence of a Nannopithex-fold on the upper molars, transverse lower molar protocristid, and the lower third molar with a weak heel), and a deep suborbital part of the maxilla (27, 47, 49, 58). To date, however, no eosimiid crania preserve the diagnostically haplorhine or anthropoid anatomy of the orbit or ear region. Cranial resemblances between the eosimiid Bahinia and adapiforms have been proposed (56), but the specimen in question is too crushed and distorted to make such claims reliable. The mounting evidence is persuasive in favor of a stem anthropoid position for eosimiids. More complete cranial material may resolve this issue.
The anthropoid status of amphipithecids also is controversial. Their status as anthropoids has long been disputed because they resemble various omomyiforms, catarrhines, and (especially) adapiforms (30, 59). Difficulty in attributing isolated postcranial elements (some of which show adapiform features) to the correct taxon has been particularly vexing. Cranial fragments from Burma that were attributed to Amphipithecus (60) and indicated the lack of postorbital closure may not belong to that taxon (61). However, a newly reconstructed face of Siamopithecus displays the highly convergent and frontated orbits and relatively short face typical of anthropoids (62). Tarsal elements thought to belong to Amphipithecus also show anthropoid features (63), and most phylogenetic analyses link amphipithecids with anthropoids (11, 13, 18).
Adaptations in Anthropoidea
Body Mass.
With the exception of the Neotropical marmosets, tamarins, and squirrel monkeys, most extant anthropoids weigh more than 1 kg. By contrast, early and middle Eocene anthropoids were all small-bodied, and no major size increases in anthropoids are detected until the late Eocene or early Oligocene (Table 1). All known eosimiid primates weighed less than a few hundred grams. Some eosimiids were extremely small (11, 47, 64)—the size of the dwarf lemur—or even smaller if some isolated middle Eocene foot bones from anthropoids are considered (65). Amphipithecids ranged from a few hundred grams up to 6 kg or more. Body mass estimates for Fayum anthropoids ranged from a few hundred grams for smaller parapithecoids up to 5–6 kg for propliopithecids (66, 67).
Table 1.
Glossary of time periods and adaptive profiles of primates discussed in text
| Adapiformes | Clade of North American, Eurasian, and African Eocene to Miocene stem strepsirrhines: >90 species. Body mass: 100–7,000 g; most >1 kg. Mostly diurnal. Diet: leaves and fruit, a few specialized in insects. Active arboreal quadrupeds, some deliberate slow-climbing quadrupeds. |
| Amphipithecidae | Clade of Asian Eocene to Oligocene stem anthropoids: six species. Body mass: 130–5,900 g. Siamopithecus diurnal; activity pattern for other taxa unknown. Diet: fruit, nuts, seeds. Slow or active arboreal quadrupeds.* |
| Catarrhini | Clade of Old World monkeys and apes and all extinct species more closely related to this clade than to living New World monkeys (Platyrrhini), their sister group. |
| Eocene Epoch | ∼56 and ∼34 Ma. Early Eocene, ∼56 to ∼49 Ma; middle Eocene, ∼49 to ∼37 Ma; late: ∼37 to ∼34 Ma. |
| Eosimiidae | Clade of Asian and African Eocene to Oligocene stem anthropoids: 11 species.† Body mass: 30–280 g. Bahinia and Phenacopithecus diurnal; activity pattern for other taxa unknown. Diet: fruit and insects. Active arboreal quadrupeds.* |
| Haplorhini | Clade of tarsiers and anthropoids and all extinct species more closely related to this clade than to Strepsirrhini, their sister group. |
| Miocene Epoch | ∼23 to ∼5 Ma |
| Oligocene Epoch | ∼34 to ∼23 Ma |
| Oligopithecidae | Clade of African late Eocene to Oligocene stem anthropoids or stem catarrhines: three species. Body mass: 770-1,200 g. Catopithecus diurnal; activity pattern of Oligopithecus unknown. Diet: leaves, fruit. Catopithecus was a slow-climbing arboreal quadruped. |
| Omomyiformes | Clade of North American, Eurasian, and African Eocene stem haplorhines: >95 species. Body mass: 35–1775 g; most <400 g. Most nocturnal. Diet: fruit and insects. Arboreal quadrupeds, some with a significant leaping component. |
| Paleocene Epoch | ∼66 to ∼56 Ma |
| Paleogene Period | Paleocene–Oligocene Epochs. |
| Parapithecoidea | Clade of African Eocene to Oligocene stem anthropoids (or stem catarrhines): 12 species. Body mass: 140–1,800 g. Most diurnal, but earliest (Biretia) nocturnal. Diet: fruit; Simonsius grangeri more folivorous. Active arboreal quadrupeds. |
| Platyrrhini | Clade of Neotropical monkeys and all extinct species more closely related to this clade than they are to living Catarrhini, their sister group. |
| Prosimii | Paraphyletic group that includes tarsiers and strepsirrhines. |
| Propliopithecidae | Clade of African Oligocene stem catarrhines: five species. Body mass: 2-4 kg,. Diurnal. Diet: fruit. Arboreal quadrupeds. |
| Proteopithecidae | Extinct clade of African late Eocene stem anthropoids: of two species. Body mass: 500-720 g. Diurnal. Diet: fruit, insects. Proteopithecus engaged in agile above-branch quadrupedalism and pronograde leaping. |
| Strepsirrhini | Clade of lemurs of Madagascar (Lemuroidea) and lorises of Africa and Asia (Lorisoidea) and all extinct species (Adapiformes) more closely related to this clade than they are to Haplorhini, their sister group. |
| Tarsier | A small, nocturnal, and insectivorous primate from Asia. The sister group of anthropoids. |
*Isolated postcrania make attribution of locomotor patterns uncertain.
†Includes Altiatlasius and Anthrasimias, whose status as eosimiids is controversial.
The small size of the earliest anthropoids has important implications for reconstructing the feeding ecology of the earliest anthropoids. Primates over ∼500 g are able to use leaves to fulfill their protein requirements, whereas those less than ∼500 g rely on insects. Leaf consumption and insect predation require very different digestive and sensory specializations, some of which are described below.
Diet.
The dietary adaptations of fossil anthropoids have been reconstructed using dental and mandibular structure, microwear analysis, and estimations of body mass (11, 25, 53, 67–69). Asian eosimiids and most of the late Eocene and Oligocene anthropoids of Egypt show dental adaptations for eating primarily fruits. The majority of the smaller-bodied taxa would have relied on insects for their source of protein, as do extant small-bodied primates (70), but were not primarily insectivorous. The larger-bodied taxa would have used leaves as their protein source, although they were not committed folivores. A few Afro-Arabian taxa might have been more predominantly folivorous. The amphipithecids appear to have had more divergent diets. Although fruit was a major food source, the larger taxa show massive jaws, deep faces, large canines, and blunt cheek teeth with heavy apical wear indicating that they ingested hard objects such as nuts and seeds.
