Abstract
Excessive activation of calmodulin kinase II (CaMKII) causes arrhythmias and heart failure, but the cellular mechanisms for CaMKII-targeted proteins causing disordered cell membrane excitability and myocardial dysfunction remain uncertain. Failing human cardiomyocytes exhibit increased CaMKII and voltage-gated Ca2+ channel (CaV1.2) activity, and enhanced expression of a specific CaV1.2 β-subunit protein isoform (β2a). We recently identified CaV1.2 β2a residues critical for CaMKII phosphorylation (Thr 498) and binding (Leu 493), suggesting the hypothesis that these amino acids are crucial for cardiomyopathic consequences of CaMKII signaling. Here we show WT β2a expression causes cellular Ca2+ overload, arrhythmia-triggering cell membrane potential oscillations called early afterdepolarizations (EADs), and premature death in paced adult rabbit ventricular myocytes. Prevention of intracellular Ca2+ release by ryanodine or global cellular CaMKII inhibition reduced EADs and improved cell survival to control levels in WT β2a-expressing ventricular myocytes. In contrast, expression of β2a T498A or L493A mutants mimicked the protective effects of ryanodine or global cellular CaMKII inhibition by reducing Ca2+ entry through CaV1.2 and inhibiting EADs. Furthermore, CaV1.2 currents recorded from cells overexpressing CaMKII phosphorylation- or binding-incompetent β2a subunits were incapable of entering a CaMKII-dependent high-activity gating mode (mode 2), indicating that β2a Thr 498 and Leu 493 are required for CaV1.2 activation by CaMKII in native cells. These data show that CaMKII binding and phosphorylation sites on β2a are concise but pivotal components of a molecular and biophysical and mechanism for EADs and impaired survival in adult cardiomyocytes.
Keywords: arrhythmias, calcium, calcium channel, calmodulin kinase, cardiac myocytes
The multifunctional Ca2+ and calmodulin-dependent protein kinase II (CaMKII) is a proarrhythmic (1) and proapoptotic (2) signaling molecule activated in failing human myocardium and in animal models of heart failure (3). The Ca2+ homeostatic proteins involved in excitation-contraction coupling (ECC) are CaMKII targets (4), and excessive CaMKII-mediated phosphorylation of ECC proteins has recently emerged as a critical transition event leading to myocardial dysfunction and arrhythmias (5). The L-type Ca2+ channel (LTCC) protein complex is the predominant entry point for Ca2+ that supplies intracellular sarcoplasmic reticulum (SR) Ca2+ stores and is an important source of inward current (ICa) for prolonging the action potential duration (APD) (6). CaMKII drives LTCCs into an active gating mode (mode 2) with frequent, prolonged openings, and mode 2 gating occurs together with an ICa property called facilitation (7). Mode 2 gating and ICa facilitation are part of a hypothesized mechanism favoring SR Ca2+ overload and early afterdepolarizations (EADs), arrhythmia-initiating oscillations in cell membrane potential (8). LTCCs contain a pore forming α-subunit (CaV1.2) and an accessory β-subunit protein (9), allowing use of an overexpression approach to reconstitute LTCCs with predominantly exogenous β-subunits in native cells (10, 11).
All LTCC β-subunits increase CaV1.2 opening probability (Po), but β2a is more effective than other isoforms for increasing LTCC Po (12). The β2a expression is increased in failing human hearts (12), a condition marked by increased LTCC Po (13), APD prolongation, loss of intracellular Ca2+ homeostasis (14), EADs (15), and excessive cardiomyocyte death (16). Furthermore, overexpression of WT β2a in adult cardiomyocytes increases ICa, induces SR Ca2+ overload, and stimulates apoptosis by a pathway that involves CaMKII (10), supporting the hypothesis that CaMKII phosphorylation of β2a is a molecular mechanism for pathological membrane excitability and cardiomyocyte death. We recently identified CaMKII phosphorylation (Thr 498) and binding (Leu 493) sites on β2a. Using this information, we tested the concept that β2a Thr 498 and Leu 493 are proarrhythmic and cardiomyopathic targets for CaMKII in native adult cardiomyocytes. Here we show that β2a CaMKII binding and phosphorylation sites are required for the biophysical actions of CaMKII on LTCCs and for EAD induction, whereas loss of Thr 498 or Leu 493 protects against cell death during overexpression of β2a. Our findings illustrate how CaMKII phosphorylation and binding to β2a can activate mode 2 gating and initiate a coherent series of integrated, but pathological cellular responses culminating in EADs and death.
