Abstract
In nonobese diabetic mice with uncontrolled type 1 diabetes, leptin therapy alone or combined with low-dose insulin reverses the catabolic state through suppression of hyperglucagonemia. Additionally, it mimics the anabolic actions of insulin monotherapy and normalizes hemoglobin A1c with far less glucose variability. We show that leptin therapy, like insulin, normalizes the levels of a wide array of hepatic intermediary metabolites in multiple chemical classes, including acylcarnitines, organic acids (tricarboxylic acid cycle intermediates), amino acids, and acyl CoAs. In contrast to insulin monotherapy, however, leptin lowers both lipogenic and cholesterologenic transcription factors and enzymes and reduces plasma and tissue lipids. The results imply that leptin administration may have multiple short- and long-term advantages over insulin monotherapy for type 1 diabetes.
Keywords: glucagon suppression, lipid-lowering, metabolomics, cholesterol regulation, glucose regulation
The discovery of insulin in 1922 (1) transformed type 1 diabetes mellitus (T1DM) from a uniformly fatal disease to a manageable disorder with a relatively normal but burdensome life for the patient. Despite the monumental dimensions of that achievement, it is now clear that, for several reasons, monotherapy with injected insulin cannot duplicate the metabolic homeostasis provided by endogenous insulin derived from pancreatic islets. First, peripherally injected insulin cannot meet the high insulin requirements of its upstream targets, the alpha cells and hepatocytes, without exceeding the insulin requirements of its downstream targets, skeletal muscle and adipocytes. Attempts to reconcile the inherent differences in insulin requirements of its target tissues may contribute to the glycemic lability that typifies the disease. Second, unopposed lipogenic action of insulin may also contribute to glycemic instability by intermittently promoting fatty acid (FA)-mediated insulin resistance (2–4), which, in the long term, contributes to lipotoxic cardiomyopathy (5). Third, chronic hyperinsulinemia may enhance cholesterologenesis (6) and account for the high incidence of coronary artery disease in patients with T1DM (7, 8). Even when plasma lipid and lipoprotein levels are normal in patients with type 1 diabetes, the apoB-lipoproteins are cholesteryl ester-enriched and are potentially more atherogenic (9).
In this report, we compare the therapeutic actions of insulin and leptin in a rodent model of T1DM, the nonobese diabetic (NOD) mouse. We found that both hormones prevent ketoacidosis, cachexia, and death and that both restore hemoglobin A1c (HbA1c) to normal. In addition, they both reverse the catabolic state, which is reflected by normalization of a wide array of intermediary metabolites that accumulated in livers of mice with uncontrolled insulin deficiency. However, the two hormones differ dramatically with respect to their effects on lipid metabolism: leptin suppresses lipogenesis, whereas insulin monotherapy enhances lipogenesis and factors involved in cholesterologenesis. We find that recombinant leptin, either alone or combined with low-dose insulin therapy, provides equivalent or superior glycemic stability without the increase in body fat and up-regulation of cholesterologenic and lipogenic transcription factors and enzymes observed with insulin monotherapy. These findings raise the possibility of a role for leptin supplementation in the treatment of human T1DM.
Results
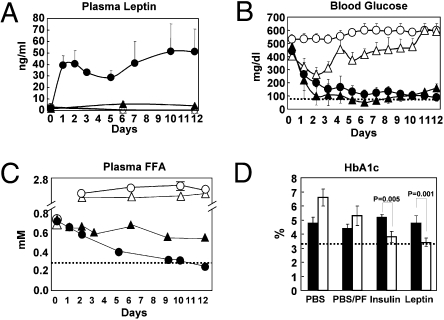
To determine if leptin monotherapy is effective in type 1 diabetes, 15 diabetic NOD mice with hyperglycemia ranging from 220 to 572 mg/dL underwent implantation of an Alzet Osmotic pump containing 3.3 mg of leptin so as to deliver 20 μg of leptin per hour for 12 days. They were compared with diabetic littermates treated with insulin by s.c. pellet. Untreated controls received PBS infusion by Alzet pump. Mean plasma leptin levels ranged between 20 and 50 ng/mL during the period of leptin infusion (Fig. 1A). Plasma glucose levels declined in all 15 leptin-treated animals and averaged 88 ± 28 mg/dL after 12 days compared with 160 ± 32 mg/dL on the insulin pellet (Fig. 1B and Table 1). Ketonuria, which ranged from 40 to 160 mg/dL in severely hyperglycemic PBS-treated control mice, disappeared with both leptin and insulin treatment (Table 1). Plasma free FA (FFA) were also dramatically lowered by leptin to 0.25 ± 0.04 mM after 12 days of treatment (P < 0.03) compared with 0.54 ± 0.1 mM in the insulin group (P = 0.07) and 1.9 ± 0.4 mM in PBS-treated controls (Fig. 1C and Table 1). Like insulin treatment, leptin infusion lowered hemoglobin A1c to 3.4 ± 0.3%, similar to the level in nondiabetic littermates (Fig. 1D and Table 1). The time required to restore normoglycemia with leptin therapy varied with the severity and duration of the disease, ranging from 1 day in the mice with the least severe diabetes to 7–9 days in mice with more severe diabetes of longer duration.
Fig. 1.
Comparisons of various parameters in plasma of type 1 diabetic NOD mice. Mice were either untreated or treated with s.c.-infused leptin (•; n = 14) through an Alzet pump or with insulin delivered from a s.c. pellet (▲; n = 6). Untreated mice were either fed ad libitum (○; n = 4) or pair-fed with the leptin-treated group (△; n = 4), and all received an infusion of PBS through an Alzet pump. (A) Plasma leptin levels. (B) Blood glucose levels. (C) Plasma FFA levels. (D) Hemoglobin A1c (day 0 =■ day 12 = □). The dotted lines mark the normal values.
Table 1.
