Abstract
Purpose
To determine whether blocking the expression of the chemokine receptor CXCR4 using siRNA inhibits chemotactic responses of human uveal melanoma cells to liver-derived factors and prevents liver metastases.
Methods
Human uveal melanoma cells were transfected with CXCR4 siRNA or control siRNA and tested in vitro for chemotactic and invasive behavior in response to soluble factors produced by human liver cells. The effect of CXCR4 siRNA transfection on the formation of liver metastases was tested by injecting transfected melanoma cells into the spleen capsules of NOD-SCID mice, and metastases were quantified by measuring the human housekeeping gene hHPRT in livers.
Results
Blocking CXCR4 interaction with its ligand using anti-CXCL12 antibody resulted in a significant reduction in the chemotactic responses of uveal melanoma cells to soluble factors produced by human liver cells. Similarly, blocking CXCR4 gene expression by transfection with CXCR4 siRNA inhibited both the chemotactic and the invasive properties of uveal melanoma cells exposed to factors produced by human livers. Uveal melanoma cells transfected with CXCR4 siRNA produced fewer liver metastases than untreated uveal melanoma cells or uveal melanoma cells transfected with control siRNA.
Conclusions
CXCR4 is a key chemokine receptor that may account for the organ-specific homing of human uveal melanomas to the liver, which contains significant quantities of CXCL2, the only known ligand for CXCR4. CXCR4 is a potential therapeutic target for preventing the initial establishment of liver metastases but has limited application for use in advanced liver tumors.
Melanomas of the uveal tract are the most common and malignant intraocular neoplasms in adults.1 In spite of improved treatment modalities for managing primary uveal melanomas, metastasis remains the leading cause of death in patients with uveal melanoma.2 In the past 30 years there has been no significant improvement in the 5-year survival of uveal melanoma patients, and there are no therapies that have been proven to be effective in treating liver metastases of uveal melanomas.1,3–5 Although skin and uveal melanomas arise from common progenitors in the neural crest, they express remarkably different properties, including their metastatic behavior. Skin melanomas can metastasize to virtually any organ, whereas uveal melanomas demonstrate a propensity to disseminate to the liver. Indeed, liver metastases are present in approximately 90% of patients with uveal melanoma at the time of death.3,6–8
Metastasis is a nonrandom process that results in a predictable pattern of tumor dissemination to specific organs, depending on the origin of the primary tumor. The tendency of tumors to preferentially form metastases in specific organs was recognized more than a century ago by Paget, who proposed the seed and soil hypothesis, which proposed that the unique milieu of some organs provides the optimal soil to support the establishment and growth of tumor cells that have disseminated from distant organs.9 A more recent hypothesis suggests that chemoattractants and cell adhesion molecules direct blood-borne tumor cells to migrate to and colonize organs in a nonrandom manner.10–14 Both hypotheses are appealing and compatible.
Recently, keen interest has surrounded the chemokine receptor family, especially in regard to the role of chemokine receptors and their ligands in organ-specific metastasis.10–12,14 Chemokines were first recognized as important factors in guiding leukocytes to regional lymph nodes during the induction of adaptive immune responses and to sites of inflammation for the expression of immunity. A growing body of evidence supports the notion that chemokine receptors expressed on tumor cells in primary neoplasms account for the nonrandom migration of blood-borne tumor cells to organs expressing the coligands for chemokine receptors expressed on the disseminated tumor cells.10–12,14 Tumors such as uveal melanoma and colon cancer demonstrate elevated expression of the CXCR4 chemokine receptor and coincidentally have a predilection to form metastases in the liver, which expresses a high level of the ligand for CXCR4 (i.e., CXCL12). In vitro studies have shown that uveal melanoma cells respond chemotactically to soluble factors produced by human liver cells and that this chemotactic response can be blocked with anti-CXCR4 antibody.15 A recent immunohistochemical study reported that approximately 60% of the primary human uveal melanomas examined expressed CXCR4. Interestingly, the most intense CXCR4 expression was found on epithelioid melanoma cells, which is the uveal melanoma cell type that is most closely associated with malignancy.16 With this in mind, we sought to determine whether downregulating CXCR4 expression with siRNA would alter the chemotactic behavior of human uveal melanoma cells and reduce their capacity to produce liver metastases.
Materials and Methods
Mice
Nonobese diabetic severe combined immune deficient (NOD-SCID) Balb/c mice were obtained from the vivarium at the University of Texas Southwestern Medical Center. All mice were housed and cared for in accordance with the National Institutes of Health Guidelines on Laboratory Animal Welfare and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Human Uveal Melanoma Cell Lines
Three primary human uveal melanoma cell lines, designated OCM3, OCM8, and MEL270, were used for in vitro studies. OCM3 and OCM8 primary uveal melanoma cell lines were kindly provided by June Kan-Mitchell (University of California, San Diego, CA). The MEL270 cell line was kindly provided by Bruce Ksander (Schepens Eye Research Institute, Boston, MA) but was originally established by Timothy Murray at the Bascom Palmer Eye Institute. Melanoma cell cultures were maintained in complete RPMI 1640 (JRH Biosciences, Lenexa, KS). The OCM3 uveal melanoma cell line was used for in vivo studies because it expresses high levels of CXCR4 and reproducibly generates metastatic liver tumors.15
Protein Extracts of Liver Parenchymal Cells and Smooth Muscle Cells
Proteins were extracted from human liver tissue and smooth muscle tissue (National Disease Research Interchange, Philadelphia, PA) with a protein extraction kit (Readyprep; Bio-Rad Laboratories, Inc., Hercules, CA) according to the manufacturer’s instructions. The protein concentration was determined with a protein assay kit (BCA; Pierce, Rockford, IL) according to the manufacturer’s instructions. For neutralization studies, cells were preincubated with 20 μg/mL azide-free mouse anti-human CXCL12 monoclonal antibody or mouse anti-human IgG isotype control (R&D Systems, Inc., Minneapolis, MN) for 24 hours.