Results
CaMKII Binding and Phosphorylation Sites on β2a Are Critical for ICa Facilitation.
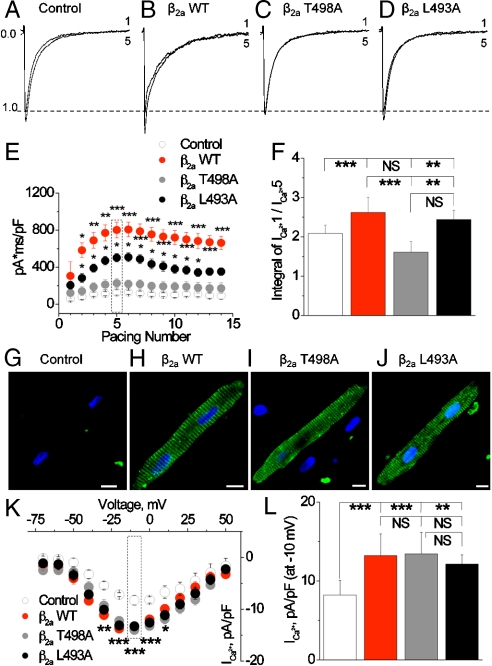
Facilitation is a CaMKII-dependent, dynamic pattern of increasing peak ICa and concomitant slowing of the fast component of ICa inactivation (Fig. 1A–F) (17–19). We measured facilitation as the integral of inward ICa (Fig. 1E and F) elicited by a train of 15 voltage clamp steps (-80 to 0 mV, 0.5 Hz). Expression of β2a WT significantly increased ICa facilitation compared to mock-infected control cells or cells expressing β2a T498A or β2a L493A (Fig. 1E and F). The β2a L493A infected cells showed an intermediate ICa facilitation phenotype that was significantly less than in β2a WT, but more than β2a T498A expressing cells. SR Ca2+ content was significantly increased in β2a WT compared to β2a mutant infected cells (Fig. S1). These findings suggest that CaMKII binding and phosphorylation sites are critical for β2a-mediated increases in ICa facilitation and Ca2+ entry that contribute to SR Ca2+ loading in adult ventricular myocytes.
Fig. 1.
CaMKII targeting to β2a is required for ICa facilitation in adult cardiomyocytes. (A–D) Examples of the first and fifth ICa recorded in response to a train of depolarizing command steps to 0 mV from cultured adult ventricular myocytes. (E) Summary data for integrated ICa in response to a train of 15 voltage command pulses. (F) Integrated ICa from the fifth command pulse normalized to ICa from the first command pulse. These data are from E. Ten through 12 cells were studied for each data point in E and F. ∗P < 0.05; ∗∗P < 0.01, ∗∗∗P < 0.001 versus empty vector controls. Confocal micrographs showing cultured rabbit ventricular myocytes after infection with an empty vector (G) WT β2a; (H) CaMKII-phosphorylation-resistant CaMKII β2a (T498A); (I) CaMKII-binding-resistant β2a (L493A); (J) β2a-encoding cDNA. The green color represents immunofluorescent detection of the FLAG-tagged exogenously expressed β2a subunits. Nuclei are stained blue with topro-3. (Scale bar: 10 μm.) (K) Current–voltage relationship showing peak L-type Ca2+ current (ICa) recorded from cells treated as in G–J. ∗P < 0.05; ∗∗P < 0.01, ∗∗∗P < 0.001 versus empty vector controls. (L) Peak ICa density in response to a voltage command pulse to 0 mV (data are from K). The vertical ticks mark groups for statistical comparison.
WT and CaMKII Resistant β2a Mutants Show Normal Membrane Targeting.
Confocal immunofluorescent detection of exogenous β2a showed that β2a WT, β2a T498A, and β2a L493A had a repetitively spaced pattern of expression (Fig. 1G–J), consistent with the known enrichment of native CaV1.2 β-subunits in adult ventricular myocyte T-tubular membranes (20). The ratio of peak ICa to peak SR Ca2+ release during cardiac ECC requires precise localization of sarcolemmal CaV1.2 and SR ryanodine receptor Ca2+ release channels. In order to test if the exogenous β2a subunits are positioned to support ECC, we measured the ratio of peak intracellular Ca2+ release triggered by ICa in voltage-clamped cells, under conditions that controlled peak ICa (by post hoc selection) and matched SR Ca2+ content using a genetically individualized conditioning protocol (SI Materials and Methods). Exogenous β2a subunits resulted in a lower ECC gain compared to mock-infected control cells (Fig. S2), potentially suggesting that β2a overexpression alters the relationship between CaV1.2 and ryanodine receptors in adult cardiomyocytes. However, there were no differences in ECC gain at any voltage command step between β2a WT, β2a T498A, or β2a L493A infected cells. Thus, the selective loss of ICa facilitation in T498A and L493A β2a infected cells indicates that concise but fundamental biophysical defects occur in LTCC channels assembled with a CaMKII binding- or phosphorylation-disabled β2a subunit.