The dietary patterns of the earliest anthropoids broadly overlapped those of sympatric omomyiform and adapiform taxa (11, 67), suggesting that gross dietary changes were not important selective factors in the origin of anthropoids. The fossil record suggests that relatively late-occurring increases in body size led to increased reliance on foods that are tough and/or hard or brittle (unripe fruits, nuts, leaves), which in turn probably selected for stronger and more stable jaws (25).
Locomotion.
Several limb bones from the Chinese middle Eocene have been attributed to eosimiids (27, 71). In the eosimiid foot, the calcaneus is short and broad, but the midcalcaneal portion is relatively long. This calcaneal morphology is similar to that of the platyrrhine Saimiri, the squirrel monkey, and implies agile, above-branch quadrupedalism and pronograde leaping. The shorter, broader calcanei of eosimiids differ from those of omomyiforms, which generally are moderately elongated like those of mouse lemurs (suggesting more leaping in omomyiforms). Likewise, the anterior bowing and prominent third trochanter of the femur and the morphology of the os coxa attributed to eosimiids are similar to late Eocene African anthropoids that are most often compared to living squirrel monkeys (31, 33). Allocation of postcrania to the Amphipithecidae has been contested. A humerus attributed to Amphipithecus suggests that it was a slow-moving arboreal quadruped (69), but a talus allocated to the same taxon indicates more active arboreal quadrupedal habits (63). Late Eocene parapithecoids and Proteopithecus often are compared to squirrel monkeys. Catopithecus differs from stem anthropoids in features of the talus, suggesting that it was a slower, more deliberate climber that may have used hind-limb suspensory postures (33).
Eye.
Most anthropoids differ from other primates and nonprimate mammals in having small corneas relative to eye size (72, 73). Reduction of cornea size in anthropoids reflects a reconfiguration of the eye's focusing apparatus (including the lens and cornea) that results in a greater focal length and an increased size of the retinal image. This eye morphology is one of several derived features supporting very high visual acuity in anthropoids (74). Furthermore, because cornea size places an upper limit on the amount of light that may be admitted to the eye, the derived eye morphologies shared by most living anthropoids almost certainly evolved in a common ancestor that was diurnal.
Consistent with their nocturnal habits, tarsiers and owl monkeys exhibit large corneas relative to eye size and thus lack the characteristic eye morphology of diurnal anthropoids (75). However, tarsiers exhibit highly unusual features for nocturnally adapted species (76). Like anthropoids, but unlike all other mammals, tarsiers possess a retinal fovea (a pit in the central retina) and a macula lutea (produced by a high concentration of carotenoid pigments around the fovea). Both features are best interpreted as adaptations for enhanced visual acuity that initially evolved in a diurnal setting. Furthermore, the tarsier central retina has surprisingly high densities of cones. In this respect, tarsiers resemble diurnal squirrels and tree shrews and differ markedly from other nocturnal mammals (74). In total, these derived retinal features provide support for the hypothesis that tarsiers are secondarily nocturnal and share a diurnal common ancestor with anthropoids. It is probable that most visual adaptations for high acuity found in anthropoids evolved initially in the haplorhine stem lineage and that this heritage is partly obscured in tarsiers through a secondary adaptation to nocturnality. Middle Eocene tarsiers from China have large orbits, suggesting that tarsiers are genuine “living fossils” that have persisted more or less in their current form for millions of years (77).
Orbit.
Anthropoids are unique among mammals in having a thin bony partition that isolates the orbit from the temporal fossa. This partition, or postorbital septum, is formed chiefly by varying contributions from the frontal, zygomatic, and alisphenoid bones. Tarsiers resemble anthropoids in exhibiting a partial postorbital septum with contact between the zygomatic and alisphenoid bones, but unlike anthropoids, tarsiers retain broad confluence between the orbit and temporal fossa inferior to the septum (21). Observational and experimental data suggest that the postorbital septum is an adaptation for preventing mechanical disturbance of the eyes by contractions of the anterior temporalis muscle (78, 79). According to this “insulation hypothesis,” haplorhines require postorbital septa because they evolved large and convergent (i.e., forward-facing) eyes and orbits, thus bringing the eyes and temporalis muscles into close proximity. Maintaining mechanical stability of the eyes is also particularly important for haplorhines, given their high visual acuity (74, 80).
The fact that no other mammals have postorbital septa has hindered comparative tests of the insulation hypothesis. However, owls resemble tarsiers and anthropoids in having a bony lamina (an expanded “postorbital process”) that shields the eye and redirects a key jaw adductor muscle posterior to the orbit (81). Owls also possess relatively large and convergent orbits, bringing the eyes and jaw adductors into close proximity, as in haplorhines. These findings lend crucial comparative support to the insulation hypothesis and suggest that haplorhines and owls evolved analogous morphological solutions to the shared problem of mechanical interference between the eyes and jaw adductor muscles.
One key question that remains unresolved is how to account for the distinctive circum-orbital morphology of tarsiers. Although most researchers have argued for homology between anthropoid and tarsier septa, others have argued that the tarsier septum represents a convergent adaptation necessitated by the evolution of extremely large eyes (82, 83). Owls are similar to tarsiers in having postorbital processes that are less extensive than anthropoid septa, in having rod-dominated retinal foveae (lost in tytonid owls), and in being predominantly nocturnal (81). As a result, the common occurrence of orbital insulating structures in owls, tarsiers, and anthropoids suggests that such structures theoretically could have evolved in either a nocturnal or a diurnal setting, provided that eye size, eye and orbit orientation, and selection to maintain high acuity were contributing factors. At present, however, it is most parsimonious to conclude that some kind of postorbital septum was present in a diurnal common ancestor of tarsiers and anthropoids because this ancestor presumably had large and convergent eyes (73, 81, 84) as well as the shared haplorhine features supporting very high visual acuity (74).
Color Vision.
