Abstract
Inhibition of blood vessel formation is a viable therapeutic approach in angiogenesis-dependent diseases. We previously used a combinatorial screening on vascular endothelial growth factor (VEGF)-activated endothelial cells to select the sequence CPQPRPLC and showed that the motif Arg-Pro-Leu targets VEGF receptor-1 and neuropilin-1. Here, we evaluated and validated D(LPR), a derivative molecule with strong antiangiogenesis attributes. This prototype drug markedly inhibits neovascularization in three mouse models: Matrigel-based assay, functional human/murine blood vessel formation, and retinopathy of prematurity. In addition to its systemic activity, D(LPR) also inhibits retinal angiogenesis when administered in an eye-drop formulation. Finally, in preliminary studies, we have showed targeted drug activity in an experimental tumor-bearing mouse model. These results show that drugs targeting extracellular domains of VEGF receptors are active, affect signal transduction, and have potential for clinical application. On a larger context, this study illustrates the power of ligand-directed selection plus retro-inversion for rapid drug discovery and development.
Keywords: peptide, cancer, VEGFR, angiogenesis, retinopathy of prematurity
The formation of blood vessels, whether de novo (vasculogenesis) or from existing blood vessels (angiogenesis), is a fundamental biological process. In the 1970s, Folkman (1) introduced the concept of angiogenesis-dependent diseases and suggested that compounds inhibiting neovascularization would find applications in medicine, particularly against cancer (1). Although this idea was entertained with skepticism at the time, several inhibitors are currently in clinical use, and others are at advanced stages of development. As such, angiogenesis stands as “an organizing principle for drug discovery” (2) and has led to converging insights into the mechanisms of pathological disorders otherwise considered dissimilar to each other (e.g., tumor growth and diabetes to rheumatoid arthritis and thalidomide-based malformations). We have previously proposed abnormal vascularization to be the one unifying feature of seemingly disparate ocular diseases such as diabetic retinopathy, age-related macular degeneration, and retinopathy of prematurity (3); indeed, abnormal blood-vessel formation in the human retina is a leading cause of blindness from children to adults to the elderly.
A balance of activators and inhibitors coordinates the angiogenic process in health and disease (2, 4, 5). Among the molecular activators, vascular endothelial growth factor (VEGF) and its receptors are considered essential contributors to angiogenesis (6), and both ligands and receptors have been targets of therapies. Since the introduction of the monoclonal antibody bevacizumab (Avastin), the first Food and Drug Administration (FDA)-approved, VEGF-targeted therapy for use in metastatic colorectal cancer in combination with chemotherapy (7), several drugs targeting VEGF-related pathways have been developed and are currently in various stages of testing (2, 8). FDA approval of pegaptanib (Macugen), an anti-VEGF pegylated aptamer, and Ranibizumab (Lucentis), a fragment of bevacizumab for the treatment of the wet type of age-related macular degeneration, are examples of anti-VEGF agents with proven efficacy toward an eye disorder with an angiogenic component (9, 10). Although VEGF-targeted therapies have shown relative success and improved cancer survival, there are still unsolved issues. For instance, many patients do not respond to VEGF-targeted therapy, and most patients who do respond initially develop resistance (11). Therefore, a better understanding of the mechanisms of action of these agents is necessary and will improve the efficacy of these therapies (12). Also, development of a new generation of agents targeted against other members of the VEGF family might overcome some of the difficulties associated with angiogenesis inhibitors (13).
VEGF belongs to a multigene family comprised of five members that bind to and selectively activate several membrane-bound tyrosine kinase receptors (VEGFR-1, -2, and -3) and neuropilins (NRP-1 and -2). VEGF (also known as VEGF-A) induces a robust proliferative response in endothelial cells by its interaction with VEGFR-2. The axis VEGF-A/VEGFR-2 is believed to be a key pathway in the angiogenic process and is the focus of current VEGF-based therapies. Moreover, other family members, such as placenta growth factor (PlGF), VEGF-B, and the receptor VEGFR-1, were initially met with less enthusiasm as potential targets, because their roles in angiogenesis were unclear; however, this view has been challenged by the development of compounds targeting these molecules. VEGFR-1 and NRP-1, as well as their specific ligands VEGF-B and PlGF, have a prominent role in angiogenesis and are promising therapeutic agents (14–20). Monoclonal antibodies directed against VEGFR-1, NRP-1, or PlGF have also shown potential as antitumor agents (18, 19, 21). In light of these studies and based on our own previous work (22), we reasoned that small molecules targeting the VEGF-receptor pathways would potentially inhibit angiogenesis and therefore, would be prospects for future clinical studies.
Results and Discussion
Rational Small-Molecule Design.