β2a Leu 493 and Thr 498 Are Required for CaMKII Activation of Mode 2 Gating.
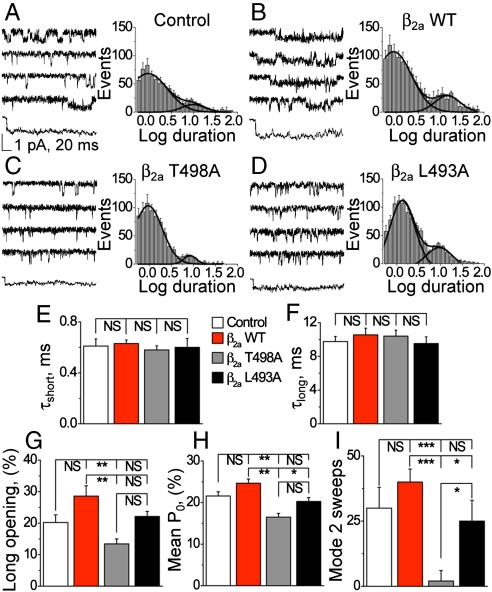
CaMKII increases mode 2 gating in LTCCs (7), but the molecular targets are unknown. In order to assess the effects of T498A and L493A mutants on LTCCs, we measured single CaV1.2 channel currents from intact infected myocytes. We identified two populations of CaV1.2 opening times (Fig. 2A–F). The duration of the short (Fig. 2E) and long (Fig. 2F) CaV1.2 opening times were not different between the experimental groups. However, β2a T498A mutants exhibited a significantly reduced relative frequency of longer opening times and reduced mean Po (Fig. 2H) compared to cells infected with β2a WT, whereas the frequency of long opening times (Fig. 2G) and mean Po (Fig. 2H) were not different between β2a T498A and β2a L493A. We next grouped CaV1.2 openings into gating modes, as previously described (7). These analyses revealed that LTCCs expressing β2a WT had significantly more mode 2 gating activity than LTCCs recorded from cells with β2a T498A or β2a L493A expression (Fig. 2I).
Fig. 2.
Reduced opening probability (Po) and mode 2 gating events in LTCC from β2a WT compared to T498A or L493A expressing ventricular myocytes. Single LTCC current tracings and modal gating analysis from (A) mock-infected control cells, (B) β2a WT infected cells, (C) β2a T498A infected cells, and (D) β2a L493A infected cells. Each panel (A–D) shows representative single LTCC current recordings and an ensemble tracing averaged from 40 sweeps (Left). A histogram in each panel shows that LTCC openings partition into short and long opening times (Right). The time constants (τ) for the short (E) and long (F) opening times are not different between experimental groups. In contrast, the percent of long openings (G), mean Po (H), and sweeps with mode 2 activity (I) are significantly less in β2a T498A compared to β2a WT infected cells. Color-coded legends are as in Fig. 1 and statistical comparisons between groups are denoted as in Fig. 1F. Eight through 10 cells in each group gave 250–300 active sweeps and were used for each data point in E–I.
We compared LTCC gating responses to global cellular CaMKII inhibition, by expression of a CaMKII inhibitory peptide (CaMKIIN) or shRNA knock down of CaMKIIδ (Fig. S3), in β2a WT expressing and mock-infected control cells. CaMKII inhibition significantly reduced ICa facilitation in control and WT β2a overexpressing cells, and significantly reduced the frequency of long LTCC opening times and Po compared to WT β2a expression or mock-infected control cells without CaMKII inhibition (Fig. S4). Finally, CaMKII inhibition with WT β2a-infected or mock-infected control cells significantly reduced the frequency of mode 2 gating to levels present in β2a T498A expressing ventricular myocytes (compare Fig. S4G to Fig. 2I). These data show that LTCCs recorded under conditions of global CaMKII inhibition or lacking the β2a CaMKII Thr 498 phosphorylation site, but in the presence of normal CaMKII activity, exhibit similar amounts of mode 2 gating.
