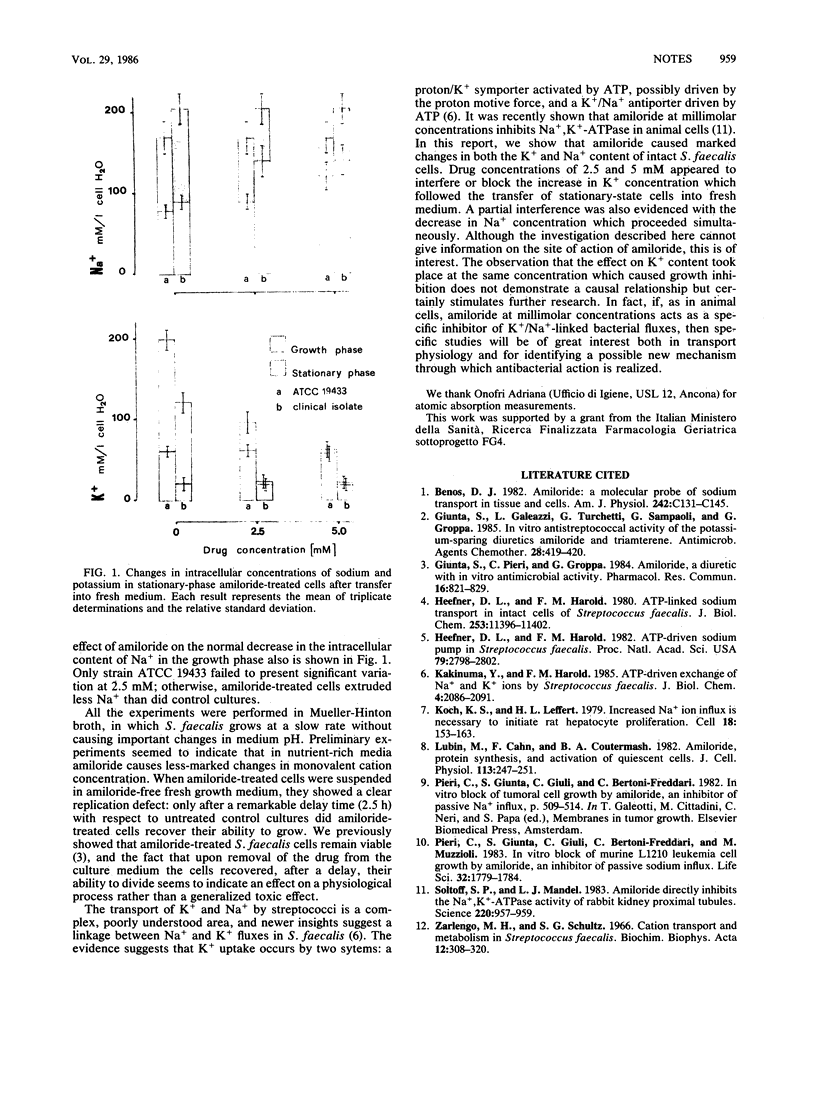
Abstract
Amiloride at millimolar concentrations caused marked changes in the growth-dependent intracellular balance of Na+ and K+ in Streptococcus faecalis. These results, whether specific to transport processes or resulting from indirect yet unknown mechanisms, constitute the first evidence of an effect of amiloride on bacterial electrolytes.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benos D. J. Amiloride: a molecular probe of sodium transport in tissues and cells. Am J Physiol. 1982 Mar;242(3):C131–C145. doi: 10.1152/ajpcell.1982.242.3.C131. [DOI] [PubMed] [Google Scholar]
- Giunta S., Galeazzi L., Turchetti G., Sampaoli G., Groppa G. In vitro antistreptococcal activity of the potassium-sparing diuretics amiloride and triamterene. Antimicrob Agents Chemother. 1985 Sep;28(3):419–420. doi: 10.1128/aac.28.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunta S., Pieri C., Groppa G. Amiloride, a diuretic with in vitro antimicrobial activity. Pharmacol Res Commun. 1984 Aug;16(8):821–829. doi: 10.1016/s0031-6989(84)80058-2. [DOI] [PubMed] [Google Scholar]
- Heefner D. L., Harold F. M. ATP-driven sodium pump in Streptococcus faecalis. Proc Natl Acad Sci U S A. 1982 May;79(9):2798–2802. doi: 10.1073/pnas.79.9.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heefner D. L., Harold F. M. ATP-linked sodium transport in Streptococcus faecalis. I. The sodium circulation. J Biol Chem. 1980 Dec 10;255(23):11396–11402. [PubMed] [Google Scholar]
- Kakinuma Y., Harold F. M. ATP-driven exchange of Na+ and K+ ions by Streptococcus faecalis. J Biol Chem. 1985 Feb 25;260(4):2086–2091. [PubMed] [Google Scholar]
- Koch K. S., Leffert H. L. Increased sodium ion influx is necessary to initiate rat hepatocyte proliferation. Cell. 1979 Sep;18(1):153–163. doi: 10.1016/0092-8674(79)90364-7. [DOI] [PubMed] [Google Scholar]
- Lubin M., Cahn F., Coutermarsh B. A. Amiloride, protein synthesis, and activation of quiescent cells. J Cell Physiol. 1982 Nov;113(2):247–251. doi: 10.1002/jcp.1041130210. [DOI] [PubMed] [Google Scholar]
- Pieri C., Giunta S., Giuli C., Bertoni-Freddari C., Muzzioli M. In vitro block of murine L 1210 leukemia cell growth by amiloride, an inhibitor of passive Na+ influx. Life Sci. 1983 Apr 11;32(15):1779–1784. doi: 10.1016/0024-3205(83)90842-1. [DOI] [PubMed] [Google Scholar]
- Soltoff S. P., Mandel L. J. Amiloride directly inhibits the Na,K-ATPase activity of rabbit kidney proximal tubules. Science. 1983 May 27;220(4600):957–958. doi: 10.1126/science.6302840. [DOI] [PubMed] [Google Scholar]
- Zarlengo M. H., Schultz S. G. Cation transport and metabolism in Streptococcus fecalis. Biochim Biophys Acta. 1966 Oct 10;126(2):308–320. doi: 10.1016/0926-6585(66)90068-9. [DOI] [PubMed] [Google Scholar]