We have previously used a subtractive phage display-library screening on activated human endothelial cells to isolate a VEGFR-1– and NRP-1–binding peptide, CPQPRPLC (22). Here, to gain insight into the specific ligand-receptor requirements, we generated a panel of mutant peptide-targeted phage clones for use in structure-functional analysis. Unexpectedly, this in tandem strategy of peptide alanine scan followed by binding assays, clearly failed to identify the individual residues in CPQPRPLC that interact with the VEGF receptors (Fig. S1), indicating that methods other than site-directed mutagenesis would be required to unveil the fine molecular basis of this receptor-ligand interaction. We assigned NMR to the minimal structural requirements for binding of CPQPRPLC to VEGFR-1 and NRP-1 to the Arg5-Pro6-Leu7 motif (RPL) embedded within the full peptide (23). Because it is established that peptide sequences selected by phage display often bind to biologically active sites in proteins (24, 25), we hypothesized that the tripeptide RPL, by its targeting of the extracellular domain of VEGFR-1 and NRP-1, might comprise the essential structure for a class of small tyrosin-kinase receptor inhibitors with antiangiogenic properties.
To develop our working hypothesis, we had to overcome a general limitation: peptides are generally considered notoriously labile and are too metabolically unstable to be suitable as drugs. We, therefore, synthesized peptidomimetic derivatives based on this VEGFR-1– and NRP-1–binding motif, and we tested their effects in angiogenesis assays in vivo.
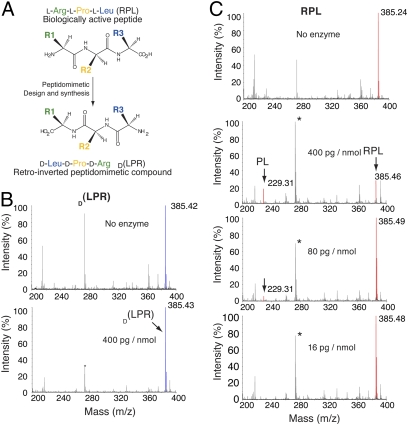
In the design of peptidomimetics, we used a method known as amino acid retro-inversion (26, 27), which consists of the reversal of the peptide backbone stereochemistry (i.e., substitution of D-amino acids for the normal L-amino acids) in conjunction with chain reversal. The resulting product is a “retro-inverso” peptidomimetic in which the side-chain topology is similar to the parent peptide (Fig. 1A). Because mammalian enzymes do not effectively recognize D-amino acid residues and their bonds, these compounds are usually less susceptible to proteolytic degradation. In this context, the corresponding RPL peptide retro-inverted DLeu-DProDArg sequence, D(LPR) peptidomimetic, was chemically produced in solid phase as a lead compound for these studies. We subsequently incubated both RPL and D(LPR) with increasing concentrations of pancreatin, and the reaction products were analyzed by mass spectrometry. No degradation of D(LPR) was observed at the highest ratio of enzyme:peptide concentration (400 pg/nmol) tested (Fig. 1B); in contrast, RPL was markedly degraded by proteases, a conclusion based on the presence of a Pro-Leu fragment and the decreased intensity of the RPL peak relative to the peak of its degradation product (Fig. 1C). We used pancreatin, because it is a harsh proteolytic mixture of digestive enzymes including amylases, lipases, and proteases (such as trypsin and chymotrypsin); we reasoned that degradation resistance to this protease cocktail would strengthen our working hypothesis that a retro-inverted sequence results in a more stable drug. However, as a cautionary note, it has long been determined that D-amino acid oxidase is the only known mammalian enzyme that metabolize D-peptidomimetics in the kidney (28). Thus, despite the apparent resistance of D(LPR) to degradation by pancreatin, detailed pharmacological studies will be required to determine whether or not gastrointestinal administration in preclinical models or ultimately, oral use in patients will be a future possibility.
Fig. 1.
Drug design and protease degradation-resistance assay. (A) Schematic representation of Arg-Pro-Leu (RPL) retro-inversion. (B and C) Mass-spectroscopy analysis (MALDI-TOF) of RPL and D(LPR) pre- and postincubation with pancreatic enzymes. Peaks corresponding to the intact peptides and enzymatic degradation products are color-coded [red, RPL; blue, D(LPR)] and indicated by arrows.
VEGF Receptor-Ligand Binding and Structure–Function Relationship.
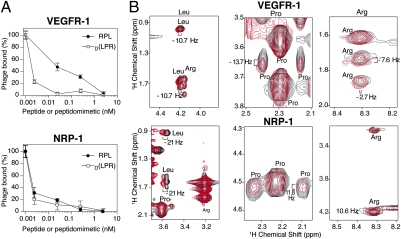
To empirically assess whether or not retro-inversion yielded an agent that binds to VEGFR-1 and NRP-1, we performed competition experiments. In this assay, VEGFR-binding phage particles (displaying CPQPRPLC) (22, 23) are incubated with receptors (VEGFR-1 or NRP-1) in the presence of increasing concentrations of RPL or D(LPR). Binding of the RPL peptide or its corresponding peptidomimetic to the immobilized VEGFR-1 or NRP-1 receptor would compete with the binding of CPQPRPLC phage particles, the affinity of which for VEGFR-1 or NRP-1 has been calculated (22). The concentration of ligand required to inhibit 50% of CPQPRPLC phage binding to the receptor (IC50) is then calculated for RPL and D(LPR). These competition experiments showed that both RPL and D(LPR) inhibited, in a concentration-dependent manner, the binding of CPQPRPLC phage to either receptor (Fig. 2A), whereas a control peptide used at the highest concentration (100 μM) had no effect on phage binding to VEGFR-1 or NRP-1. The IC50 of RPL for VEGFR-1 (30 nM) and NRP-1 (4 pM) and the IC50 of D(LPR) for VEGFR-1 (2 pM) and NRP-1 (2 pM) were reproducibly determined.
