Abstract
Understanding the pathogenesis of cancer-related bone disease is crucial to the discovery of new therapies. Here we identify activin A, a TGF-β family member, as a therapeutically amenable target exploited by multiple myeloma (MM) to alter its microenvironmental niche favoring osteolysis. Increased bone marrow plasma activin A levels were found in MM patients with osteolytic disease. MM cell engagement of marrow stromal cells enhanced activin A secretion via adhesion-mediated JNK activation. Activin A, in turn, inhibited osteoblast differentiation via SMAD2-dependent distal-less homeobox–5 down-regulation. Targeting activin A by a soluble decoy receptor reversed osteoblast inhibition, ameliorated MM bone disease, and inhibited tumor growth in an in vivo humanized MM model, setting the stage for testing in human clinical trials.
Keywords: osteoblasts, osteoclasts, tumor niche
Tumor-related bone disease, specifically osteolytic disease, represents a major clinical burden in many cancers, including multiple myeloma (MM) (1, 2). Osteolysis is the consequence of a pathological imbalance between osteoblast (OB) and osteoclast (OC) activity in the bone marrow (BM) niche. Tumor cells activate OC through several well characterized cytokines and signaling pathways (3). However, relatively little is known about the effects of tumor cells on OB differentiation. Consequently, although the majority of clinical interventions have targeted OCs in osteolytic disease, therapeutic interventions that target OBs have been far less successful (4).
To identify potential pathways with immediate relevance to human cancer-induced bone disease, we performed broad cytokine profiling of primary BM samples of MM patients with or without osteolytic disease. As a result, we identified an association between presence of osteolytic lesions (OLs) and levels of activin A, a TGF-β superfamily member.
Activin A is involved in bone remodeling as a promoter of osteoclastogenesis. However, controversial data have been reported on its role on OB differentiation (5, 6). Pharmacological modulation of activin A was made possible recently by means of a soluble form of activin receptor, RAP-011 (Acceleron Pharma), that increased bone formation in a mouse osteoporotic model (7). This anabolic reagent allowed us to probe the role of activin A inhibition on osteolytic disease of MM.
Our studies revealed that MM cells induce activin A expression from BM stromal cells (BMSCs), in part via activation of the JNK pathway. In turn, activin A inhibits OB differentiation by stimulating SMAD2 activity and inhibiting distal-less homeobox (DLX)–5 expression. More importantly, inhibition of activin A signaling rescued MM-induced OB impairment in vitro and in vivo while reducing MM burden in a humanized myeloma model. Our study therefore identifies activin A as a critical pathway in tumor-induced osteolysis and establishes it as a therapeutic target with direct relevance to human cancer-related bone disease.
Results
Activin A Correlates with Osteolytic Disease in MM Patients.
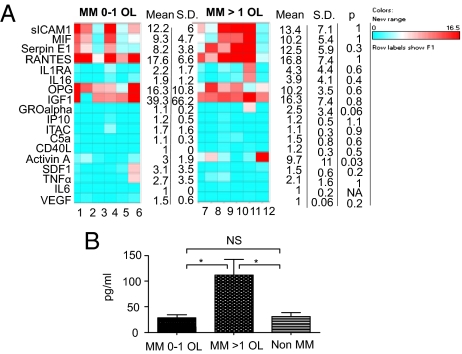
To identify pathways that might be involved in the pathogenesis of bone disease in MM, we performed broad cytokine profiling of BM plasma derived from MM patients with and without osteolytic bone disease. Forty-three cytokines associated with tumor development or involved in bone remodeling were profiled. We could not detect any expression for 25 cytokines independent of the presence of osteolysis. Of the 18 cytokines with detectable levels, only activin A demonstrated a significantly higher expression in patients with more than one OL versus patients with one or no OLs (9.7- and threefold increase, respectively; P = 0.03; Fig. 1A). Interestingly, we observed that IL-16 and CD40 ligand were preferentially expressed in patients with osteolysis (five of six patients with osteolysis vs. three of six patients without osteolysis expressed IL-16 and two of six vs. none of six expressed CD40 ligand), whereas VEGF expression was associated with absence of osteolysis (one of six patients with osteolysis vs. three of six patients without osteolysis), but the differences did not reach statistical significance.
Fig. 1.
Activin A correlates with osteolytic disease in MM patients. (A) Cytokine profile of BM plasma from 12 MM patients with ≤1 OL (n = 6) or >1 OL (n = 6). The levels of 18 cytokines are represented as fold increase over the background. Average, SD, and P values are provided. (B) BM plasma levels of activin A were assessed by ELISA in MM patients with osteolytic disease (n = 15), MM patients with ≤1 OL (n = 13), and non-MM patients (n = 10). Error bars represent SEM. *P < 0.05, **P < 0.01.