Over the last two decades, studies of opsin genes and the photopigments that they encode have revealed that most mammals, including tarsiers and some lemurs, have color vision that is based on two cone types (85). This type of color vision—in which one cone class is maximally sensitive to blue-violet light and another to red-green light—is called “dichromacy.” Dichromats are able to use these two cone types to discriminate between short and long wavelength light and thus differ from a smaller assortment of mammals (including lorises, bushbabies, and owl monkeys among primates) that are truly color-blind because they possess only one cone type (“monochromacy”). Anthropoids are not uniform in their color vision, but all diverge somewhat from the typical mammalian pattern of dichromacy (85). Due to an ancient gene duplication event, catarrhines have three cone types that are each maximally sensitive to different regions of the visible spectrum. This type of color vision (“routine trichromacy”) is rare among mammals and gives catarrhines the ability to discriminate between short, middle, and long wavelengths of light. With the exception of howler monkeys, platyrrhines lack the key gene duplication that confers routine trichromacy on catarrhines. However, most platyrrhines do exhibit polymorphism in the opsin gene expressed in the class of cones that is maximally sensitive to red-green light. Because this gene is on the X chromosome, most female platyrrhines inherit different alleles for the red-green opsin locus and are functionally trichromats. Males, by contrast, receive only one red-green allele and are dichromats. The most recent genetic data suggest that such “polymorphic trichromacy” has evolved multiple times among diurnal and cathemeral lemuriform primates as well (86, 87), although the functionality of color vision in lemurs needs to be more fully explored (88). Similarly, studies of Australian marsupials suggest that many are routine trichromats like catarrhine primates (89). These data reveal that excellent color vision based on the presence of three cone types is not as rare among mammals as was recently thought.
Olfaction.
As with color vision, studies of the primate olfactory system have been revolutionized by the study of genes that code for olfactory receptor proteins (ORPs) and vomeronasal receptor proteins. These proteins are expressed in specialized sensory epithelia and initiate a transduction cascade when they bind with target molecules (odorants). Like most mammals, many primates have both a “main” olfactory system (MOS) and an “accessory” olfactory system (AOS), with receptors localized in the nasal mucosa and vomeronasal organ (VNO), respectively. The MOS is often characterized as detecting odors associated with a wide range of stimuli such as food or predators, whereas the AOS is more narrowly specialized to detect pheromones or other sociosexual odors (90). The functional roles of the MOS and AOS, however, are probably far more complex than this generalization implies (90, 91).
Living haplorhines have small olfactory bulbs compared with most mammals and lack the primitive “olfactory recess” in which olfactory mucosa is separated from the main respiratory airway. As a result, haplorhines have generally been characterized as lacking well-developed olfactory abilities. However, recent comparative studies of the MOS suggest that platyrrhines resemble strepsirrhines and many other mammals in retaining a large proportion of functional ORP genes (92–94). Because the olfactory bulb is primarily a relay between MOS receptors in the nasal fossa and olfactory centers in the brain, the small size of the platyrrhine olfactory bulb probably reflects a decrease in the total number of MOS receptor cells rather than a decrease in the number of distinct ORP types. These considerations suggest that although platyrrhines may not be able to detect volatile odorants at very low concentrations, they probably resemble strepsirrhines in retaining a keen ability to discriminate between families of odorants. As with color vision, it is mainly catarrhines that stand out as unusual among primates in terms of their ORP gene complement. Up to 50% of the ORP genes in Old World monkeys, apes, and humans no longer code for functional receptor proteins (92–95). Apparently, loss of olfactory discriminative ability is not an anthropoid characteristic generally but is instead restricted mainly to catarrhines. However, we still know very little about the precise relationship between olfactory bulb size and olfactory abilities in primates. Likewise, the specific odorants detected by individual ORPs remain unknown in most cases, and thus we do not yet fully understand the functionality of ORP genes.
As with variation in the MOS, the state of the anthropoid AOS is more complicated than might be expected on the basis of the evident gross reductions of the olfactory periphery. The VNO is the primary receptor organ of the AOS, and accordingly much attention recently has been devoted to the presence or absence of this structure among primates. Catarrhines are unique among primates in lacking functional VNOs (91, 96). Early reports of a functional VNO in humans are controversial and have not been supported by subsequent anatomical research (97, 98). Although some adult catarrhines (including humans) may express a rudimentary VNO, such structures do not contain a sensory epithelium, often do not communicate with the nasal fossa, and lack vomeronasal nerves to transmit information to the brain. More importantly, the gene coding for the TRP2 ion channel (which is critical for transduction by V1R receptor cells in the VNO) is nonfunctional in extant catarrhines (99).
Together with data on the MOS, these findings suggest that the reduced emphasis on olfaction that is generally characteristic of anthropoids evolved in two major steps. First, the complexity of the nasal cavity and the size of the main olfactory epithelium were reduced in the haplorhine stem lineage. This anatomical reorganization was correlated with a reduction in the size of the MOS receptor cell population, a reduction in the size of the central targets of olfactory input in the brain (e.g., main olfactory bulb and olfactory cortex), and a corresponding reduction in olfactory sensitivity. Second, stem catarrhines lost a functional VNO as well as the ability to produce a large fraction of their ORPs. Although it is unclear why catarrhines became less dependent on olfactory stimuli than other primates, some researchers have suggested that this de-emphasis on olfaction was tied to the evolution of routine trichromacy (93, 99; but see ref. 100). Irrespective of the causal agent, these findings provide further evidence that catarrhine primates are highly derived in lacking the well-developed olfactory apparatus and sophisticated mechanisms of pheromonal communication that are commonly found in other primates and nonprimate mammals. In light of these distinct changes in their olfactory systems, it is intriguing that catarrhines, including humans, are known to respond to certain sociosexual odor cues and to exhibit a limited repertoire of pheromonal responses (101, 102). The mechanisms through which catarrhines sense these cues are not yet well understood but may partly involve trace-amine–associated receptors expressed in the main olfactory epithelium (103).
Brain Size.
Extant anthropoids exhibit an upward “grade shift” in relative brain size compared to tarsiers and living strepsirrhines (104). A number of adaptive explanations have been proposed for this shift, including enhanced environmental mapping, dietary shifts, changes in the visual system, changes in social structure, and enhanced domain–general cognition. Most of these proposals are bolstered by correlative distributions of brain size versus behavioral traits in living species. The emergence of a more detailed fossil record is beginning to serve as an important test of these hypotheses. For example, we now know that relative increases in brain size occurred independently in catarrhines and platyrrhines (8, 105, 106) and that stem anthropoids (e.g., Simonsius) and even stem catarrhines (Aegyptopithecus) and stem platyrrhines (Chilecebus and Homunculus) had brains broadly comparable in size to living strepsirrhines (105, 107, 108). Thus, the larger brains of living anthropoids evolved gradually and potentially could have been influenced by different selective factors in platyrrhines and catarrhines. We can now also establish that high visual acuity was well developed in early anthropoids before a large brain evolved (109).