Fig. 2.
The tripeptide RPL and the drug D(LPR) target VEGFR-1 and NRP-1. (A) In a phage-competition assay, increasing concentrations of RPL (black circles) or D(LPR) (open squares) inhibit binding of CPQPRPLC-displaying phage to the immobilized receptors VEGFR-1 or NRP-1. (B) Chemical-shift changes induced on the D(LPR) peptidomimetic resonances by its binding to VEGFR-1 or NRP-1 at 25 °C are shown. 2D TOCSY spectra of D(LPR) alone (black color) or in the presence of the individual receptors (red color) are shown. Different regions of the spectra of D(LPR) are shown to indicate chemical-shift changes in individual D(LPR) residues.
To understand the ligand attributes of our lead compound, we analyzed the direct interaction of D(LPR) to VEGF receptors by NMR, the methodology originally used to yield the functional RPL motif (23). Here, the VEGF receptor-ligand behavior of D(LPR) was first analyzed by total correlation spectroscopy (TOCSY) and nuclear overhauser enhancement apectroscopy (NOESY), and all resonances were unambiguously assigned (Table S1). To further identify the specific residues in D(LPR) that participate in the binding to either VEGFR-1 or NRP-1, we next incubated D(LPR) with each individual receptor and measured changes in NMR parameters (chemical shift changes) of D(LPR) resonances in the fast-exchange regime (the resonances in the peptidic NMR spectrum reflect the average parameters between the free and bound states). We observed that both VEGFR induced side-chain chemical shift changes in the resonances of the peptidomimetic (Fig. 2B and Table S2). Leu and Arg side-chain hydrogen atoms showed chemical shift changes in several residues (Table S2) as a consequence of incubation with VEGFR-1 and NPR-1, a result consistent with our studies of the RPL motif (23). In previous work, we showed that the interaction of RPL with NRP-1, but not with VEGFR-1, requires the key participation of the Pro residue, likely representing an evolutionary gain-of-function mutation (23). However, differently from the RPL motif, the Pro residue in D(LPR) also participates in the binding to VEGFR-1, which is shown by the chemical shift change of 13.7 Hz in the Pro.HD1 hydrogen. These results confirm that D(LPR) binds to VEGFR-1 and NRP-1, but, in contrast to RPL, binding of D(LPR) to each individual receptor involves all amino acids in the peptidomimetic, including the Pro residue; these data could explain the similar affinity of D(LPR) observed for either VEGF receptor. Consistently, a differential binding pattern to each VEGF receptor was not observed with the D(LPR) peptidomimetic, which has a similar IC50 for either VEGFR-1 or NRP-1.
Together, we show (i) that the mimic D(LPR) is less prone to proteolytic degradation, (ii) that both RPL and D(LPR) have a higher affinity for NRP-1, but not for VEGFR-1, than that observed with the original CPQPRPLC (22), and (iii) that retro-inversion confers superior ligand binding to VEGF receptors; indeed, the IC50 of CPQPRPLC for NRP-1 (∼50–100 nM) (22) is ∼1.2 × 104-fold to 5 × 104-fold higher than the IC50 of RPL and D(LPR) for either receptor, a functional result suggesting that they are much stronger VEGF receptor ligands than the CPQPRPLC peptide.
Effect of D(LPR) in Angiogenesis Models.
Given its favorable affinity to the targeted VEGF receptors and resistance to degradation in vitro, we concluded that D(LPR) is a suitable candidate for evaluation of angiogenesis assays in vivo. To that end, we used three standard animal models to evaluate the effect of D(LPR) on angiogenesis (29–33).
First, immunocompetent mice were implanted s.c. with VEGF165-containing Matrigel, with or without either D(LPR) or a negative control. On postimplantation day 7, neovascularization within each Matrigel plug was determined by quantification of hemoglobin. Matrigel plugs that had been preloaded with D(LPR) and VEGF165 exhibited significantly fewer new vessels than Matrigel plugs that contained only VEGF165; no detectable effect on blood-vessel formation was observed in the VEGF165-containing Matrigel plugs plus a control peptidomimetic (Fig. 3A). These results indicate that D(LPR) markedly inhibits VEGF-induced neovascularization in vivo.
Fig. 3.
