Abstract
The Wnt/β-catenin signaling pathway is activated in breast cancer, a leading cause of cancer mortality in women. Because mutations in the key intracellular components of this pathway are rare, identifying the molecular mechanisms of aberrant Wnt activation in breast cancer is critical for development of pathway-targeted therapy. Here, we show that expression of the Wnt signaling coreceptor LRP6 is up-regulated in a subpopulation of human breast cancers. LRP6 silencing in breast cancer cells reduces Wnt signaling, cell proliferation, and in vivo tumor growth. In vivo administration of an LRP6 antagonist, Mesd, markedly suppressed growth of MMTV-Wnt1 tumors without causing undesirable side effects. These results demonstrate that Wnt activation at the cell surface contributes to breast cancer tumorigenesis. Together, our studies highlight LRP6 as a potential therapeutic target in breast cancer, and introduce Mesd as a promising antitumor agent for treating breast cancer subtypes with Wnt activation at the cell surface.
Keywords: Wnt signaling, gene silencing, tumorigenesis, proliferation, Mesd
Breast cancer causes more than 40,000 deaths annually, making this disease the second leading cause of cancer mortality among American women. It is a complex disease that comprises at least 18 distinct histopathological entities (1). In addition to chemotherapeutic agents, tamoxifen, an antiestrogen agent used for treating estrogen receptor (ER)–positive breast tumors, and trastuzumab, a monoclonal antibody targeting human epidermal growth factor receptor 2 (HER2)–overexpressing tumors, have benefited specific subsets of breast cancer patients (2, 3). However, the enormous variation in cellular pathways driving breast cancer initiation and growth complicate biological approaches to this malignancy and limit the effectiveness of current therapies. For example, breast cancers that are ER, progesterone receptor (PR) and HER2 triple negative are highly aggressive and exhibit poor prognosis (4).
The Wnt/β-catenin signaling pathway is involved in various differentiation events during embryonic development and can lead to tumor formation when aberrantly activated (5–7). Activation of the canonical Wnt pathway involves the stabilization of β-catenin through the binding of Wnt ligands to cell surface receptors: Frizzled (Fz) family receptors and low-density lipoprotein receptor (LDLR)–related protein 5 (LRP5) and LRP6. In the absence of Wnt ligands, β-catenin is phosphorylated by a multiprotein degradation complex, which marks it for ubiquitination and degradation by the proteasome. In the presence of appropriate Wnt ligands, β-catenin is stabilized and can translocate to the nucleus and act as a transactivator of TCF/LEF transcription factors, regulating crucial target genes that promote cell proliferation, differentiation, and tissue development (8). In the mammary tissues, Wnt signaling plays an important role in stem cell self-renewal and mammary gland development. Compelling evidence indicates that when the Wnt/β-catenin pathway is aberrantly activated, it may lead to mammary carcinogenesis (9–12). Specifically, enhanced nuclear/cytoplasmic β-catenin staining was found in ∼60% of human breast cancer specimens (11, 13). However, it is surprising that classical mutations in Wnt pathway components, such as Adenomatous polyposis coli (APC), Ctnnb1 (encoding β-catenin), and Axin, which are frequent and responsible for the development of several types of human cancers, are rarely detected in human breast cancer (10, 14). Because of the lack of mutations in the intracellular components of this pathway, the underlying cause of aberrant Wnt activation in breast cancer remains unexplained (10, 12, 14). LRP5/6, type I transmembrane proteins of the LDLR family, are essential coreceptors for canonical Wnt signaling. A truncated LRP5 is implicated in breast tumor formation, and increased LRP6 expression is sufficient to trigger Wnt activation, cell proliferation, and tumorigenesis (15–17). Therefore, we hypothesized that overexpression of components upstream of the intracellular signaling cascade, in particular the Wnt receptors LRP5/6, contribute to breast cancer tumorigenesis. Here, we demonstrate that expression of LRP6, but not LRP5, is frequently up-regulated in a subset of human breast carcinomas, and that down-regulation of LRP6 is sufficient to inhibit breast cancer tumorigenesis. In addition, we also identify Mesd (mesoderm development), a specialized chaperone for LRP5/6 (18), as an LRP6 antagonist capable of blocking breast cancer tumor growth in vivo without significant toxicity.
Results
LRP6 Expression Is Frequently Up-Regulated in Human Breast Cancer.