Overall increase in brain size is, of course, not the whole story. The anthropoid brain also has been reorganized, although only the outlines of this story have been gleaned from fossil endocasts. Reduction in the size of the olfactory bulb has already been mentioned. The size of the neocortex also has increased. Although the size of the brain of Aegyptopithecus was smaller than that of living anthropoids, the area of the visual cortex exhibited an anthropoid-like expansion (106). Homunculus had an enlarged optic canal, indicating improved visual acuity in the small-brained stem platyrrhine (107). Aegyptopithecus and Simonsius (108) appear to have had smaller frontal neocortexes than do living anthropoids.
These independent increases in brain size documented in the fossil record of anthropoid evolution accords well with the underlying genetic mechanisms that may be associated with anthropoid brain evolution. The anthropoid clade has experienced positive selection in genes that have a strong phenotypic effect on brain anatomy. For example, phylogenetically independent increases in anthropoid neocortex size are positively correlated with increases in amino acid substitution in the ASPM gene in a broad array of primates (110). Differences in gene expression or enzyme function in ASPM remain to be demonstrated directly among species examined (111), but the ASPM work highlights the need to extend comparisons beyond simply those within humans or between humans and chimpanzees (112).
Genes that affect proteins important in the machinery of aerobic energy production also experienced positive selection in the anthropoid stem and its descendant clades, whereas these genes are highly conserved in tarsiers, strepsirrhines, and mammals generally (1). It has been hypothesized that changes to genes influencing aerobic metabolism may have fueled the emergence of an enlarged, energetically expensive anthropoid neocortex (1, 113). Another proposed explanation for these changes involves provision of more energy for prolongation of the time to reach maturity or reductions in the release of free radicals in aerobic metabolic processes that may reduce tissue damage and prolong life span (114).
Biogeography
The continent of origin of anthropoid primates remains uncertain because early fossil occurrences are documented in both Afro-Arabia and Asia and because the Tethys seaway between the two continents was evidently a permeable barrier to mammalian dispersal at various times during the Paleogene. The limited state of our current knowledge about anthropoids from the Paleocene and Eocene also contributes to our uncertainty about where various anthropoid groups originated. The strongest evidence for stem anthropoid origins is documented from middle Eocene deposits in China and Southeast Asia, but eosimiids may also be known from the early Eocene of India. Crown anthropoids certainly were present in Africa by the late Eocene.
Biogeographic hypotheses must be tempered by the discrepancy between molecular and fossil-based estimates of branch times for the divergence of primates from other mammals, haplorhines from strepsirrhines, and tarsiers from anthropoids. All molecularly based estimates for primate origins fall in the late Cretaceous (83.3–70.6 Ma) and those for the haplorhine–strepsirrhine split and anthropoid–tarsier split are older than ∼56 Ma (115–118). In contrast, the oldest known fossil evidence for a “primate of modern aspect” is early Eocene (∼55.8 Ma) or late Paleocene if Altiatlasius is a euprimate. The great antiquity of these molecular branch times is discordant with the evidence of the fossil record (14). Molecular clock estimates of the basal splits among the major lineages of living primates predates all known primates of modern aspect by up to 25 million years. This discrepancy has given rise to suggestions that anthropoids had an African or Indo-Madagascan origin (57). So far this hypothesis, like many others, lacks concrete support. Recent fossils supporting the extension of the stem primate lineage to 65 million years (40) lessen this discrepancy.
Summing Up
In the next decade, paleontological exploration in late Paleocene and early Eocene deposits in Asia and Africa will likely produce important new fossils that will extend our understanding of the earliest branches of the anthropoid radiation. Discordance between the fossil record and molecular divergence estimates is likely to decrease as we focus our efforts on the Asian and African Paleocene deposits. In the meantime, data accumulated over the last 15 years reveal many details about the early origins of monkeys, apes, and humans. Africa no longer holds the distinction of being the only likely center for anthropoid origins, and a diverse assemblage of early fossil anthropoids are now known from China, India, and Southeast Asia.
All of the available fossil evidence suggests that anthropoids originated at very small size. Many of the key features of anthropoid sensory ecology and anatomy, such as highly acute diurnal vision and retinal foveae, are best interpreted as having first evolved in stem haplorhines rather than in stem anthropoids. Other sensory features long thought to characterize all anthropoids, such as an extreme reduction of olfactory abilities, are characteristic mainly of a more restricted group—the catarrhines. Similarly, postorbital septa (found in all haplorhines) and routine trichromacy (characteristic of catarrhines) are known to have evolutionary analogs among other vertebrates. Finally, fossil evidence reveals that major increases in brain size occurred independently in New World and Old World anthropoids. These revelations hold the key to understanding the initial evolution of many distinctive human characteristics—from our keen eyesight to our poor sense of smell and large brain. Like many human features, the first adaptive shifts responsible for the emergence of these characteristics are truly ancient, substantially predating the appearance of a human evolutionary lineage distinct from other primates.