To further test the concept that β2a Thr 498 and Leu 493 were crucial for CaMKII-induced mode 2 gating, we designed experimental conditions where CaMKII activity was directly controlled. We measured CaV1.2 single channel currents from membrane patches excised from noninfected cultured ventricular myocytes and from ventricular myocytes infected with β2a WT, β2a T498 or β2a L493A. We enriched the bath (cytoplasmic) solution with exogenous, constitutively active CaMKII to test the ability of native and β2a WT, β2a T498A, and β2a L493A reconstituted channels to respond to CaMKII (Fig. S5). Mode 2 gating was observed significantly less in β2a T498A (P < 0.001 versus mock-infected controls and β2a WT) and β2a L493A (P < 0.05 versus mock-infected controls and P < 0.001 versus β2a WT) compared to β2a WT or endogenous channels. We interpret these findings to mean that CaMKII actions on LTCCs are predominantly controlled by β2a Thr 498 and Leu 493. Because Thr 498 is conserved on all major β-subunit isoforms in heart (21), it seems likely that this Thr is similarly critical for CaMKII effects on all CaV1.2 complexes.
Loss of CaMKII Sites on β2a Reduces EADs During APD Prolongation.
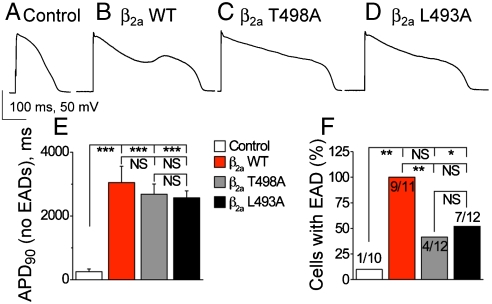
Expression of β2a WT, β2a L493A, and β2a T498A significantly and equivalently prolonged the APD compared to control vector only infected ventricular myocytes (Fig. 3A–E). The striking increases in APD are consistent with the known potential for ICa to slow membrane repolarization in adult ventricular myocytes (6). APD prolongation by β2a WT expression favored EADs, but APD prolongation by β2a T498A expression did not significantly increase EADs compared to control (Fig. 3F). β2a L493A expressing cells showed a reduction in EAD frequency compared to β2a WT expressing cells that was not significantly different than β2a T498A expressing cells (Fig. 3F). Cells with global CaMKII inhibition and infected with β2a WT were also resistant to EADs, despite significant APD prolongation (Fig. S6). These results indicate a reduced proarrhythmic potential from APD prolongation due to β2a expression lacking Leu 493 or Thr 498, and suggest that CaMKII binding and phosphorylation sites on β2a are required for efficient EAD induction during APD prolongation in adult ventricular myocytes.
Fig. 3.
CaMKII sites are required for frequent EADs but not for APD prolongation. Representative action potentials recorded from (A) mock-infected control cells, (B) β2a WT infected cells, (C) β2a T498A infected cells, and (D) β2a L493A infected cells. (E) APD recorded at 90% repolarization to baseline (APD90) are significantly and equivalently increased in all groups. (F) EAD frequency is significantly increased over mock-infected cells only in β2a WT infected cells, while β2a T498A and L493A infected cells are protected. Color-coded legends are as in Fig. 1 and statistical comparisons between groups are denoted as in Fig. 1F. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
SR Ca2+ Release and CaMKII Effects on β2a Contribute to Cytotoxicity.