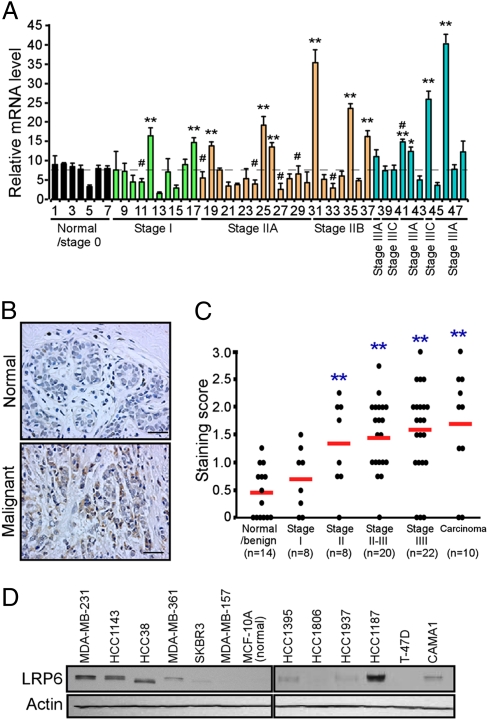
To explore the role of LRP6 in breast cancer, we first analyzed LRP6 expression in human breast cancer tissues using a real-time PCR–based tissue array. Of 41 breast cancer cases with disease stages ranging from Stage I to IIIC, 10 exhibited significant increases in LRP6 transcripts compared to normal mammary tissues (Fig. 1A). LRP6 was up-regulated more frequently in ER- or HER2-negative tissues (Fig. S1 A and B). Because we did not observe significant up-regulation of LRP5 in human breast cancer tissues (Fig. S1C), we primarily investigated the role of LRP6 in breast cancer tumorigenesis. To extend this study, we next used immunostaining to analyze LRP6 expression levels in a tissue array containing common types of breast carcinoma and nonmalignant mammary tissues. The specificity of LRP6 immunostaining was first confirmed in control and LRP6 knocked down (KD) tumor tissues derived from MDA-MB-231 cells. Significantly, moderate to strong staining for LRP6 was observed in subsets of breast carcinomas, resulting in higher mean scores compared with normal/benign tumor tissues (Fig. 1 B and C). LRP6 is up-regulated more frequently in triple-negative, ER-negative, or HER2-negative tumors (Fig. S1 D–F). To further investigate LRP6 expression in breast cancer, we examined 14 human breast cancer cell lines (nine ER-negative and five ER-positive) using SuperArray to profile the expression of 84-Wnt-related genes, including LRP5, LRP6, Wnt ligands and several Wnt target genes. LRP6 expression is more than 6-fold higher in seven of 14 breast cancer cell lines compared with nontransformed MCF-10A cells (Table S1). Furthermore, LRP6 was overexpressed at the protein level in six of 12 breast cancer cell lines (Fig. 1D). Together, these results demonstrate that up-regulation of LRP6 expression is a common event among defined subsets of human breast cancers.
Fig. 1.
LRP6 expression is frequently up-regulated in a subset of human breast cancer tissues and cell lines. (A) Breast cancer TissueScan Real-Time qPCR array, containing seven normal/Stage 0 cDNAs and 41 human breast cancer cDNAs, was analyzed for LRP6 expression by real-time PCR. Averages of relative LRP6 expression from three independent plates are plotted with clinical status indicated. LRP6 mRNA levels are markedly up-regulated in a subset of human breast cancer tissues. #Samples with elevated HER2 transcripts. (B and C) Breast cancer tissue microarray was used for IHC staining of LRP6. (B) Representatives of LRP6 staining in normal and malignant breast tissue are shown. LRP6 antibody (C-term T1546, Abgent), which specifically recognizes human LRP6, was used for IHC staining. (C) The quantification of LRP6 IHC staining was determined from three independent experiments. Staining intensity was scored as absent (0), weak (1), moderate (2), or strong (3). Four observations were made on each slide by independent investigators, and a mean score was recorded. (D) Expression of LRP6 in human mammary epithelial cell (MCF-10A) and indicated breast cancer cell lines analyzed by Western blot analysis. *P < 0.05; **P < 0.01.
Down-Regulation of LRP6 in Breast Cancer Cells Attenuates Wnt/β-Catenin Signaling and Inhibits Cell Proliferation.
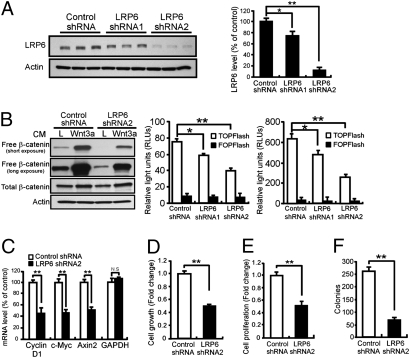
We next examined the effects of modulating LRP6 expression on Wnt signaling and tumorigenesis in breast cancer cells. Using two independent lentiviral shRNAs targeting distinct regions of LRP6, we knocked down LRP6 expression in MDA-MB-231 (Fig. 2A and Fig. S3A) and HCC1187 cells (Fig. S2A), which display relatively high levels of endogenous LRP6. Free β-catenin pool and TCF-dependent TOPFlash reporter activity, measures of Wnt/β-catenin signaling strength, were significantly reduced when LRP6 was knocked down in MDA-MB-231 and HCC1187 cells (Fig. 2B and Fig. S2 B and C). Finally, expression of Cyclin Dl and c-Myc, Wnt target genes critical for cell cycle regulation, was significantly decreased in LRP6-KD cells (Fig. 2C and Fig. S2D). Expression of Axin2, a well-recognized Wnt target, as well as several other Wnt target genes, was also suppressed when LRP6 expression was knocked down (Fig. 2C and Figs. S2D and S3B). These results demonstrate that decreased LRP6 expression is sufficient to down-regulate Wnt signaling in breast cancer cells.
Fig. 2.