Metabolic profiles of NOD mice treated with PBS, PBS/pair-fed, recombinant leptin pump, and insulin pellet as well as nondiabetic controls
| PBS pump (n = 4) | PBS/pair-fed (n = 4) | Leptin (n = 14) | Insulin (n = 6) | Nondiabetic (n = 5) | ||||||
| Measurement | Day 0 | Day 12 | Day 0 | Day 12 | Day 0 | Day 12 | Day 0 | Day 12 | ||
| Body weight (g) | 23.9 ± 0.65 | 21.93 ± 1.4* | 23.0 ± 0.95 | 18.63 ± 1.24 | 24.6 ± 2.0 | 22.13 ± 1.6† | 24.24 ± 0.91 | 25.94 ± 0.63 | 22.88 ± 0.88 | |
| Food intake (g/day) | 10.1 ± 1.6* | 2.8–3.0 | 2.8 ± 0.8† | 5.9 ± 0.73 | 3.32 ± 0.71 | |||||
| Body fat (%) | 11.02 ± 4.72 | 4.71 ± 1.62* | 10.29 ± 1.72 | 3.82 ± 1.07 | 13.49 ± 2.90 | 3.23 ± 1.35† | 8.0 ± 0.99 | 13.2 ± 1.22 | 12.30 ± 2.11 | |
| Body temperature (°C) | 37.48 ± 0.76 | 38.15 ± 0.50 | 37.67 ± 0.13 | 37.88 ± 0.33 | 37.24 ± 0.33 | 37.84 ± 0.40† | 37.32 ± 0.38 | 37.06 ± 0.61 | 37.64 ± 0.44 | |
| Urine glucose (mg/dL) | 250–500 | 1,000–2,000* | 250–500 | 1,000–2,000 | 250–500 | Negative | 250–500 | Negative | Negative | |
| Urine ketone (mg/dL) | Negative | 40–160 | Negative | 15–80 | Negative | Negative | Negative | Negative | Negative | |
| Blood glucose (mg/dL) | 531 ± 27 | > 600* | 403 ± 98 | 591 ± 14 | 443 ± 97 | 88 ± 28† | 477 ± 91 | 160 ± 32 | 102 ± 12 | |
| Hemoglobin A1c | 4.8 ± 0.4 | 6.6 ± 0.6* | 4.4 ± 0.3 | 5.3 ± 0.7 | 4.8 ± 0.5 | 3.4 ± 0.3† | 5.2 ± 0.2 | 3.8 ± 0.4 | 3.3 ± 0.1 | |
| Leptin (ng/mL) | 2.38 ± 0.99 | 0.25 ± 0.04* | 2.98 ± 1.38 | 0.27 ± 0.01 | 3.9 ± 2.1 | 35.6 ± 11.1† | 1.37 ± 0.88 | 4.19 ± 0.88 | 2.43 ± 0.94 | |
| Insulin (ng/mL) | 1.54 ± 0.76 | 0.04 ± 0.02 | 1.01 ± 0.11 | 0.05 ± 0.03 | 2.00 ± 1.18 | 0.06 ± 0.05† | 1.51 ± 0.79 | 11.45 ± 0.47 | 1.32 ± 0.42 | |
| Glucagon (pg/mL) | 391.8 ± 41.6* | 463.4 ± 111.6 | 78.5 ± 40.1 | 53.6 ± 17.6 | 76.5 ± 11.6 | |||||
| TG (mg/dL) | 63.5 ± 20.5 | 1,118.5 ± 165.0* | 51.4 ± 6.4 | 406.7 ± 79.4 | 42.8 ± 7.7 | 6.7 ± 5.0† | 77.5 ± 30.5 | 47.5 ± 23.1 | 30.9 ± 7.1 | |
| FFA (mM) | 0.75 ± 0.14 | 1.88 ± 0.42* | 0.68 ± 0.05 | 1.62 ± 0.05 | 0.72 ± 0.09 | 0.25 ± 0.04† | 0.71 ± 0.14 | 0.54 ± 0.12 | 0.49 ± 0.10 | |
| Liver TG (mg/g) | 6.36 ± 0.85* | 7.07 ± 0.63 | 4.61 ± 0.47† | 7.50 ± 2.14 | 8.65 ± 1.41 | |||||
| Liver glycogen (mg/g) | 7.29 ± 1.68* | 8.47 ± 6.18 | 20.77 ± 5.18 | 19.33 ± 5.04 | 12.80 ± 4.49 | |||||
*Significant difference between PBS and leptin groups at day 12.
†Significant difference between leptin and insulin groups at day 12.
Leptin treatment profoundly reduced food intake from 10 ± 1.5 g/day in untreated hyperphagic diabetic mice to 2.8 ± 0.8 g/day, which was not significantly different from the 3.3 ± 0.7 g/day intake of normal nondiabetic mice (Table 1). The leptin-treated mice lost 2.5 g of body weight and 76% of body fat during the 12 days, whereas the PBS-treated mice on ad libitum feeding lost 2.0 ± 1.7 g of body weight in 12 days, 57% of which was body fat. When pair-fed to the leptinized group, the PBS-treated mice lost 4.4 ± 0.5 g. Insulin-treated mice gained 1.7 ± 1.2 g, and their body fat rose 72% (Table 1). Body length in leptin-treated mice measured 8.7 ± 0.5 cm vs. 8.4 ± 0.2 cm in mice with insulin therapy [not significant (NS)] and 7.95 ± 0.05 cm in PBS-treated controls (P < 0.035). These findings suggest that leptin-induced weight loss was at the expense of body fat rather than lean body mass. Furthermore, with leptin, liver glycogen increased from 7.3 ± 1.7 mg/g to 20 ± 5 mg/g, which was not different from insulin therapy; this suggests that catabolic actions of leptin are confined to lipids and that proteins and carbohydrates are exempt.
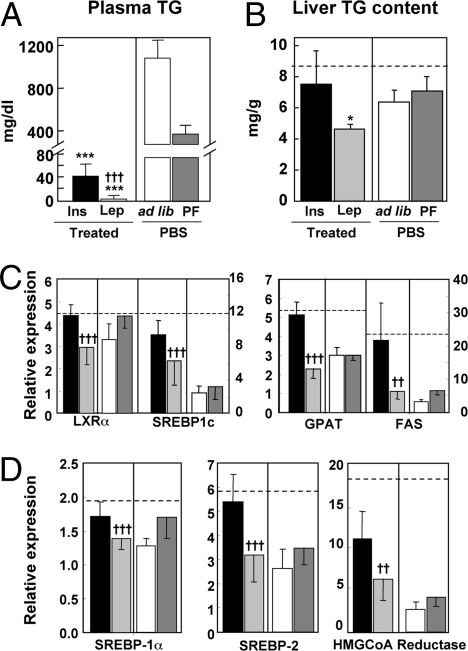
Indeed, the most striking differences between leptin and insulin therapies were in lipid metabolism. In addition to the lowering of FFA, other lipid abnormalities associated with insulin-treated T1DM were corrected by leptin therapy. Plasma triacylglycerol (TG), which averaged 1,118 ± 165 mg/dL in ad libitum fed PBS-treated diabetic mice and 406 ± 79 mg/dL in the pair-fed PBS group, measured 7 ± 5 mg/dL after 12 days of leptin therapy (Fig. 2A and Table 1) compared with 48 ± 23 mg/dL in the insulin-treated group and 31 ± 7 mg/dL in nondiabetic mice. Liver TG averaged 6.4 ± 0.9 mg/g wet weight in PBS-treated diabetic mice compared with 4.6 ± 0.5 mg/g after 12 days of leptin treatment, which was significantly less (P < 0.05) than the 7.5 ± 2 mg/g content after insulin treatment. TG averaged 8.7 ± 1 mg/g in nondiabetic livers (Fig. 2B and Table 1).
Fig. 2.
