Abstract
Many excitatory synapses express Group 1, or Gq coupled, metabotropic glutamate receptors (Gp1 mGluRs) at the periphery of their postsynaptic density. Activation of Gp1 mGluRs typically occurs in response to strong activity and triggers long-term plasticity of synaptic transmission in many brain regions including the neocortex, hippocampus, midbrain, striatum and cerebellum. Here we focus on mGluR-induced long-term synaptic depression (LTD) and review the literature that implicates Gp1 mGluRs in the plasticity of behavior, learning and memory. Moreover, recent studies investigating the molecular mechanisms of mGluR-LTD have discovered links to mental retardation, autism, Alzheimer’s disease, Parkinson’s disease and drug addiction. We discuss how mGluRs lead to plasticity of neural circuits and how the understanding of the molecular mechanisms of mGluR plasticity provides insight into brain disease.
Cellular mechanisms of Group 1 mGluR-dependent synaptic plasticity
Group 1 mGluRs are comprised of mGluR1 and mGluR5 and constitute a subclass of metabotropic glutamate receptors that are canonically linked to the Gαq/11 heterotrimeric G-proteins. Immunoreactivity for mGluR1 and mGluR5 are largely complementary in the CNS (figure 1, for a review see (Ferraguti and Shigemoto, 2006)). mGluR1 staining is most intense in Purkinje cells of the cerebellar cortex and mitral/tufted cells of the olfactory bulb. Strong expression is also observed in neurons of the lateral septum, the pallidum and in the thalamus. mGluR5 on the other hand is observed in the cerebral cortex, hippocampus, subiculum, olfactory bulb, striatum, nucleus accumbens and lateral septal nucleus. In the hippocampus, mGlu5 is mainly expressed in dendritic fields of the stratum radiatum, whereas mGluR1 is mostly found on cell bodies. Subcellularly, group 1 mGluRs are localized postsynaptically in a perisynaptic zone surrounding the ionotropic receptors (Lujan et al., 1996). At excitatory synapses mGluRs are thus well positioned for rapid and selective regulation of excitatory synaptic strength for example by redistribution of AMPA and NMDA receptors. Hence, Gp1 mGluRs are known to facilitate or induce both long-term depression (LTD) and potentiation (LTP) of synaptic strength (Anwyl, 1999; Bellone et al., 2008). Gp1 mGluRs also trigger plasticity of non-synaptic conductances that lead to enhanced neuronal excitability (Wong et al., 2004). The best-characterized synaptic plasticity induced by Gp1 mGluRs is a long-term depression (LTD) of excitatory synaptic strength. MGluR-LTD was first described at the granule cell parallel fiber (PF) synapses onto Purkinje cells (PC) in the cerebellum and subsequently has been demonstrated in diverse brain regions such as the hippocampus, neocortex, dorsal and ventral striatum and spinal cord (reviewed in (Bellone et al., 2008; Gladding et al., 2009; Jorntell and Hansel, 2006)). Consequently, much of the detailed molecular mechanisms of cerebellar mGluR-LTD are known and there is strong evidence for its role in cerebellar-dependent learning (Jorntell and Hansel, 2006). The study of hippocampal mGluR-LTD has lead to the discovery of novel cellular mechanisms with implications for disease, but its contribution to normal hippocampal function remains elusive. mGluR-LTD has also been demonstrated in medium spiny neurons (MSNs) of the striatum and dopamine neurons of the midbrain where there is some overlap among the underlying molecular mechanisms. At the systems level, there is strong evidence for a role of mGluR-LTD in goal directed learning, Parkinson’s disease and drug addiction (Fig. 1). For the purpose of this review, we will focus on the conserved mGluR-LTD mechanisms, their role in normal brain function and their implication for neurological diseases and drug addiction.
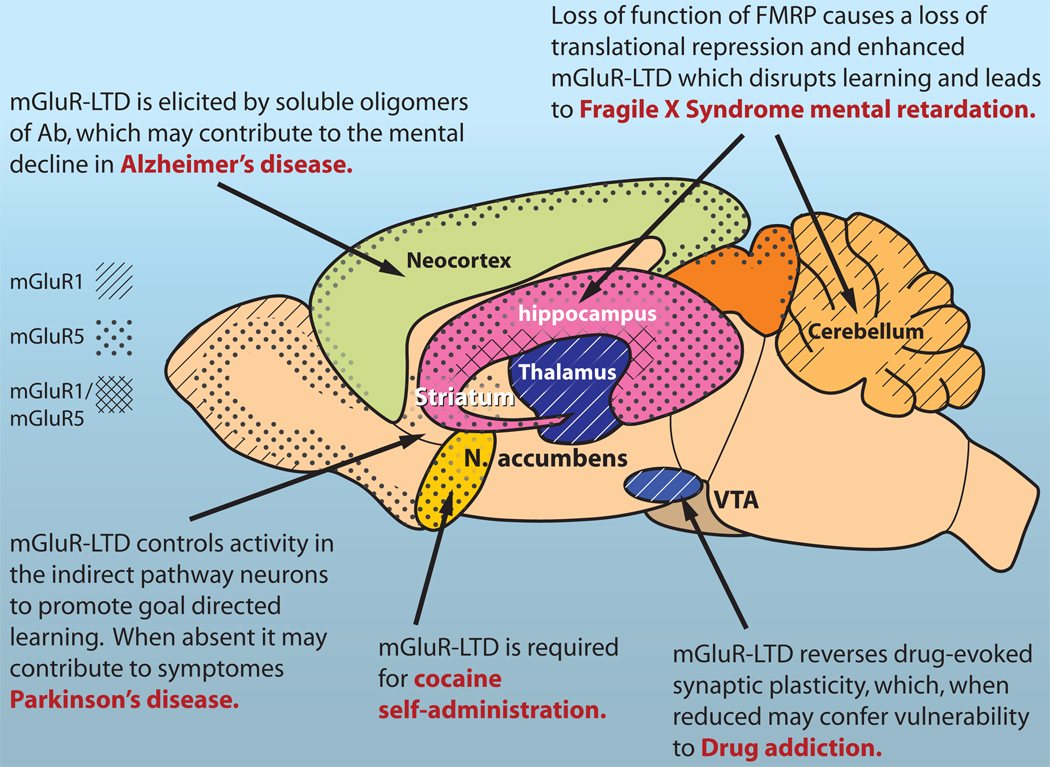
Figure 1. mGluR-LTD in health and disease.
Experimental evidence suggest the involvement of mGluR-LTD in goal directed learning, and cerebellar circuit adjustment during motor learning. Excessive mGluR-LTD has been linked to Alzheimer disease and Fragile X syndrome, while a loss of mGluR-LTD in the striatum may contribute to Parkinson’s symptoms. Finally, reduced mGluR-LTD in the midbrain has been suggested to confer a vulnerability of drug addiction. The group 1 mGluRs, mGluR1 and mGluR5 are differentially expressed in most brain regions, and co-expressed in some, based on (Ferraguti and Shigemoto, 2006). mGluR1 expression is indicated by the diagonal lines and mGluR5 expression pattern is indicated by the dotted stipple. Gray stipple indicates a lower level of expression.