Inhibition of neovascularization in vivo by D(LPR) treatment. (A) Representative pictures of Matrigel plugs containing 500 μg/mL of D(LPR) or control after 7 days of implantation (Lower). Matrigel plugs were excised, and angiogenesis was quantified by measurement of the hemoglobin content within the matrix. The bar graph shows representative mice from the experiment (Upper). (B) Immunostaining with anti-human factor VIII antibody of scaffolds containing human microvascular endothelial cells (HDMEC) implanted into SCID mice that received 25 mg/kg i.p. daily of D(LPR) or control. (C) Number of human factor VIII-positive blood vessels at 200× magnification. *Student’s t test (P < 0.01).
Second, we checked for a systemic therapeutic effect in a SCID-mouse model of human angiogenesis in which human endothelial cells, cultured in vivo within polymer implants, grow to form a microvasculature; then, this merges with the host (i.e., mouse) capillaries. These functional neovessels are lined with human endothelial cells that express angiogenesis markers and serve to transport the murine circulation. To evaluate the effect of D(LPR), we maintained mice implanted with scaffolds containing human endothelial cells for 9 days. The endothelial cells formed nonfunctional tubular structures containing empty lumens that slowly matured to fully functional human blood vessels containing the murine hematic cells and other elements (30). The implanted mice received i.p. administration of D(LPR) or control peptidomimetic daily on days 12–21. The scaffolds were removed, and the number of functional blood vessels was determined by Factor VIII immunostaining. On day 21, all animals developed functional blood vessels with the expected cell density; these chimeric blood vessels also expressed the angiogenesis marker von Willebrand factor (vWF), a receptor for Factor VIII (Fig. 3B). We observed a significant reduction (37%; t test with P < 0.001) in the number of blood vessels in mice treated with D(LPR) [32.1 ± 3.8 per field in the control peptidomimetic versus 20.3 ± 3.8 in the presence of D(LPR)]. A typical experiment is shown in Fig. 3.
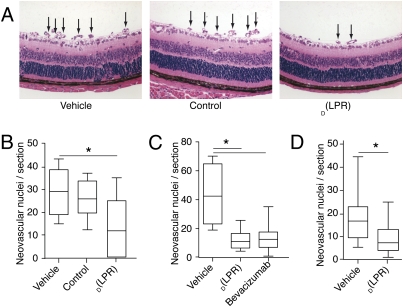
Third, we used a mouse model of retinopathy of prematurity (3, 31–34). This model is generally accepted not only as representative of the human condition, but also as the best available model of selected features of other retinal angiogenic diseases, including diabetic retinopathy and perhaps, age-related macular degeneration (3, 33). In brief, mice are exposed to 75% O2 from postnatal day 7 (P7) to P12, a treatment that inhibits the formation of retinal blood vessels that are otherwise a normal developmental process at this age. The pups are returned to room air (21% O2). Their retinas are analyzed at P17–P21, by which time pathological neovascularization has supervened on the inner retinal surface (31–33). On their return to room air (P12), neonatal mice received either D(LPR) or control daily for 7 days; another negative control cohort of mice received daily i.p. administration of the vehicle alone (PBS). At the end of treatment (P19), the eyes were examined histologically, and neovascularization was quantified by counting the number of blood vessels and endothelial cells protruding from the retina; a typical experiment is shown (Fig. 4A). Overall, a significant reduction in angiogenesis (59%; t test with P < 0.05) was observed in D(LPR)-treated mice (n = 7; median = 12; range = 0–5) relative to mice treated with the negative control (n = 8; median 26; range = 12–37) or the vehicle (n = 8; median = 29; range = 15–43) alone (Fig. 4B). We also used this model to compare the efficacy of D(LPR) with bevacizumab, a human monoclonal antibody. One should note that although bevacizumab has been reported to have lower (or no) reactivity with murine than with human VEGF (35), it has still shown antiangiogenic activity in ocular and tumor mouse models (36) and has later served as a positive control for new compounds in other experimental settings (37). At P12, mice received D(LPR) daily (20 mg/kg per day) or bevacizumab every other day (1 mg/kg per dose). A significant reduction in angiogenesis (72%; t test with P < 0.05) was observed in either D(LPR)-treated mice (n = 9; median = 11; range = 4–26) or bevacizumab-treated mice (n = 9; median = 12; range = 1–35) relative to mice that received vehicle alone (n = 4; median = 42; range = 19–70); this direct comparison in a side-by-side experiment (Fig. 4C) does suggest that the magnitude of the systemic therapeutic effect of D(LPR) may be in a similar range of other FDA-approved agents in clinical use.
Fig. 4.