We further studied the association of activin A with osteolysis by comparing a larger group of MM patients at diagnosis with variable degree of bone disease versus non-MM patients. The average expression level of activin A was 112.07 pg/mL (SEM, 30.4) in MM patients with osteolytic disease (n = 15), versus 28.62 pg/mL (SEM, 6.2) in MM patients with one or fewer OLs (n = 13) and 30.6 pg/mL (SEM, 7.9) in the non-MM group (n = 10), respectively (P < 0.05; Fig. 1B). Importantly, activin A levels did not correlate with parameters reflecting tumor burden (Table S1), suggesting that activin A has a specific role in bone disease.
JNK Activation Is Associated with MM-Induced Stromal Cell Secretion of Activin A.
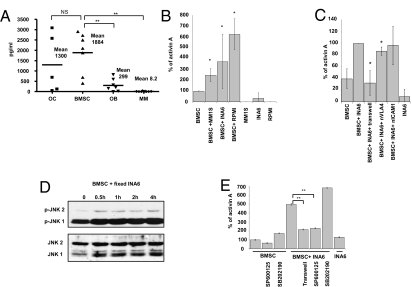
To identify the main sources of activin A in the tumor niche, we analyzed activin A secretion by BMSCs, OBs, and OCs from MM patients, as well as MM cell lines and MM primary cells (Fig. 2A). BMSCs secreted high levels of activin A (average 1.8 ng/mL), whereas activin A secretion by OC was variable over a wide range. Differentiation of BMSCs to OBs markedly decreased activin A expression (6). MM primary cells and cell lines secreted very low or undetectable levels of activin A (P < 0.01). Of note, we observed that increased expression of activin A was a specific feature of tumor-conditioned BMSCs derived from MM patients compared with tumor-naive BMSCs derived from healthy donors (P < 0.01; Fig. S1), suggesting that MM-conditioned stromal cells retain activin A secretion after ex vivo culture.
Fig. 2.
BMSC secretion of activin A is induced by MM cells via JNK pathway activation. (A) Ex vivo–derived OCs (n = 5), BMSCs (n = 7), OBs (n = 7), and MM patient cells, as well as MM cell lines (n = 2 and n = 6, respectively) were cultured for 72 h, and ELISA for activin A was performed on the supernatant. (B) MM1.S, INA6, and RPMI cells were cocultured with BMSCs for 24 h and the supernatant was analyzed for activin A expression levels by ELISA. The graph shows a quantification of three independent experiments. (C) INA6 and BMSCs were cocultured for 24 h with or without a transwell system, neutralizing antibody against VLA-4 (5 μg/mL), and ICAM-1 (10 μg/mL). The supernatant was analyzed for activin A expression levels by ELISA. The graph shows a quantification of three independent experiments. (D) BMSC were cocultured with fixed INA6 MM cells and harvested at the indicated time points to analyze JNK phosphorylation by Western blotting. (E) INA6 and BMSCs were cocultured for 24 h with or without a transwell system. In the last 15 h of culture, JNK inhibitor (SP600125, 20 μM), p38 inhibitor (SP202190, 20 μM), or DMSO 0.1% were added. The supernatant was analyzed for activin A expression levels by ELISA. Error bars represent SD. *P < 0.05, **P < 0.01.
The enhanced expression of activin A in patients with MM bone disease and in tumor-conditioned BMSC (compared with tumor-naive BMSCs or MM cells alone) led us to investigate whether activin A expression was being affected by the engagement of MM with BMSCs. We cultured several MM cell lines with tumor-naive BMSCs expressing low basal levels of activin A. The engagement of MM cells with healthy donor-derived BMSCs significantly increased activin A levels in the coculture supernatant by 2.5- to 6-fold (P < 0.05; Fig. 2B). By analyzing the expression levels of inhibin-βA subunits, of which activin is a dimer, and inhibin-α subunits, which form a heterodimer inhibin A, we confirmed that activin A is up-regulated in the stromal compartment by coculturing with MM cells (2.5-fold; P < 0.05; Fig. S2A).
To determine whether the induction of activin A expression was adhesion or cytokine-mediated, we used a transwell system that significantly inhibited activin A secretion (Fig. 2C), suggesting that direct MM–BMSC contact is necessary to induce activin A secretion. To further elucidate the ligand/receptor interactions leading to activin A secretion in coculture, we tested several molecules previously studied in the context of MM cell–BMSC adhesion, including CD40 ligand, osteopontin, intercellular adhesion molecule (ICAM)–1, and very late antigen (VLA)–4. Only neutralizing antibody against VLA-4 inhibited activin A secretion (20%; P < 0.05, Fig. 2C), albeit modestly. Of note, recombinant VLA-4 induced BMSC secretion of activin A, which was completely inhibited in the presence of neutralizing vascular cell adhesion molecule (VCAM)–1 antibody (Fig. S2B). These data suggests that the VLA4/VCAM-1 axis mediates activin A secretion, although the activation of other adhesion-mediated pathways likely contribute to activin A up-regulation in the presence of MM cells.