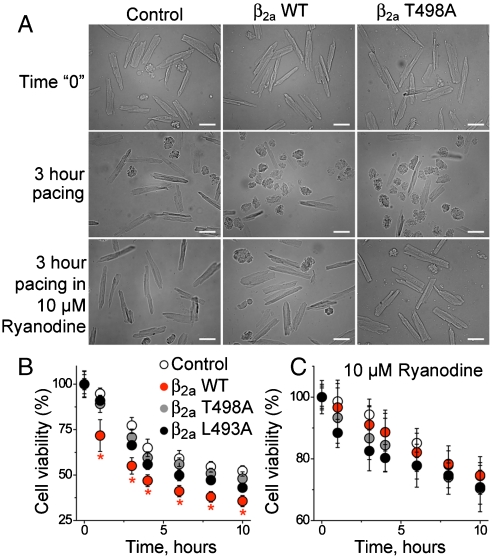
Ventricular myocytes with β2a WT expression die prematurely by a CaMKII-dependent process (10). We paced cultured ventricular myocytes and measured cell viability at various time points over 24 h (Fig. 4A and B) to test if CaMKII regulated cardiomyocyte survival through β2a Leu 493 or Thr 498. β2a WT infected cell cultures showed a significantly higher proportion of dead cells, assessed by morphological criteria (Fig. 4B) or Trypan blue exclusion (Fig. S7A and B), after 3 h of pacing, compared to β2a T498A or vector control infected ventricular myocytes. The β2a L493A expressing ventricular myocytes showed an intermediate viability pattern that was not significantly different than for β2a T498A infected cells (Fig. 4B). Loss of cell viability was similar in all groups after pacing for 24 h. CaMKII enhances SR Ca2+ release (5) and preventing SR Ca2+ release improves viability in ventricular myocytes expressing β2a WT (10). In order to test if reduced survival by β2a WT expression required SR Ca2+ release in our model, we repeated the cell viability assays in ventricular myocytes infected with each of the β2a constructs in the presence of ryanodine (10 μM). Ryanodine treatment abolished the differences between genotypes and significantly increased the percentage of viable cells at each time point (Fig. 4C). Finally, we measured cardiomyocyte viability in response to pacing during global cellular CaMKII inhibition in the absence (Fig. S7C) or presence of ryanodine (Fig. S7D). Surprisingly, global CaMKII inhibition or ryanodine treatment equivalently improved viability to control levels for β2a WT expressing cells at each of the time points. These data suggest a model where CaMKII phosphorylation of β2a can initiate a pathological cascade of enhanced cellular Ca2+ entry causing excessive SR Ca2+ release that leads to reduced cardiomyocyte survival.
Fig. 4.
Maximal survival by ryanodine treatment and improved survival with β2a T498A and L493A compared to WT expressing cardiomyocytes. (A) Representative micrographs of mock-infected, β2a WT and β2a T498A infected ventricular myocytes (left to right) at baseline and after 3 h of pacing in the absence and presence of ryanodine (top to bottom). (Calibration bar: 50 μm.) Summary data for cell viability based on morphological criteria in the absence (B) or presence (C) of ryanodine (10 μM). Each data point is from an average of ≥50 cells. ∗P < 0.05 compared to vector-infected controls. Color-coded legends in B and C are as in Fig. 1.
SR Ca2+ Release Enhances EADs by Activating CaMKII Targeted to β2a.
We repeated the AP recordings in the presence of ryanodine to test if reduced SR Ca2+ release affected APD or EADs. Ryanodine significantly prolonged APD but reduced EADs (Fig. S7E and F) to levels previously observed in β2a T498A expressing cells (Fig. 3F). EAD suppression by ryanodine suggested that SR Ca2+ release is critical for activation of β2a-targeted CaMKII. To test this concept, we infected adult cardiomyocytes with a constitutively active T286D CaMKIIδ mutant. We observed APD prolongation and spontaneous EADs in cells infected with constitutively active CaMKII (Fig. S8), but EADs in the T286D CaMKIIδ infected cells were not inhibited by ryanodine (Fig. S8C). The CaV1.2 antagonist nifedipine (500 nM) eliminated EADs and significantly and equivalently shortened APDs in myocytes infected with the T286D CaMKIIδ mutant (Fig. S8D), suggesting that LTCCs were critical for the proarrhythmic actions of CaMKII. In order to establish the role of β2a L493 and T498 in transducing EADs by constitutively active CaMKII, we coinfected cardiomyocytes with T286D CaMKIIδ and each of the β2a constructs. CaMKIIδ T286D caused EADs in 9/10 control cells, but was significantly less effective at inducing EADs in myocytes infected with β2a L493A or T498A (Fig. S8E), despite similar APD prolongation in each of these groups (Fig. S8F). We interpret these findings to mean that SR Ca2+ release is necessary for activating CaMKII that ultimately triggers EADs by targeting β2a L493 and T498. Constitutively active CaMKII expression circumvents the need for activating SR Ca2+, but nevertheless requires β2a L493 and T498 in order to effectively induce EADs.
New Mathematical Model of Increased Mode 2 Gating and EADs.