Knockdown of LRP6 in MDA-MB-231 breast cancer cells decreases Wnt signaling, breast cancer cell viability, proliferation, and colony formation. MDA-MB-231 cells were transduced with lentivirus expressing control or LRP6 shRNA. Cells were then subjected to the indicated analysis 48 h postinfection. (A) Western blot and densitometric analysis show that both LRP6 shRNAs reduce LRP6 expression in MDA-MB-231 cells compared with control shRNA. (B) LRP6 down-regulation suppressed Wnt signaling examined by free β-catenin pull-down (Left) and TOPFlash reporter assays in the absence (Center) and presence (Right) of Wnt3a ligands. (C) Quantitative real-time PCR shows that expression of Wnt target genes (Cyclin D1, c-Myc, and Axin2) is down-regulated in cancer cells expressing LRP6 shRNA2. GAPDH was included as a control gene. (D) Knockdown of LRP6 decreased cell viability assessed by MTT assay. (E) Proliferation of breast cancer cells expressing LRP6 shRNA2 was suppressed by ∼50% as measured by BrdU incorporation. (F) Soft agar colony formation assay demonstrating reduced colony formation when LRP6 expression was knocked down. Data are mean ± SD from three independent experiments. *P < 0.05; **P < 0.01.
We next examined whether the tumorigenic properties of breast cancer cells are affected in LRP6-KD breast cancer cells. Cell growth slowed when LRP6 expression was knocked down in MDA-MB-231 cells (Fig. 2D). MDA-MB-231 and HCC1187 cells expressing LRP6 shRNA exhibited significantly decreased proliferation (Fig. 2E and Fig. S2E), whereas apoptosis was not affected (Fig. S3C). LRP5/6 inhibitors Dkk1 (Dickkopf1) or Mesd suppressed cell growth in HCC1187 cells (Fig. S2F). Consistent with this notion, Wnt3a CM significantly increased HCC1187 cell growth and this effect was abolished by Dkk1 or Mesd (Fig. S2F). LRP6-KD cells displayed markedly lower frequencies of colony formation and smaller colony size (Fig. 2F and Fig. S3D), indicating that LRP6 down-regulation has a strong inhibitory effect on anchorage-independent growth of MDA-MB-231 cells. Furthermore, we found that down-regulation of LRP6 suppresses Wnt signaling and cell growth in T-47D breast cancer cells, which express low levels of endogenous LRP6 (Fig. 1D and Fig. S4 A–D). Together, these results suggest that inhibition of LRP6 might be an effective strategy to suppress the growth of breast cancer with aberrant Wnt signaling activation.
Overexpression of shRNA-Resistant LRP6 or Constitutively Active β-Catenin Rescues Wnt Signaling and Breast Cancer Cell Growth.
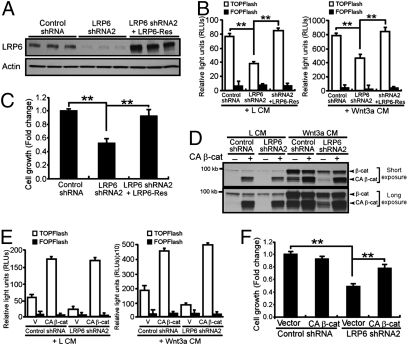
To confirm that the observed effects on breast cancer cell growth were attributable specifically to LRP6 knockdown, we generated an shRNA-resistant LRP6 (LRP6-Res) construct for rescue experiments. Transfection of LRP6-Res construct into LRP6-KD cells markedly increased LRP6 expression in MDA-MB-231 cells (Fig. 3A). More importantly, overexpression of LRP6-Res restored Wnt/β-catenin activation (Fig. 3B and Fig. S5 A and B) and cell growth (Fig. 3C). To determine whether the phenotypes resulted from LRP6 knockdown depend on β-catenin, we infected MDA-MB-231 control and LRP6-KD cells with a constitutively active form of β-catenin (CA β-catenin) or its corresponding vector-control retrovirus (Fig. S5C). CA β-catenin expression significantly increased Wnt signaling and rescued MDA-MB-231 cell growth (Fig. 3 D–F).
Fig. 3.
shRNA-resistant LRP6 and CA β-catenin rescue Wnt signaling and cell growth in MDA-MB-231 cells. (A and B) MDA-MB-231 cells expressing control or LRP6 shRNA were transfected with vector control or shRNA-resistant LRP6 (LRP6-Res). (A) Levels of LRP6 expression were examined by Western blot analysis. (B) Cells were then treated with L or Wnt3a CM. Expression of LRP6-Res in MDA-MB-231 cells restored Wnt signaling, detected by TOPFlash reporter assay. (C) Expression of LRP6-Res in MDA-MB-231 cells rescued cell growth detected by MTT assay. (D–F) MDA-MB-231 cells expressing control or LRP6 shRNA were transduced with retrovirus expressing IRES-GFP vector control or CA β-catenin. (D) Free β-catenin levels were analyzed by GST–E-cadherin pull-down. (E) CA β-catenin promoted Wnt activation independent of Wnt3a ligand. (F) CA β-catenin expression restored breast cancer cell growth determined by MTT assay. All results are the mean ± SD of three independent experiments. *P < 0.05; **P < 0.01.
Overexpression of LRP6 Is Sufficient to Induce Wnt Signaling and to Promote Breast Cancer Cell Growth.
To investigate whether overexpression of LRP6 enhances Wnt signaling in breast cancer cells, LRP6 cDNA was transfected into T-47D cells, which express low levels of endogenous LRP6. Wnt signaling and Wnt target expressions were significantly increased in T-47D-LRP6 cells compared with T-47D-vector control cells (Fig. S4 E–H). In addition, overexpression of LRP6 is sufficient to promote cell growth in these T-47D breast cancer cells (Fig. S4I). These results further demonstrate the importance of LRP6 in controlling Wnt signaling and cell growth in breast cancer cells.