Activin A secretion by other cell types is known to occur via p38-dependent and JNK-dependent pathways (8). Because previous reports have particularly implicated activation of the JNK signaling pathway by cell-to-cell contact (9), and a highly conserved c-Jun–binding sequence is present in the INHβA promoter (10), we next investigated whether the JNK pathway was associated with activin A induction by coculture. Cell contact between BMSCs and MM cells was sufficient to activate the JNK pathway, evidenced by JNK phosphorylation in BMSCs by fixed MM cells (Fig. 2D). Additionally, treatment with specific JNK inhibitor (SP600125, 20 μM) reduced activin A secretion by BMSCs alone and completely inhibited MM-induced secretion of activin A (Fig. 2E). Inhibition of the p38 pathway (SP202190) served as a positive control.
Activin A Inhibits Osteoblastic Differentiation via SMAD2-Mediated DLX5 Down-Regulation.
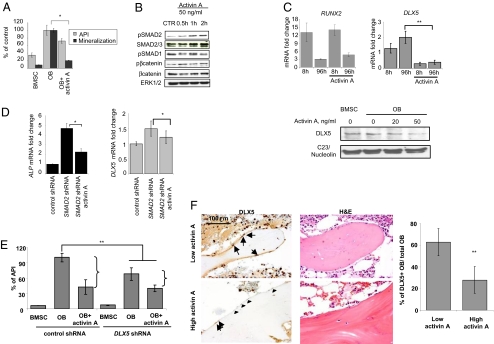
Activin A is a TGF-β family member whose role in maintaining the balance of osteoclastogenesis and osteoblastogenesis remains controversial (5, 11, 12). Consistent with recent results, we found that activin A activated OC differentiation (Fig. S3) (13) while inhibiting OB differentiation from tumor-naive BMSCs, evidenced by decreased alkaline phosphatase (ALP) enzyme activity and by decreased mineralization (P < 0.05; Fig. 3A) (6).
Fig. 3.
Activin A inhibits OB differentiation via DLX5 down-regulation. (A) Healthy donor–derived OBs were differentiated in the presence of activin A (50 ng/mL). ALP activity was detected after 2 weeks of differentiation with a chromogenic substrate and corrected for the number of viable cells quantified via AlamarBlue assay (i.e., API). OB activity was assessed at d 21 of differentiation by quantification of calcium deposits stained with alizarin red. (B) OBs were differentiated for 1 week and then incubated with activin A (50 ng/mL) for the indicated time points. Protein expression of phosphoSMAD2, SMAD2/3 phosphoSMAD1, phosphoß-catenin, β-catenin, and ERK1/2 were assessed by Western blot. (C) OBs were differentiated in the presence of activin A (50 ng/mL) for the indicated time points and the mRNA expression levels of RUNX2 (Left) and DLX5 (Middle) were assessed by quantitative-PCR. (Right) HS27-derived OBs were differentiated in the presence of various concentrations of activin A for 24 h. Nuclear protein extracts were performed to assess DLX5 and nucleolin expression. (D) After SMAD2 knockdown, BMSCs were stimulated with activin A (50 ng/mL) for 48 h and expression levels of ALP and DLX5 were assessed by quantitative PCR. (E) BMSCs were transduced with shRNA targeting DLX5 or control shRNA, and stimulated with activin A (50 ng/mL) in the presence of osteogenic media. ALP activity was detected after 10 d of differentiation with a chromogenic substrate and corrected for the number of viable cells quantified via AlamarBlue assay (i.e., API). (F) BM biopsies from MM patients with low (<50 pg/mL; n = 10) or high (≥50 pg/mL; n = 10) BM plasma levels of activin A were analyzed for DLX5 expression by IHC (counterstained with hematoxylin). The arrows indicate DLX5+ OBs and the arrowheads point at DLX5-OB. H&E staining of a corresponding field is also represented (Left). OB with nuclear expression of DLX5 were quantified and normalized against the total OB number (Right). Error bars represent SD. *P < 0.05, **P < 0.01.
We next investigated the signaling pathways responsible for activin A–mediated OB inhibition. Activin A induced SMAD2 phosphorylation in OB after 30 min of stimulation without affecting SMAD1 or β-catenin phosphorylation (Fig. 3B). Although SMAD2 downstream targets include RUNX2 and DLX5 genes (14), only DLX5 expression was markedly down-regulated in the presence of activin A by both mRNA and protein expression levels (Fig. 3C).