Systemic and topical treatment with D(LPR) inhibits retinal angiogenesis in a mouse retinopathy-of-prematurity model. Retinal neovascularization was induced in C57BL/6 neonatal mice by exposure to 75% oxygen (P7–P12) followed by daily i.p. administration of D(LPR) or control (20 mg/kg per day). (A) H&E-stained retinal sections (P19) showed new blood vessels at the retinal inner surface (arrows) in control animals (Top and Center). There was marked reduction in D(LPR)-treated mice (Bottom). (B) P19 quantification of neovascular nuclei protruding into the vitreous space. Serial sections (n > 5) of eyes (n > 10) were quantified in each group. Treatment with D(LPR) yielded a significant reduction in nuclei relative to vehicle or control. (C) In systemic (i.p.) delivery, the magnitude of D(LPR)-induced neovascularization inhibition was similar to that observed by treatment with the monoclonal antibody bevacizumab. (D) Topical delivery of D(LPR) in an eye-drop formulation (200 μg e.d. three times daily) also induced a significant reduction in abnormal retinal angiogenesis. Shown is mean ± SEM in each experiment.*, Student’s t test (P < 0.05).
The recent report, in which the inhibitor of Src and Yes administered topically blocked pathological angiogenesis in animal models (37), stimulated our reasoning to determine if D(LPR) would also penetrate the vitreous humor and be effective as an eye-drop (e.d.) formulation for topical application (Fig. 4D and Fig. S2 A–C). We first found that topically administered D(LPR) to the eyes of mice resulted in accumulation of the drug (n = 6; median = 1.25 μM; range = 0.65–2.14 μM) within the vitreous humor (Fig. S2 A–C). Then, to evaluate the effect of topically administered D(LPR), we put P7 mice in the oxygen chamber; at P12, animals were treated by laying D(LPR)-containing eye drops on the cornea (200 μg e.d. three times a day) for 7 days. At the end of treatment on P19, the eyes were examined histologically, and neovascularization was quantified. Again, despite minimal optimization in dose and schedule, a significant reduction in angiogenesis (53%; t test with P < 0.05) was observed in mice treated topically with D(LPR) (n = 8; median = 7; range = 2–23) relative to those receiving vehicle (Systane) alone (n = 9; median = 16; range = 5–43; Fig. 4D). These results suggest that D(LPR) may serve as a lead for the development of soluble and permeable small drug molecules that can be administered in eye drops. If so, an eye-drop drug formulation would represent a great improvement for many patients with blinding diseases who now must receive intraocular injections of agents with larger molecular weights, such as monoclonal antibodies.
Finally, in preliminary studies, we have begun to evaluate whether or not D(LPR) might also have effects against tumors. As an initial tumor model, we have used an isogenic mouse mammary cancer that is both aggressive and angiogenic (38). We first confirmed the ability of the CPQPRPLC-targeted phage to home i.v. to established tumors in vivo relative to the control nontargeted phage (Fig. 5A). We next treated tumor-bearing mice with vehicle, control peptide, or D(LPR). A significant reduction in tumor volume was detected in the cohort receiving D(LPR) relative to the control cohorts (Fig. 5B). Future preclinical studies will determine the full translational value of this drug prototype against cancer in addition to angiogenic retinopathies.
Fig. 5.
CPQPRPLC-targeted phage homed to tumors, and D(LPR) treatment reduced tumor growth. (A) Tumor-bearing mice received 1010 transducing units (TU) of either CPQPRPLC phage or insertless phage (negative control). After 24 h of circulation, phage homing to tissues was evaluated by counting the relative TU. The brain served as a negative control organ. (B) Tumor-bearing mice (n = 7 per cohort) were treated with vehicle alone, D(LPR), or control peptide (50 mg/kg/day for 5 days). Two independent experiments were performed with similar results. A representative experiment is shown. *Student’s t test (P = 0.02).
Mechanism of Action of D(LPR) on VEGF Receptor Pathways.
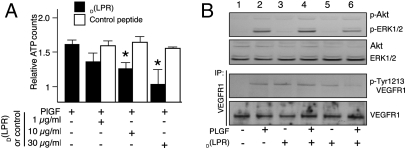
The well-established involvement of VEGFR-1 and NRP-1 in endothelial cell proliferation (15, 39) has prompted us to evaluate the effects of the drug D(LPR) in this setting. However, several experimental challenges are evident in this complex ligand-receptor systematic analysis. VEGF165 also binds to VEGFR-2, an interaction that mediates most of its mitogenic activity; moreover, PlGF, a VEGFR-1– and NRP-1–specific ligand that induces VEGFR-1 phosphorylation, extracellular signal-regulated kinase (ERK) activation, and endothelial cell proliferation (15, 39), is another potential target in angiogenesis (13, 21). Thus, given the similar receptor-binding profiles of the RPL motif and PlGF (22, 23), we chose to first study the effect of D(LPR) on this specific pathway. Human umbilical-vein endothelial cells (HUVEC) incubated with PlGF in the presence of increasing concentrations of D(LPR) showed a marked growth reduction relative to HUVEC cultured with no D(LPR); a control peptide had no detectable effect (Fig. 6A). Consistently, in contrast to its effect on PlGF-induced HUVEC proliferation, D(LPR) had no detectable effect on cell growth in the presence of VEGF165, which acts mainly through the VEGFR-2 pathway.
Fig. 6.