Transfection of Uveal Melanoma Cells with Small Interfering RNA
Small interfering RNA (siRNA) was constructed and directed against CXCR4 (gene accession no. NM_001008540; sense, reverse, CAGCUAACACAGAUGUAAA, dTdT; antisense, reverse, UUUACAUCUGUGUUAGCUG, dGdA). We determined the efficiency of CXCR4 siRNA transfection by analyzing mRNA inhibition and protein by RT-PCR and FACS analysis, respectively, for 24, 48, 72, and 96 hours after transfection. Our results show that CXCR4 expression by siRNA-transfected OCM3 cells was suppressed 60% to 70% compared with control siRNA-transfected OCM3 cells at 24 and 48 hours. In addition, CXCR4 expression by siRNA-transfected OCM3 cells was suppressed 40% to 50% compared with control siRNA-transfected OCM3 cells at 72 and 96 hours after transfection. We performed siRNA concentration transfection efficiency assays using varying concentrations of siRNA (5–30 nM siRNA) and determined that 20 nM siRNA provided optimal transfection efficiency. Consequently, we used a concentration of 20 nM siRNA for all subsequent experiments. OCM3 uveal melanoma cells were transiently transfected at 80% confluence with 20 nM CXCR4 and control siRNA using transfection reagent (HiPerFect; Qiagen, Cambridge, MA). Optimum transfection was achieved in RPMI 1640 medium supplemented with 5% FCS. The viability of tumor cells was assessed by [3H]thymidine incorporation assay. Transfection efficacy was analyzed using flow cytometry and fluorescence microscopy. At the 20 nM siRNA level, it demonstrated the highest inhibitory effect (~70%) at 24 to 48 hours after transfection. Even at 72 hours after transfection, they still have 50% to 60% inhibitory effects (data not shown).
Real-Time RT-PCR
mRNA was isolated with an mRNA mini kit (Oligotex Direct; Qiagen). First-strand cDNA synthesis and amplification were performed with a reagent kit (RT2 First Strand Kit; SA Biosciences, Frederick, MD). Primers used in the real-time RT-PCR were specific for human hypoxanthine phosphoribosyl transferase (hHPRT) sense primer 5′-GACCAGTCAACAGGGGACAT-3′, antisense primer 5′-AAGCAGATGGCCACAGAACT-3′, mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH) sense 5′-ACTCACGGCAAATTCAACGGC-3′, and antisense 5′-ATCACAAACATGGGGGCATCG-3′. Real-time RT-PCR amplifications were performed on a real-time PCR detection system (iCycler IQ; Bio-Rad, Hercules, CA) using a standard protocol (95°C, 10 minutes, 40 cycles; 95°C, 15 seconds, 60°C, 1 minute). Each analysis was performed in triplicate, in a 96-well PCR plate sealed with an optical adhesive cover. Nontemplate controls (water) were included in each assay. A mix for real-time PCR applications (IQ SYBR Green SuperMix 2× Reaction System kit; Bio-Rad) was used, with a final volume of 25 μL containing fluorescein for dynamic well factor collection on a detection system (iCycler iQ; Bio-Rad). For each analysis, 5 μL diluted DNA was used. After several trials, the primer concentrations were fixed at 0.2 μM. The ΔCt data were collected automatically. The average ΔCt of each group was calculated with the following formula: ΔCt = average hHPRT gene Ct − average mGAPDH gene Ct. ΔΔCt was calculated by ΔΔCt = ΔCt of control SiRNA group − ΔCt CXCR4 SiRNA group. The fold-change for hHPRT expression level was calculated using 2−ΔΔC117,18
Melanoma Cell Migration and Invasion Assays
Melanoma cell migration in response to protein extracts of human liver were performed using 24-well transwell chambers (Costar, Corning Inc., Corning, NY). Nontransfected or siRNA-transfected uveal melanoma cells (1 × 105) were collected 24 hours after in vitro transfection with siRNA and placed in the top chambers and either medium alone or medium containing protein extract (40 μg/mL) isolated from either human liver or smooth muscle cells was added to the bottom chambers. Upper and lower chambers were separated by 8-μm pore membranes. In some experiments, anti-CXCL12 or an isotype control antibody was added to the lower chamber (20 μg/mL). Plates were incubated for 24 hours at 37°C in 5% CO2. The top chambers were removed, and the number of melanoma cells that migrated to the bottom chambers was determined by counting the number of cells in 10 random fields using direct light microscopy. The results were expressed as the mean number of cells per high-powered field (HPF). All assays were performed in quadruplicate.
Transwell chambers were used for invasion assays to evaluate the capacity of melanoma cells to penetrate a synthetic basement membrane. The top chamber of the transwell 8-μm pore membrane was coated with 60 μL basement membrane matrix (Matrigel; Collaborative Biomedical Products, Bedford, MA) for 30 minutes at 37°C. Excess basement membrane matrix (Matrigel; Collaborative Biomedical Products) was removed, and the membranes were allowed to dry at room temperature. Melanoma cells (2 × 105) were added to the top chamber, and protein extracts (40 μg/mL) of human liver were added to the lower chambers. Anti-CXCL12 and isotype control antibodies were added to the bottom chambers, as described, and the number of melanoma cells that penetrated the basement membrane matrix (Matrigel; Collaborative Biomedical Products) was determined in the same manner as the chemotaxis assays described.