To determine the role of SMAD2 in OB inhibition, we transduced BMSCs with a lentivirus construct carrying a validated shRNA against human SMAD2. SMAD2 knockdown induced ALP gene expression (4.7-fold; P < 0.05) in pre-OB and addition of activin A partially reduced ALP expression but was unable to return expression to the baseline (2.26-fold increase; P < 0.05; Fig. 3D Right). Furthermore, SMAD2 knockdown derepressed DLX5 expression (1.5-fold; P < 0.05) whereas addition of exogenous activin A was unable to fully inhibit DLX5 expression (1.22-fold increase; P < 0.05; Fig. 3D Left).
We then evaluated whether DLX5 might be a crucial component of activin A–mediated impairment of OB differentiation. Osteogenic differentiation of cells transduced with control shRNA stimulated the ALP activity index (API), whereas addition of exogenous activin A decreased API by 58%. In contrast, DLX5 knockdown inhibited OB differentiation (32.3% inhibition of API) and the inhibitory effect of exogenous activin A on DLX5 knocked-down OBs was relatively attenuated (41% inhibition; Fig. 3E) and no synergy was observed. Taken together, these data suggest that activin A affects OB differentiation mainly via SMAD2-mediated DLX5 inhibition, although other pathways may also be involved.
Finally, to determine whether activin A–induced DLX5 repression observed in our in vitro studies was directly relevant to the pathogenesis of human MM osteolysis, we performed immunohistochemistry (IHC) for DLX5 on BM biopsies from MM patients and correlated with activin A levels. Patients with high activin A levels (>50 ng/mL, n = 5) had an average of 27.8% (range, 8.9–41%) OB stained for DLX5, whereas patients with low activin A levels (<50 ng/mL; n = 5) showed 62.7% OB staining (range, 47.9–75.3%, P < 0.01; Fig. 3F). Thus, the repression of DLX5 by activin A observed in vitro was corroborated in vivo by the reduction of DLX5-positive OBs in human MM BM samples with high activin levels and osteolytic disease.
Activin A Inhibition by the Soluble Decoy Receptor RAP-011 Rescues MM-Induced Impairment of OB Differentiation.
As shown in Fig. S1, tumor-conditioned BMSCs secrete high levels of activin A compared with naive BMSCs. To verify whether activin A might represent a potential therapeutic target against MM-induced osteolysis, we treated MM-derived BMSCs with an anti–activin A neutralizing antibody. A significant increase in OB differentiation was observed, confirmed by up-regulated ALP mRNA levels and enhanced API. This was also associated with a significant increase in bone mineralization assayed by alizarin red staining (three- and fivefold increase by 0.1 and 1 μg/mL, respectively; P < 0.05; Fig. S4).
Although activin A expression is down-regulated by OB differentiation (Fig. 2A) (6), we observed that MM cells stimulated activin A release in OB culture (Fig. S5), confirming our previous results (Fig. 2B).
We therefore hypothesized that if activin A was involved in coupling MM–OB interactions, its inhibition should reverse the OB impairment induced by MM cells, resulting in restoration of the pospho-SMAD2 and DLX5 axis. RAP-011 is a soluble activin A receptor that binds to activin A with high affinity (3.39 pM). Previous work in nonmalignant systems has shown that RAP-011 enhances OB mineralization and increases bone density in an osteoporotic mouse model (7). The humanized counterpart (ACE-011) is currently in clinical trials in patients with osteoporosis (15). Other than activin A, soluble activin A receptors bind four other ligands—bone morphogenic proteins (BMPs) 2, 3, 7 and inhibin A—at micromolar concentration (Fig. S6A) (7, 16, 17). As shown in Fig. S6B, activin A could be efficiently immunodepleted from MM-derived stromal supernatant, but we were unable to show any binding to BMP3, inhibin, BMP2, and BMP7. We therefore chose to use RAP-011 to achieve activin A inhibition in our models.
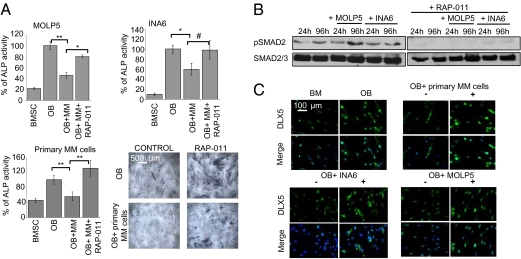
Exposure of BMSCs to both MM cell lines (MOLP5, INA6) and MM primary cells decreased OB differentiation, evident by decreased ALP activity; conversely, RAP-011 restored osteoblastic differentiation, suggesting implication of activin A in this inhibition (Fig. 4A). Several reports have also suggested that OBs reciprocally inhibit the proliferation and survival of MM cells (18, 19). We also observed that, compared with untreated BMSCs and OBs, OBs differentiated in the presence of RAP-011 did not support MM cell proliferation (Fig. S7). Therefore, RAP-011 stimulatory effects on OBs translated in inhibition of MM cell growth.