TG concentrations in plasma and liver and expression levels of transcription factors and enzymes involved in lipogenesis and cholesterologenesis in livers of type 1 diabetic NOD mice treated either with s.c.-infused leptin ( ) or insulin delivered from an s.c. pellet (■). Untreated controls infused with PBS were either fed ad libitum (□) or pair-fed (PF) to the leptin-treated group (
) or insulin delivered from an s.c. pellet (■). Untreated controls infused with PBS were either fed ad libitum (□) or pair-fed (PF) to the leptin-treated group ( ). (A) Plasma TG concentration. (B) Liver TG content. (C) Hepatic expression of a lipogenic transcription factor, LXRα and SREBP-1c, and lipogenic enzymes, FAS and GPAT. (D) Hepatic expression of transcription factors, SREBP-1a and -2, and enzyme, HMG CoA reductase, involved in cholesterologenesis. Relative expression signifies the ratio of the mRNA of interest to the mRNA of 36B4, the invariant control. The broken horizontal lines indicate the relative expression level of the mRNA in nondiabetic control mice. [*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 vs. ad libitum–fed untreated (PBF) controls; †P ≤ 0.05; ††P ≤ 0.01; †††P ≤ 0.001 leptin treatment vs. insulin.]
). (A) Plasma TG concentration. (B) Liver TG content. (C) Hepatic expression of a lipogenic transcription factor, LXRα and SREBP-1c, and lipogenic enzymes, FAS and GPAT. (D) Hepatic expression of transcription factors, SREBP-1a and -2, and enzyme, HMG CoA reductase, involved in cholesterologenesis. Relative expression signifies the ratio of the mRNA of interest to the mRNA of 36B4, the invariant control. The broken horizontal lines indicate the relative expression level of the mRNA in nondiabetic control mice. [*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 vs. ad libitum–fed untreated (PBF) controls; †P ≤ 0.05; ††P ≤ 0.01; †††P ≤ 0.001 leptin treatment vs. insulin.]
To determine the mechanism of the antilipogenic effect of leptin, we compared the expression of the lipogenic transcription factor, sterol regulatory element binding protein (SREBP)-1c, and several of its lipogenic target enzymes (Fig. 2C and Table 2). The expression of both SREBP-1c and liver X receptor-α (LXRα), which responds to insulin by activating the SREBP-1c promoter (10), was significantly lower in leptin-treated mice than in insulin-treated or nondiabetic mice (P < 0.025 and P < 0.0001, respectively). Expression of two lipogenic enzymes, fatty acyl CoA synthetase (FAS) and glycerophosphate acyl transferase (GPAT), was also far below the levels in nondiabetic or insulin-treated diabetic livers (P < 0.00035) (Fig. 2C and Table 2). Reduced insulin receptor substrate 2 (IRS-2) mRNA is reportedly associated with insulin-stimulated lipogenesis and hepatic insulin resistance (11); indeed, it was almost twice as high in insulin-treated mice (NS) (12) (Table 2).
Table 2.
Quantitative PCR analysis of mRNAs in liver from NOD mice treated with PBS, PBS/pair-fed, leptin, and insulin as well as nondiabetic controls after 12 days of treatment
| Genes | PBS (n = 4) | P value (leptin vs. PBS) | PBS/pair-fed (n = 4) | Leptin (n = 6) | Insulin (n = 6) | P value (leptin vs. insulin) | Nondiabetic (n = 4) |
| (A) FA metabolism | |||||||
| LXRα | 3.3 ± 0.7 | 4.3 ± 0.6 | 2.9 ± 0.8 | 4.4 ± 0.5 | ††† | 4.4 ± 0.4 | |
| SREBP1c | 2.4 ± 0.8 | ** | 3.2 ± 1.4 | 6.2 ± 3.3 | 9.3 ± 1.6 | † | 12.2 ± 3.8 |
| FAS | 3.3 ± 0.5 | ** | 6.7 ± 1.0 | 6.4 ± 3.8 | 21.8 ± 12.2 | ††† | 24.1 ± 6.6 |
| GPAT | 3.0 ± 0.4 | ** | 3.0 ± 0.2 | 2.3 ± 0.5 | 5.1 ± 0.7 | ††† | 5.4 ± 0.4 |
| SCD-1 | 16.1 ± 20.5 | *** | 8.4 ± 2.4 | 394 ± 136 | 313 ± 205 | 484 ± 141 | |
| PPARα | 3.3 ± 1.3 | *** | 3.6 ± 0.6 | 6.1 ± 1.5 | 7.4 ± 2.7 | †† | 6.8 ± 0.5 |
| Foxo1 | 1.8 ± 0.2 | *** | 1.6 ± 0.4 | 1.3 ± 0.2 | 1.5 ± 0.2 | 1.8 ± 0.3 | |
| PGC1α | 9.5 ± 4.2 | *** | 6.0 ± 1.8 | 3.0 ± 1.0 | 4.5 ± 2.0 | † | 3.6 ± 1.0 |
| CD36 | 16.9 ± 5.3 | *** | 17.7 ± 5.0 | 3.1 ± 1.3 | 3.6 ± 2.2 | 2.1 ± 0.4 | |
| SPT1 | 5.2 ± 0.6 | * | 5.7 ± 0.8 | 4.3 ± 1.1 | 5.9 ± 0.9 | ††† | 6.3 ± 0.6 |
| SIRT1 | 2.6 ± 0.3 | * | 2.2 ± 0.4 | 3.2 ± 0.7 | 3.8 ± 0.6 | † | 3.2 ± 0.6 |
| (B) Gluconeogenesis | |||||||
| PEPCK | 5.5 ± 0.5 | *** | 4.7 ± 0.2 | 3.4 ± 0.6 | 3.2 ± 0.9 | 2.7 ± 0.4 | |
| (C) Cholesterologenesis | |||||||
| SREBP1a | +1.3 ± 0.1 | 1.7 ± 0.3 | 1.4 ± 0.2 | 1.7 ± 0.2 | ††† | 1.9 ± 0.1 | |
| SREBP2 | 2.6 ± 0.8 | 3.5 ± 0.7 | 3.2 ± 1.1 | 5.4 ± 1.2 | ††† | 5.9 ± 0.5 | |
| HMG-CoA R | 2.9 ± 0.9 | ** | 4.3 ± 1.3 | 6.6 ± 3.7 | 11.8 ± 3.4 | †† | 19.2 ± 9.5 |
Leptin vs. PBS:
*P values < 0.05;
**P value < 0.01;
***P value < 0.001. Leptin vs. insulin:
†P value < 0.05;
††P value < 0.01;
†††P value < 0.001. P values > 0.05 (insulin vs. leptin): MCD, CPT1, IGF1, IGFBP2, BCl2, BAX, Foxc2, PPARγ, IRS-2, ACCα, ACCβ, ACO, and UCP2. 36B4 was the invariant control.
There were two unexpected results. First, in leptin- and insulin-treated mice, the mRNA of stearoyl CoA desaturase 1 (SCD1), which had declined to 3% of normal in untreated T1DM controls, rose 27-fold higher with leptin therapy and 20-fold higher with insulin therapy. Second, CD36, the fatty acid transporter, was elevated in the PBS-treated group and lowered to near-normal values with insulin and leptin therapies (Table 2).