Our data support a model where a single CaV1.2 phosphorylation event at β2a Thr 498 can initiate a cascade of CaMKII-triggered cellular events orchestrated by ECC proteins, including increased CaV1.2 mode 2 gating, augmented ICa facilitation, and cellular Ca2+ entry, increased SR Ca2+ release and EADs. We developed a mathematical model of CaMKII regulation of ECC proteins that incorporates our experimental findings (Fig. S9). Computer simulations in the Luo-Rudy ventricular cell model show that β2a overexpression results in greater CaMKII activity during the action potential plateau compared to the T498A mutant. CaMKII activation, in turn, increases the number of LTCCs exhibiting mode 2 gating, which promotes reactivation of the ICa and EADs during AP repolarization. Consistent with our experimental observations, block of SR Ca2+ release eliminates EADs by reducing CaMKII and mode 2 gating activity. To determine whether the protective effects of the T498A mutant required reduced SR Ca2+ content (Fig. S1), we computed T498A and WT action potentials under reversed steady-state SR Ca2+ loading conditions (relatively high SR [Ca2+] in T498A and low SR [Ca2+] in WT). These computer simulations demonstrate that WT β2a expressing cells develop EADs, whereas T498A mutant expressing cells are resistant to EADs (Fig. S9), independent of SR Ca2+ load, suggesting that EADs were ultimately dependent on Thr 498 phosphorylation and consistent with our findings in ryanodine-treated cells expressing constitutively active CaMKII (Fig. S8).
Discussion
β2a-Initiated Molecular Pathway for EADs and Cell Death.
Our results indicate that expression of WT β2a is highly effective at leveraging the ECC protein machinery in adult ventricular myocytes by connecting cellular Ca2+ entry with excessive SR Ca2+, EADs, and impaired cell survival. Arrhythmias and heart failure occur together, in part, because they are both consequences of hyperphosphorylated ECC proteins (22). CaMKII catalyzes phosphorylation of each of the major Ca2+ homeostatic proteins, which regulate cellular Ca2+ entry and SR Ca2+ uptake and release. CaMKII catalyzes the phosphorylation of β Thr 498 (11), additional sites on the CaV1.2 α-subunit (23, 24), phospholamban (25), and ryanodine receptors (26). CaV1.2 is a critical control point for cellular Ca2+ entry in ventricular myocardium and this Ca2+ entry due to ICa ultimately sustains the SR Ca2+ content necessary for SR Ca2+ release and myofilament contraction. Thus, the hyperactive CaV1.2 channels that occur in failing human ventricular myocytes (13) are well positioned to provoke EADs and intracellular Ca2+ overload. Expression of β2a increases in heart failure, relative to other β-subunit isoforms (12), while expression of ryanodine receptors and the phospholamban-regulated SR Ca2+ ATPase (SERCa2a) are generally reduced or unchanged in failing myocardium (27). Studies in a rabbit model of heart failure show increased CaMKII activation, afterdepolarizations, arrhythmias, and sudden death, but without an increase in peak ICa (28). Thus, CaMKII signaling to the ryanodine receptor (28) and other ion channels is also important for arrhythmias in structural heart disease. Our findings are consistent with a concept that CaMKII actions at β2a can initiate and orchestrate CaMKII-dependent actions on membrane excitability and cell survival that are important for heart failure progression, because loss of CaMKII binding or phosphorylation sites completely disrupts the connection between β2a WT expression and EADs and partially protects against premature cell death.
β-Subunit is Critical for CaMKII Signaling to CaV1.2.
Our data show that CaMKII targeting β2a is preeminent over other CaMKII actions at CaV1.2 for modulating LTCC mode 2 gating, ICa facilitation, and EADs in native adult cardiomyocytes. Although our findings do not rule out a role for CaMKII phosphorylation of other LTCC sites, including amino acids identified on CaV1.2 (23, 24), they strongly suggest that Thr 498 and Leu 493 on β2a are dominant over other CaMKII regulatory LTCC sites in native adult heart cells. Our model uses overexpression to probe the role of CaMKII at β2a for regulating membrane excitability and cell survival, and it is possible that β2a overexpression could bias our findings toward LTCC and away from alternative CaMKII ECC protein targets. However, in our model, expression of all β2a backgrounds and peak ICa were closely matched, allowing us to assign clear roles to Thr 498 and Leu 493 for CaV1.2 gating and facilitation.
What is the Relationship Between APD and Arrhythmias?
Our experimental and computational data add to a growing body of evidence suggesting that CaMKII activation is a critical step linking increased APD with EADs and arrhythmias. Rabbits with prolongation of the electrocardiographic QT interval showed significantly reduced proarrhythmia in the presence of systemic calmodulin inhibition (29), and Langendorff-perfused rabbit hearts with APD prolongation had reduced EAD induction during infusion of calmodulin or CaMKII inhibitory drugs (30). In these studies, calmodulin and CaMKII inhibition suppressed arrhythmias and EADs without significantly affecting QT interval or APD, suggesting that APD prolongation, per se, was insufficient for proarrhythmia in vivo. Our present studies provide fresh molecular details to the concept that CaMKII enables APD prolongation-dependent proarrhythmia by showing that the APD prolongation due to increased ICa requires SR Ca2+ to activate CaMKII and that activated CaMKII binds to and phosphorylates β2a to trigger EADs.