Fig. 4.
Inhibition of activin A reverses MM-induced OB inhibition. (A) OBs from healthy donor or HS27 cell line were differentiated in the presence of MM cell lines MOLP5 (Upper Left) and INA6 (Upper Right), and MM primary cells (Lower Left), respectively, with or without the soluble receptor for activin A, RAP-011 (50 μg/mL). After 2 weeks of differentiation, ALP activity was assessed with a chromogenic substrate. Cells were stained for ALP and methyl green was used as nuclear counterstaining (Lower Right). (B) OBs were differentiated in the presence of MM cell lines MOLP5 and INA6 for the indicated time-points with or without RAP-011 (50 μg/mL) as demonstrated. After magnetic bead depletion of CD38+ MM cells, phosphoSMAD2 and SMAD2/3 expression levels were assessed in the OB fraction by Western blot. (C) OBs were differentiated in the presence of MM cell lines MOLP5 and INA6 as well as MM primary cells either in the absence (minus signs) or in the presence of RAP-011 50 μg/mL (plus signs). After 1 week of differentiation, cells were fixed and stained with antibodies against DLX5 and counterstained with DAPI. Error bars represent SD. #P = 0.05, *P < 0.05, **P < 0.01.
Consistent with our studies, MM engagement of BMSCs was associated with increased SMAD2 phosphorylation in OB that was inhibited by RAP-011 (Fig. 4B). Additionally, whereas MM cell lines and primary cells down-regulated DLX5 expression in cocultured OBs to levels found in undifferentiated BMSCs, treatment with RAP-011 rescued DLX5 expression to comparable levels as noted in OBs (Fig. 4C).
Taken together, these results suggest that RAP-011, an inhibitor of activin A, enhances OB differentiation in the presence of MM cells via rescuing DLX5 expression, and this results in an indirect inhibition of MM cell proliferation.
RAP-011 Improves Osteolytic Disease and Exerts Antitumor Activity in a Humanized Myeloma Model.
To investigate whether activin A inhibition could overcome MM osteolytic disease and impact tumor growth in vivo, we used a well established mouse model of MM bone disease (SCID-hu model) (20).
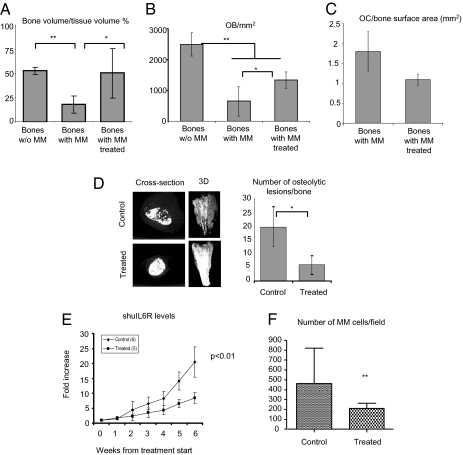
In the presence of MM cells, bone volume per tissue volume (BV/TV%) was significantly decreased compared with tumor-naive bone chips [mean, 17.6% ± 9 (SD) vs. 52.5% ± 3.7, respectively; P < 0.01). Treatment with RAP-011 markedly restored BV/TV% nearly back to tumor-naive levels, although bone fraction remained somewhat decreased even upon RAP-011 treatment (50.1% ± 25.6; P < 0.05; Fig. 5A). Similarly, OB number per mm2 was markedly decreased in the bone chips injected with MM cells compared tumor-naive bones (645.4 ± 476.1 vs. 2,490 ± 377, respectively; P < 0.01); conversely, RAP-011 treatment reversed this negative trend, although we did not observe a complete rescue (1,336.2 ± 270.48; P < 0.05; Fig. 5B). Of note, we observed a decreased OC number/bone area, suggesting that RAP-011 has a negative effect on OC (Fig. 5C).
Fig. 5.