Coronary artery disease (CAD), a common event in longstanding T1DM (8), is generally attributed to the hyperglycemia of diabetes rather than to the hyperinsulinemia caused by its treatment. However, because hyperinsulinemia is a risk factor for CAD, we compared the hepatic expression of two cholesterologenic transcription factors, SREBP1a and SREBP2 (13), in mice treated with leptin or insulin and the rate-limiting enzyme of cholesterol synthesis, HMG CoA reductase. All three were significantly lower with leptin than with insulin treatment (P < 0.001 for SREBP1a, P < 0.0001 for SREBP2, and P < 0.0035 for HMG CoA reductase) (Fig. 2D and Table 2).
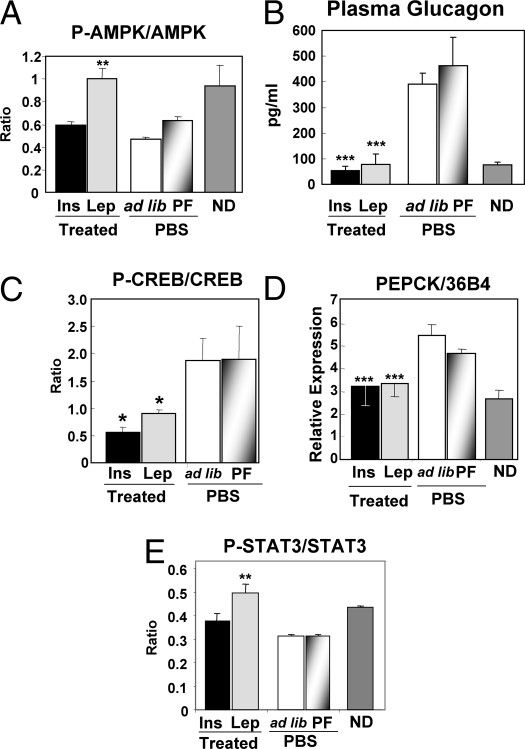
To determine if increased FA oxidation might have contributed to the lipid-lowering action of leptin, we measured liver peroxisome proliferator-activated receptor-α (PPARα) expression and activation of AMP-activated protein kinase (AMPK). PPARα was low in PBS-treated diabetic mice but was increased to normal by treatment with both leptin (P < 0.0002) and insulin (P < 0.007) (Table 2). Phosphorylated AMPK, a master regulator of β oxidation of FA (14), was significantly higher in leptin-treated livers than in insulin-treated and untreated mice, confirming earlier work (15) (Fig. 3A).
Fig. 3.
Plasma glucagon levels and activation of its transcription factor, CREB, and a target enzyme in livers of type 1 diabetic NOD mice treated with s.c.-infused leptin ( ) or PEPCK insulin delivered from an s.c. pellet (■); untreated controls were infused with PBS and fed ad libitum (□) or PF to the leptin-treated group (
) or PEPCK insulin delivered from an s.c. pellet (■); untreated controls were infused with PBS and fed ad libitum (□) or PF to the leptin-treated group ( ). (A) Ratio of phosphorylated to total AMPK. (B) Plasma glucagon. (C) Ratio of phosphorylated to total CREB. (D) mRNA of phosphoenol pyruvate carboxykinase. (E) Ratio of phosphorylated to total STAT-3. ND, nondiabetic. (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 vs. ad libitum fed untreated PBF controls.)
). (A) Ratio of phosphorylated to total AMPK. (B) Plasma glucagon. (C) Ratio of phosphorylated to total CREB. (D) mRNA of phosphoenol pyruvate carboxykinase. (E) Ratio of phosphorylated to total STAT-3. ND, nondiabetic. (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 vs. ad libitum fed untreated PBF controls.)
We had previously shown that hepatic overproduction of glucose, ketones, and other catabolic manifestations of insulin deficiency cannot occur in the absence of hyperglucagonemia (16). To determine if the antidiabetic effects of leptin might be mediated by glucagon suppression, which was suggested by Tuduri et al. (17), we compared plasma glucagon in the four groups of diabetic mice. Plasma glucagon averaged 392 ± 42 pg/mL and 463 ± 112 pg/mL, respectively, in ad libitum fed and pair-fed PBS-treated diabetic controls, and it was suppressed to 79 ± 40 pg/mL by leptin therapy and 54 ± 18 pg/mL by insulin treatment (Fig. 3B and Table 1). Thus, like insulin, leptin is a potent suppressor of glucagon. It remains to be determined if, like insulin, leptin acts directly on α cells or if it involves a hypothalamic effect.
Glucagon suppression was associated with a reduction in phosphorylated cAMP response element binding protein (CREB) in the liver (18), which was consistent with less glucagon action (Fig. 3C). The mRNA of phosphoenol pyruvate carboxykinase, a prime gluconeogenic target of glucagon, was also reduced by leptin as well as insulin (Fig. 3D and Table 2). P-STAT3, an indicator of direct leptin action, was increased in the livers of the leptin-treated but not insulin-treated mice, suggesting that leptin was acting directly on the liver (Fig. 3E). However, it is not clear how much of the hepatic action of leptin therapy is the result of glucagon suppression and how much is a direct insulin-like effect on the liver.
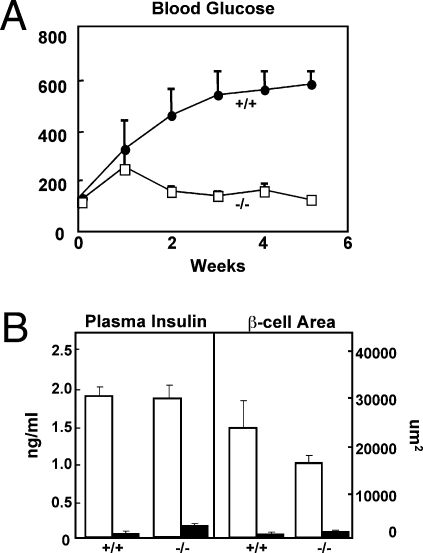
To determine if the suppression of the hyperglucagonemia itself could explain the leptin-induced improvement in the metabolic condition, we induced total insulin deficiency by treating glucagon receptor null mice (Gcgr−/−) mice (19) with two 80-mg/kg doses of alloxan administered 1 week apart. Alloxanized wild-type controls became severely hyperglycemic, hyperketonemic, and cachectic unless treated with insulin. By contrast, the untreated Gcgr−/− mice remained normoglycemic without any clinical or laboratory evidence of insulin deficiency (Fig. 4A), despite the fact that their plasma insulin levels were undetectable and that immunocytochemical analysis of their pancreata indicated destruction of 93% of its insulin-positive pancreatic β-cells (Fig. 4B). Because preproinsulin mRNA could not be detected in their pancreata [cycle threshold (Ct) value ∼ 40 vs. 36B4 mRNA of 18], it seems unlikely that the cells were producing any insulin. These findings indicate that the laboratory and clinical aberrations of severe insulin deficiency cannot occur in the absence of glucagon action. It further suggests that the leptin-mediated reversal of the insulin-deficiency phenotype in T1DM rodents could be caused by glucagon suppression alone.