Liver Metastasis Model and Histopathologic Analysis
Liver metastases were induced by injecting OCM3 uveal melanoma cells, OCM3 cells transfected with CXCR4 siRNA, or OCM3 cells transfected with control SiRNA. OCM3 uveal melanoma cells (2 × 105) were injected under the spleen capsule as a facile method for inducing the development of liver metastases.17 Based on pilot studies in NOD-SCID mice, we found that the left liver lobe consistently contained metastasizes 18 days after intrasplenic injection of 2 × 105 OCM3 uveal melanoma cells. Accordingly, mice were subjected to necropsy 18 days after intrasplenic injection of OCM3 uveal melanoma cells, and the entire left lobe of the liver (0.2 g) from each mouse was collected for real time RT-PCR analysis of hHPRT gene expression (five mice for each group). At day 35, liver tissues were removed from euthanatized mice (seven mice for each group). The tissues were fixed in formalin, embedded in paraffin, and cut into 5-μm sections. Sections were deparaffinized, and conventional hematoxylin-eosin staining was performed. The histopathologic changes of the liver metastasis among the different groups were analyzed by three independent masked observers.
Statistical Analysis
Student’s t-test was used to determine significance in differences between experimental and control groups.
Results
Effect of Blocking CXCR4/CXCL12 Interactions on Uveal Melanoma Cell Migration to Liver Chemoattractants
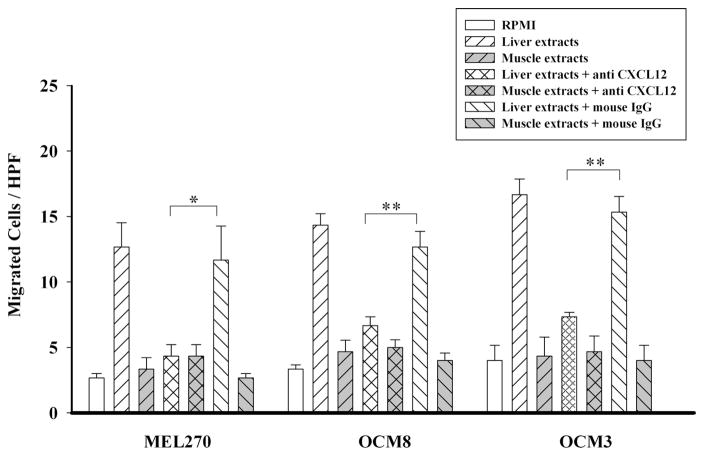
We have previously shown that uveal melanoma cells express CXCR4 and respond chemotactically to factors elaborated by human liver cells.15 The liver contains large amounts of CXCL12, which is the only known ligand for CXCR4 and is a potent chemoattractant for many types of cancer cells.15 Moreover, CXCL12 is highly conserved among species, with 99% homology between mouse and human.10 Accordingly, we sought to determine whether the chemotactic response of uveal melanoma cells to human liver cells was CXCL12 dependent. OCM3, OCM8, and MEL270 uveal melanoma cells were examined in vitro for their chemotactic responses to aqueous extracts from human liver, the most common site for uveal melanoma metastases, and extracts from smooth muscle, a tissue that is not known to harbor uveal melanoma metastases. Chemotaxis assays were performed in the presence of either anti-CXCL12 or an isotype control antibody. The results demonstrate that as previously reported,15 human uveal melanoma cells responded positively to extracts of human liver cells but not to extracts of human smooth muscle. Moreover, addition of the anti-CXCL12 antibody significantly inhibited the chemotactic responses of all three uveal melanoma cell lines toward liver extracts but had no effect on responses to smooth muscle extracts (Fig. 1).
Figure 1.
Blockade of CXCR4/CXCL12 interactions with anti-CXCL12 antibody inhibits uveal melanoma chemotactic responses to liver-derived chemoattractants. Uveal melanoma cells were placed in the top chambers of 24-well transwell culture plates, and protein extracts of either human liver or human smooth muscle (40 μg/mL) were added to the bottom chambers. Top and bottom chambers were separated by a membrane with 8-μm pore size. In some experiments, anti-CXCL12 or isotype control antibody was added to the lower chamber (20 μg/mL). Twenty-four hours later, the number of melanoma cells that migrated to the bottom chamber was determined by counting the melanoma cells in 10 random HPFs using a compound microscope. Mean ± SEM. *P < 0.05; **P < 0.01.
Effects of CXCR4 siRNA Inhibition on CXCR4 Message and Protein Expression in Human Uveal Melanoma Cells
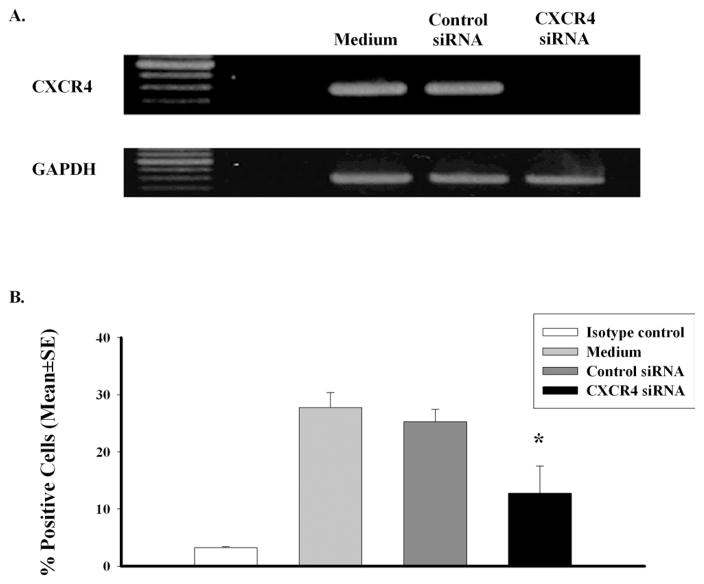
The capacity of anti-CXCL12 antibody to block the chemotactic responses of uveal melanoma cells to liver-derived chemoattractants suggested that blocking CXCR4/CXCL12 interactions might be a feasible therapeutic strategy. Therefore, OCM3 human uveal melanoma cells were selected for further study and were transfected with either CXCR4 siRNA or control siRNA. Inhibition of CXCR4 expression by CXCR4 siRNA was confirmed by RT-PCR. The results demonstrate that transfection with CXCR4 siRNA produced a profound reduction in CXCR4 gene expression in OCM3 uveal melanoma cells (Fig. 2A). By contrast, transfection with control siRNA had no detectable effect on CXCR4 message (Fig. 2A). Flow cytometric analysis revealed that transfection with CXCR4 siRNA produced a significant reduction in the number of OCM3 uveal melanoma cells expressing CXCR4, whereas transfection with control siRNA had no effect on CXCR4 expression (Fig. 2B).