Materials and Methods
Ventricular Myocyte Isolation and Viral Infection.
The cardiomyocyte isolation from adult male New Zealand white rabbits and viral infections were performed as described with minor modifications (31). Virus was added to the cells at a multiplicity of infection of 1–3, and cell cultures were maintained for 24–36 h. See SI Materials and Methods for full details.
β-subunits, CaMKII Protein and CaMKII Inhibition.
Expression of β-subunits (11, 21), CaMKII protein, and CaMKII inhibition were performed according to our published methods. See SI Materials and Methods for full details (7).
Confocal Microscopy and Immunofluorescence.
Confocal Ca2+ measurement and immunofluorescent studies were performed according to established methods (11), with minor modifications. See SI Materials and Methods for full details.
Electrophysiology.
Whole cell mode current and voltage clamp studies, and single channel LTCC recordings, were performed using our previously published methods, with minor modifications. We selected β2a infected cells with peak ICa 13–16 pA/pF and mock-infected cells with peak ICa from 6–8 pA/pF for analysis in current clamp and whole cell mode voltage clamp studies to avoid potential confounding effects of variable CaV1.2 expression amongst the β2a-infected cells. See SI Materials and Methods for full details.
Cell Pacing and Viability Measurements.
Dissociated ventricular myocytes were cultured on a 12-mm-diameter (glass #1) cover glasses placed into four-well Nunclon Delta Treated dishes (10 coverslips in each well). We used morphology and Trypan blue staining to estimate cell viability (10). See SI Materials and Methods for full details.
Modeling.
The Luo-Rudy model of the mammalian ventricular action potential including a Markov representation of ICa is used as the basis for all computer simulations. See SI Materials and Methods for full details.
Statistical Analysis.
Data are presented as mean ± SEM. P values were assessed with a paired Student’s t test or ANOVA, as appropriate, for continuous data. The Bonferroni test was used for post hoc testing. The Fisher exact test was used to analyze EAD induction frequency. The null hypothesis was rejected for P ≤ 0.05.
Supplementary Material
Acknowledgments.
We are grateful for assistance from the University of Iowa Gene Transfer Vector Core, a National Institutes of Health (NIH) funded resource. This work was supported by NIH Grants R01 HL079031, R01 HL070250, and R01 HL096652 (to M.E.A.); R01 HL084583, R01 HL083422, and the Pew Scholars Trust (to P.J.M). This work was supported by the Fondation Leducq Award to the Alliance for Calmodulin Kinase Signaling in Heart Disease.
Footnotes
Conflict of interest statement: M.E.A. is a named inventor on a patent owned by Stanford University claiming to treat arrhythmias by CaMKII inhibition.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the National Center for Biotechnology Information database (β2a accession no. NM_053851.1; CaMKII accession no. NM_001025438.1).
This article contains supporting information online at www.pnas.org/cgi/content/full/0913760107/DCSupplemental.