Cerebellar and hippocampal mGluR-LTD: Conservation of a postsynaptic LTD expression mechanism
At both hippocampal CA1 synapses and cerebellar parallel fiber to Purkinje cell (PF-PC) synapses brief activation of Gp1 mGluRs either by pharmacological or synaptic stimulation induces LTD. mGluR1 is the primary Gp1 mGluR expressed in cerebellar PCs and consequently is solely responsible for PF-PC LTD (Romano et al., 1995; Shigemoto et al., 1992). Coincident synaptic activation of mGluR1 at PF inputs onto PCs together with PC depolarization provided by climbing fibers from the inferior olive, is required to induce LTD. Climbing fiber-mediated depolarization activates voltage-dependent Ca2+ channels and increases intracellular Ca2+ concentration in PCs, which together with PF mediated mGluR1 activation, induces LTD specifically of the active PF inputs. The requirement for coincident parallel and climbing fiber activation in the induction of long-term synaptic depression of PF-PC synapses was predicted by learning theorists Marr and Albus as well as the neuroscientist Ito and is a critical component of the learning mechanism ((Ito, 1982); reviewed in (Jorntell and Hansel, 2006) (Kano et al., 2008). In hippocampal CA1 pyramidal neurons, mGluR-LTD is typically induced with either prolonged low frequency synaptic stimulation (1–3 Hz, 5–15 min) of the CA3 Schaffer collateral axons or brief application of the Gp1 mGluR agonist, R,S-Dihydroxyphenylglycine (5–10 min; DHPG) and is observed in slice preparations as well as in vivo in awake, behaving rodents (Bolshakov and Siegelbaum, 1994; Huber et al., 2000; Kemp and Bashir, 1999; Manahan-Vaughan, 1997; Naie and Manahan-Vaughan, 2005; Palmer et al., 1997; Volk et al., 2007). Because the induction and expression mechanisms differ across development (Nosyreva and Huber, 2005) reviewed in (Bellone et al., 2008), here we will focus on mGluR-LTD mechanisms in mature CA1 neurons (>2nd postnatal week in rodents) where most recent work has focused. Although mGluR5 is highly expressed in area CA1, activation of either mGluR1 or mGluR5 appears to be sufficient for agonist-induced LTD (Fitzjohn et al., 1999; Hou and Klann, 2004; Palmer et al., 1997; Volk et al., 2006). Interestingly, mGluR1 activity is also required for LTD expression in CA1, but the cellular mechanism by which mGluR1 mediates LTD expression is unknown (Volk et al., 2006). Paired pulses of low frequency synaptic stimulation (1 Hz; 50 msec interstimulus interval; PP-LFS), induce LTD through activation of Gp1 mGluRs (mGluR1 and 5) in conjunction with the Gq-coupled M1 muscarinic acetylcholine receptors (mAChRs). In other words, mGluR1/5 antagonists alone do not block LTD induced by PP-LFS, but only when combined with an M1 mAChR antagonist (Kemp and Bashir, 1999; Volk et al., 2006; Volk et al., 2007). Deafferented cholinergic fibers from the septal nucleus maintain the capacity to release acetylcholine when stimulated extracellularly in the stratum radiatum of acute hippocampal slices (Cole and Nicoll, 1983; Shinoe et al., 2005), thus explaining the specific contribution of M1 mAChRs to LTD induced with extracellular stimulation, in contrast to chemically induced mGluR-LTD.
Gp1 mGluRs are canonically linked to activation of phospholipase C (PLCβ), inositol trisphosphate (IP3) generation, release of Ca2+ from intracellular stores and Protein Kinase C (PKC) activation, of which all are required for cerebellar mGluR-LTD (reviewed in (Kano et al., 2008). In contrast, hippocampal mGluR-LTD requires Gαq,, but occurs independently of postsynaptic Ca2+ increases, IP3 sensitive Ca2+ stores, PLC or PKC activity (Fitzjohn et al., 2001; Kleppisch et al., 2001; Moult et al., 2006) (Huber and Bear, unpublished} implicating distinct Gαq dependent signaling pathways. However it is important to emphasize that most of these findings were obtained using bath application of the Gp1 mGluR agonist DHPG to induce LTD. Recent work using glutamate uncaging onto individual spines of CA1 neurons suggests a role for mGluR-induced Ca2+ increases in the spine in mGluR-LTD (see (Holbro et al., 2009). Therefore, it will be important to test the requirement for postsynaptic Ca2+ and PLC using more physiological, mGluR-LTD paradigms in response to synaptically released glutamate.
Although cerebellar and hippocampal mGluR-LTD rely on two distinct mGluR signaling pathways, both ultimately trigger endocytosis of ionotropic AMPA receptor subunits (GluR1 and 2 in CA1 and GluR2 only in PCs) and a long-term reduction in the number of postsynaptic surface AMPAR (Moult et al., 2006; Snyder et al., 2001; Steinberg et al., 2004; Wang and Linden, 2000) (Fig. 4). As discussed below, mGluR-LTD in other brain regions such as the midbrain and the striatum can be expressed by other pre- or postsynaptic mechanisms including insertion of lower conductance GluR2-containing AMPARs and the retrograde signaling of endocannabinoids. Therefore, mGluR-LTD can come about through a number of expression mechanisms (Bellone et al., 2008; Gladding et al., 2009). The mechanisms of mGluR-triggered AMPAR endocytosis are best understood in cerebellar PCs. At PCs, mGluR-LTD and AMPAR endocytosis are induced by Ca2+-dependent activation of Protein kinase C (PKC) and phosphorylation of GluR2 at Ser880 (Chung et al., 2003; Steinberg et al., 2006). This GluR2 phosphorylation reduces its affinity for the AMPAR scaffold GRIP, which leads to increased AMPAR endocytosis and reduced surface AMPAR expression (Chung et al., 2003). In contrast, mGluR-induced LTD and AMPAR endocytosis in CA1 do not require PKC, but instead rely on tyrosine dephosphorylation and the tyrosine phosphatase STEP (Striatal-enriched tyrosine phosphatase). mGluR activation results in dephosphorylation of GluR2 on Tyr residues; a process which requires STEP (Moult et al., 2006; Moult et al., 2002; Schnabel et al., 1999; Zhang et al., 2008) (Fig. 4). Recent exciting work implicates the matrix metalloproteinase (MMP) TACE (tumor necrosis factor-α-converting enzyme) in mGluR-triggered AMPAR endocytosis underlying both hippocampal and cerebellar mGluR-LTD. Gp1 mGluRs stimulate the enzymatic activity of TACE, which in turn cleaves the intramembrane protein neuronal pentraxin receptor (NPR) to release its extracellular pentraxin domain. The NPR ectodomain cleavage product clusters AMPARs through extracellular interactions and stimulates their endocytosis (Cho et al., 2008) (Fig. 4). The discovery of mGluR induced regulation of TACE and NPR cleavage revealed a novel mechanism by which mGluRs invoke synaptic plasticity. Therefore, many questions remain such as: How mGluRs regulate or couple to TACE activation? How NPR-induced clustering of AMPARs interacts with Tyr dephosphorylated GluR2 or other endocytic proteins implicated in mGluR-LTD such as Arc (see below)? Furthermore, determining if mGluRs activate other MMPs or intramembrane cleavage of other known TACE substrates such as TNFα or amyloid precursor protein (APP) will be important to understand the complexities of mGluR regulation of synaptic function and its involvement in disease (Black, 2002; Blobel, 2000).