Figure 2.
CXCR4 siRNA inhibits CXCR4 message and protein expression in human uveal melanoma cells. RT-PCR results of OCM3 melanoma cells transfected with CXCR4 siRNA compared with control siRNA and nontransfected OCM3 melanoma cells. CXCR4 gene expression was downregulated in the CXCR4 siRNA group (A). FACS analysis of CXCR4 protein expression (B). Compared with the control siRNA group, the CXCR4 protein expression was 50% lower in the CXCR4 siRNA group. *P < 0.05.
Effect of CXCR4 siRNA on the Chemotactic Responses and Invasiveness of Human Uveal Melanoma Cells
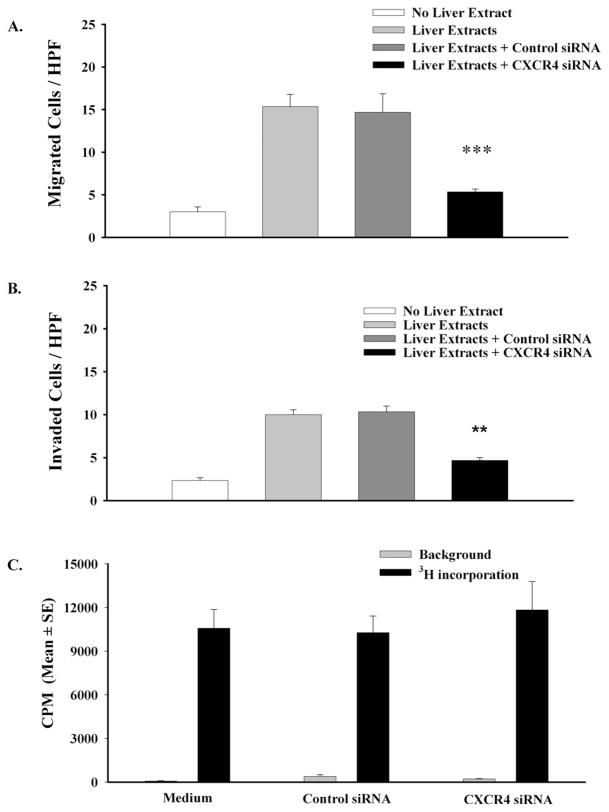
The prevailing paradigm proposes that CXCR4 is the most widely expressed chemokine receptor among many categories of cancers, including uveal melanoma, and that the organ-specific patterns of metastasis of CXCR4-positive cancers is due in large part to the preferential expression of the only known ligand for CXCR4 (i.e., CXCL12) in the lungs, lymph nodes, and liver. As shown earlier, blockade of CXCR4/CXCL12 interactions with anti-CXCL12 antibody significantly reduced the chemotactic responses of uveal melanoma cells to soluble factors released from human hepatocytes. Additional in vitro experiments were performed to determine whether inhibiting the expression of CXCR4 on uveal melanoma cells with siRNA would be as effective as blocking CXCL12 in reducing the chemotactic responses of uveal melanoma cells to human liver cells. OCM3 uveal melanoma cells transfected with CXCR4 siRNA demonstrated significantly (P < 0.001) reduced chemotactic responses to soluble factors produced by human liver cells (Fig. 3A). By contrast, the chemotactic responses of OCM3 uveal melanoma cells treated with the control siRNA were not affected, testifying that the siRNA transfection procedure itself did not nonspecifically affect the chemotactic responses of uveal melanoma cells (P > 0.05).
Figure 3.
CXCR4 siRNA inhibits chemotactic and chemoinvasive responses of human uveal melanoma cells but does not affect melanoma cell proliferation. Untreated and siRNA-transfected OCM3 uveal melanoma cells were placed in the top chambers of transwell culture plates. Protein extracts of either human liver or human smooth muscle (40 μg/mL) were added to the bottom chambers and served as chemoattractants to stimulate uveal melanoma cell migration to the bottom chambers. For chemotaxis assays, the top and bottom chambers were separated by a membrane with 8-μm pore size (A). For invasion assays, chambers were separated by a synthetic basement membrane created by coating an 8-μm pore membrane with basement membrane matrix (B). Twenty-four hours later, the number of melanoma cells that migrated to the bottom chamber was determined by counting the melanoma cells in 10 random HPFs using a compound microscope. This experiment was performed three times with similar results. Bars represent mean ± SEM. ***P < 0.001; **P < 0.01. OCM3 melanoma cells were transfected with CXCR4 siRNA or control siRNA. Melanoma cell proliferation was assessed by uptake of [3H]thymidine after 48 hours in culture (C). P > 0.05 in all groups.