Inhibition of activin A with a soluble receptor, RAP-011, improves MM-related osteolytic disease and impairs MM cell growth in vivo. (A) Midsections of non–tumor-injected bones (n = 6) and tumor-injected bones harvested from, respectively, control (n = 4) and treated mice (n = 4) were stained for DAPI and H&E and photographed through its entire length at low power (40×). Bone volume was then quantified and corrected for the total tissue areas. (B) OBs were counted on H&E–stained slides and cell number expressed per tissue area (in mm2). (C) OCs were counted after TRAP staining and number expressed per bone surface area (in mm2). (D) A representative cross-section image and 3D reconstruction of the harvested human bones obtained performing high-resolution CT scan is shown. Osteolytic lesions, identified as low bone density, discrete, punctuate areas, were quantified on the 3D images from control (n = 4) and treated mice (n = 4). (E) Tumor growth was monitored as levels of circulating sHuIL6R, secreted by INA6 MM cells. (F) Midsections of each bone were photographed in GFP channel through its entire length at low power (40×) and GFP+ INA6 MM cells were counted. Error bars represent SD. *P < 0.05, **P < 0.01.
High-resolution CT scan performed on the human bones demonstrated increased bone mass (a representative image is shown in Fig. 5D Left). A 75% decrease in the number of OLs was observed in the RAP-011–treated group (P < 0.02; Fig. 5D Right). Importantly, DLX5 expression analyzed by IHC was increased along the bone surface in the RAP-011 treated bones, whereas almost no DLX5 staining was evident on the trabecular surface of the untreated bones (Fig. S8A).
As increasing OB number may result in inhibition of MM proliferation, we next evaluated the effects of modifying the OC/OB niche on the tumor cell compartment. We quantified MM cell growth in our in vivo model using soluble human IL-6 receptor (sHuIL6R) secreted by INA-6 MM cells. Treatment with RAP-011 showed significant anti-MM effects as seen by a decrease in sHuIL6R levels observed as early as week 2 that persisted over the 4-week treatment period (n = 11; P < 0.01; Fig. 5E). Additionally, direct quantification of MM burden in the human bones by counting GFP+ cells in midsection bones through the entire bone area also revealed a reduction of MM cells in the RAP-011–treated animals (P < 0.01; Fig. 5F and Fig. S8B).
These data support the conclusion that activin A is a promising target for the treatment of MM-induced OLs.
Discussion
Bone disease represents a significant clinical problem in the management of MM patients, yet current knowledge of the MM–microenvironment interactions leading to osteolysis is limited, as are the treatment options available to our patients. In this study we have identified activin A among a broad panel of cytokines as a critical player in MM-induced bone disease. We show that activin A is a stromally derived OB inhibitor induced by MM cells via the JNK pathway. MM-induced activin A down-regulates DLX5 gene expression via SMAD2 activation that can be effectively blocked by activin A inhibition with a soluble receptor, RAP-011. Our in vivo animal studies confirmed the anabolic effects of activin A inhibition. Furthermore, the antitumor activity observed with RAP-011 supports the notion that targeting the tumor niche cross-talk via activin A inhibition is a promising therapeutic strategy in tumor-related bone disease.
Activin A is a TGF-β superfamily member most commonly associated with embryogenesis and gonadal hormone signaling (21). In addition, activin A is involved in bone remodeling with growth stimulatory effects on OCs (13). In contrast, the effects on OB differentiation are still unclear; both inhibition and promotion of OB differentiation by exogenous activin have been reported (5, 6, 11). However, in vivo evidence supports the hypothesis of an inhibitory role on OBs for activin A. Both transgenic expression of inhibin or treatment with a soluble receptor for activin, RAP-011, result in enhanced bone formation rate and bone mass in vivo (7, 22). Our data confirmed these findings and demonstrate DLX5 down-regulation as the main mechanism of action for activin A. Indeed, DLX5 is a critical transcription factor in OB differentiation, regulating the expression of osterix (23). DLX5 is also a common gene target for other TGF-β family members as well as the β-catenin signaling pathway (24, 25). For example, differential effects on DLX5 transcription account for the opposing effects of Wnt10b, BMP2, and TGF-β on OB differentiation (14, 25). Importantly, our data show that forced DLX5 expression via SMAD2 knockdown only partially rescues activin A–induced OB inhibition, suggesting that other signaling pathways may be influenced by activin A. Importantly, SMAD2 is also a downstream mediator of TGF-β signaling. As TGF-β plays an important role in cancer metastasis and osteolysis (26, 27), SMAD2-mediated OB inhibition may represent an additional pathogenic mechanism in TGF-β–induced bone disease. Future studies will address this hypothesis.