Effect of D(LPR) on VEGFR-1–meditated endothelial cell proliferation and signaling. (A) Dose-dependent effect of D(LPR) on PlGF-induced HUVEC proliferation. Results are presented as values relative to BSA. (B) Immunoblot analysis of phosphorylated and total forms of VEGFR-1, ERK, and Akt. P1GF was used at 100 ng/mL, and D(LPR) or control was used at 10 μg/mL.
To evaluate whether or not the inhibition of endothelial cell proliferation by D(LPR) is mediated through its binding to VEGFR-1 and/or NRP-1, we analyzed the phosphorylation of VEGFR-1 and ERK. D(LPR) inhibited VEGFR-1 Tyr1213 phosphorylation and reduced ERK phosphorylation by ∼55% (Fig. 6B; Fig. S3 A and B), again in agreement with the previous results (Fig. 6A). VEGFR-1 and ERK phosphorylation were not altered by a negative control peptide. Similarly, we observed that the treatment of cells with D(LPR) affected neither PlGF-induced Akt phosphorylation nor ERK phosphorylation if endothelial cells were stimulated by VEGF instead of PlGF; both of these results suggest specificity. D(LPR) behaved similarly to other NRP-1–binding peptides with anti-angiogenic activity. Tuftsin (TKPPR) (40) and hexapeptide A7R (ATWLPPR) (41) both bind to NRP-1 and inhibit neovascularization in different assays; another peptide derived from the VEGF-E C-terminal domain (RPPR) also binds to NRP-1 and inhibits angiogenesis (42). Of note, all of these NRP-1–binding sequences share the terminal dipeptide motif Pro–Arg. Recently, a consensus sequence R/KXXR/K has also been reported as an NRP-1–binding motif (43). Because D(LPR) does not functionally affect VEGF165-stimulated endothelial cell activation and proliferation through VEGFR-2, we conclude that D(LPR) likely exerts its antiangiogenic effects through VEGFR-1– and/or NRP-1–specific pathways; to our knowledge, RPL and D(LPR) are the only known reagents that target both NRP-1 and VEGFR-1. In summary, these signal-transduction data establish the basis of a specific mechanism of action for the RPL motif and its degradation-resistant counterpart, the peptidomimetic D(LPR).
Conclusion
Based on a subtractive combinatorial screening on human angiogenic endothelial cells and structure-function studies (22, 23), here we rationally designed, synthesized, and functionally validated small-molecule targeting VEGF receptors, namely VEGFR-1– and NRP-1–specific pathways. This antiangiogenic drug, D(LPR), showed promising attributes in vitro, in cellulo, and in vivo in several mouse models. The data presented in this study support the working hypothesis that drugs against members of the VEGF family that do not signal through the VEGFR-2 axis may also have important biomedical applications. This fact reinforces recent reports of the roles of VEGFR-1 and NRP-1 in abnormal angiogenesis (18, 19, 44).
Currently available inhibitors of receptor tyrosine-kinase receptors belong to one of two classes of drugs: small molecules affecting the intracellular kinase domain or large antibodies binding to the extracellular domain. Our results show that small molecules targeting an extracellular receptor ligand-binding domain comprise an unrecognized candidate class of receptor tyrosine-kinase inhibitors.
Even without much optimization in dose and schedule, we show that D(LPR) is water-soluble and degradation-resistant, which are favorable biochemical features for possible translation into an orally or topically administered drug. Of course, although small molecules might have a few inherent theoretical advantages with respect to tissue permeability, biodistribution, and cost effectiveness, it remains to be determined whether or not the prototype introduced here will prove at least as effective as currently available drugs (7–10) or newly engineered antibodies (45). If so, development of D(LPR) or its derivatives as antiangiogenic drugs in an eye-drop formulation would represent an improvement in quality of life for patients with retinopathies. Unequivocally, using eye drops to avoid distinctly unpleasant, risky, and expensive repetitive injections into the eye would have universal appeal.
Material and Methods
Reagents and Cell Culture.
All peptides and peptidomimetics were synthesized and purified by HPLC to a purity greater than 95% by Polypeptide Laboratories. The parental peptide sequence CPQPRPLC, along with its motifs [i.e., linear or cyclic RPL peptide, linear or cyclic negative control Ala-Pro-Ala (APA) peptide, linear or cyclic D(LPR) peptidomimetic, and linear or cyclic negative control peptidomimetic D(APA)], were used correspondingly as appropriate, unless otherwise specified.
VEGF receptors and ligands were purchased from R&D Systems or Leinco Technologies. Heparin, Drabkin’s reagent, hemoglobin, and Brij-35 were obtained from Sigma-Aldrich. Phospho-tyrosine monoclonal antibody (sc-7020) was purchased from Santa Cruz Biotechnology, and VEGFR-1 polyclonal sera were purchased from R&D Systems (AF321). Anti-pERK1/2, pAkt (Ser473), anti-Akt, and anti-p44/42 MAPK (ERK1/2) were obtained from Cell Signaling Technology. HUVEC (Lonza) was grown on gelatin-coated dishes in endothelial-cell basal medium (EBM) containing endothelial growth factors (Lonza), 2% heat-inactivated FBS, 50 U/mL penicillin-streptomycin, and 2 mM L-glutamine. Cells were maintained at 37 °C and 5% CO2.