To develop metastases, uveal melanoma cells must also be able to respond to chemotactic stimuli that arise behind the basement membrane, penetrate the basement membrane, and thereby enter the parenchyma of the liver. Accordingly, we examined the capacity of OCM3 uveal melanoma cells to respond to soluble factors produced by human hepatocytes and placed behind a synthetic basement membrane (Matrigel; Collaborative Biomedical Products). The results demonstrate that OCM3 melanoma cells not only responded to soluble factors elaborated by human liver cells but were able to penetrate the synthetic basement membrane (Fig. 3B). Blockade of CXCR4 with siRNA significantly inhibited OCM3 melanoma cell invasion of the basement membrane (50% inhibition; P < 0.01). By contrast, treatment with control siRNA had no effect on the capacity of OCM3 uveal melanoma cells to respond to liver-derived chemotactic factors and penetrate the synthetic basement membrane (P > 0.05).
Effect of Transfection with CXCR4 siRNA on the Proliferation of Human Uveal Melanoma Cells
One might argue that the reduced chemotactic and invasive properties of OCM3 uveal melanoma cells that were transfected with CXCR4 siRNA might be attributed to poor viability or reduced proliferative capacities of the transfected melanoma cells. For example, Hong et al.19 reported that downregulation of CXCR4 by siRNA resulted in approximately 30% reduction in the in vitro proliferation of human oral squamous cell carcinoma cells.19 To rule out this explanation, the proliferative properties of CXCR4 siRNA-treated OCM3 melanoma cells were assessed in vitro. Untreated OCM3 uveal melanoma cells and OCM3 melanoma cells transfected with CXCR4 siRNA or control siRNA were cultured for 48 hours, and cell proliferation was assessed by incorporation of tritiated thymidine. The results show that transfection with either CXCR4 siRNA or control siRNA had no affect on the proliferation of OCM3 uveal melanoma cells (Fig. 3C).
Inhibition of Liver Metastasis of Human Uveal Melanoma Cells by siRNA Blockade of CXCR4 Expression
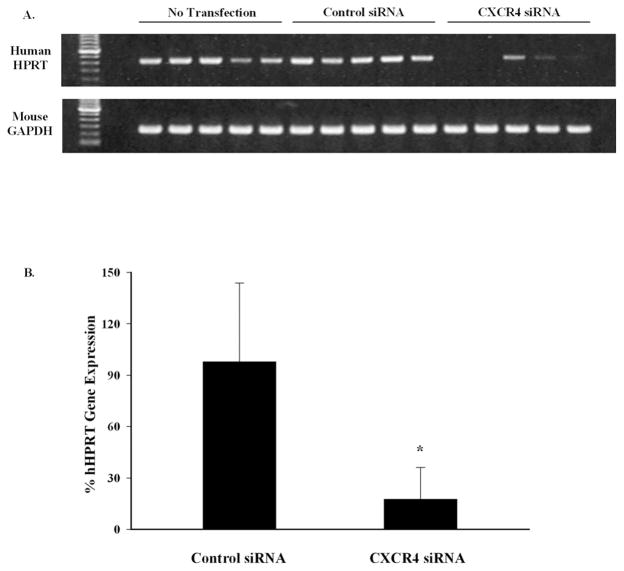
The capacity of CXCR4 siRNA to inhibit the chemotactic and invasive properties of OCM3 uveal melanoma cells suggested that siRNA might be effective in preventing the development of liver metastases. Injecting tumor cells into the spleen capsules of mice is a facile method for producing experimental liver metastases.17 Accordingly, OCM3 uveal melanoma cells were injected into the spleen capsules of NOD-SCID mice as a means of inducing melanoma liver metastases. Mice underwent necropsy 18 days later, and 0.2 g of the left lobe of each liver was collected from each mouse. The liver samples were assessed by RT-PCR to determine the amount of the human housekeeping gene hHPRT present. Given that OCM3 uveal melanoma cells were the only human cells present in the NOD-SCID mice, assessing hHPRT gene expression is a sensitive method for quantifying liver metastases of human uveal melanoma cells.17,18 Mice challenged with OCM3 uveal melanoma cells transfected with CXCR4 siRNA had a significantly lighter load of liver metastases, as reflected by a >70% reduction in human hHPRT gene expression (Fig. 4). Histopathologic examination of livers from the same mice confirmed that mice challenged with CXCR4 siRNA-treated OCM3 uveal melanoma cells had a reduced burden of liver metastases than mice challenged with either untreated OCM3 melanoma cells or OCM3 melanoma cells transfected with control siRNA (Fig. 5).
Figure 4.
CXCR4 siRNA treatment inhibits liver metastasis of human uveal melanomas in NOD-SCID mice. OCM3 uveal melanoma cells were transfected with either CXCR4 siRNA or control siRNA. OCM3 uveal melanoma cells (2 × 105) were injected into the spleen capsules of NOD-SCID mice. Mice underwent necropsy 18 days later, and the metastatic tumor burden was quantified by gel electrophoresis (A) and real time RT-PCR (B) of the human housekeeping gene hHPRT. There were five mice in each group. Mean ± SEM. *P = 0.027
Figure 5.
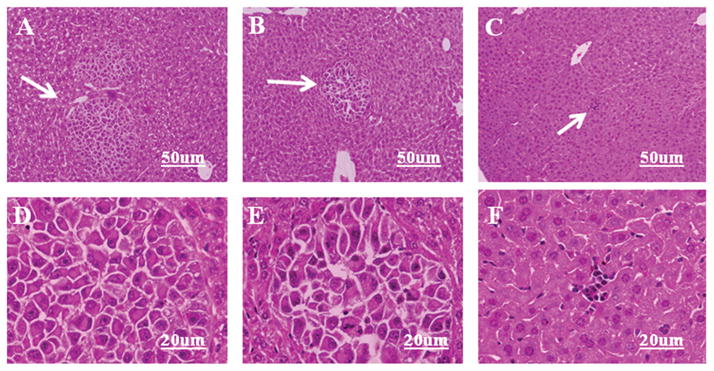
Histopathology of liver metastases arising from CXCR4 siRNA-transfected OCM3 uveal melanoma cells. OCM3 uveal melanoma cells were transfected with either CXCR4 siRNA or control siRNA. OCM3 uveal melanoma cells (2 × 105) were injected into the spleen capsules of NOD-SCID mice. Mice underwent necropsy 35 days later, and livers were processed for conventional hematoxylin and eosin staining. (A, D) Untreated. (B, E) siRNA control-treated OCM3 uveal melanoma cells. (C, F) CXCR4 siRNA-treated OCM3 melanoma cells. Arrow: metastatic tumor nodules. (A–C) Low power and (D–F) high-power photographs of A–C, respectively.