Tumor cells rely on their microenvironment for growth and survival. In turn, they shape their microenvironment by creating a milieu promoting OC and inhibiting OB formation, resulting in the creation of a permissive cancer niche. These changes are mainly induced by cytokines directly released by MM cells, such as B-cell activating factor, CCL3, and Dickoppf -1 (28–30). Activin is normally synthesized and secreted by BMSCs and mediates an autocrine regulatory loop on stromal differentiation into OB (31). Here, we show that malignant plasma cells disrupt the normal regulatory pathway of bone homeostasis by inducing BMSC secretion of activin A via JNK pathway. MM-induced activin A secretion mediates OB inhibition both in vitro and in vivo and is regulated in part by SMAD2-DLX5 signaling. Targeting this unique pathway by using RAP-011, a soluble activin A receptor, we have demonstrated reversal of these effects. RAP-011 treatment prevents the development of OLs and inhibits tumor growth in a humanized model of MM bone disease. These results suggest that targeting the MM–microenvironment interactions with the purpose of restoring bone homeostasis and creating a hostile niche for tumor cell growth may provide an alternative approach for the development of anticancer therapies. Indeed, interventions that alter the malignant cell niche may be a promising dimension of anticancer therapeutics.
Experimental Procedures
Patients.
We studied BM plasma from 28 patients with MM at diagnosis and 10 non-MM patients as control subjects, including patients with acute leukemia (n = 5), non-Hodgkin lymphoma (n = 1), thyroid cancer (n = 1), primary amyloidosis (n = 1), osteoporosis (n = 1), and anemia of chronic disease (n = 1). Additionally, 10 BM biopsy samples were obtained in MM patients to perform IHC analysis for DLX5. All patients provided written informed consent per the Declaration of Helsinki, and approval was obtained by the institutional review board of the Massachusetts General Hospital Cancer Center (Boston, MA).
Mouse Model.
All animal studies were conducted according to protocols approved by the Institutional Animal Care and Use Committee. The SCID-hu model was generated as previously described (20). Four weeks after INA6 injection, we started s.c. injections of RAP-011 (10 mg/kg twice per week) for 28 d. Two weeks after the end of the treatment schedule, the mice were killed and eight matching bone chips harvested. Each bone was sectioned in half and processed for either cryosectioning or paraffin-embedding. Six non–tumor-injected bones were obtained from fetal bones of similar age and processed like the tumor-injected bones, except that animals were not implanted with MM. These bones were used as controls. See SI Experimental Procedures for more information.
Supplementary Material
Acknowledgments
Grant support for this study was received in the form of an International Myeloma Foundation junior award (S.V., L.S., S.P.), K08 (S.M.), NIH (D.T.S.), ASCO CDA, MMRF, LLS CDA, and NIH SPORE (N.R.).
Footnotes
*This Direct Submission article had a prearranged editor.
Conflict of interest statement: J.S.S. is an employee of Acceleron Pharma. He provided RAP-011 but was not involved with the experimental design.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911929107/DCSupplemental.
References
- 1.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Natl Rev. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 3.Michigami T, et al. Cell-cell contact between marrow stromal cells and myeloma cells via VCAM-1 and alpha(4)beta(1)-integrin enhances production of osteoclast-stimulating activity. Blood. 2000;96:1953–1960. [PubMed] [Google Scholar]
- 4.Roodman GD. New potential targets for treating myeloma bone disease. Clin Cancer Res. 2006;12:6270s–6273s. doi: 10.1158/1078-0432.CCR-06-0845. [DOI] [PubMed] [Google Scholar]
- 5.Ikenoue T, Jingushi S, Urabe K, Okazaki K, Iwamoto Y. Inhibitory effects of activin-A on osteoblast differentiation during cultures of fetal rat calvarial cells. J Cell Biochem. 1999;75:206–214. doi: 10.1002/(sici)1097-4644(19991101)75:2<206::aid-jcb3>3.3.co;2-k. [DOI] [PubMed] [Google Scholar]
- 6.Eijken M, et al. The activin A-follistatin system: potent regulator of human extracellular matrix mineralization. FASEB J. 2007;21:2949–2960. doi: 10.1096/fj.07-8080com. [DOI] [PubMed] [Google Scholar]
- 7.Pearsall RS, et al. A soluble activin type IIA receptor induces bone formation and improves skeletal integrity. Proc Natl Acad Sci USA. 2008;105:7082–7087. doi: 10.1073/pnas.0711263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funaba M, Ikeda T, Ogawa K, Abe M. Calcium-regulated expression of activin A in RBL-2H3 mast cells. Cell Signal. 2003;15:605–613. doi: 10.1016/s0898-6568(02)00150-x. [DOI] [PubMed] [Google Scholar]