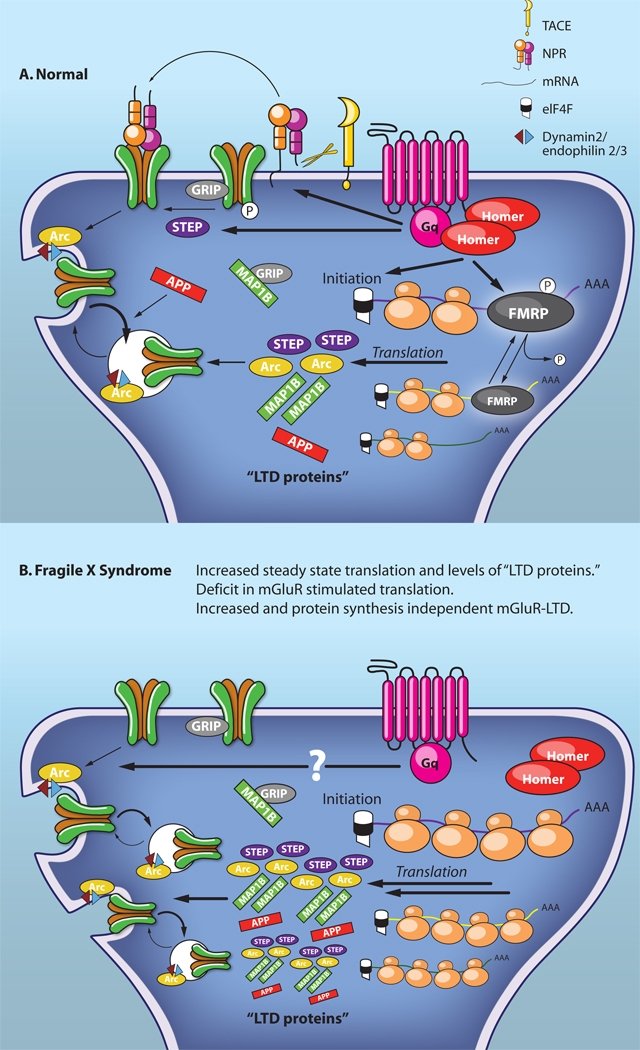
Figure 4. Mechanisms of translation-dependent mGluR-LTD: Implications for Fragile X Syndrome.
A. In normal (or wildtype) hippocampal CA1 neurons brief activation of mGluR1/5 triggers rapid endocytosis of AMPARs through TACE mediated intramembrane cleavage of NPR. mGluR-stimulated AMPAR endocytosis requires activity of the Tyr phosphatase STEP as well as existing Arc protein. MGluRs also trigger translation of proteins through activation of translation initiation, as well as dephosphorylation of the RNA binding protein, FMRP. Known proteins whose synthesis is stimulated by mGluRs as well as play a role in mGluR-LTD include Step, Map1b, Arc and APP. These proteins are known to regulate and/or stimulate AMPAR endocytosis. B. In the absence of FMRP, as in Fragile X Syndrome, mGluRs stimulate endocytosis of AMPARs, but it is unknown if the mechanisms are similar to those at normal synapses. In Fmr1 KO mice, there are increased steady state translation rates and protein levels of MAP1b and APP, as well as a deficit in mGluR stimulation of translation. mGluR-LTD in the Fragile X Syndrome mouse model (Fmr1 KO mice) is enhanced and independent of translation suggesting that the “LTD proteins” are available to maintain persistent decreases in AMPARs and LTD.
It is important to point out that there are distinct forms of LTD, independent of mGluRs that coexist at CA1 synapses and the nucleus accumbens. These forms typically rely on activation of NMDA receptors (NMDARs). Like mGluR-LTD, NMDAR-dependent LTD is mediated by decreases in postsynaptic AMPAR number, but has distinct molecular mechanisms. mGluR-LTD and NMDAR-LTD may also be induced at distinct synapses or affect distinct populations of surface AMPARs. In support of the former, 2-photon uncaging of glutamate onto individual spines of CA1 neurons expressing fluorescent endoplasmic reticulum (ER) proteins revealed that spines with an ER were susceptible to mGluR-LTD; whereas spines without an ER were refractory to LTD induction (Holbro et al., 2009). ER-containing spines were larger in volume and responded to glutamate with larger synaptic currents as well as mGluR-mediated Ca2+ transients, suggesting a role for Ca2+ released from ER-stores in hippocampal mGluR-LTD. Importantly, this data provides evidence that mGluR-LTD may function to selectively weaken strong synaptic inputs and/or destabilize stable, mature spines. A corollary of this hypothesis is that NMDAR-dependent LTD is more prevalent at smaller spines and/or weaker synapses without an ER. Recent evidence supports this view. A structural correlate of NMDAR-LTD is the separation of spines from their associated presynaptic boutons, the latter of which selectively occurs at smaller spines (Bastrikova et al., 2008; Becker et al., 2008). Interestingly, although mGluR-mediated Ca2+ transients spread to neighboring spines, these synapses were not depressed functionally, suggesting an additional role for the ER in maintaining spine-specific mGluR-LTD. The fact that the ER is also a site of ribosome localization supports data implicating local, synaptic translation in mGluR-LTD (see below).
Striatal and mesolimbic mGluR-LTD: Cell type specific plasticity and circuit function
In the striatum, excitatory synaptic inputs from cortical neurons can undergo mGluR-LTD. Such cortico-striatal afferents impinge on both the neurons of the direct and the indirect striatal pathways. In both cases excitatory afferents synapse on the so-called medium spiny neurons (MSN) that send out GABAergic projections. While direct pathway MSNs monosynaptically project to the output neurons of the basal ganglia, indirect pathway neurons first connect to the medial globus pallidus and the subthalamic nucleus, before reaching the output nuclei (Fig. 2). The two types of neurons also express distinct sets of receptors. D1 and M4 muscarinic receptors mark direct pathway MSNs, while D2 receptors are exclusively expressed on indirect pathway MSNs. Recently, expressing GFP under the control of M4 receptor (M4-GFP) or dopamine D2 receptor (D2-GFP) promoter allowed the visualization of direct and indirect pathway neurons respectively in living tissues (Gerfen, 2006). Meanwhile several similar mouse lines that express large constructs have been generated, thanks to bacterial artificial chromosome (BAC) technology, and improved our understanding of the role in plasticity for shaping the striatal networks (for a recent review see (Kreitzer and Malenka, 2008)).