Discussion
More than 100 years ago, Paget proposed the seed and soil hypothesis to explain the nonrandom nature of metastasis.9 According to this hypothesis, blood-borne tumor cells (seeds) migrate to and enter organs that attract disseminated tumor cells and provide the most hospitable growth conditions (soil) for the development of metastases. The seminal studies by Muller et al.13 demonstrated the role of chemokine receptors and their ligands on the organ-specific metastasis of breast cancer. Since this report, hundreds of studies have examined the effect of chemokine receptors in the metastases of multiple tumor types in humans and experimental animals.10–12,14 Although more than 40 chemokines and 19 chemokine receptors have been identified, CXCR4 stands out as the most widely expressed chemokine receptor.10–12,14 High expression of CXCR4 on primary tumors is associated with poor prognosis in numerous types of cancers.13,20–24 We and others15,16 have shown that human uveal melanoma cells express CXCR4 and that expression was highest on uveal melanoma cells displaying epithelioid morphology, which is the histologic category associated with the greatest malignancy. However, our previous findings using cell lines derived from metastases in patients with uveal melanoma and liver metastases arising from human uveal melanoma cells transplanted into the eyes of nude mice indicated that CXCR4 expression was virtually absent in liver metastases yet remained high in the primary melanomas.15 This suggests that CXCR4 is involved in the initial homing and colonization of the liver but is not likely to participate in the subsequent growth or angiogenic steps, as has been reported with other tumors in which CXCR4 expression is maintained or even elevated in metastases.10–12,14
Considerable enthusiasm surrounds the possibility of targeting chemokine receptors, especially CXCR4, for preventing metastases in several tumor categories.25,26 Inhibition of CXCR4 with antibodies or peptide antagonists has been reported to reduce tumor metastasis.13,18,27–29 Numerous investigations have examined the feasibility of pharmacologic inhibitors of CXCR4 expression, such as AMD3100, and have shown that they produce promising antimetastatic effects in animal studies and are well tolerated in human subjects.18,27–34 However, a recent in vivo study in nude mice reported that instead of producing an antitumor effect, treatment with AMD3100 stimulated the growth of human epithelial carcinoma cells that were stably transfected with a mutant form of CXCR4.35 By contrast, studies in mice have shown that the blockade of CXCR4 expression via siRNA or RNAi knockdown produced significant reductions in the metastasis of breast cancer.36,37 Our results demonstrate the importance of CXCR4 expression on uveal melanoma cells in the development of liver metastases and establish the proof-of-principle in targeting CXCR4 for preventing the initial stages in liver metastasis. Transfection with CXCR4 siRNA can be maintained for 7 days, as determined by RT-PCR (data not shown); the OCM3 uveal melanoma cells used in this study were not stable transfectants. We have not determined the level of CXCR4 expression in CXCR4 siRNA-transfected cells at days 18 and 35 after transfection, but it would be reasonable to assume that CXCR4 expression is not suppressed at these later time points. Given that we are specifically targeting and suppressing CXCR4 expression with CXCR4 siRNA and it appears that control siRNA vector-transfected OCM3 cells show no suppression of metastasis formation, it would be unlikely that other processes related to in vitro siRNA transfer affect metastasis of the transfected cells into the liver.
Presently, there is a groundswell of interest in cancer stem cells. It has long been recognized that malignant tumors are composed of heterogeneous populations of cancer cells that are endowed with different properties. The subpopulation of tumor cells with tumorigenic properties and the capacity for self-renewal have been termed the cancer stem cell (CSC) and are believed by some to be responsible for the formation of metastases.38 Hermann et al.39 have reported that putative CD133+ CSCs from a human pancreatic cancer could be subdivided into CD133+CXCR4+ and CD133+CXCR4neg populations. Interestingly, depletion of the CD133+CXCR4+ subset prevented the development of metastases. These findings provide even further impetus for focusing on CXCR4 as a therapeutic target for preventing the development of metastases in many categories of cancer, especially in the context of the highly malignant CSC population.