Acknowledgments
We thank Kari Allen, Nate Dominy, Christine Drea, Daniel Gebo, Yasuhoro Go, Laurent Marivaux, Erik Seiffert, Mary Silcox, Derek Wildman, Greg Wray, and an anonymous reviewer for advice, helpful conversations, and/or suggestions on the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Goodman M, Grossman LI, Wildman DE. Moving primate genomics beyond the chimpanzee genome. Trends Genet. 2005;21:511–517. doi: 10.1016/j.tig.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Hedges DJ, Batzer MA. From the margins of the genome: Mobile elements shape primate evolution. Bioessays. 2005;27:785–794. doi: 10.1002/bies.20268. [DOI] [PubMed] [Google Scholar]
- 3.Shedlock AM, Okada N. SINE insertions: Powerful tools for molecular systematics. Bioessays. 2000;22:148–160. doi: 10.1002/(SICI)1521-1878(200002)22:2<148::AID-BIES6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 4.Xing J, Witherspoon DJ, Ray DA, Batzer MA, Jorde LB. Mobile DNA elements in primate and human evolution. Am J Phys Anthropol. 2007;45(Suppl):2–19. doi: 10.1002/ajpa.20722. [DOI] [PubMed] [Google Scholar]
- 5.Hartwig W, editor. The Primate Fossil Record. Cambridge, UK: Cambridge University Press; 2002. [Google Scholar]
- 6.Beard KC, Dagosto M, Gebo DL, Godinot M. Interrelationships among primate higher taxa. Nature. 1988;331:712–714. doi: 10.1038/331712a0. [DOI] [PubMed] [Google Scholar]
- 7.Szalay FS, Delson E. Evolutionary History of the Primates. New York: Academic Press; 1979. [Google Scholar]
- 8.Kay RF, Ross CF, Williams BA. Anthropoid origins. Science. 1997;275:797–804. doi: 10.1126/science.275.5301.797. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen DT. The phylogenetic position of Mahgarita stevensi: Protoanthropoid or lemuroid? Int J Primatol. 1990;11:437–467. [Google Scholar]
- 10.Franzen JL, et al. Complete primate skeleton from the middle Eocene of Messel in Germany: Morphology and paleobiology. PLoS One. 2009;4:e5723. doi: 10.1371/journal.pone.0005723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajpai S, et al. The oldest Asian record of Anthropoidea. Proc Natl Acad Sci USA. 2008;105:11093–11098. doi: 10.1073/pnas.0804159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose KD, et al. Early Eocene primates from Gujarat, India. J Hum Evol. 2009;56:366–404. doi: 10.1016/j.jhevol.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Seiffert ER, et al. Basal anthropoids from Egypt and the antiquity of Africa's higher primate radiation. Science. 2005;310:300–304. doi: 10.1126/science.1116569. [DOI] [PubMed] [Google Scholar]
- 14.Godinot M. Lemuriform origins as viewed from the fossil record. Folia Primatol (Basel) 2006;77:446–464. doi: 10.1159/000095391. [DOI] [PubMed] [Google Scholar]
- 15.Seiffert ER, Perry JGM, Simons EL, Boyer DM. Convergent evolution of anthropoid-like adaptations in Eocene adapiform primates. Nature. 2009;461:1118–1121. doi: 10.1038/nature08429. [DOI] [PubMed] [Google Scholar]
- 16.Beard KC. The oldest North American primate and mammalian biogeography during the Paleocene-Eocene Thermal Maximum. Proc Natl Acad Sci USA. 2008;105:3815–3818. doi: 10.1073/pnas.0710180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross C, Williams BA, Kay RF. Phylogenetic analysis of anthropoid relationships. J Hum Evol. 1998;35:221–306. doi: 10.1006/jhev.1998.0254. [DOI] [PubMed] [Google Scholar]
- 18.Marivaux L. The eosimiid and amphipithecid pri-mates (Anthropoidea) from the Oligocene of the Bugti Hills (Balochistan, Pakistan): New insight into early higher primate evolution in South Asia. Palaeovertebrata. 2006;34:29–109. [Google Scholar]
- 19.Luckett WP. Cladistic relationships among primate higher categories: Evidence of fetal membranes and placenta. Folia Primatol (Basel) 1976;25:245–276. doi: 10.1159/000155719. [DOI] [PubMed] [Google Scholar]
- 20.Ross CF. Allometric and functional influences on primate orbit orientation and the origins of the Anthropoidea. J Hum Evol. 1995;29:210–228. [Google Scholar]
- 21.Cartmill M. Morphology, function and evolution of the anthropoid postorbital septum. In: Ciochon R, Chiarelli B, editors. Evolutionary Biology of the New World Monkeys and Continental Drift. New York: Plenum Press; 1980. pp. 243–274. [Google Scholar]
- 22.Martin RD. Primate Origins and Evolution: A Phylogenetic Reconstruction. Princeton, NJ: Princeton University Press; 1990. [Google Scholar]
- 23.MacPhee RDE, Cartmill M. Basicranial structures and primate systematics. In: Swindler DR, Erwin J, editors. Comparative Primate Biology, Vol 1: Systematics, Evolution, and Anatomy. New York: Alan R. Liss; 1986. pp. 219–275. [Google Scholar]
- 24.Cartmill M, Kay RF. Craniodental morphology, tarsier affinities, and primate suborders. In: Chivers DJ, Joysey KA, editors. Recent Advances in Primatology: Evolution. Vol. 3. London: Academic Press; 1978. pp. 205–214. [Google Scholar]
- 25.Ravosa MJ, Hogue A. Function and fusion of the mandibular symphysis in mammals: A comparative and experimental perspective. In: Ross C, Kay RF, editors. Anthropoid Origins: New Visions. New York: Kluwer/Plenum Publishing; 2003. [Google Scholar]
- 26.Beard KC, Wang B, Dawson M, Huang X, Tong Y. Earliest complete dentition of an anthropoid primate from the late middle Eocene of Shanxi Province, China. Science. 1996;272:82–85. [Google Scholar]
- 27.Gebo DL, Dagosto M, Beard KC, Ni X. New primate hindlimb elements from the middle Eocene of China. J Hum Evol. 2008;55:999–1014. doi: 10.1016/j.jhevol.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Gebo DL, Dagosto MK, Beard KC, Ni X, Qi T. A haplorhine first metatarsal from the middle Eocene of China. In: Fleagle JG, Gilbert CC, editors. Elwyn Simons: A Search for Origins. New York: Springer; 2008. pp. 229–242. [Google Scholar]
- 29.Seiffert ER. Revised age estimates for the later Paleogene mammal faunas of Egypt and Oman. Proc Natl Acad Sci USA. 2006;103:5000–5005. doi: 10.1073/pnas.0600689103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaeger JJ, et al. New Myanmar middle Eocene anthropoids. An Asian origin for catarrhines? C R Acad Sci Gen. 1998;321:953–959. [Google Scholar]