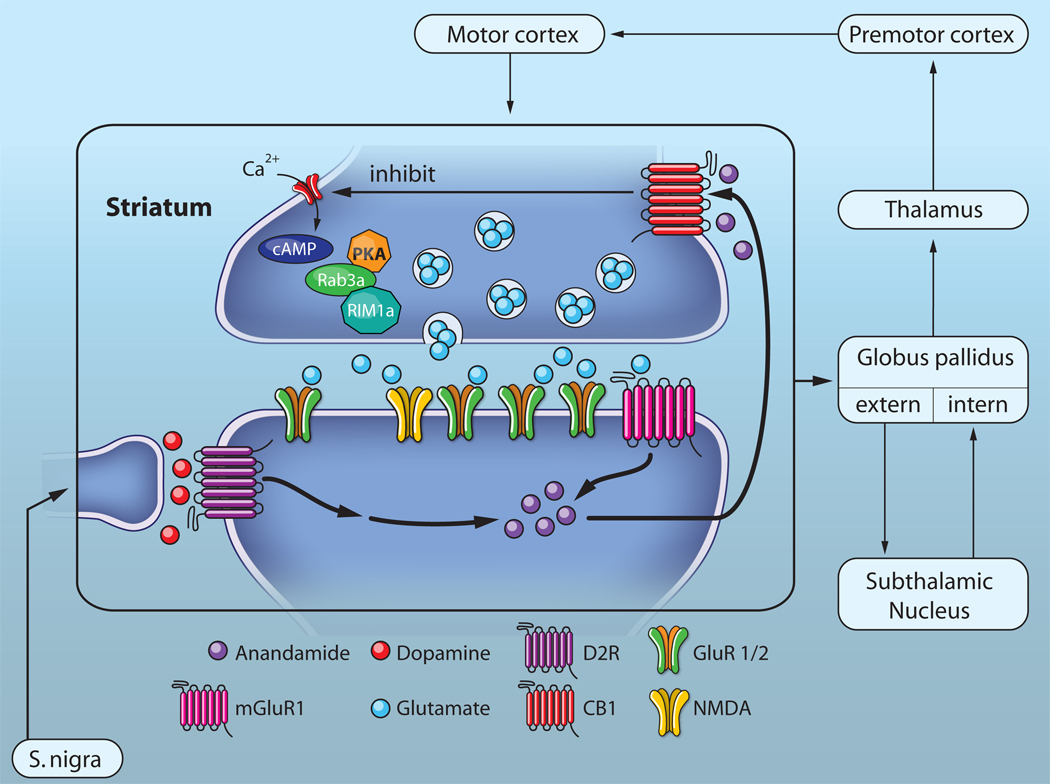
Figure 2. Striatal mGluR-LTD controls indirect pathway activity.
In medium spiny neurons of the dorsal striatum, excitatory afferents from the cortex and dopamine fiber arising from the substantia nigra converge. If Gp1 mGluRs and D2Rs are activated concomitantly endocannabinoids. most likely anandamide, are synthesized and released from the MSN. This retrograde messenger activates presynaptic CB1 receptors, and reduced release probability via inhibition of calcium channels, cAMP and PKA, such that Rab3a and Rim1a dependent exocytosis is reduced. In Parkinson’s disease dopamine afferents are absent, and mGluR-LTD as a consequence reduced. This leads to increased indirect pathway activity (i.e. more inhibitory output from the GABAergic MSNs) and a reduction of spontaneous movements.
Previous work has shown that MSNs in general are capable of expressing forms of mGluR-LTD independent of NMDA receptor activation, but suggested a dependence of D2 receptors (Calabresi et al., 1997). Thanks to BAC-transgenic mice, which express GFP selectively in D1/M4- or D2-expressing neurons, two distinct forms of LTD have recently been extensively characterized. They both are induce by the activation of postsynaptic mGluRs, require endocannabinoids as retrograde messenger, and are expressed by presynaptic mechanisms. A striking difference is that mGluR-LTD in MSNs of the indirect pathway requires D2 receptor activation, while LTD in direct neurons is blocked when D1 receptors are activated (Kreitzer and Malenka, 2007; Shen et al., 2009). In all MSNs, LTD is typically initiated by high frequency stimulation (10–15 Hz; HFS; or strong pharmacological activation of the mGluRs), which triggers an induction mechanism in the postsynaptic neuron, followed by the presynaptic expression of the plasticity (Choi and Lovinger, 1997) (Fig. 2). An endocannabinoid, most likely anandamide, serves as the retrograde signal originating in the postsynaptic cell and affecting transmitter release in the presynaptic partner. This mechanism has been formally demonstrated only for indirect pathway MSNs (Gerdeman et al., 2002). Other authors have therefore classified striatal LTD of glutamatergic transmission among the eCB-LTDs (Heifets and Castillo, 2009). Here, we will keep with a nomenclature that makes reference to the induction mechanism; mGluR-LTD (Bellone et al., 2008). When LTD is elicited in slice preparations of the dorsal striatum, extracellular HFS concomitantly activates glutamatergic axons from the cortex and dopaminergic fibers arising from the midbrain. This activates Gp I mGluRs along with D2 receptors in indirect pathway MSNs. Several experiments led to the conclusion that D2 receptor activation gates the induction of mGluR-LTD in these neurons. In fact, mGluR agonists alone will only transiently depress synaptic transmission, while in the presence of a D2 agonist LTD is observed (Kreitzer and Malenka, 2007). D2R are Gio coupled, hence liberating Gβγ dimers, which can recruit and activate PLCb (Hernandez-Lopez et al., 2000), eventually triggering the synthesis and release of endocannabinoids. In addition, L-type voltage-gated calcium channels may also positively modulate the mobilization of the endocannabinoids. Clearly pharmacological stimulation of CB1 receptors alone is not enough to cause mGluR-LTD in the dorsal striatum, and there is a requirement for low-frequency presynaptic activity during CB1R activation (Singla et al., 2007). The concomitant activation of CB1 receptors and presynaptic activity has been proposed to confer synapse specificity to mGluR-LTD thus limiting volume transmission of endocannabinoids. Although MSNs express higher levels of mGluR5 relative to mGluR1, pharmacological evidence suggests that the mGluR1 is the primary receptor to drive mGluR-LTD in the striatum (Gubellini et al., 2001), but these findings await confirmation in genetic mouse models.
For direct pathway MSNs, in contrast, a precisely timed activation of the pre- and the postsynaptic neurons (i.e. a spike-time dependent plasticity (STDP) protocol) is very efficient to induce mGluR-LTD, provided D1 receptors are pharmacologically blocked (Shen et al., 2008). As mentioned above, in both types of MSNs, endocannabinoids diffuse retrogradely to the presynaptic terminal where they bind to CB1 receptors and reduce the release probability, presumably through inhibition of calcium channels and inhibition of adenylyl cyclase and PKA. It is believed that mGluR-LTD is maintained by a steady production of endocannabinoids, although the experimental evidence remains controversial. Alternatively, CB1 signaling may permanently reduce the release probability. Although not directly tested in the striatum, in other parts of the brain once LTD is established, washing in a CB1 antagonist typically does not reverse the depression (Heifets and Castillo, 2009). For example, mGluR and eCB dependent LTD of GABAergic synaptic transmission onto hippocampal CA1 neurons is expressed by an alteration of the release machinery through an effect on the active-zone protein RIM1a (Chevaleyre et al., 2007; Schoch et al., 2002).