The present findings demonstrate the importance of CXCR4 expression on uveal melanoma cells in the development of liver metastases and establish the proof of principle in targeting CXCR4 for preventing the initial stages in liver metastasis. However, we are less sanguine about the potential of CXCR4 as a therapeutic target in managing established liver metastases. We have previously reported that cell lines derived from liver metastases in patients with uveal melanoma do not express detectable CXCR4 protein.15 Moreover, CXCR4+ uveal melanoma cells retain their expression of CXCR4 while growing in the eyes of nude mice, yet metastasizing uveal melanoma cells rapidly downregulate CXCR4 after entering the livers of the same mice. Moreover, uveal melanoma cells exposed to soluble factors produced by human liver cells rapidly downregulate CXCR4 message and protein expression.15 Other evidence suggesting the limited efficacy of CXCR4-targeted therapy comes from studies showing that blockade of CXCR4/CXCL12 interactions can significantly reduce the initial seeding of pulmonary metastases of skin melanomas in mice but does not affect the growth of lung metastases once they have become established.28,29
We have previously demonstrated that intravenously injected radiolabeled human uveal melanoma cells preferentially home to and enter the livers of nude mice.40 The present results help to explain the homing of blood-borne uveal melanoma cells to the livers in experimental mice and the development of liver metastases in patients with uveal melanoma. However, the swift downregulation of CXCR4 on uveal melanoma cells that enter the liver suggests that anti-CXCR4-targeted therapy has limited usefulness. Moreover, it has been suggested that hepatic micrometastases are present in at least some of the patients at the time of initial diagnosis of uveal melanoma.41,42 In these patients, anti-CXCR4 therapy would most likely be ineffective. However, the seed and soil hypothesis holds that other factors may contribute to the growth and survival of liver metastases. One candidate molecule is c-Met, which is the receptor for hepatocyte growth factor-scatter factor (HGF/SF). Interestingly, c-Met is found on murine skin melanoma cells that preferentially produce liver metastases.43–45 The ligand for c-Met, HGF/SF, is present in the liver, and when it engages c-Met, it stimulates the growth and invasiveness of melanoma cells in the liver. Importantly, human uveal melanoma cells also express c-Met, which is highest in invasive cell lines that also display the greatest chemotactic responses to HGF/SF.46 Thus, at least two cell membrane-bound molecules contribute to the preferential development of liver metastases in patients with uveal melanoma. We propose that CXCR4 directs blood-borne uveal melanoma cells to home to the liver and that, after entering the liver parenchyma, chemokine receptor expression dissipates. Uveal melanoma cells also express c-Met, which engages HGF/SF in the liver and serves as the soil that promotes the growth and progression of liver metastases. Both these molecules are potential therapeutic targets for managing liver metastases of uveal melanoma. Although such therapy is highly theoretical, the stagnation in the 5-year survival rate for patients with uveal melanoma and the complete lack of any proven therapy for liver metastases of uveal melanoma indicate that new therapeutic paradigms are desperately needed.
Acknowledgments
The authors thank Elizabeth Mayhew and Jessamee Mellon for technical assistance.
Supported by National Institutes of Health Grant CA30276 and by an unrestricted grant from Research to Prevent Blindness.
Footnotes
Disclosure: H. Li, None; W. Yang, None; P.W. Chen, None; H. Alizadeh, None; J.Y. Niederkorn, None
References
- 1.Egan KM, Seddon JM, Glynn RJ, Gragoudas ES, Albert DM. Epidemiologic aspects of uveal melanoma. Surv Ophthalmol. 1988;32:239–251. doi: 10.1016/0039-6257(88)90173-7. [DOI] [PubMed] [Google Scholar]
- 2.Bedikian AY, Legha SS, Mavligit G, et al. Treatment of uveal melanoma metastatic to the liver: a review of the M. D. Anderson Cancer Center experience and prognostic factors. Cancer. 1995;76:1665–1670. doi: 10.1002/1097-0142(19951101)76:9<1665::aid-cncr2820760925>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Albert DM. The ocular melanoma story: LIII Edward Jackson Memorial Lecture, part II. Am J Ophthalmol. 1997;123:729–741. doi: 10.1016/s0002-9394(14)71119-5. [DOI] [PubMed] [Google Scholar]
- 4.Gombos DS, Mieler WF. Therapy of uveal melanoma: methods and risk factors associated with treatment. In: Albert DM, Polans A, editors. Ocular Oncology. New York: Marcel Dekker, Inc; 2003. pp. 321–352. [Google Scholar]
- 5.Johnson JP, Stade BG, Holzmann B, Schwable W, Riethmuller G. De novo expression of intercellular-adhesion molecule 1 in melanoma correlates with increased risk of metastasis. Proc Natl Acad Sci USA. 1989;86:641–644. doi: 10.1073/pnas.86.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Augsburger JJ, Mullen D, Kleineidam M. Planned combined I-125 plaque irradiation and indirect ophthalmoscope laser therapy for choroidal malignant melanoma. Ophthalmic Surg. 1993;24:76–81. [PubMed] [Google Scholar]
- 7.COMS. Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch Ophthalmol. 2001;119:670–676. doi: 10.1001/archopht.119.5.670. [DOI] [PubMed] [Google Scholar]
- 8.Kath R, Hayungs J, Bornfeld N, Sauerwein W, Hoffken K, Seeber S. Prognosis and treatment of disseminated uveal melanoma [see comments] Cancer. 1993;72:2219–2223. doi: 10.1002/1097-0142(19931001)72:7<2219::aid-cncr2820720725>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- 10.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 11.Kakinuma T, Hwang ST. Chemokines, chemokine receptors, and cancer metastasis. J Leukoc Biol. 2006;79:639–651. doi: 10.1189/jlb.1105633. [DOI] [PubMed] [Google Scholar]
- 12.Koizumi K, Hojo S, Akashi T, Yasumoto K, Saiki I. Chemokine receptors in cancer metastasis and cancer cell-derived chemokines in host immune response. Cancer Sci. 2007;98:1652–1658. doi: 10.1111/j.1349-7006.2007.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 14.Zlotnik A. Chemokines and cancer. Int J Cancer. 2006;119:2026–2029. doi: 10.1002/ijc.22024. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Alizadeh H, Niederkorn JY. Differential expression of chemokine receptors on uveal melanoma cells and their metastases. Invest Ophthalmol Vis Sci. 2008;49:636–643. doi: 10.1167/iovs.07-1035. [DOI] [PubMed] [Google Scholar]