- 31.Simons EL, Seiffert ER. A partial skeleton of Proteopithecus sylviae (Primates Anthropoidea): First associated dental and postcranial remains of an Eocene anthropoidean. C R Acad Sci Gen. 1999;329:921–927. [Google Scholar]
- 32.Seiffert ER, Simons EL, Fleagle JG. Anthropoid humeri from the late Eocene of Egypt. Proc Natl Acad Sci USA. 2000;97:10062–10067. doi: 10.1073/pnas.97.18.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seiffert ER, Simons EL. Astragular morphology of late Eocene anthropoids from the Fayum Depression (Egypt) and the origin of catarrhine primates. J Hum Evol. 2001;41:577–606. doi: 10.1006/jhev.2001.0508. [DOI] [PubMed] [Google Scholar]
- 34.Takai M, Anaya F, Shigehara N, Setoguchi T. New fossil materials of the earliest New World monkey, Branisella boliviana, and the problem of platyrrhine origins. Am J Phys Anthropol. 2000;111:263–281. doi: 10.1002/(SICI)1096-8644(200002)111:2<263::AID-AJPA10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Kay RF, Ross J, Simons EL. The basicranial anatomy of African Eocene/Oligocene anthropoids. Are there any clues for platyrrhine origins? In: Fleagle JG, Gilbert CG, editors. Elwyn L. Simons: A Search for Origins. New York: Springer; 2008. pp. 125–158. [Google Scholar]
- 36.Seiffert ER, Simons EL, Simons CVM. Phylogenetic, biogeographic, and adaptive implications of new fossil evidence bearing on crown anthropoid origins and early stem catarrhine evolution. In: Ross C, Kay RF, editors. Anthropoid Origins: New Visions. New York: Kluwer/Plenum Publishing; 2004. pp. 157–182. [Google Scholar]
- 37.Kay RF, Fleagle JG, Simons EL. A revision of the African Oligocene apes of the Fayum Province, Egypt. Am J Phys Anthropol. 1981;55:293–322. [Google Scholar]
- 38.Sigé B, Jaeger J-J, Sudre J, Vianey-Liaud M. Altiatlasius koulchii n. gen. et sp., primate omomyidé du Paléocène supérieur du Moroc, et les origines des Euprimates. Paleontographica. 1990;212:1–24. [Google Scholar]
- 39.Godinot M. Early North African primates and their significance for the origin of Simiiformes (=Anthropoidea) In: Fleagle JG, Kay RF, editors. Anthropoid Origins: The Fossil Evidence. New York: Plenum Press; 1994. pp. 235–296. [Google Scholar]
- 40.Bloch JI, Silcox MT, Boyer DM, Sargis EJ. New Paleocene skeletons and the relationship of plesiada-piforms to crown-clade primates. Proc Natl Acad Sci USA. 2007;104:1159–1164. doi: 10.1073/pnas.0610579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ginsburg L, Mein P. Tarsius thailandica nov. sp., premier Tarsiidae (Primates, Mammalia) fossile d'Asie. C R Acad Sci. 1987;304:1213–1214. [Google Scholar]
- 42.Rasmussen DT, Conroy G, Simons EL. Tarsier-like locomotor specializations in the Oligocene primate Afrotarsius. Proc Natl Acad Sci USA. 1998;95:14848–14850. doi: 10.1073/pnas.95.25.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tabuce R, et al. Anthropoid vs. strepsirhine status of the African Eocene primates Algeripithecus and Azibius: Craniodental evidence. Proc R Soc B Biol Sci. 2009;276:4087–4094. doi: 10.1098/rspb.2009.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabuce R, Mahboubi M, Tafforeau P, Sudre J. Discovery of a highly specialized Plesiadapiformes (Mammalia, Primates) in the Eocene of Africa. J Hum Evol. 2004;47:305–321. doi: 10.1016/j.jhevol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Silcox MT. The biogeographic origins of primates and euprimates: East, west, north or south of Eden? In: Sargis EJ, Dagosto M, editors. Mammalian Evolutionary Morphology: A Tribute to Fredrick S. Szalay. The Netherlands: Springer, Dordrecht; 2008. pp. 199–231. [Google Scholar]
- 46.Beard KC, Qi T, Dawson M, Wang B, Li C. A diverse new primate fauna from middle Eocene fissure-fillings in Southeastern China. Nature. 1994;369:604–609. doi: 10.1038/368604a0. [DOI] [PubMed] [Google Scholar]
- 47.Beard KC, Wang J. The eosimiid primates (Anthropoidea) of the Heti Formation, Yuanqu Basin, Shanxi and Henan Provinces, People's Republic of China. J Hum Evol. 2004;46:401–432. doi: 10.1016/j.jhevol.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Takai M, et al. A new eosimiid from the latest middle Eocene in Pondaung, central Myanmar. Anthropol Sci. 2005;113:17–25. [Google Scholar]
- 49.Jaeger J-J, et al. A new primate from the middle Eocene of Myanmar and Asian early origin of Anthropoids. Science. 1999;286:528–530. doi: 10.1126/science.286.5439.528. [DOI] [PubMed] [Google Scholar]
- 50.Marivaux L, et al. Anthropoid primates from the Oligocene of Pakistan (Bugti Hills): Data on early anthropoid evolution and biogeography. Proc Natl Acad Sci USA. 2005;102:8436–8441. doi: 10.1073/pnas.0503469102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gunnell GF, et al. New primates (Mammalia) from the early and middle Eocene of Pakistan and their paleobiogeographical implications. Contributions from Museum of Paleontology. University of Michigan. 2008;32:1–14. [Google Scholar]
- 52.Takai M, et al. A new anthropoid from the latest middle Eocene of Pondaung, central Myanmar. J Hum Evol. 2001;40:393–409. doi: 10.1006/jhev.2001.0463. [DOI] [PubMed] [Google Scholar]
- 53.Beard KC, et al. A new primate from the Eocene Pondaung Formation of Myanmar and the monophyly of Burmese amphipithecids. Proc R Soc B Biol Sci. 2009;276:3285–3294. doi: 10.1098/rspb.2009.0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaimanee Y, Suteethorn V, Jaeger J-J, Ducroq S. A new late Eocene anthropoid primate from Thailand. Nature. 1997;385:429–431. doi: 10.1038/385429a0. [DOI] [PubMed] [Google Scholar]
- 55.Rasmussen DT, Simons EL. Paleobiology of the oligopithecines, the earliest known anthropoid primates. Int J Primatol. 1992;13:1–32. [Google Scholar]
- 56.Rosenberger AL, Hogg RL. On Bahinia pondaungensis, an alleged early anthropoid. PaleoAnthropology. 2007 2007:26–30. [Google Scholar]
- 57.Miller ER, Gunnell GF, Martin RD. Deep time and the search for anthropoid origins. Am J Phys Anthropol. 2005;48:60–95. doi: 10.1002/ajpa.20352. [DOI] [PubMed] [Google Scholar]
- 58.Kay RF, Williams BA, Ross CF, Takai M, Shigehara N. Anthropoid origins: A phylogenetic analysis. In: Ross CF, Kay RF, editors. Anthropoid Origins: New Visions. Plenum, New York: Kluwer; 2004. pp. 91–135. [Google Scholar]