References
- 1.Khoo MS, et al. Death, cardiac dysfunction, and arrhythmias are increased by calmodulin kinase II in calcineurin cardiomyopathy. Circulation. 2006;114:1352–1359. doi: 10.1161/CIRCULATIONAHA.106.644583. [DOI] [PubMed] [Google Scholar]
- 2.Erickson JR, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang T, Brown JH. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc Res. 2004;63:476–486. doi: 10.1016/j.cardiores.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 4.Couchonnal LF, Anderson ME. The role of calmodulin kinase II in myocardial physiology and disease. Physiology. 2008;23:151–159. doi: 10.1152/physiol.00043.2007. [DOI] [PubMed] [Google Scholar]
- 5.Ling H, et al. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alseikhan BA, DeMaria CD, Colecraft HM, Yue DT. Engineered calmodulins reveal the unexpected eminence of Ca2+ channel inactivation in controlling heart excitation. Proc Natl Acad Sci USA. 2002;99:17185–17190. doi: 10.1073/pnas.262372999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat Cell Biol. 2000;2:173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, MacMillan LB, McNeill RB, Colbran RJ, Anderson ME. CaM kinase augments cardiac L-type Ca2+ current: A cellular mechanism for long Q-T arrhythmias. Am J Physiol. 1999;276:H2168–H2178. doi: 10.1152/ajpheart.1999.276.6.H2168. [DOI] [PubMed] [Google Scholar]
- 9.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, et al. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 11.Grueter CE, et al. L-type Ca2+ channel facilitation mediated by phosphorylation of the beta subunit by CaMKII. Mol Cell. 2006;23:641–650. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Hullin R, et al. Increased expression of the auxiliary beta2-subunit of ventricular L-type Ca2+ channels leads to single-channel activity characteristic of heart failure. PLoS One. 2007;2:e292. doi: 10.1371/journal.pone.0000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroder F, et al. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation. 1998;98:969–976. doi: 10.1161/01.cir.98.10.969. [DOI] [PubMed] [Google Scholar]
- 14.Piacentino V, et al. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92:651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 15.Priebe L, Beuckelmann DJ. Simulation study of cellular electric properties in heart failure. Circ Res. 1998;82:1206–1223. doi: 10.1161/01.res.82.11.1206. [DOI] [PubMed] [Google Scholar]
- 16.Olivetti G, et al. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 17.Anderson ME, Braun AP, Schulman H, Premack BA. Multifunctional Ca2+/calmodulin-dependent protein kinase mediates Ca(2+)-induced enhancement of the L-type Ca2+ current in rabbit ventricular myocytes. Circ Res. 1994;75:854–861. doi: 10.1161/01.res.75.5.854. [DOI] [PubMed] [Google Scholar]
- 18.Yuan W, Bers DM. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am J Physiol. 1994;267:H982–H993. doi: 10.1152/ajpheart.1994.267.3.H982. [DOI] [PubMed] [Google Scholar]
- 19.Xiao RP, Cheng H, Lederer WJ, Suzuki T, Lakatta EG. Dual regulation of Ca2+/calmodulin-dependent kinase II activity by membrane voltage and by calcium influx. Proc Natl Acad Sci USA. 1994;91:9659–9663. doi: 10.1073/pnas.91.20.9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun XH, et al. Molecular architecture of membranes involved in excitation-contraction coupling of cardiac muscle. J Cell Biol. 1995;129:659–671. doi: 10.1083/jcb.129.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grueter CE, Abiria SA, Wu Y, Anderson ME, Colbran RJ. Differential regulated interactions of calcium/calmodulin-dependent protein kinase II with isoforms of voltage-gated calcium channel beta subunits. Biochemistry. 2008;47:1760–1767. doi: 10.1021/bi701755q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 23.Lee TS, et al. Calmodulin kinase II is involved in voltage-dependent facilitation of the L-type Cav1.2 calcium channel: Identification of the phosphorylation sites. J Biol Chem. 2006;281:25560–25567. doi: 10.1074/jbc.M508661200. [DOI] [PubMed] [Google Scholar]
- 24.Hudmon A. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171:537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bilezikjian LM, Kranias EG, Potter JD, Schwartz A. Studies on phosphorylation of canine cardiac sarcoplasmic reticulum by calmodulin-dependent protein kinase. Circ Res. 1981;49:1356–1362. doi: 10.1161/01.res.49.6.1356. [DOI] [PubMed] [Google Scholar]
- 26.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 27.Yano M, Ikeda Y, Matsuzaki M. Altered intracellular Ca2+ handling in heart failure. J Clin Invest. 2005;115:556–564. doi: 10.1172/JCI24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo T, Ai X, Shannon TR, Pogwizd SM, Bers DM. Intra-sarcoplasmic reticulum free [Ca2+] and buffering in arrhythmogenic failing rabbit heart. Circ Res. 2007;101:802–810. doi: 10.1161/CIRCRESAHA.107.152140. [DOI] [PubMed] [Google Scholar]
- 29.Mazur A, Roden DM, Anderson ME. Systemic administration of calmodulin antagonist W-7 or protein kinase A inhibitor H-8 prevents torsade de pointes in rabbits. Circulation. 1999;100:2437–2442. doi: 10.1161/01.cir.100.24.2437. [DOI] [PubMed] [Google Scholar]
- 30.Anderson ME. KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J Pharmacol Exp Ther. 1998;287:996–1006. [PubMed] [Google Scholar]
- 31.Thiel WH, et al. Proarrhythmic defects in Timothy syndrome require calmodulin kinase II. Circulation. 2008;118:2225–2234. doi: 10.1161/CIRCULATIONAHA.108.788067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.