Down-Regulation of LRP6 in Breast Cancer Cells Suppresses Tumor Growth.
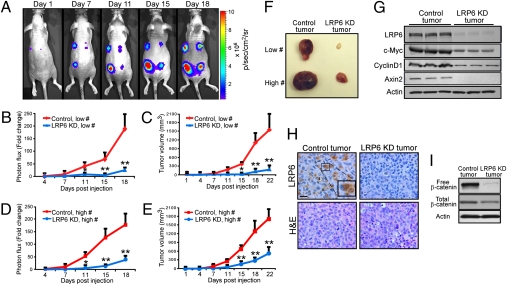
To investigate whether down-regulation of LRP6 affects breast cancer tumorigenesis in vivo, we established a tumor xenograft model by generating stable pools of either control or LRP6-KD MDA-MB-231 cells expressing the firefly luciferase reporter (MDA-MB-231-Luc). MDA-MB-231-Luc control or LRP6-KD cells were s.c. injected into female immunodeficient mice. Each mouse received LRP6-KD cells in one flank of the back and control cells in the other, providing intra-animal comparisons. Mice bearing xenograft tumors were subjected to in vivo imaging twice weekly (Fig. 4A). Strikingly, tumors derived from MDA-MB-231-Luc cells stably expressing LRP6 shRNA grew substantially slower than those derived from control cells as evaluated by both live-animal imaging and standard external calipers (Fig. 4 B–E). In the xenograft experiments, gross examinations of control and LRP6-KD tumors at necropsy (Fig. 4F) demonstrated that LRP6-KD tumors were significantly smaller than control tumors (490 ± 195mm3 vs. 1,785 ± 418 mm3, respectively). Importantly, LRP6-KD tumors showed decreased levels of Wnt signaling and target gene expression as a direct result of LRP6 knockdown (Fig. 4 G–I and Fig. S3 E and F). Taken together, these results demonstrate that LRP6 plays a crucial role in breast cancer tumorigenesis of MDA-MB-231 cells and that down-regulation of LRP6 is sufficient to inhibit tumor growth in vivo.
Fig. 4.
Down-regulation of LRP6 significantly inhibits breast tumor growth in vivo. MDA-MB-231 cells (pooled clones) stably expressing control or LRP6 shRNA were injected s.c. into female BNX immunocompromised mice. Tumors initiated from 5 × 105 or 2 × 106 cancer cells were indicated as Low # or High #, respectively. Low #, thoracic pair; High #, caudal pair; control tumors, left; LRP6-KD tumors, right. Tumor growth was monitored over time for 3 weeks by in vivo bioluminescent imaging and caliper measurements. (A) Representative bioluminescence images over time from the same mouse bearing MDA-MB-231 xenografts. (B and D) Growth of tumors over time for control and LRP6-KD xenografts is shown as fold changes of bioluminescence photon flux values over initial value (1 day postinjection). Data are mean ± SEM (six animals, four tumors each) from two independent experiments. (C and E) Tumor volume was monitored for 3 weeks by caliper measurement. (LxWxD). *P < 0.05; **P < 0.01. (F) Gross examination of xenograft tumors. (G) Levels of LRP6 and Wnt target gene expressions (Cyclin D1, c-Myc, and Axin2) in control and LRP6-KD xenograft tumors detected by Western blot analysis. (H) Immunohistochemical analysis of LRP6 level in control and LRP6-KD xenograft tumors with anti-LRP6 antibody (Abgent). (Scale bars, 50 μm.) (I) Western blot analysis showing total and free β-catenin in LRP6-KD tumors were decreased.
LRP6 Antagonist Mesd Suppresses Tumor Growth in Vivo.