In the ventral striatum, a similar form of mGluR-LTD can be observed. In MSNs of the NAc Gp1 mGluRs are also expressed postsynaptically, and their activation eventually leads to the release of endocannabinoids and a long-lasting decrease of the presynaptic release probability (Robbe et al., 2002). Efficient protocols to induce LTD require sustained high frequency stimulation (e.g. 10–15 Hz for 1 minute), presumably so that enough glutamate is released to reach the perisynaptically located mGluRs by diffusion. Pharmacological stimulation mGluRs is sufficient to induce LTD. However the in vivo pre-exposure of the animal to a single injection of tetrahydrocannabinol (THC) or cocaine will block the expression of LTD through an unknown mechanism (Fourgeaud et al., 2004). Future studies will have to address the question whether heterogeneity in the neuronal population (and therefore plasticity) of the NAc exists, similar to the one described for the dorsal striatum.
Taken together, mGluR.LTD is expressed throughout the striatum. In the dorsal part, dopamine gates mGluR-LTD, albeit with opposing polarity. In the indirect pathway the presence of dopamine promotes LTD, while in the direct pathway dopamine prevents LTD. Whether a similar segregation also exists in the ventral striatum remains to be shown. mGluR-LTD contributes to balance direct and indirect pathways, dominated by a long-lasting inhibitory effect on the indirect pathway (Surmeier et al., 2007). The crucial role of dopamine in controlling plasticity has received much attention, because studies in awake non-human primates suggest that dopamine release from midbrain neurons represent a learning signal shaping goal directed actions (see below, (Schultz, 2006).
In dopamine neurons of the VTA, normally no mGluR-LTD is observed, even when Gp 1 mGluRs are strongly stimulated. The plasticity can be unmasked, after an exposure to an addictive drug, such as cocaine. In fact mGluR-LTD has been identified as the mechanism by which cocaine-evoked potentiation of excitatory afferents onto dopamine neurons is reversed (Fig. 3) (Bellone and Lüscher, 2006) and could therefore also be referred to as a mGluR-depotentiation. In drug-naïve mice, pharmacological activation of GpI mGluRs only transiently affects synaptic transmission. This short-term depression involves retrograde release of endocannabinoids, which in the VTA unlike the NAc does not seem to permanently affect excitatory transmission, but may be involved in LTD of GABAergic transmission (Pan et al., 2008). Pharmacological as well as experiments in KO mice suggest that the short-term depression is mediated by mGluR5, while mGluR-LTD is induced by mGluR1. At the same synapse another form of LTD coexists that can be observed acute midbrain slices. When excitatory afferents onto DA neurons are stimulated with a low frequency (e.g. 1 Hz for 15 minutes) a form of LTD is triggered that depends neither on mGluRs nor NMDAR but is regulated by PKA (Gutlerner et al., 2002).
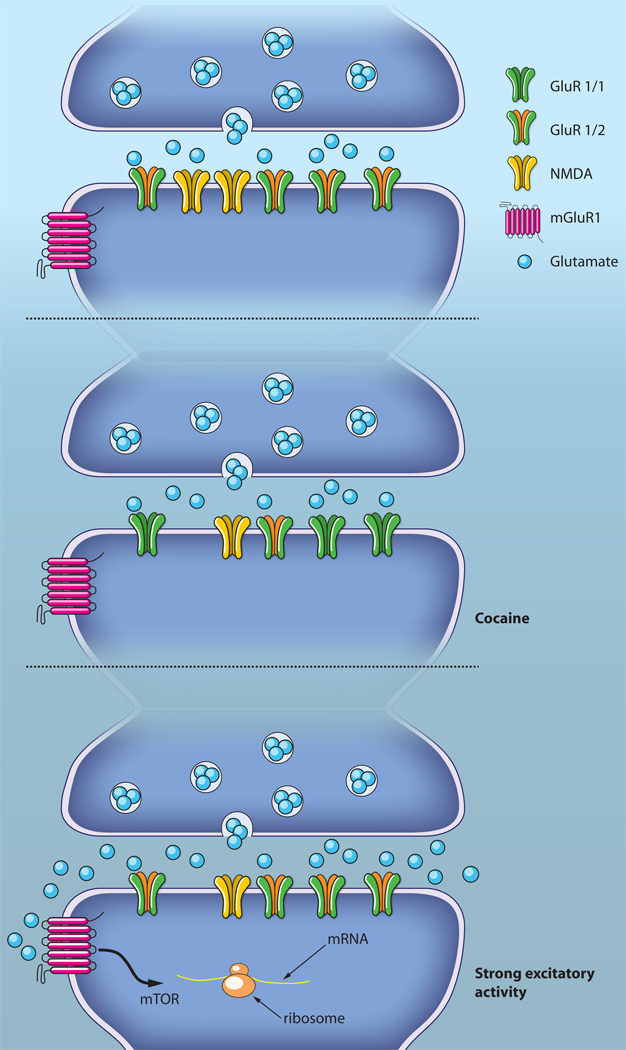
Figure 3. mGluR reverses cocaine-evoked synaptic plasticity.
Excitatory afferents onto dopamine neurons of the VTA are potentiated after cocaine exposure by the insertion of GluR2-lacking AMPARs. This drug-evoked plasticity can be reversed by mGluR-LTD elicited by strong activation of the excitatory afferents.
The expression mechanism of mGluR-LTD in the VTA is unique. Rather than reducing the number of AMPA receptors (see above), mGluR-LTD in the VTA relies on an exchange of receptors with a distinct subunit composition (Fig. 3). Typically, in naïve rodents, AMPARs are GluR2-containing heteromers, as in many other parts of the brain. Within hours of a single exposure to cocaine a substantial fraction of AMPAR become GluR2-lacking, and excitatory transmission therefore calcium permeable. Activation of mGluR1 causes the selective removal of the GluR2-lacking AMPARs that are then replaced with GluR2 containing ones.
mGluR-dependent LTD: A model of rapid translational control of synaptic function
A common cellular mechanism for mGluR-LTD in many brain regions including cerebellar PCs, hippocampus, VTA and probably also striatum is the reliance on rapid (in minutes) protein synthesis (Huber et al., 2000; Karachot et al., 2001; Mameli et al., 2007; Waung and Huber, 2009). The rapid requirement for translation in mGluR-LTD predicts a role for locally synthesized proteins (Fig. 4). This was first demonstrated in the CA1 pyramidal neurons, where the protein synthesis required for LTD occurs in dendrites (Huber et al., 2000). As mentioned above, activation of Gq coupled, M1 muscarinic acetylcholine receptors (mAChRs) either with synaptic stimulation (PP-LFS) or an M1 mAChR agonist, induces LTD onto CA1 neurons that shares similar mechanisms as mGluR-LTD (Volk et al., 2007). Therefore, Gq coupled receptors activate a common postsynaptic, protein synthesis-dependent LTD mechanism that is mediated by a persistent decrease in AMPAR number. It has been hypothesized that Gq coupled receptors stimulate the rapid local synthesis of new proteins, which participate in the regulation of AMPAR endocytosis and/or trafficking after endocytosis (Fig.1). For the purpose of this review, these newly synthesized proteins will be referred to as “LTD proteins”. Although mGluR-LTD in the striatum and the VTA is mediated by distinct pre- or postsynaptic mechanisms from hippocampal and cerebellar mGluR-LTD, these forms of synaptic plasticity also rely on rapid protein synthesis (Mameli et al., 2007; Yin et al., 2006). From the study of translation-dependent mGluR-LTD, researchers have gained knowledge of how synaptic activity regulates dendritic translation, what and how newly synthesized proteins alter synaptic function and discovered how disease linked proteins with translational control functions impact synaptic plasticity (Costa-Mattioli et al., 2009; Waung and Huber, 2009). It is important to note that mGluR-LTD occur independently of protein synthesis under some circumstances, for example, in hippocampal area CA1 in the Fragile X Syndrome disease model (Hou et al., 2006; Nosyreva and Huber, 2006) (discussed below) and in older rodents (3–4 months) (Moult et al., 2008), but see (Kumar and Foster, 2007). Interestingly, mGluR-LTD under these conditions is mediated by a similar postsynaptic mechanism as translation dependent LTD (i.e. tyrosine dephosphorylation and/or AMPAR endocytosis) (Kumar and Foster, 2007; Moult et al., 2008; Nosyreva and Huber, 2006). This result suggests that protein synthesis is dispensable for mGluR-LTD if existing levels of “LTD proteins” are sufficient to maintain decreases in surface AMPARs and LTD.