Fig. 4.
(A) Comparison of blood glucose levels in glucagon receptor null (Gcgr−/−) mice (□) and wild-type (Gcgr+/+) controls (•) after treatment with double-dose alloxan. (B) Comparison of severity of insulin deficiency in the alloxan-treated Gcgr−/− and Gcgr+/+ and nondiabetic Gcgr−/− and Gcgr+/+ controls based on plasma insulin levels and morphometric values for insulin-immunostained β-cells in pancreata. The results indicate no significant difference in residual insulin that could explain the absence of hyperglycemia in the alloxan-treated Gcgr−/− group. Nondiabetic, □; alloxan diabetic, ■.
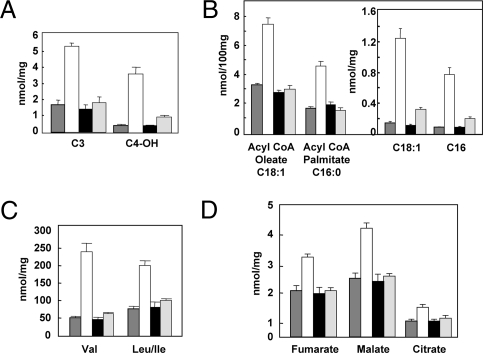
Nevertheless, other actions of leptin in addition to glucagon suppression may well have contributed to the normalization of the metabolic state in the absence of insulin. To understand more completely the intracellular metabolic effects of leptin vs. insulin therapy in NOD mice, we used targeted mass spectrometry-based metabolic profiling (“metabolomics”) to measure the levels of a large number of intermediary metabolites from four chemical classes in the liver of treated and untreated mice—fatty acyl CoAs, amino acids, organic acids [tricarboxylic acid (TCA) cycle intermediates and related analytes], and acylcarnitines (representing products of lipid, glucose, and amino acid catabolism in mitochondria). Untreated NOD mice with uncontrolled diabetes exhibited large increases in many of these metabolites in liver compared with prediabetic NOD animals, including 3 carbon (C3, reflecting the propionyl CoA pool) and 5 carbon (C5, representing α-methylbutyryl CoA and isovaleryl CoA species) acylcarnitines (byproducts of amino acid catabolism), β-hydroxybutryl acyl carnitine (C4-OH, reflective of ketone accumulation), multiple long-chain acylcarnitines and fatty acyl CoAs, branched-chain amino acids (valine and leucine/isoleucine), and organic acids/TCA cycle intermediates, including citrate, fumarate, and malate. Remarkably, despite the enormous differences in lipid partitioning and metabolism described for the two hormone therapies, leptin and insulin were virtually identical in their abilities to reduce to normal the elevations of hepatic metabolite levels across all of the classes of analytes surveyed (Fig. 5 and Table S1). Taken together, our findings suggest that restoration to normal of intermediates of lipid metabolism was achieved by reactivation of lipogenesis in the case of insulin and by stimulation of lipid oxidation in the case of leptin. Leptin also mimicked many other anticatabolic effects of insulin in the NOD mouse.
Fig. 5.
Examples of metabolomic patterns of four classes of metabolites in livers of nondiabetic ( ), untreated diabetic (□), diabetic treated with insulin pellets (■), and diabetic leptin-treated mice (
), untreated diabetic (□), diabetic treated with insulin pellets (■), and diabetic leptin-treated mice ( ). (A) Acyl carnitines reflecting branch-chain amino acid metabolism and ketone production. (B) Long-chain acyl Co As and acyl carnitines (Inset). (C) Branch-chain amino acids. (D) Organic acids. Of 121 metabolites measured, 67 were abnormal in untreated diabetes. Of these, 55 were corrected or improved by both hormones. Three were corrected by leptin but not insulin. Three were corrected by insulin but not leptin. The remainder were not corrected.
). (A) Acyl carnitines reflecting branch-chain amino acid metabolism and ketone production. (B) Long-chain acyl Co As and acyl carnitines (Inset). (C) Branch-chain amino acids. (D) Organic acids. Of 121 metabolites measured, 67 were abnormal in untreated diabetes. Of these, 55 were corrected or improved by both hormones. Three were corrected by leptin but not insulin. Three were corrected by insulin but not leptin. The remainder were not corrected.
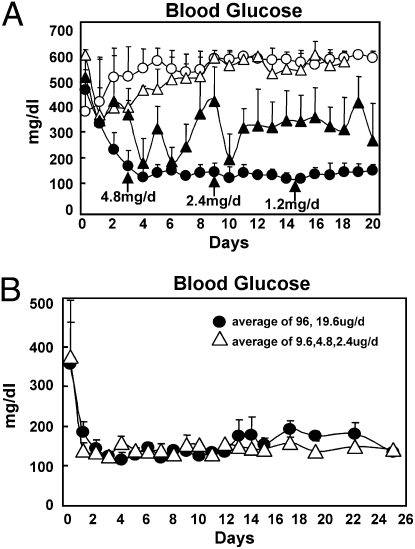
Although leptin monotherapy normalized the metabolic dysfunction in NOD mice with T1DM, a regimen without any insulin would not be approved for treatment in humans with this disease. We, therefore, compared the effects of insulin alone or supplemented with insulin at 0.02 U/day or 10% of the optimal insulin dose of 0.2 U/day s.c. twice daily. Fig. 6A compares the glycemia of diabetic NOD mice treated with 0.2 U insulin (Levemir; Novo Nordisk) twice daily with mice treated with 0.02 U insulin twice daily with and without twice daily leptin injections at decreasing doses from 4.8 to 0.3 mg per day for 20 days. With insulin monotherapy at the 0.2 U dose, HbA1c was normal at 3.9%, but glucose levels varied widely. The SEM of the mean of each mouse’s glucose measurements during 20 days, an index of glycemic lability, was 229 ± 52 mg/dL in the mice treated with insulin monotherapy compared with 47 ± 32 mg/dL in the bihormonally treated mice. With the low 0.02 U/day insulin dose plus leptin, HbA1c was 3.2%, the same as in nondiabetic mice, and plasma glucose over the 20 days averaged 136 mg/dL with an SEM of 32 mg/dL, indicating far less glucose variability than with insulin alone. Liver TG content on this bihormonal regimen was 6.2 ± 0.2 mg/g compared with 10.3 ± 0.8 mg/g on the low 0.02 U insulin dose and 11.2 ± 1.8 mg/g on the high 0.2 U insulin dose. Plasma TG on this bihormonal regimen measured 34 ± 3 mg/dL compared with 314 ± 45 mg/dL on the low 0.02 U without leptin and 51 ± 7 mg/dL on the high 0.2 U insulin dose.