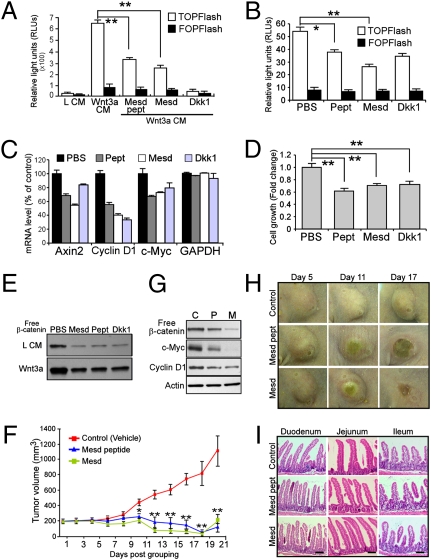
Mesd was discovered because of its requirement in the proper folding of LRP5/6 (18). We have previously demonstrated that exogenously administrated Mesd binds specifically to mature LRP5/6 at the cell surface and antagonizes ligand binding (19, 20). A 38-amino acid region in Mesd C terminus is both necessary and sufficient for its binding to LRP6. Here, we further confirmed that Mesd and Mesd peptide inhibit Wnt signaling (Fig. 5A). Because LRP6 knockdown is sufficient to suppress Wnt signaling and to inhibit breast cancer tumorigenesis, we next explored whether LRP6 could be a target of inhibition by Mesd in breast cancer cells. We found that Mesd and Mesd peptide effectively inhibit Wnt signaling, target gene expression, and cell growth in unstimulated HCC1187 breast cancer cells in which LRP6 is overexpressed (Fig. 5 B–E and Fig. S6 A and B). We further investigated the effects of targeting cell surface Wnt receptors for breast cancer therapy in a well-characterized Wnt1-driven tumor model. Transgenic mice in which Wnt1 is overexpressed under the control of the mouse mammary tumor virus (MMTV) promoter develop spontaneous mammary adenocarcinoma (21), making it a suitable model for examining the therapeutic effects of Mesd and Mesd peptide. The pharmacokinetics and bioavailability of Mesd and Mesd peptide were first investigated (Fig. S7). To take advantage of the established techniques and to overcome the wide range of tumor latency in MMTV-Wnt1 mice (4–6 months) (21, 22), cells from MMTV-Wnt1 tumors were injected into the mammary fat pads of female nude mice, and the resultant mice were subsequently randomly grouped when the mean tumor volume reached ca. 200 mm3. Mice were then treated with recombinant Mesd (10 mg/kg), Mesd peptide (10 mg/kg), or vehicle control (PBS) for 3 weeks. Remarkably, administration of Mesd or Mesd peptide inhibited Wnt1-induced Wnt signaling activation and resulted in significant suppression of tumor growth even though LRP6 expression was unchanged compared with control mammary glands (Fig. 5 F–H and Fig. S6C). Consistent with this notion, a growth inhibitory effect was also observed in T-47D cells (Fig. S6D). These results suggest that targeting of LRP5/6 with the antagonist Mesd warrants further exploration as a potential therapy for breast cancer.
Fig. 5.
Therapeutic effect of Mesd treatment on human breast cancer cells and MMTV-Wnt1 tumor xenografts. (A) Wnt inhibitory effect was analyzed by measuring luciferase activity in Wnt3a-stimulated HEK293 cells stably expressing TOPFlash reporter treated with indicated reagents. Cells were incubated with L CM, Wnt3a CM, or Wnt3a CM together with Mesd protein (1 μM), Mesd peptides (1 μM) or Dkk1 (10 nM) for 16 h at 37°C. (B and C) Treatment with Mesd (5 μM), Mesd peptide (5 μM), or Dkk1 (50 nM) decreased Wnt signaling in unstimulated HCC1187 cells examined by TOPFlash reporter assay (B) and Wnt target gene expression assessed by qRT-PCR (C). (D and E) Mesd treatment suppressed free β-catenin accumulation (E) and cell growth (D) in HCC1187 cells. (F) Mesd and its peptide significantly inhibited tumor growth. Representative pictures of tumors upon treatment are shown in H. Mice bearing established MMTV-Wnt1 tumor transplants were divided into three groups (five animals per group) and were i.p. injected with PBS (vehicle), Mesd, or Mesd peptide every other day. Tumor volume was analyzed using caliper measurement. Data represent at least three independent experiments; each time point represents the mean tumor volume ± SEM. *P < 0.05; **P < 0.01. (G) Mesd peptide (P) and Mesd (M) treatment decreased Wnt signaling compared with control (C) treatment as confirmed by GST–E-cadherin pull-down and target gene expressions in MMTV-Wnt1 tumors. (I) No significant adverse effect on small intestine with Mesd and Mesd peptide administration is apparent upon gross examination. (Scale bar, 50 μm.)
Numerous studies have demonstrated crucial roles of the Wnt signaling pathway in regulating self-renewal and differentiation of various stem cells in regenerating tissues (23). Previous studies have shown that a complete blockage of Wnt pathway by Dkk1, a potent LRP5/6 antagonist, inhibits proliferation in small intestine and colon, accompanied by progressive architectural degeneration with the loss of crypts, villi, and glandular structure by 7 days (24, 25). Ectopic expression of the Dkk1 or ablation of β-catenin induces loss of hair follicles in adult mice (25, 26). To explore whether there are significant side effects from Mesd and Mesd peptide treatment, we examined the architectural integrity of murine regenerating tissues, including intestinal system and skin. We found that mice treated with Mesd or Mesd peptide are grossly healthy and maintain their weights. Gross examination of tissues by H&E staining revealed that the architecture of various gastrointestinal compartments and skin were morphologically normal after 10 treatments with Mesd or Mesd peptide (Fig. 5I and Fig. S8 A and B). Moreover, there were no significant bone lesions or outgrowths observed in tail vertebrae of mice under the conditions in which antitumor efficacy was achieved (Fig. S8 C and D), a concern prompted because loss of LRP5 and LRP6 function diminishes bone density (27). Overall, these results demonstrate that systemic administration of Mesd and Mesd peptide allows for inhibition of breast tumor growth without generating apparent adverse effects on nontumor tissues.