Work in the last year has made great progress in determining the identity of the “LTD proteins” with regard to hippocampal mGluR-LTD. From these studies, a picture is emerging in which mGluRs synthesize a number of functionally related proteins that impact AMPAR trafficking. Recent work has implicated Activity-regulated cytoskeletal associated protein (Arc) in mGluR-LTD (Park et al., 2008; Waung et al., 2008). Arc associates directly with dynamin 2 and endophilin, components of AMPAR endocytosis machinery, and functions to increase AMPAR endocytosis and decrease surface AMPARs (Chowdhury et al., 2006; Rial Verde et al., 2006; Shepherd et al., 2006). Consistent with this role, existing Arc protein is necessary for mGluRs to trigger AMPAR endocytosis and LTD (Fig. 4). The Arc gene is well known as an activity-dependent immediate early gene that upon induction the Arc mRNA rapidly localizes to dendrites (Link et al., 1995; Steward et al., 1998; Steward and Worley, 2001). In dendrites, Arc is rapidly (~5 min) translated in response to group 1 mGluRs and this rapid synthesis is required to maintain decreases in surface AMPARs and LTD. Interestingly, Arc levels remain elevated for the duration of LTD (at least one hour) and evidence suggests that Arc actively maintains LTD through a persistent increase in the endocytosis rate of AMPARs (Park et al., 2008; Waung et al., 2008). Because Arc transcription is induced by neuronal activity associated with salient experiences and learning (Guzowski et al., 1999; Guzowski et al., 2006; Link et al., 1995; Lyford et al., 1995). This suggests that mGluR-LTD may participate in the encoding of Arc-inducing experience.
MGluRs induce rapid translation other proteins that regulate AMPAR trafficking, such as microtubule associated protein 1b (MAP1b) and STEP. STEP and MAP1b negatively regulate AMPAR surface expression and are required for mGluRs to reduce AMPAR surface expression, suggesting that they contribute to mGluR-LTD (Davidkova and Carroll, 2007; Zhang et al., 2008). STEP synthesis may function in LTD to dephosphorylate GluR2 and to maintain an increased endocytosis rate (Zhang et al., 2008) (Moult et al., 2006). MAP1b interacts with a GluR scaffold, GRIP, and may function to sequester GRIP and associated AMPARs from the synaptic surface (Davidkova and Carroll, 2007; Seog, 2004). In summary, mGluRs stimulate a coordinated translation of proteins that together reduce surface AMPAR expression. The “LTD proteins” that underlie cerebellar mGluR-LTD are unknown. Because the late phase of cerebellar LTD relies on newly translated proteins to maintain surface AMPAR decreases, Arc and MAP1b may play a similar role in the cerebellum.
Although mGluR-LTD in the VTA and striatum is expressed via distinct postsynaptic or presynaptic mechanisms, it also requires rapid protein synthesis suggesting a common mechanism by which mGluR invoke plasticity. As mentioned above, in dopamine neurons of the VTA, mGluR-LTD is expressed by insertion of lower conductance, GluR2 containing AMPARs (Bellone and Lüscher, 2005). Interfering selectively with the synthesis of GluR2 through the diffusion of siRNA into the postsynaptic dopaminergic neuron blocked mGluR-LTD (Mameli et al., 2007). In other words one of the proteins that needs to be synthesized in order to express mGluR-LTD in these neurons is GluR2, which is then reinserted into AMPARs that are built from scratch within minutes (Fig. 3). Only one report has examined the protein-synthesis dependence of striatal mGluR-LTD, albeit without distinguishing direct and indirect pathway neurons (Yin et al., 2006). They find that in a slice preparation, where presynaptic cell bodies (i.e. the soma of the cortical neurons) had been surgically removed, the bath application but not postsynaptic filling of the MSN with the protein synthesis inhibitor anisomycin blocked mGluR-LTD. These results would suggest a presynaptic or astroglial locus of the protein synthesis.
mGluR regulated translational activation in neurons
Translational regulation is a major mechanism by which mGluRs induce plasticity, and therefore defining the signaling pathways by which mGluRs control translation provides insight into the plasticity mechanisms. Current evidence indicates that Gp1 mGluRs regulate translation at both the level of translation initiation and elongation as reviewed (Costa-Mattioli et al., 2009; Waung and Huber, 2009). Briefly, mGluRs appear to stimulate translation initiation through 2 major signaling pathways, the ERK-MAPK and PI3K–mTOR pathways. To initiate translation, mGluRs trigger phosphorylation of eukaryotic initiation factor 4E (eIF4E), and eIF4E binding protein (4EBP) as well as stimulate formation of the translation initiation (eIF4F) complex via the ERK and PI3K–mTOR signaling pathways (Banko et al., 2006; Ronesi and Huber, 2008a; Waung and Huber, 2009). MGluR activation of PI3K, mTOR and ERK also stimulates phosphorylation of p70 ribosomal S6 kinase (RSK) and ribosomal S6 in hippocampal slices which functions to increase translation of a subset of mRNAs (those with a 5’ terminal oligopyrimidine tract; 5’TOP) that encode ribosomes, translation factors, thus increasing the overall translational capacity of the neuron (Antion et al., 2008; Ronesi and Huber, 2008a). mGluR-LTD in CA1 relies on activation of both ERK and the PI3K–mTOR pathway (Gallagher et al., 2004; Hou and Klann, 2004). Similarly, mGluR-LTD in other brain regions such as the cerebellum and bed nucleus of the stria terminalis (BNST) also rely on ERK (Grueter et al., 2006; Ito-Ishida et al., 2006); whereas mTOR is required for LTD in the VTA (Mameli et al., 2007).