Fig. 6.
(A) Comparison of plasma glucose levels in type 1 diabetic NOD mice treated with twice daily injection of insulin alone at a total dose of 0.2 U/day (▲), twice daily insulin alone at a total dose of 0.02 U/day fed ad libitum (○), twice daily insulin PF to the leptin-treated group (□), or a total daily dose of insulin of 0.02 U/day plus leptin injected at the doses and times indicated by arrows (•). (B) Evidence that much lower doses of infused leptin without insulin are also effective in reducing the hyperglycemia of T1DM. Blood glucose levels of diabetic NOD mice treated for 20 days with leptin monotherapy by osmotic infusion pump are shown. Priming doses during the first 7 days ranged from 1/500 to 1/2,000 of the doses injected with low-dose insulin, and maintenance doses of infused leptin thereafter ranged from 1/500 to 1/2,500 of the doses of injected leptin employed.
The human equivalent of doses of leptin employed in the foregoing rodent studies (Fig. 6A) would be far too costly to employ in patients with T1DM. However, these high doses had been selected to simulate the adenovirally induced hyperleptinemia employed by Yu et al. (20). To determine if lower leptin doses equivalent to those that could be practically employed in humans could also effectively reverse the metabolic abnormalities of insulin deficiency, we infused much lower doses through an infusion pump. We found that after 1 week of priming doses of leptin ranging from 96 to 9.6 μg/day, normoglycemia of NOD mice with T1DM could be maintained for 26 days with as little as 2.4 μg/day or 1/2,000 of the dose of injected leptin (Fig. 6B).
Discussion
Monotherapy with insulin has transformed a previously fatal illness into a livable one, but injected hormone has not approached endogenous insulin in either the quality or quantity of life that it makes possible. Quality of life is impaired by the frequent glucose monitoring and multiple insulin injections required to minimize the glycemic instability and its clinical consequences. This is, at least in part, the result of the disparity between the much higher insulin requirements of the upstream targets of insulin, such as α cells and liver, relative to downstream targets, such as adipocytes and muscle. Peripherally injected insulin can meet all of these disparate needs only with insulin doses that far exceed the needs of peripheral sites, causing hypoglycemia. Another contributing factor to glycemic instability is lability of FFA levels (21). A surge of FFA, released whenever insulin falls beneath the antilipolytic threshold, can flood the targets of insulin and make them resistant to its action (21–23), thus promoting hyperglycemia.
As for quantity of life, life expectancy with T1DM is reduced, not only by diabetes-specific microvascular diseases but also by macrovascular complications that are not specific to diabetes. Of the latter, CAD and other atheromatous disorders have been linked to hyperinsulinemia, both endogenous and exogenous (24). In addition, chronic hyperinsulinemia could contribute to lipotoxic complications present in the late stages of the diabetes.
The ability of leptin to potentiate insulin in diabetes has been previously shown (25, 26). Recently, we reported that adenovirally induced hyperleptinemia could restore rodents with total insulin deficiency to good health (27). Although this showed that there was an alternative to insulin in the treatment of insulin deficiency, it did not disclose the mechanism of the benefits or set the stage for translation to humans with T1DM, in whom adenoviral gene therapy is not an option. This report indicates that injection of recombinant leptin can not only prevent the metabolic consequences of insulin deficiency like adenovirally induced hyperleptinemia but that it may eliminate certain underappreciated adverse consequences of insulin monotherapy. Most strikingly, it shows in T1DM mice that leptin inhibits lipogenesis, increases FA oxidation, and thus, reduces tissue lipids while reducing factors involved in cholesterol biosynthesis. Furthermore, it suppresses hyperglucagonemia and mimics the anabolic effects of insulin on protein and carbohydrate metabolism, which restores a wide array of metabolic intermediates to normal in the liver. Between 2 and 8 days of starting the infusion of recombinant leptin, the severe hyperglycemia and ketonuria are corrected, and the relentless catabolic course is reversed in every mouse that was treated (Fig. 1A and Table 1). Within 2 days, the markedly elevated FFA levels fall to one-half the levels of insulin-treated mice. Within 12 days, plasma TG plummets from over 1,000 md/dL to 6.7 mg/dL, 85% below the level in the insulin-treated group and 94% below PBS-treated controls. Total body fat is 25% lower and liver TG content is 60% lower than in the insulin-treated group (Table 1).
The lipopenic action of leptin can be explained by the significant down-regulation of lipogenic transcription factor SREBP1c, its activator LXRα, and two of its lipogenic target enzymes, FAS and GPAT, compared with insulin treatment. The increase in activated AMPK in the liver of leptin-treated mice points to enhanced FA oxidation as a third factor contributing to the lipopenia. Given the key role of FA in insulin resistance, the lipopenic state induced by leptin treatment should stabilize glycemia by eliminating FFA surges into insulin’s target tissues. The addition of low-dose insulin to the leptin therapy provides physiological insulin levels for peripheral targets and avoids the unphysiologically high insulin doses required to suppress the hyperglucagonemia by assigning this task to leptin. The result was a more stable glycemic profile. We suspect that the lipopenic action, coupled with the correction of hyperglucagonemia, accounts for much of the metabolic improvement. The initial results in this study were obtained using high doses of injected leptin intended to simulate the marked hyperleptinemia induced by adenovirus in the report by Yu et al. (27). It was, therefore, gratifying to find that much lower doses delivered by constant infusion seemed equally effective, which indicates that equivalent doses in humans may be feasible.
Finally, we used a metabolomics approach to characterize abnormal liver metabolism in uncontrolled T1DM and to compare actions of leptin and insulin on these abnormalities. A total of 121 metabolites were measured in normal, untreated diabetic mice and diabetic mice treated with insulin or leptin. Of the 67 abnormalities identified in untreated diabetes, 55 were corrected by both hormones; three were corrected by leptin but not insulin, three were corrected by insulin but not leptin, and four were not corrected by either hormone. Not only did leptin therapy normalize indices of lipid metabolism such as long-chain acylcarnitine and acyl CoA metabolites, but it also corrected other features of the uncontrolled catabolic state of untreated type 1 diabetes, such as increases in amino acids, their catabolic byproducts such as C3 and C5 acylcarnitines, and several TCA cycle intermediates. The anticatabolic effects of leptin on processes such as protein catabolism may be related to its strong ability to suppress otherwise uncontrolled glucagon secretion. The importance of glucagon suppression in the leptin-induced amelioration of uncontrolled insulin deficiency was confirmed by showing that Gcgr−/− mice remained completely nondiabetic and clinically normal after induction of near-total insulin deficiency.