- 9.Snider JL, Allison C, Bellaire BH, Ferrero RL, Cardelli JA. The beta1 integrin activates JNK independent of CagA, and JNK activation is required for Helicobacter pylori CagA+-induced motility of gastric cancer cells. J Biol Chem. 2008;283:13952–13963. doi: 10.1074/jbc.M800289200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanimoto K, et al. Human activin betaA gene. Identification of novel 5′ exon, functional promoter, and enhancers. J Biol Chem. 1996;271:32760–32769. doi: 10.1074/jbc.271.51.32760. [DOI] [PubMed] [Google Scholar]
- 11.Gaddy-Kurten D, Coker JK, Abe E, Jilka RL, Manolagas SC. Inhibin suppresses and activin stimulates osteoblastogenesis and osteoclastogenesis in murine bone marrow cultures. Endocrinology. 2002;143:74–83. doi: 10.1210/endo.143.1.8580. [DOI] [PubMed] [Google Scholar]
- 12.Kawabata N, Kamiya N, Suzuki N, Matsumoto M, Takagi M. Changes in extracellular activin A:follistatin ratio during differentiation of a mesenchymal progenitor cell line, ROB-C26 into osteoblasts and adipocytes. Life Sci. 2007;81:8–18. doi: 10.1016/j.lfs.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Fuller K, Bayley KE, Chambers TJ. Activin A is an essential cofactor for osteoclast induction. Biochem Biophys Res Commun. 2000;268:2–7. doi: 10.1006/bbrc.2000.2075. [DOI] [PubMed] [Google Scholar]
- 14.Lee MH, et al. BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J Biol Chem. 2003;278:34387–34394. doi: 10.1074/jbc.M211386200. [DOI] [PubMed] [Google Scholar]
- 15.Ruckle J, et al. Single-dose, randomized, double-blind, placebo-controlled study of ACE-011 (ActRIIA-IgG1) in postmenopausal women. J Bone Miner Res. 2009;24:744–752. doi: 10.1359/jbmr.081208. [DOI] [PubMed] [Google Scholar]
- 16.del Re E, Sidis Y, Fabrizio DA, Lin HY, Schneyer A. Reconstitution and analysis of soluble inhibin and activin receptor complexes in a cell-free system. J Biol Chem. 2004;279:53126–53135. doi: 10.1074/jbc.M408090200. [DOI] [PubMed] [Google Scholar]
- 17.Allendorph GP, Isaacs MJ, Kawakami Y, Izpisua Belmonte JC, Choe S. BMP-3 and BMP-6 structures illuminate the nature of binding specificity with receptors. Biochemistry. 2007;46:12238–12247. doi: 10.1021/bi700907k. [DOI] [PubMed] [Google Scholar]
- 18.Yaccoby S, et al. Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica. 2006;91:192–199. [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards CM, et al. Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood. 2008;111:2833–2842. doi: 10.1182/blood-2007-03-077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tassone P, et al. A clinically relevant SCID-hu in vivo model of human multiple myeloma. Blood. 2005;106:713–716. doi: 10.1182/blood-2005-01-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodruff TK. Regulation of cellular and system function by activin. Biochem Pharmacol. 1998;55:953–963. doi: 10.1016/s0006-2952(97)00477-2. [DOI] [PubMed] [Google Scholar]
- 22.Perrien DS, et al. Inhibin A is an endocrine stimulator of bone mass and strength. Endocrinology. 2007;148:1654–1665. doi: 10.1210/en.2006-0848. [DOI] [PubMed] [Google Scholar]
- 23.Samee N, et al. Dlx5, a positive regulator of osteoblastogenesis, is essential for osteoblast-osteoclast coupling. Am J Pathol. 2008;173:773–780. doi: 10.2353/ajpath.2008.080243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holleville N, Quilhac A, Bontoux M, Monsoro-Burq AH. BMP signals regulate Dlx5 during early avian skull development. Dev Biol. 2003;257:177–189. doi: 10.1016/s0012-1606(03)00059-9. [DOI] [PubMed] [Google Scholar]
- 25.Bennett CN, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 27.Kominsky SL, Doucet M, Brady K, Weber KL. TGF-beta promotes the establishment of renal cell carcinoma bone metastasis. J Bone Miner Res. 2007;22:37–44. doi: 10.1359/jbmr.061005. [DOI] [PubMed] [Google Scholar]
- 28.Choi SJ, et al. Antisense inhibition of macrophage inflammatory protein 1-alpha blocks bone destruction in a model of myeloma bone disease. J Clin Invest. 2001;108:1833–1841. doi: 10.1172/JCI13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian E, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 30.Neri P, et al. Neutralizing B-cell activating factor antibody improves survival and inhibits osteoclastogenesis in a severe combined immunodeficient human multiple myeloma model. Clin Cancer Res. 2007;13:5903–5909. doi: 10.1158/1078-0432.CCR-07-0753. [DOI] [PubMed] [Google Scholar]
- 31.Shao LE, Frigon NL, Jr, Yu A, Palyash J, Yu J. Contrasting effects of inflammatory cytokines and glucocorticoids on the production of activin A in human marrow stromal cells and their implications. Cytokine. 1998;10:227–235. doi: 10.1006/cyto.1997.0282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.