Animals.
The Institutional Animal Care and Use Committees at the University of Texas M.D. Anderson Cancer Center and the University of Michigan approved all animal experimentation. This study adhered to the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research. Mice were bought from Harlan.
Protease Degradation-Resistance Assay.
D(LPR) or RPL was diluted to 500 μg/mL in PBS and incubated with serially increasing concentrations of pancreatin (Sigma-Aldrich) for 2 h at 37 °C. Samples were analyzed by MALDI-TOF.
Peptide-Targeted Phage Binding and Ligand-Competition Assay.
A phage-binding assay on purified proteins and ligand-competition assays were carried out as described (22) in SI Materials and Methods.
NMR Spectroscopy.
NMR experiments were performed as described (23, 46) in SI Materials and Methods.
Cell Proliferation, Signal Transduction, and Phosphorylation Analysis.
We analyzed HUVEC proliferation, signal-transduction pathways, and VEGF receptor phosphorylation; for details, see SI Materials and Methods.
Mass Spectrometry Analysis of Drug Penetration.
D(LPR) was detected and quantified in the vitreous humor after topical e.d. application. D(LPR) (2 μL of a 100 mg/mL solution in Systane) was dropped topically on the external corneal surface of C57BL/6 mice (e.d. every 2 h; n = 3 doses). Two hours after the third and final dose, mice were killed, eyes were enucleated, and vitreous humor was gently released and collected by centrifugation (10,000 × g for 1 minute). Vitreous humor samples or D(LPR) standards were diluted 1:10 in α-cyano-4-hydroxyl cinnamic acid (10 mg/mL in 50:50 acetonitrile:water; 0.1% trifluoracetic acid final concentration), spotted on the target, and analyzed in a MALDI-TOF/TOF System [4700 Proteomics Analyzer; Applied Biosystems (ABI)].
Angiogenesis Assays.
We used an in vivo Matrigel angiogenesis assay as described (29). For the model of human angiogenesis, SCID mice were implanted with 106 human dermal microvascular endothelial cells (HDMEC) highly porous poly-L (lactic) acid scaffolds. Two scaffolds were used per mouse, one on each flank (30). For the retinal neovascularization angiogenesis assay, we used C57BL/6 mouse pups with their nursing mothers (33). For details, see SI Materials and Methods.
Tumor Targeting.
Selective phage homing to tumors and peptide treatment were performed as described (38). See SI Materials and Methods for details.
Statistics.
The appropriate statistical test was used for the analysis of assays as indicated. Statistical significance was set at P < 0.05. For details, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Bih-Fang Pan for technical assistance and E. Helene Sage for critical reading of the manuscript. This work was supported by grants from the Department of Defense (DOD) and National Institutes of Health (J.E.N., R.L.S., R.P., and W.A.) and by awards from the Gillson-Longenbaugh Foundation, the Marcus Foundation, and AngelWorks (W.A. and R.P.); M.C.-V. received a postdoctoral fellowship award from the Susan G. Komen Breast Cancer Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0915141107/DCSupplemental.
References
- 1.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis: An organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 3.Arden GB, Sidman RL, Arap W, Schlingemann RO. Spare the rod and spoil the eye. Br J Ophthalmol. 2005;89:764–769. doi: 10.1136/bjo.2004.062547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 5.Loges S, Roncal C, Carmeliet P. Development of targeted angiogenic medicine. J Thromb Haemost. 2009;7:21–33. doi: 10.1111/j.1538-7836.2008.03203.x. [DOI] [PubMed] [Google Scholar]
- 6.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 7.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 8.Crawford Y, Ferrara N. VEGF inhibition: Insights from preclinical and clinical studies. Cell Tissue Res. 2009;335:261–269. doi: 10.1007/s00441-008-0675-8. [DOI] [PubMed] [Google Scholar]
- 9.Gragoudas ES, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeld PJ, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 11.Ellis LM, Hicklin DJ. VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 12.Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14:6371–6375. doi: 10.1158/1078-0432.CCR-07-5287. [DOI] [PubMed] [Google Scholar]
- 13.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: Drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 14.Carmeliet P, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 15.Autiero M, et al. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9:936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- 16.Luttun A, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y, et al. Anti-vascular endothelial growth factor receptor-1 antagonist antibody as a therapeutic agent for cancer. Clin Cancer Res. 2006;12:6573–6584. doi: 10.1158/1078-0432.CCR-06-0831. [DOI] [PubMed] [Google Scholar]