A potential advantage of leptin therapy over insulin monotherapy is the significant reduction in the cholesterologenic transcription factors, SREBP1a and SREBP2, and in HMG CoA reductase, the rate-limiting enzyme in cholesterol biosynthesis. Leptin-mediated down-regulation of the expression of LXRα, SREBP-1c, and its target enzymes, FAS and GPAT, would seem to account for the observed lowering of TG and FFA levels. In humans, this could possibly translate into long-term benefits, such as a reduction in the high incidence of CAD in T1DM (7, 8). There is evidence that insulin therapy in T1DM mice promotes ectopic fat deposition in liver and muscle and causes insulin resistance (28), actions that are prevented by leptin. Life-long insulin monotherapy in man may, thus, create late-life health problems that might be avoided by the addition of leptin.
Whether or not the benefits of leptin therapy in T1DM mice can be translated to humans with T1DM will be important to determine. Since its discovery in 1994 (29), leptin therapy in humans with diabetes has been limited to patients with generalized lipodystrophy (30), including two patients with coexisting acquired generalized lipodystrophy and T1DM (31). It will now be of great interest to assess its efficacy in the more common T1DM.
It should be pointed out that the mice in this study were hypoleptinemic because of uncontrolled diabetes at the start of leptin monotherapy. Hypoleptinemia is presumed to be the result of depletion of adipocyte fat secondary to insulin deficiency. The inverse relationship between leptin expression and the expression of its receptor (32) raises the possibility that well-controlled diabetic patients with normal or increased adipocyte fat content may be less responsive to leptin therapy because of down-regulation of the leptin receptor.
Materials and Methods
Animals.
Eight-week-old NOD (LtJ) mice were purchased from the Jackson Lab and housed in individual cages in a temperature-controlled environment with ad libitum access to water and Tekla pelleted 6% fat mouse/rat chow (Teklad) and a standard light/dark cycle (light: 6:00 AM to 6:00 PM; dark: 6:00 PM to 6:00 AM). Glucose was measured in conscious animals from a hand-held glucose meter on tail vein blood between 10:00 and 12:00 AM at ∼5-day every day. Animals were killed under sodium pentobarbital anesthesia. Nonfasting blood samples were obtained from the inferior vena cava. All tissues were rapidly excised, frozen in liquid nitrogen, and stored at −70 °C until use. Institutional guidelines for animal care and use were followed.
Gcgr(−/−) mice (M. J. Charron, Bronx, NY) were housed in individual cages with constant temperature and 12 h of light alternating with 12 h of darkness; they were fed Teklad 6% mouse/rat diet (Teklad) and were given free access to water.
Chemical Destruction of β-Cells.
β-cells were destroyed in 10- to 12-week-old Gcgr−/− and Gcr+/+ mice with two i.v. injections of alloxan (80 mg/kg at 7-day intervals). Food intake, body weight, and blood glucose were measured weekly for 6 weeks.
The animal protocols were approved by the Institutional Animal Care and Research Advisory Committee of the University of Texas Southwestern Medical Center.
Metabolic Profiling Studies.
Acylcarnitines, fatty acyl CoAs, and amino acids were analyzed in liver homogenates by tandem MS/MS, and organic acids were analyzed by gas GC/MS as described previously (33–38). All MS analyses employed stable-isotope dilution with internal standards from Isotec, Cambridge Isotope Laboratories, and CDN Isotopes. A list of all internal standards used in these studies has been published (34, 37).
s.c. Leptin Infusion.
Miniosmotic pumps (Alzet model 2001) were loaded with recombinant leptin (Amylin Pharmaceuticals) at a concentration of 20 mg/mL and delivered at a rate of 1 μL/h over a 7-day period. Pumps were implanted s.c. between the scapulae under ketamine/xylazine anesthesia (0.1 mL per 20–30 g body weight) and replaced after 6 days. The untreated control group received PBS delivered by the same pump, whereas the insulin-treated control group received sustained-release insulin implants for mice (Linshin Canada, Ltd.). Food intake, body weight, and blood glucose were monitored daily. Blood samples were collected on days 0, 1, 2, 3, 5, 7, 10, and 12.
s.c. Leptin and Insulin Injections.
Diabetic NOD mice received twice daily s.c. injections of recombinant leptin (Amylin Pharmaceuticals and Sigma-Aldrich, Inc.) at varying doses plus twice daily s.c. injections of the long-acting insulin analog levemir (Novo Nordisk) at a dose of 0.01 U. Other mice were treated with insulin monotherapy using levemir at 0.01 or 0.1 U s.c. twice daily.
Quantitative Real-Time PCR.
Total RNA was extracted from tissues by TRIzol isolation method (Life Technologies). All PCRs were done in triplicate, as previously described (39). mRNA was calculated by using the standard curve method; 36B4 RNA was used as the invariant control. Primer sequences of genes used for quantification of mRNA by quantitative real-time PCR (QRT-PCR) are shown in Table S2.
Immunohistochemistry and β-Cell Mass Measurement.
The distal 20% of pancreata dissected from adult mice was fixed in Bouin’s solution (Sigma) and dehydrated before embedding in paraffin; 5-μ contiguous paraffin sections were prepared on a Leica RM2155 rotary microtome for insulin and glucagon immunohistochemical staining, as previously described (40). Morphometric analysis for insulin cells was performed using Image-J image-analysis software and particle-analysis macro (Scion). The area of insulin staining in three sections of liver from 4 to 6 animals, relative to total sectional area examined, was quantified by monochromatic thresholding.
Plasma Measurements.
Plasma leptin and insulin were measured by using ELISA kits (Crystal Chem). Plasma glucagon was measured using a rat glucagon RIA kit (Linco Research). Plasma insulin-like growth factor-1 (IGF1) was measured by a rat/mouse IGF1 ELISA kit (Gropep Limited; IDS Inc.). Plasma TG were measured using a glycerol phosphate oxidase-Trinder triglyceride kit (Sigma). Plasma-free FA were measured using the Wako nonesterified fatty acid kit (Wako Chemical USA). Plasma cholesterol profiles were performed in the laboratory of Jay Horton (Dallas, TX). Glycated hemoglobin A1c was measured by HPLC in the laboratory of Philip Raskin (Dallas, TX).
TG Content of Tissues.
Mice were anesthetized with pentobarbital sodium. Tissues were rinsed with PBS (pH 7.4), dissected, and placed in liquid nitrogen immediately. Total lipids from tissues were extracted and dried under N2 gas. TG content was assayed as previously described (41).
Immunoblotting.
Total cell extracts prepared from tissues of mice were resolved by SDS/PAGE; they were transferred to a poly(vinylidene difluoride) membrane (Amersham Pharmacia) and analyzed as previously described (39) for total and phosphorylated STAT-3, AMPK, and CREB using primary antibodies antiphospho-STAT-3 (Tyr-705), anti–STAT-3, antiphospho-AMPK (Thr-172), anti-AMPK, antiphospho-CREB (Ser-133), anti-CREB (Cell Signaling Technology), and anti–γ-tubulin (Sigma). The proteins of interest were detected by an enhanced chemiluminescence detection system (Amersham Pharmacia).
Statistical Analysis.