Interaction of the mGluR5 C-terminal tail with the scaffold and signaling molecule Homer forms a critical link between mGluRs and activation of the translational apparatus as well as mGluR-LTD induction. Multimerization of Homer molecules scaffolds mGluR5 to PI3K enhancer (PIKE), a small GTPase that binds PI3K and stimulates its lipid kinase activity (Rong et al., 2003). Acute disruption of mGluR5-Homer interactions in hippocampal slices blocks mGluR-LTD, as well as mGluR stimulation of PI3K–mTOR, translation initiation and synthesis of Elongation factor 1a (EF1a), a 5’TOP mRNA (Ronesi and Huber, 2008a). As discussed below in the context of mGluR-LTD and addiction, mGluR1-Homer interactions in the VTA in vivo are required for mGluR1- LTD and reversal of cocaine induced plasticity in this brain region (Mameli et al., 2009). MGluRs control translation elongation which also occurs through Homer interactions (Davidkova and Carroll, 2007; Park et al., 2008). MGluR5 forms direct and indirect (via Homer) association with Ca2+/calmodulin-dependent eukaryotic elongation factor 2 kinase (EF2K) (Park et al., 2008). Although somewhat counterintuitive, evidence indicates that mGluR5 inhibits translation elongation through stimulation of EF2K which, as the name implies, phosphorylates eukaryotic elongation factor 2 (EF2). Although phosphorylation of EF2 generally inhibits elongation, this is thought to make available more initiation factors for poorly initiated mRNAs such as Arc and MAP1b. In support of this hypothesis, mGluR stimulated Arc and MAP1b synthesis, as well as mGluR-LTD, are abolished with EF2K knockdown or KO; effects that are rescued by low concentrations of cycloheximide, a translation elongation inhibitor (Davidkova and Carroll, 2007; Park et al., 2008). Therefore, mGluR5 activation, through a Homer scaffold, concurrently stimulates translation initiation while slightly inhibiting elongation, which coordinates translational activation of specific mRNAs required for LTD. The RNA binding protein Fragile X Mental Retardation Protein (FMRP) also contributes to the specificity of Gp1 mGluR translational activation of specific mRNAs as discussed below (Ronesi and Huber, 2008b; Waung and Huber, 2009).
MGluR-dependent LTD, goal-directed learning and neurological disease of the striatum
As developed above, striatal dopamine may represent a learning signal that is released when an unexpected rewarding outcome signals the need to change a behavior (Schultz, 2006). In such as model, striatal synaptic plasticity under the modulatory control by dopamine may serve as the mechanism underlying reward-driven learning. Under normal conditions DA neurons of midbrain exhibit a firing pattern that reflects a prediction error signal: when an unexpected reward is given, firing increases, when reward occurs as expected that firing does not change and when a promised reward is omitted, DA neurons are inhibited (Schultz, 2006). It has been argued that dopamine facilitates the learning of the circumstances under which rewards are obtained.
In general, activity in direct pathway neurons is believed to select appropriate actions while indirect pathway connections would inhibit unnecessary actions or movements. Changes in the balance between these two pathways, caused by synaptic plasticity, will therefore help to shape the most appropriate action. While most empirical evidence to support this conclusion is based on experiments aiming at interfering with LTP (e.g. local infusion of NMDA receptors antagonists), LTD may also shape goal-directed learning, and when deficient, explain key symptoms of Parkinson’s (PD) and addiction.
Parkinson’s disease (PD)
In PD, dopamine levels in the striatum are reduced because midbrain neurons degenerate. This first affects the DA neurons of the substantia nigra compacta, and as a consequence, a characteristic motor phenotype develops that is dominated by the reduction of spontaneous movements. Patients also have an increased muscle tone, referred to as rigidity, execute movements much slower than normal (bradykinesia) and tremble (tremor). Several mouse models for PD exist. For example, neurotoxins selectively targeting dopamine neurons (e.g. 5-OH-dopamine) cause their degeneration and leads to a motor phenotype in rodents that mimics core components of the human disease. Obviously, these models have been used to study the underlying cellular mechanism and one of the most prominent functional alterations is the absence of indirect pathway mGluR-LTD (Kreitzer and Malenka, 2007; Shen et al., 2008). As a result, the balance is shifted towards LTP within the striatum, and the activity in the indirect pathway may actually be enhanced, ultimately excessively inhibiting movements. Support for this interpretation comes from rescue experiments with dopamine agonists in the mouse model, but also from the clinical observation that D2 receptor agonists are among the most efficient symptomatic treatments of PD. Of particular interest here is the observation that a partial restoral of indirect pathway mGluR-LTD by inhibition of the degradation of endocannabinoids in combination with D2 receptor activation enhances locomotor activity in dopamine-depleted mice (Kreitzer and Malenka, 2007). The situation may however be more complicated because dopamine obviously does much more that controlling LTD. For example it can hyperpolarize MSNs through D2 receptors and G-protein gated inwardly rectifiying potassium (GIRK) channel opening, and dopamine also modulates direct pathway activity. To further investigate the role of mGluR-LTD in PD, optogenetic tools may turn out to be very helpful, which will allow for cell type-specific intervention in freely moving rodents. A first report recently appeared that deconstructed of the parkinsonian circuitry by transfecting neurons in the striatum and the basal ganglia with the excitatory Channelrhadopsin or the inhibitory Halorhodopsin (Gradinaru et al., 2009). The results suggest that manipulations of synaptic transmission (and therefore also synaptic plasticity) rather than direct modulation of neuronal excitability are the most efficient manipulations to improve the motor deficit. Moreover, light-activated control of G-protein signaling, by transfection of the so-called OptoXRs (Airan et al., 2009) may also allow to directly probe the role of mGluRs in a more physiological setting in the future.
Addiction
mGluR1 as well as mGluR5 have been implicated in addiction and recent evidence suggests that this effect may be mediated via synaptic plasticity. On the other hand, direct and indirect modulation of synaptic plasticity by addictive drugs has received much attention, because there is an emerging consensus that addiction ultimately is a disease of goal-directed learning (Hyman, 2005; Redish et al., 2008). In brief, this model posits that drugs promote the learning of drug-related behaviors with such efficiency that they become compulsive. Excessive levels of dopamine in response to the exposure to an addictive drug would be permissive for a pathological form of synaptic plasticity of glutamatergic transmission. With normal rewards, the learning signal becomes quiescent (no dopamine release) once the behavior is predictive of the outcome, and addictive drugs override this mechanism (Redish, 2004). On the cellular level there is a myriad of studies that indicate that addictive drugs evoke long-term alterations of synaptic transmission in the mesolimbic dopaminergic system (Kauer and Malenka, 2007). Such pathologic plasticity has been observed in many parts of the brain, and starts with a LTP-like potentiation of excitatory synapses formed onto dopamine neurons in the midbrain hours after a single injection of cocaine (Ungless et al., 2001) or other addictive drugs (Saal et al., 2003). In vitro as well as in vivo studies now show that mGluR-LTD allows reversing the early synaptic effects of cocaine (Bellone and Lüscher, 2006; Mameli et al., 2007). It is important to realize that these initial changes certainly do not constitute the molecular mechanism of addiction, but only represent first steps in the induction of the disease. In fact, a recent study shows that the persistence of the cocaine-evoked synaptic potentiation in the VTA is required for subsequent synaptic adaptations in the NAc (Mameli et al., 2009). mGluR-LTD may therefore serve as a defense mechanism that limits synaptic adaptations to the VTA. In other words, when mGluR1-function is overruled by repeated exposure to cocaine, one observes much more persistent changes, first in the ventral striatum that may then be followed by adaptations in the dorsal striatum (Belin and Everitt, 2008) following established anatomical connections (Haber et al., 2000). In the VTA, mGluR1 needs to bind to Homer isoforms to induce LTD. When this protein-protein interaction is disrupted through the introduction of a TAT-conjugated dominant negative peptide (to allow for cell-membrane permeation) plasticity in response to a single injection of cocaine becomes persistent and drives synaptic adaptations in the NAc that normally require several injections.