- 59.Gunnell GF, Ciochon R, Gingerich PD, Holroyd PA. New assessment of Pondaungia and Amphipithecus (Primates) from the late middle Eocene of Myanmar, with comments on ‘Amphipithecidae’. Contributions from Museum of Paleontology. University of Michigan. 2002;30:337–372. [Google Scholar]
- 60.Shigehara N, Takai M. Facial fragments of Amphipithecus. In: Ross C, Kay RF, editors. Anthropoid Origins: New Visions. Plenum, New York: Kluwer; 2004. pp. 323–340. [Google Scholar]
- 61.Beard KC, et al. Taxonomic status of purported primate frontal bones from the Eocene Pondaung Formation of Myanmar. J Hum Evol. 2005;49:468–481. doi: 10.1016/j.jhevol.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 62.Zollikofer CPE, et al. The face of Siamopithecus: new geometric-morphometric evidence for its anthropoid status. Anat Rec (Hoboken) 2009;292:1734–1744. doi: 10.1002/ar.20998. [DOI] [PubMed] [Google Scholar]
- 63.Marivaux L, et al. The anthropoid status of a primate from late middle Eocene Pondaung Formation (central Myanmar): Tarsal evidence. Proc Natl Acad Sci USA. 2003;100:13173–13178. doi: 10.1073/pnas.2332542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Egi N, Takai M, Shigehara N, Tsubamoto T. Body mass estimates for Eocene eosimiid and amphipithecid primates using prosimian and anthropoid scaling models. Int J Primatol. 2004;25:211–236. [Google Scholar]
- 65.Gebo DL, Dagosto M, Beard KC, Tao Q. The smallest primates. J Hum Evol. 2000;38:585–594. doi: 10.1006/jhev.2000.0395. [DOI] [PubMed] [Google Scholar]
- 66.Fleagle JG. Primate Adaptation and Evolution. San Diego: Academic Press; 1999. [Google Scholar]
- 67.Kirk EC, Simons EL. Diet of fossil primates from the Fayum Depression of Egypt: A quantitative analysis of molar shearing. J Hum Evol. 2000;40:203–229. doi: 10.1006/jhev.2000.0450. [DOI] [PubMed] [Google Scholar]
- 68.Teaford MF, Maas MC, Simons EL. Dental microwear and microstructure in early Oligocene primates from the Fayum, Egypt: Implications for diet. Am J Phys Anthropol. 1996;101:527–543. doi: 10.1002/(SICI)1096-8644(199612)101:4<527::AID-AJPA7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 69.Kay RF, et al. The paleobiology of Amphipithecidae, South Asian late Eocene primates. J Hum Evol. 2004;46:3–25. doi: 10.1016/j.jhevol.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 70.Kay RF, Covert HH. Anatomy and behaviour of extinct primates. In: Chivers DJ, Wood BA, Bilsborough A, editors. Food Acquisition and Processing in Primates. New York: Plenum Press; 1984. pp. 467–508. [Google Scholar]
- 71.Gebo DL, Dagosto M, Beard KC, Tao Q, Wang J. The oldest known anthropoid postcranial fossils and the early evolution of higher primates. Nature. 2000;404:276–278. doi: 10.1038/35005066. [DOI] [PubMed] [Google Scholar]
- 72.Kirk EC. Eye morphology in cathemeral lemurids and other mammals. Folia Primatol (Basel) 2006;77:27–49. doi: 10.1159/000089694. [DOI] [PubMed] [Google Scholar]
- 73.Ross CF, Kirk EC. Evolution of eye size and shape in primates. J Hum Evol. 2007;52:294–313. doi: 10.1016/j.jhevol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Kirk EC, Kay RF. The evolution of high visual acuity in the Anthropoidea. In: Ross CF, Kay RF, editors. Anthropoid Origins: New Visions. New York: Kluwer/Plenum Publishing; 2004. pp. 539–602. [Google Scholar]
- 75.Kirk EC. Comparative morphology of the eye in primates. Anat Rec. 2004;281A:1095–1103. doi: 10.1002/ar.a.20115. [DOI] [PubMed] [Google Scholar]
- 76.Collins CE, Hendrickson A, Kaas JH. Overview of the visual system of Tarsius. Anat Rec. 2005;287A:1013–1025. doi: 10.1002/ar.a.20263. [DOI] [PubMed] [Google Scholar]
- 77.Rossie JB, Ni X, Beard KC. Cranial remains of an Eocene tarsier. Proc Natl Acad Sci USA. 2006;103:4381–4385. doi: 10.1073/pnas.0509424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ross CF, Hall MI, Heesy CP. Were basal primates nocturnal? Evidence from eye and orbit shape. In: Ravosa M, Dagosto M, editors. Primate Origins: Adaptations and Evolution. New York: Springer; 2007. [Google Scholar]
- 79.Heesy CP, Ross CF. Evolution of activity patterns and chromatic vision in primates: Morphometrics, genetics and cladistics. J Hum Evol. 2001;40:111–149. doi: 10.1006/jhev.2000.0447. [DOI] [PubMed] [Google Scholar]
- 80.Veilleux CC, Kirk EC. Visual acuity in the cathemeral strepsirrhine Eulemur macaco flavifrons. Am J Primatol. 2009;71:1–10. doi: 10.1002/ajp.20665. [DOI] [PubMed] [Google Scholar]
- 81.Menegaz RA, Kirk EC. Septa and processes: Convergent evolution of the orbit in haplorhine primates and strigiform birds. J Hum Evol. 2009 doi: 10.1016/j.jhevol.2009.04.010. 57:–687. [DOI] [PubMed] [Google Scholar]
- 82.Le Gros Clark WE. The Antecedents of Man. 1st Ed. Edinburgh: Edinburgh University Press; 1959. pp. 1–374. [Google Scholar]
- 83.Simons EL, Rasmussen DT. Cranial anatomy of Aegyptopithecus and Tarsius and the question of the tarsier-anthropoidean clade. Am J Phys Anthropol. 1989;79:1–23. doi: 10.1002/ajpa.1330790103. [DOI] [PubMed] [Google Scholar]
- 84.Heesy CP, Ross C. Mosaic evolution of activity pattern, diet, and color vision in haplorhine primates. In: Ross C, Kay RF, editors. Anthropoid Origins: New Visions. New York: Kluwer Academic/Plenum Publishers; 2004. pp. 649–682. [Google Scholar]
- 85.Jacobs GH. Primate color vision: A comparative perspective. Vis Neurosci. 2008;25:619–633. doi: 10.1017/S0952523808080760. [DOI] [PubMed] [Google Scholar]
- 86.Tan Y, Li WH. Trichromatic vision in prosimians. Nature. 1999;402:36. doi: 10.1038/46947. [DOI] [PubMed] [Google Scholar]
- 87.Veilleux CC, Bolnick DA. Opsin gene polymorphism predicts trichromacy in a cathemeral lemur. Am J Primatol. 2008;71:86–90. doi: 10.1002/ajp.20621. [DOI] [PubMed] [Google Scholar]
- 88.Leonhardt SD, Tung J, Camden JB, Leal M, Drea CM. Seeing red: Behavioral evidence of trichromatic color vision in strepsirrhine primates. Behav Ecol. 2009;20:1–12. [Google Scholar]
- 89.Cowing JA, Arrese CA, Davies WL, Beazley LD, Hunt D M. Cone visual pigments in two marsupial species: The fat-tailed dunnart (Sminthopsis crassicaudata) and the honey possum (Tarsipes rostratus) Proc R Soc B Biol Sci. 2008;275:1491–1499. doi: 10.1098/rspb.2008.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Keverne EB. The vomeronasal organ. Science. 1999;286:716–720. doi: 10.1126/science.286.5440.716. [DOI] [PubMed] [Google Scholar]