- 19.Pan Q, et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Zhang F, et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci USA. 2009;106:6152–6157. doi: 10.1073/pnas.0813061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer C, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 22.Giordano RJ, Cardó-Vila M, Lahdenranta J, Pasqualini R, Arap W. Biopanning and rapid analysis of selective interactive ligands. Nat Med. 2001;7:1249–1253. doi: 10.1038/nm1101-1249. [DOI] [PubMed] [Google Scholar]
- 23.Giordano RJ, et al. Structural basis for the interaction of a vascular endothelial growth factor mimic peptide motif and its corresponding receptors. Chem Biol. 2005;12:1075–1083. doi: 10.1016/j.chembiol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Falciani C, Lozzi L, Pini A, Bracci L. Bioactive peptides from libraries. Chem Biol. 2005;12:417–426. doi: 10.1016/j.chembiol.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Sergeeva A, Kolonin MG, Molldrem JJ, Pasqualini R, Arap W. Display technologies: Application for the discovery of drug and gene delivery agents. Adv Drug Deliv Rev. 2006;58:1622–1654. doi: 10.1016/j.addr.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chorev M. The partial retro-inverso modification: A road traveled together. Biopolymers. 2005;80:67–84. doi: 10.1002/bip.20219. [DOI] [PubMed] [Google Scholar]
- 27.Fischer PM. The design, synthesis and application of stereochemical and directional peptide isomers: A critical review. Curr Protein Pept Sci. 2003;4:339–356. doi: 10.2174/1389203033487054. [DOI] [PubMed] [Google Scholar]
- 28.Meister A. Biochemistry of the Amino Acids. 2nd Ed. Vol. 1. New York: Academic; 1965. p. 364. [Google Scholar]
- 29.Passaniti A, et al. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992;67:519–528. [PubMed] [Google Scholar]
- 30.Nör JE, et al. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest. 2001;81:453–463. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 31.Smith LE, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 32.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lahdenranta J, et al. An anti-angiogenic state in mice and humans with retinal photoreceptor cell degeneration. Proc Natl Acad Sci USA. 2001;98:10368–10373. doi: 10.1073/pnas.181329198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heidary G, Vanderveen D, Smith LE. Retinopathy of prematurity: Current concepts in molecular pathogenesis. Semin Ophthalmol. 2009;24:77–81. doi: 10.1080/08820530902800314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerber HP, et al. Mice expressing a humanized form of VEGF-A may provide insights into the safety and efficacy of anti-VEGF antibodies. Proc Natl Acad Sci USA. 2007;104:3478–3483. doi: 10.1073/pnas.0611492104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorrell MI, Aguilar E, Scheppke L, Barnett FH, Friedlander M. Combination angiostatic therapy completely inhibits ocular and tumor angiogenesis. Proc Natl Acad Sci USA. 2007;104:967–972. doi: 10.1073/pnas.0607542104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheppke L, et al. Retinal vascular permeability suppression by topical application of a novel VEGFR2/Src kinase inhibitor in mice and rabbits. J Clin Invest. 2008;118:2337–2346. doi: 10.1172/JCI33361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajitou A, et al. A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell. 2006;125:385–398. doi: 10.1016/j.cell.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 39.Landgren E, Schiller P, Cao Y, Claesson-Welsh L. Placenta growth factor stimulates MAP kinase and mitogenicity but not phospholipase C-gamma and migration of endothelial cells expressing Flt 1. Oncogene. 1998;16:359–367. doi: 10.1038/sj.onc.1201545. [DOI] [PubMed] [Google Scholar]
- 40.von Wronski MA, et al. Tuftsin binds neuropilin-1 through a sequence similar to that encoded by exon 8 of vascular endothelial growth factor. J Biol Chem. 2006;281:5702–5710. doi: 10.1074/jbc.M511941200. [DOI] [PubMed] [Google Scholar]
- 41.Starzec A, et al. Antiangiogenic and antitumor activities of peptide inhibiting the vascular endothelial growth factor binding to neuropilin-1. Life Sci. 2006;79:2370–2381. doi: 10.1016/j.lfs.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Cébe-Suarez S, et al. Orf virus VEGF-E NZ2 promotes paracellular NRP-1/VEGFR-2 coreceptor assembly via the peptide RPPR. FASEB J. 2008;22:3078–3086. doi: 10.1096/fj.08-107219. [DOI] [PubMed] [Google Scholar]
- 43.Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc Natl Acad Sci USA. 2009;106:16157–16162. doi: 10.1073/pnas.0908201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luttun A, Autiero M, Tjwa M, Carmeliet P. Genetic dissection of tumor angiogenesis: Are PlGF and VEGFR-1 novel anti-cancer targets? Biochim Biophys Acta. 2004;1654:79–94. doi: 10.1016/j.bbcan.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Dennis MS, et al. Imaging tumors with an albumin-binding Fab, a novel tumor-targeting agent. Cancer Res. 2007;67:254–261. doi: 10.1158/0008-5472.CAN-06-2531. [DOI] [PubMed] [Google Scholar]
- 46.Sklenar V, Piotto M, Leppik R, Saudek V. Gradient-tailored water suppression for 1H-15N HSQC experiments optimized to retain full sensitivity. J Magn Reson Series A. 1993;102:241–245. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.