Results are presented as means ± SEM and were evaluated by using Student’s t test for two groups or by ANOVA for comparisons of more than two groups. The ANOVA test is used to compare mRNA expression data.
Acknowledgments
We thank Jay Horton, M.D., and Philipp Scherer, Ph.D., for helpful suggestions. S. Kay McCorkle created the figures, and Kay Naughton and Christie Fisher helped organize the manuscript. Xiu Quan Du in the Charron laboratory was responsible for breeding the glucagon-receptor null mice. This work was supported by the VA North Texas Health Care System, National Institute of Diabetes and Digestive and Kidney Diseases, UT Southwestern Medical Center at Dallas High Impact/High Risk Award, and private donors.
Footnotes
The authors declare no conflict of interest.
This Feature Article is part of a series identified by the Editorial Board as reporting findings of exceptional significance.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909422107/DCSupplemental.
See Commentary on page 4793.
References
- 1.Banting FG, Best CH. Pancreatic extracts. 1922. J Lab Clin Med. 1990;115:254–272. [PubMed] [Google Scholar]
- 2.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: Defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 3.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 4.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou YT, et al. Lipotoxic heart disease in obese rats: Implications for human obesity. Proc Natl Acad Sci USA. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ness GC, Zhao Z, Wiggins L. Insulin and glucagon modulate hepatic 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity by affecting immunoreactive protein levels. J Biol Chem. 1994;269:29168–29172. [PubMed] [Google Scholar]
- 7.Larsen J, et al. Silent coronary atheromatosis in type 1 diabetic patients and its relation to long-term glycemic control. Diabetes. 2002;51:2637–2641. doi: 10.2337/diabetes.51.8.2637. [DOI] [PubMed] [Google Scholar]
- 8.Orchard TJ, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2003;26:1374–1379. doi: 10.2337/diacare.26.5.1374. [DOI] [PubMed] [Google Scholar]
- 9.Chahil TJ, Ginsberg HN. Diabetic dyslipidemia. Endocrinol Metab Clin North Am. 2006;35:491–510. vii–viii. doi: 10.1016/j.ecl.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci USA. 2004;101:11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimomura I, et al. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6:77–86. [PubMed] [Google Scholar]
- 12.Hedbacker K, et al. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 2010;11:11–22. doi: 10.1016/j.cmet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Briggs MR, Yokoyama C, Wang X, Brown MS, Goldstein JL. Nuclear protein that binds sterol regulatory element of low density lipoprotein receptor promoter. I. Identification of the protein and delineation of its target nucleotide sequence. J Biol Chem. 1993;268:14490–14496. [PubMed] [Google Scholar]
- 14.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: Metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 15.Minokoshi Y, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 16.Dobbs R, et al. Glucagon: Role in the hyperglycemia of diabetes mellitus. Science. 1975;187:544–547. doi: 10.1126/science.1089999. [DOI] [PubMed] [Google Scholar]
- 17.Tudurí E, et al. Inhibitory effects of leptin on pancreatic alpha-cell function. Diabetes. 2009;58:1616–1624. doi: 10.2337/db08-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montminy MR, Gonzalez GA, Yamamoto KK. Regulation of cAMP-inducible genes by CREB. Trends Neurosci. 1990;13:184–188. doi: 10.1016/0166-2236(90)90045-c. [DOI] [PubMed] [Google Scholar]
- 19.Gelling RW, et al. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci USA. 2003;100:1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci USA. 2008;105:14070–14075. doi: 10.1073/pnas.0806993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bevilacqua S, et al. Acute elevation of free fatty acid levels leads to hepatic insulin resistance in obese subjects. Metabolism. 1987;36:502–506. doi: 10.1016/0026-0495(87)90051-5. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins AB, Storlien LH, Chisholm DJ, Kraegen EW. Effects of nonesterified fatty acid availability on tissue-specific glucose utilization in rats in vivo. J Clin Invest. 1988;82:293–299. doi: 10.1172/JCI113586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258:766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- 24.Unger RH. Reinventing type 2 diabetes: Pathogenesis, treatment, and prevention. JAMA. 2008;299:1185–1187. doi: 10.1001/jama.299.10.1185. [DOI] [PubMed] [Google Scholar]
- 25.Chinookoswong N, Wang JL, Shi ZQ. Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes. 1999;48:1487–1492. doi: 10.2337/diabetes.48.7.1487. [DOI] [PubMed] [Google Scholar]
- 26.Miyanaga F, et al. Leptin as an adjunct of insulin therapy in insulin-deficient diabetes. Diabetologia. 2003;46:1329–1337. doi: 10.1007/s00125-003-1193-6. [DOI] [PubMed] [Google Scholar]
- 27.Yu X, et al. Leptinomimetic effects of the AMP kinase activator AICAR in leptin-resistant rats: Prevention of diabetes and ectopic lipid deposition. Diabetologia. 2004;47:2012–2021. doi: 10.1007/s00125-004-1570-9. [DOI] [PubMed] [Google Scholar]
- 28.Liu HY, et al. Insulin is a stronger inducer of insulin resistance than hyperglycemia in mice with type 1 diabetes mellitus (T1DM) J Biol Chem. 2009;284:27090–27100. doi: 10.1074/jbc.M109.016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 30.Oral EA, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 31.Park JY, et al. Type 1 diabetes associated with acquired generalized lipodystrophy and insulin resistance: The effect of long-term leptin therapy. J Clin Endocrinol Metab. 2008;93:26–31. doi: 10.1210/jc.2007-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang MY, Orci L, Ravazzola M, Unger RH. Fat storage in adipocytes requires inactivation of leptin’s paracrine activity: implications for treatment of human obesity. Proc Natl Acad Sci USA. 2005;102:18011–18016. doi: 10.1073/pnas.0509001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.An J, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med. 2004;10:268–274. doi: 10.1038/nm995. [DOI] [PubMed] [Google Scholar]
- 34.Ferrara CT, et al. Genetic networks of liver metabolism revealed by integration of metabolic and transcriptional profiling. PLoS Genet. 2008;4:e1000034. doi: 10.1371/journal.pgen.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monetti M, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Newgard CB, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ronnebaum SM, et al. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem. 2006;281:30593–30602. doi: 10.1074/jbc.M511908200. [DOI] [PubMed] [Google Scholar]
- 38.Wu JY, et al. ENU mutagenesis identifies mice with mitochondrial branched-chain aminotransferase deficiency resembling human maple syrup urine disease. J Clin Invest. 2004;113:434–440. doi: 10.1172/JCI19574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang MY, et al. Adipogenic capacity and the susceptibility to type 2 diabetes and metabolic syndrome. Proc Natl Acad Sci USA. 2008;105:6139–6144. doi: 10.1073/pnas.0801981105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stefan Y, et al. Quantitation of endocrine cell content in the pancreas of nondiabetic and diabetic humans. Diabetes. 1982;31:694–700. doi: 10.2337/diab.31.8.694. [DOI] [PubMed] [Google Scholar]
- 41.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]