Discussion
Aberrant Wnt activation is found in 40–60% of breast cancers (11, 13). However, because mutations in the classical intracellular components of the Wnt signaling pathway are rare, the underlying cause of this aberrant activation remains elusive and may involve deregulation of upstream elements. Here, we show that LRP6, an essential Wnt signaling coreceptor, is significantly up-regulated in 20–36% of human breast carcinomas. Although overexpression of LRP6 is sufficient to increase Wnt signaling and cell growth, we also found that one or more Wnt ligands were significantly up-regulated in breast cancer cells including those in which LRP6 is overexpressed (Table S1). Wnt1 and Wnt4 ligands are also up-regulated in a subset of breast cancers (28). Therefore, it is possible that overexpression of LRP6 and Wnt ligands additively or synergistically initiate mammary transformation. We demonstrate that suppression of LRP6 expression and function is sufficient to inhibit Wnt signaling and breast tumor growth even though Wnt ligands are still overexpressed. These findings are significant because identifying specific therapeutic targets for distinct breast tumor subtypes will be critical for the development of novel targeted therapies.
Aberrant Wnt signaling is often associated with triple-negative breast cancers (9, 28, 29). Interestingly, our results suggest that LRP6 overexpression is also highly represented in a subset of breast cancers that are negative for ER and/or HER2, the subclass that often exhibits poor prognosis. We found that the majority of tested tumor samples exhibiting increased LRP6 transcript are distinct from those with increased HER2 transcript, suggesting that Wnt/LRP6 signaling is potentially an independent diagnostic marker. By inhibition of LRP6 expression and/or functions in MDA-MB-231 human breast cancer cells or the MMTV-Wnt1 mouse model, our results also demonstrate that LRP6 might be a novel, independent cell surface target for breast cancer therapy.
LRP5 and LRP6 are highly homologous; however, they may not be equivalent in their ability to transduce Wnt signals (30). LRP6 independently induces axis duplication in Xenopus embryos, whereas LRP5 does not (31). LRP6 knockout in mice is embryonic lethal, whereas Lrp5-deficient mice are viable and fertile (32, 33). Furthermore, LRP5 and LRP6 exhibit overlapping as well as distinct tissue- and cell-type–specific expression patterns. Overall, LRP5 and LRP6 exhibit some functional redundancy, most likely acting with different efficiencies in a context-dependent manner (30). In this study, we observed a significant up-regulation of LRP6, but not LRP5, in a subset of human breast cancer tissues. LRP5 deletion delays Wnt1-induced tumorigenesis even though LRP6 is still expressed (34). Similarly, we found that silencing of LRP6 inhibits human breast cancer tumorigenesis in MDA-MB-231 cells in which LRP5 is expressed. Therefore, it is possible that these two receptors synergistically contribute to breast cancer formation and that more pronounced inhibition of tumor growth might be observed when the expression or function of both LRP5 and LRP6 is suppressed. Consistent with this notion, we showed that Mesd/Mesd peptide suppress the growth of mammary tumors by targeting both LRP5 and LRP6 in MMTV-Wnt1 tumor model.
Mounting evidence suggests that cancers are initiated from stem and/or progenitor cells (CSCs) by deregulation of self-renewal processes that are normally strictly regulated. Deregulation of Wnt signaling may be an early event in mammary epithelial transformation, which predisposes mice to breast cancer by amplifying stem/progenitor populations (28). Supporting this possibility, mice expressing MMTV-Wnt1 or MMTV-∆N89β-catenin develop mammary tumors that are enriched in CSC populations (35). We speculate that knockdown of LRP6 in breast cancer cells suppresses CSCs, the function of which is regulated by Wnt signaling (36, 37). Consistent with this notion, LRP6 is also uniquely overexpressed in human embryonic carcinoma cells, rather than in embryonic stem cells, suggesting a role for this receptor in CSC self-renewal and tumor growth (38). The connection between LRP6 function and CSC population in breast tumors warrants further investigation.
Because Wnt signaling plays essential roles in several physiological functions, in particular stem cell survival and maintenance, therapeutic targeting of the Wnt pathway has become a challenging task in breast cancer treatment. For example, the Wnt pathway regulates proliferation and self-renewal of intestinal and skin epithelial stem cells and plays indispensable roles in gastrointestinal and skin homeostasis (24–26). Therefore, an ideal Wnt inhibitor should reduce Wnt signaling sufficiently to have an impact on cancer development/progression without generating significant toxicity. Here, we demonstrate that Mesd, an LRP5/6 inhibitor, profoundly inhibits the growth of Wnt1-driven mammary tumors in vivo without causing undesirable side effects. We speculate that by partially inhibiting Wnt signaling, Mesd-based therapy might allow sufficient signaling for essential functions (e.g., stem cell maintenance) while inhibiting tumor growth. These results further suggest that LRP6 and, more generally, Wnt signaling at the cell surface, are potential therapeutic targets in breast cancer treatment. Importantly, our work also introduces Mesd as a promising antitumor agent that can be further developed for breast cancer targeted therapy.
Materials and Methods
Cell Culture, Human Breast Cancer Tissue, Transfection, and Lentiviral Infections.
MDA-MB-231, MDA-MB-157, SKBR3, MCF-7, MDA-MB-435s, MDA-MB-361, HCC1187, HCC1143, HCC1806, HCC38, HCC1937, HCC1395, T-47D, and CAMA1 breast cancer cell lines and MCF-10A nontransformed cells were all from the American Type Culture Collection (ATCC) and grown according to ATCC recommendations. MDA-MB-231-Luc cells were a kind gift from Dr. Katherine Weilbaecher (Washington University). Real-time PCR-based TissueScan Breast Cancer Disease Panels (OriGene) were used to screen for LRP6 expression. A breast cancer and normal tissue microarray containing 96 independent cores (Biomax) was used for LRP6 immunohistochemical staining (IHC). Human LRP6 was knocked down using LRP6-specific lentiviral shRNA (MISSION, Sigma-Aldrich). Virus was produced at the Viral Core Facility at Washington University, and virus infection was performed as described (39). Stably transfected cells for in vivo studies were generated from heterogeneous pools of puromycin-resistant clones. Detailed methods for viral production and LRP6-res plasmid construction are provided in SI Materials and Methods.