- 91.Smith TD, Siegel MI, Bhatnagar KP. Reappraisal of the vomeronasal system of catarrhine primates: Ontogeny, morphology, functionality, and persisting questions. Anat Rec. 2001;265:176–192. doi: 10.1002/ar.1152. [DOI] [PubMed] [Google Scholar]
- 92.Rouquier S, Blancher A, Giorgi D. The olfactory receptor gene repertoire in primates and mouse: Evidence for reduction of the functional fraction in primates. Proc Natl Acad Sci USA. 2000;97:2870–2874. doi: 10.1073/pnas.040580197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gilad Y, Przeworski M, Lancet D. Loss of olfactory receptor genes coincides with the acquisition of full trichromatic vision in primates. PLoS Biol. 2004;2 doi: 10.1371/journal.pbio.0020005. and erratum (2007) 5:e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Niimura Y, Nei M. Extensive gains and losses of olfactory receptor genes in mammalian evolution. PLoS One. 2007;2:e708. doi: 10.1371/journal.pone.0000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Go Y, Niimura Y. Similar numbers but different repertoires of olfactory receptor genes in humans and chimpanzees. Mol Biol Evol. 2008;25:1897–1907. doi: 10.1093/molbev/msn135. [DOI] [PubMed] [Google Scholar]
- 96.Smith TD, et al. Histological definition of the vomeronasal organ in humans and chimpanzees, with a comparison to other primates. Anat Rec. 2002;267A:166–176. doi: 10.1002/ar.10095. [DOI] [PubMed] [Google Scholar]
- 97.Swaney WT, Keverne EB. The evolution of pheromonal communication. Behav Brain Res. 2009;200:239–247. doi: 10.1016/j.bbr.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 98.Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: An update. Prog Neurobiol. 2003;70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- 99.Liman ER, Innan H. Relaxed selective pressure on an essential component of pheromone transduction in primate evolution. Proc Natl Acad Sci USA. 2003;100:3328–3332. doi: 10.1073/pnas.0636123100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nei M, Niimura Y, Nozawa M. The evolution of animal chemosensory receptor gene repertoires: Roles of chance and necessity. Nat Rev Genet. 2008;9:951–963. doi: 10.1038/nrg2480. [DOI] [PubMed] [Google Scholar]
- 101.Wedekind C, Seebeck T, Bettens F, Paepke AJ. Mhc-dependent mate preferences in humans. Proc R Soc Lond B Biol Sci. 1995;260:245–249. doi: 10.1098/rspb.1995.0087. [DOI] [PubMed] [Google Scholar]
- 102.Stern K, McClintock MK. Regulation of ovulation by human pheromones. Nature. 1998;392:177–179. doi: 10.1038/32408. [DOI] [PubMed] [Google Scholar]
- 103.Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- 104.Isler K, et al. Endocranial volumes of primate species: Scaling analyses using a comprehensive and reliable data set. J Hum Evol. 2008;55:967–978. doi: 10.1016/j.jhevol.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 105.Sears KE, Finarelli JA, Flynn JJ, Wyss A. Estimating body mass in New World “monkeys” (Platyrrhini, Primates), with a consideration of the Miocene platyrrhine, Chilecebus carrascoensis. Am Mus Novit. 2008;3617:1–29. [Google Scholar]
- 106.Radinsky L. Primate brain evolution. Am Sci. 1975;63:656–663. [PubMed] [Google Scholar]
- 107.Kay RF, Kirk EC, Malinzak M, Colbert MW. Brain size, activity pattern, and visual acuity in Homunculus patagonicus, an early Miocene stem platyrrhine: The mosaic evolution of brain size and visual acuity in Anthropoidea. J Vertebr Paleontol. 2006;26:83A–84A. [Google Scholar]
- 108.Bush EC, Simons EL, Allman J. High-resolution computed tomography study of the cranium of a fossil anthropoid primate, Parapithecus grangeri: New insights into the evolutionary history of primate sensory systems. Anat. Rec. A Discov Mol Cell Evol Biol. 2004;281A:1083–1087. doi: 10.1002/ar.a.20113. [DOI] [PubMed] [Google Scholar]
- 109.Kirk EC. Visual influences on primate encephalization. J Hum Evol. 2006;51:76–90. doi: 10.1016/j.jhevol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 110.Ali F, Meier R. Positive selection in ASPM is correlated with cerebral cortex evolution across primates but not with whole-brain size. Mol Biol Evol. 2008;25:2247–2250. doi: 10.1093/molbev/msn184. [DOI] [PubMed] [Google Scholar]
- 111.Haygood R, Fedrigo O, Hanson B, Yokoyama K-D, Wray GA. Promoter regions of many neural- and nutrition-related genes have experienced positive selection during human evolution. Nat Genet. 2007;39:1140–1144. doi: 10.1038/ng2104. [DOI] [PubMed] [Google Scholar]
- 112.Vallender EJ. Exploring the origins of the human brain through molecular evolution. Brain Behav Evol. 2008;72:168–177. doi: 10.1159/000151476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Uddin M, et al. Molecular evolution of the cytochrome c oxidase subunit 5A gene in primates. BMC Evol Biol. 2008;8:8. doi: 10.1186/1471-2148-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schmidt TR, et al. Rapid electrostatic evolution at the binding site for cytochrome c on cytochrome c oxidase in anthropoid primates. Proc Natl Acad Sci USA. 2005;102:6379–6384. doi: 10.1073/pnas.0409714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Janecka JE, et al. Molecular and genomic data identify the closest living relative of primates. Science. 2007;318:792–794. doi: 10.1126/science.1147555. [DOI] [PubMed] [Google Scholar]
- 116.Murphy WJ, Pringle TH, Crider TA, Springer MS, Miller W. Using genomic data to unravel the root of the placental mammal phylogeny. Genome Res. 2007;17:413–421. doi: 10.1101/gr.5918807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Phillips MJ. Branch-length estimation bias misleads molecular dating for a vertebrate mitochondrial phylog-eny. Gene. 2009;441:132–140. doi: 10.1016/j.gene.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 118.Martin RD, Soligo C, Tavare S. Primate origins: Implications of a Cretaceous ancestry. Folia Primatol (Basel) 2007;78:277–296. doi: 10.1159/000105145. [DOI] [PubMed] [Google Scholar]
- 119.Smith AG, Smith DG, Funnell BM. Atlas of Mesozoic and Cenozoic Coastlines. Cambridge, UK: Cambridge University Press; 1994. [Google Scholar]