- 16.Scala S, Ierano C, Ottaiano A, et al. CXC chemokine receptor 4 is expressed in uveal malignant melanoma and correlates with the epithelioid-mixed cell type. Cancer Immunol Immunother. 2007;56:1589–1595. doi: 10.1007/s00262-007-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinmuth N, Liu W, Ahmad SA, et al. αvβ3 integrin antagonist S247 decreases colon cancer metastasis and angiogenesis and improves survival in mice. Cancer Res. 2003;63:2079–2087. [PubMed] [Google Scholar]
- 18.Murakami T, Maki W, Cardones AR, et al. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002;62:7328–7334. [PubMed] [Google Scholar]
- 19.Hong JS, Pai HK, Hong KO, et al. CXCR-4 knockdown by small interfering RNA inhibits cell proliferation and invasion of oral squamous cell carcinoma cells. J Oral Pathol Med. 2009;38:214–219. doi: 10.1111/j.1600-0714.2008.00671.x. [DOI] [PubMed] [Google Scholar]
- 20.Jiang YP, Wu XH, Shi B, Wu WX, Yin GR. Expression of chemokine CXCL12 and its receptor CXCR4 in human epithelial ovarian cancer: an independent prognostic factor for tumor progression. Gynecol Oncol. 2006;103:226–233. doi: 10.1016/j.ygyno.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 21.Kim J, Takeuchi H, Lam ST, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–2753. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 22.Koshiba T, Hosotani R, Miyamoto Y, et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res. 2000;6:3530–3535. [PubMed] [Google Scholar]
- 23.Li YM, Pan Y, Wei Y, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–469. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Scala S, Ottaiano A, Ascierto PA, et al. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 2005;11:1835–1841. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- 25.Giles R, Loberg RD. Can we target the chemokine network for cancer therapeutics? Curr Cancer Drug Targets. 2006;6:659–670. doi: 10.2174/156800906779010245. [DOI] [PubMed] [Google Scholar]
- 26.Khan A, Greenman J, Archibald SJ. Small molecule CXCR4 chemokine receptor antagonists: developing drug candidates. Curr Med Chem. 2007;14:2257–2277. doi: 10.2174/092986707781696618. [DOI] [PubMed] [Google Scholar]
- 27.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 28.Kim SY, Lee CH, Midura BV, et al. Inhibition of the CXCR4/CXCL12 chemokine pathway reduces the development of murine pulmonary metastases. Clin Exp Metastasis. 2008;25:201–211. doi: 10.1007/s10585-007-9133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee CH, Kakinuma T, Wang J, et al. Sensitization of B16 tumor cells with a CXCR4 antagonist increases the efficacy of immunotherapy for established lung metastases. Mol Cancer Ther. 2006;5:2592–2599. doi: 10.1158/1535-7163.MCT-06-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arya M, Ahmed H, Silhi N, Williamson M, Patel HR. Clinical importance and therapeutic implications of the pivotal CXCL12-CXCR4 (chemokine ligand-receptor) interaction in cancer cell migration. Tumour Biol. 2007;28:123–131. doi: 10.1159/000102979. [DOI] [PubMed] [Google Scholar]
- 31.De Clercq E. The bicyclam AMD3100 story. Nat Rev Drug Discov. 2003;2:581–587. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- 32.De Clercq E. Potential clinical applications of the CXCR4 antagonist bicyclam AMD3100. Mini Rev Med Chem. 2005;5:805–824. doi: 10.2174/1389557054867075. [DOI] [PubMed] [Google Scholar]
- 33.Liles WC, Broxmeyer HE, Rodger E, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 34.Rubin JB, Kung AL, Klein RS, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci USA. 2003;100:13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ierano C, Giuliano P, D’Alterio C, et al. A point mutation (G574A) in the chemokine receptor CXCR4 detected in human cancer cells enhances migration. Cell Cycle. 2009;8:1228–1237. doi: 10.4161/cc.8.8.8250. [DOI] [PubMed] [Google Scholar]
- 36.Liang Z, Yoon Y, Votaw J, Goodman MM, Williams L, Shim H. Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res. 2005;65:967–971. [PMC free article] [PubMed] [Google Scholar]
- 37.Smith MC, Luker KE, Garbow JR, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–8612. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 38.Dalerba P, Clarke MF. Cancer stem cells and tumor metastasis: first steps into uncharted territory. Cell Stem Cell. 2007;1:241–242. doi: 10.1016/j.stem.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 39.Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Ma D, Niederkorn JY. Role of epidermal growth factor receptor in the metastasis of intraocular melanomas. Invest Ophthalmol Vis Sci. 1998;39:1067–1075. [PubMed] [Google Scholar]
- 41.Donoso LA, Shields JA, Augsburger JJ, Orth DH, Johnson P. Metastatic uveal melanoma: diffuse hepatic metastasis in a patient with concurrent normal serum liver enzyme levels and liver scan. Arch Ophthalmol. 1985;103:758. doi: 10.1001/archopht.1985.01050060016002. [DOI] [PubMed] [Google Scholar]
- 42.Feldman ED, Pingpank JF, Alexander HR., Jr Regional treatment options for patients with ocular melanoma metastatic to the liver. Ann Surg Oncol. 2004;11:290–297. doi: 10.1245/aso.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Rusciano D, Lin S, Lorenzoni P, Casella N, Burger MM. Influence of hepatocyte growth factor/scatter factor on the metastatic phenotype of B16 melanoma cells. Tumour Biol. 1998;19:335–345. doi: 10.1159/000030026. [DOI] [PubMed] [Google Scholar]
- 44.Rusciano D, Lorenzoni P, Burger M. Murine models of liver metastasis. Invasion Metast. 1994;14:349–361. [PubMed] [Google Scholar]
- 45.Rusciano D, Lorenzoni P, Burger MM. Paracrine growth response as a major determinant in liver-specific colonization by in vivo selected B16 murine melanoma cells. Invasion Metast. 1993;13:212–224. [PubMed] [Google Scholar]
- 46.Hendrix MJ, Seftor EA, Seftor RE, et al. Regulation of uveal melanoma interconverted phenotype by hepatocyte growth factor/scatter factor (HGF/SF) Am J Pathol. 1998;152:855–863. [PMC free article] [PubMed] [Google Scholar]