Western Blot Analysis and Immunohistochemistry.
Western blot analysis was performed as described previously (19). Details of Western blot analysis and immunohistochemical staining can be found in SI Materials and Methods.
Antibodies.
The following antibodies were used in this study: LRP6 antibodies (Cell Signaling; Abgent; Abcam), β-catenin (BD Pharmingen, Cell Signaling), c-Myc and Cyclin D1 (Santa Cruz), Axin2 (Cell Signaling) and actin antibodies (Sigma). All antibodies were used according to the manufacturers’ instructions. Polyclonal rabbit anti-Mesd antibody was produced by immunizing rabbits with purified Mesd protein. HRP-conjugated antirabbit and antimouse secondary antibodies were used (Amersham Pharmacia).
Quantitative Real-Time PCR.
Real-time PCR–based TissueScan Breast Cancer Panel containing 48 tissues covering four disease stages and normal tissues (SA Biosciences) was used to evaluate LRP6 expression levels in human breast cancers. Details can be found in SI Materials and Methods.
Cell Growth, Cell Proliferation, and Soft Agar Tumorigenicity Assays.
Cell growth and cell proliferation were measured by MTT assay (Promega) and BrdU incorporation using the BrdU ELISA kit (Roche Molecular Systems) according to the manufacturers’ instructions. The colony formation ability of cancer cells was analyzed by soft agar assay (SI Materials and Methods).
Xenograft Tumor Model and Bioluminescent Imaging of Mice.
Animal protocols were approved by the Animal Studies Committee of Washington University School of Medicine. Stable pool clones expressing control or LRP6 shRNA were generated in MDA-MB-231-Luc cells. Tumor xenografts were established by s.c. injection of 5 × 105 or 2 × 106 cancer cells into 6-week-old female BNX mice (Taconic). Bioluminescence imaging of tumors was performed as previously described using IVIS 100 (Caliper Life Sciences; exposure time, 1–60 s; binning 8; field of view 15 cm; f/stop 1; open filter) (40). The first mouse images were obtained 24 h after s.c. inoculation of tumor cells. Total photon flux (photons per second) was determined from tumor region-of-interest (ROI) using LivingImage (Xenogen) and IgorPro (Wave metrics) image analysis software. Data were normalized by plotting as fold-enhancement on a given imaging day over bioluminescence on the first day. Tumor sizes were also measured with calipers.
Mesd Therapeutic Studies.
Recombinant Mesd protein was prepared as described previously (19). Mesd peptide, KGGGSKEKNKTKQDKGKKKKEGDLKSRSSKEENRAGNK, was manufactured by Abgent. Female athymic nude mice (Taconic), 6–8 weeks old, were used for passaging tumors from MMTV-Wnt1 mice (Taconic). MMTV-Wnt1 tumors were serially passaged in mice by implantation in the mammary fat pad as described (22). Therapeutic agents (200 μL Mesd protein, Mesd peptide, or PBS) were administrated i.p. with a first dose of 15 mg/kg, followed by 10 mg/kg (nine more doses). Three groups of mice were treated every other day for 3 weeks and tumor volumes were measured three times weekly.
Statistical Analysis.
All quantified data represent an average of at least three independent experiments. Error bars represent mean ± SD (or mean ± SEM) as indicated in figure legends. Statistical significance was determined by Student’s t test, and P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Todd Zankel (Raptor Pharmaceutical) for performing pharmacokinetics studies of Mesd and Mesd peptide; Dr. Snehal Naik for technical assistance on bioluminescence imaging; Christian Nowak and Juan Zhang for purifying Mesd protein, Dr. Xiaolin Tu for providing CA β-catenin virus and Dr. Katherine Weilbaecher for providing MDA-MB-231-Luc cells. We are grateful to Dr. Phillip Tarr, Julie Trausch-Azar (Washington University), and Dr. Todd Zankel for critical reading of this manuscript. This work was supported by National Institutes of Health Grants R01CA100520 (to G.B.) and P50CA94056 (to D.P-W.). C-C.L. is partially supported by a Cancer Biology Pathway fellowship from Washington University Siteman Cancer Center.
Footnotes
Conflict of interest statement: Guojun Bu serves as a consultant for Raptor Pharmaceutical, which has licensed a patent from the Washington University on Mesd as a potential agent to treat bone disease and cancer.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911220107/DCSupplemental.
References
- 1.Ellis IO, et al. In: World Health Organization Classification of Tumours Pathology and Genetics of Tumors of the Breast and Female Genital Organs. Tavassoli FA, Devilee P, editors. Lyon, France: IARC Press; 2003. pp. 13–59. [Google Scholar]
- 2.Carter P, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. 1992;89:4285–4289. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arteaga CL. Trastuzumab, an appropriate first-line single-agent therapy for HER2-overexpressing metastatic breast cancer. Breast Cancer Res. 2003;5:96–100. doi: 10.1186/bcr574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee S, et al. Basal-like breast carcinomas: Clinical outcome and response to chemotherapy. J Clin Pathol. 2006;59:729–735. doi: 10.1136/jcp.2005.033043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 6.Lustig B, Behrens J. The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol. 2003;129:199–221. doi: 10.1007/s00432-003-0431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: Arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 8.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci USA. 2003;100:15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan KR, Brown AMC. Wnt proteins in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2004;9:119–131. doi: 10.1023/B:JOMG.0000037157.94207.33. [DOI] [PubMed] [Google Scholar]
- 11.Lin SY, et al. Beta-catenin, a novel prognostic marker for breast cancer: Its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA. 2000;97:4262–4266. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlosshauer PW, et al. APC truncation and increased beta-catenin levels in a human breast cancer cell line. Carcinogenesis. 2000;21:1453–1456. [PubMed] [Google Scholar]
- 13.Nakopoulou L, et al. Study of phospho-beta-catenin subcellular distribution in invasive breast carcinomas in relation to their phenotype and the clinical outcome. Mod Pathol. 2006;19:556–563. doi: 10.1038/modpathol.3800562. [DOI] [PubMed] [Google Scholar]
- 14.Brown AM. Wnt signaling in breast cancer: Have we come full circle? Breast Cancer Res. 2001;3:351–355. doi: 10.1186/bcr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brennan K, Gonzalez-Sancho JM, Castelo-Soccio LA, Howe LR, Brown AM. Truncated mutants of the putative Wnt receptor LRP6/Arrow can stabilize beta-catenin independently of Frizzled proteins. Oncogene. 2004;23:4873–4884. doi: 10.1038/sj.onc.1207642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Björklund P, Svedlund J, Olsson AK, Akerström G, Westin G. The internally truncated LRP5 receptor presents a therapeutic target in breast cancer. PLoS One. 2009;4:e4243. doi: 10.1371/journal.pone.0004243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Lu W, He X, Schwartz AL, Bu G. LRP6 expression promotes cancer cell proliferation and tumorigenesis by altering beta-catenin subcellular distribution. Oncogene. 2004;23:9129–9135. doi: 10.1038/sj.onc.1208123. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh JC, et al. Mesd encodes an LRP5/6 chaperone essential for specification of mouse embryonic polarity. Cell. 2003;112:355–367. doi: 10.1016/s0092-8674(03)00045-x. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, et al. Mesd binds to mature LDL-receptor-related protein-6 and antagonizes ligand binding. J Cell Sci. 2005;118:5305–5314. doi: 10.1242/jcs.02651. [DOI] [PubMed] [Google Scholar]
- 20.Liu CC, Pearson C, Bu G. Cooperative folding and ligand-binding properties of LRP6 beta-propeller domains. J Biol Chem. 2009;284:15299–15307. doi: 10.1074/jbc.M807285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- 22.DeAlmeida VI, et al. The soluble wnt receptor Frizzled8CRD-hFc inhibits the growth of teratocarcinomas in vivo. Cancer Res. 2007;67:5371–5379. doi: 10.1158/0008-5472.CAN-07-0266. [DOI] [PubMed] [Google Scholar]
- 23.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 24.Kuhnert F, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 2009;174:715–721. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 27.Holmen SL, et al. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res. 2004;19:2033–2040. doi: 10.1359/JBMR.040907. [DOI] [PubMed] [Google Scholar]
- 28.Ayyanan A, et al. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl Acad Sci USA. 2006;103:3799–3804. doi: 10.1073/pnas.0600065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zardawi SJ, O’Toole SA, Sutherland RL, Musgrove EA. Dysregulation of Hedgehog, Wnt and Notch signalling pathways in breast cancer. Histol Histopathol. 2009;24:385–398. doi: 10.14670/HH-24.385. [DOI] [PubMed] [Google Scholar]
- 30.Kelly OG, Pinson KI, Skarnes WC. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131:2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- 31.Tamai K, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 32.Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- 33.Kato M, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindvall C, et al. The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. J Biol Chem. 2006;281:35081–35087. doi: 10.1074/jbc.M607571200. [DOI] [PubMed] [Google Scholar]
- 35.Cho RW, et al. Isolation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem Cells. 2008;26:364–371. doi: 10.1634/stemcells.2007-0440. [DOI] [PubMed] [Google Scholar]
- 36.Woodward WA, et al. WNT/β-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci USA. 2007;104:618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flahaut M, et al. The Wnt receptor FZD1 mediates chemoresistance in neuroblastoma through activation of the Wnt/beta-catenin pathway. Oncogene. 2009;28:2245–2256. doi: 10.1038/onc.2009.80. [DOI] [PubMed] [Google Scholar]
- 38.Dormeyer W, et al. Plasma membrane proteomics of human embryonic stem cells and human embryonal carcinoma cells. J Proteome Res. 2008;7:2936–2951. doi: 10.1021/pr800056j. [DOI] [PubMed] [Google Scholar]
- 39.Stewart SA, et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9:493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gross S, Piwnica-Worms D. Monitoring proteasome activity in cellulo and in living animals by bioluminescent imaging: Technical considerations for design and use of genetically encoded reporters. Methods Enzymol. 2005;399:512–530. doi: 10.1016/S0076-6879(05)99035-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.