Conversely, enhancing mGluR1-function with a positive modulator prevents synaptic adaptations in the NAc even when cocaine is given daily for a week (Knoflach et al., 2001; Mameli et al., 2009). Finally, preventing the induction of cocaine-evoked plasticity in dopamine neurons of the VTA by the conditional cell type specific removal of the NR1 subunit of the NMDA receptors normally required for its induction, leads to reduced reinstatement of conditioned place preference and attenuated cue-induced cocaine seeking (Engblom et al., 2008). While this implicates cocaine-evoked plasticity in the mesolimbic system, it remains to be shown whether mGluR-LTD will also reverse the behavioral changes. It is possible that once the information has been relayed to the NAc, reversal in the VTA becomes inefficient.
Using behavioral rodent models of the disease, several studies link mGluR-LTD in the NAc to core components of drug addiction such as locomotor sensitization, conditioned place preference or cue-induced reinstatement (for a recent review see (Goto and Grace, 2008)). As discussed above, mGluR-LTD in the NAc follows the scheme of postsynaptic induction - presynaptic expression (see above). Ex vivo studies on brain slices of rats that have been exposed to several injections of cocaine show a depression of AMPA-mediated transmission (Thomas et al., 2008) that is expressed by a reduction of receptors and occlusion of further mGluR-dependent LTD. This LTD via removal of AMPARs does probably not require mGluR activity in vivo, but the role of these receptors has not been directly investigated. Cocaine-evoked synaptic plasticity may therefore represents the cellular correlate of a metaplasticity, where cocaine exposure precludes subsequent induction of LTP and LTD in the NAc. Synaptic plasticity can be restored by in vivo-application of n-acetyl cysteine, an activator of the cysteine-glutamate exchanger. This effect relies on the increase of ambient glutamate levels, which then ensure basal mGluR5 activation required for LTD (Moussawi et al., 2009). Once LTD in the NAc is restored, rats are again able to self-administer cocaine. This model is supported by the observation that a blockade of mGluR5 inhibits cocaine-induced lever pressing, whereas a positive allosteric modulator of mGluR5 occluded the effects of n-acetyl cysteine. These recent findings in rats are in line with earlier observations in mGluR5 knockout mice which display reduced self-administration. Once these models have been investigated at the cellular level these interpretations will certainly need refinement.
While the idea of mGluR-LTD as an endogenous defense mechanism against drug-evoked plasticity is appealing, it is important to realize that other types of vulnerability have been postulated that may interfere downstream of the early changes in the VTA (Redish et al., 2008). Clearly additional tools will be required to probe its role. Conditional-inducible gene deletion may be of help. In addition, direct activation of G-protein coupled receptors in neurons of the NAc through optogenetic tools as reported recently has only scratched the surface (OptoXR, (Airan et al., 2009). In a first step viral vectors were used to transduce chimeric proteins made of the light sensitive rhodopsin and the cytoplastic portion of either a Gq or Gs coupled receptor in neurons of the NAc in vivo. The mice were then put in a chamber with two compartments, and OptoXRs were activated in only one of the two. Interestingly, Gq activation caused the strongest place preference, followed by Gs pathway activation, while increased excitability (transfection of ChR2) did not lead to any preference. These data suggest that activation of G-protein dependent signaling pathways rather than cellular excitability are required for the behavioral changes. Although not directly addressed in these studies, G-proteins mediate their effects most likely through synaptic plasticity such as the mGluR-LTD.
Role of Gp 1 mGluR-LTD in cerebellar and hippocampal learning and diseases of cognition
Computational and empirical evidence suggests that memories are encoded by a combination of synapse strengthening and weakening that create unique patterns of neuronal network activity to encode experience (Bear and Linden, 2001) (Kemp and Manahan-Vaughan, 2007). mGluR-LTD at the PF-PC synapse in the cerebellar cortex was first predicted to mediate cerebellar-dependent learning which culminated in the Marr-Albus-Ito theory of cerebellar learning. mGluR-LTD at this synapse is one of the best understood examples of how synaptic plasticity contributes to a learned behavior in the mammalian brain and has therefore been extensively reviewed (Jorntell and Hansel, 2006; Kano et al., 2008). In brief, as mentioned above, mGluR-LTD of PF-PC synapse occurs when PF inputs are coactive with the climbing fiber inputs (CF) onto PCs. CF activity relays the occurrence of an "error signal" during learning or adaptation of a reflex. The contribution of PF-PC LTD to learning is best understood in the context of the adaptation of the vestibular ocular reflex (VOR). Briefly, the VOR functions to maintain stability of a retinal image during head movement. The basic reflex consists of vestibular sensory afferents that activate vestibular nuclei and in turn, oculomotor neurons. Vestibular afferents also activate the mossy fiber inputs onto granule cells, which give rise to PF inputs on PCs. PCs provide inhibitory input to the vestibular nuclei and therefore can modify the VOR. Importantly, the CF inputs to PCs are activated during a retinal slip; that is when a stable retinal image is not maintained during head movement and thus constitutes an error signal. Therefore, an associative LTD mechanism weakens PF synapses that were active during the inappropriate vestibular signal (coded by CF activity) which in turn modifies the PC inhibitory output to vestibular nuclei and ultimately oculomotor output and eye movement to maintain a stable retinal image reviewed in (Jorntell and Hansel, 2006). There are other forms and sites of plasticity in the cerebellum that modify cerebellar circuitry during learning, but the wealth of evidence indicates that mGluR-LTD at the PF-PC synapse is crucial for cerebellar mediated learning (Jorntell and Hansel, 2006; Kano et al., 2008).
Hippocampal and perirhinal mGluR-dependent LTD: Role in encoding novelty?
Although much is known of the cellular mechanisms of hippocampal mGluR-LTD, little is known about the contribution of this form of plasticity to hippocampal-dependent learning. Antagonism or genetic deletion of mGluR5 impairs both acquisition and extinction of hippocampal dependent learning tasks, such as radial arm maze or Morris water maze (Lu et al., 1997; Manahan-Vaughan and Braunewell, 2005; Naie and Manahan-Vaughan, 2004; Xu et al., 2009). However, mGluR5 antagonism also impairs the late phase of LTP in both CA1 and dentate gyrus making the contribution of mGluR-LTD to hippocampal learning and memory unclear (Lu et al., 1997; Manahan-Vaughan and Braunewell, 2005; Naie and Manahan-Vaughan, 2004). A specific role for hippocampal LTD in encoding novelty has been suggested from work by Manahan-Vaughan and colleagues using chronic in vivo recordings in awake behaving rats. Exploration of novel objects placed in a hole board facilitates induction of LTD in both CA1 and dentate gyrus in response to baseline synaptic stimulation, whereas exploration of an empty holeboard facilitates LTP (Kemp and Manahan-Vaughan, 2004, 2007; Manahan-Vaughan and Braunewell, 1999). Interestingly, LTD can be specifically facilitated in either dentate gyrus or CA1 depending on the nature of the novel cues. CA1 LTD is enhanced by small novel features, whereas large novel orientational cues facilitate LTD in the dentate gyrus (Kemp and Manahan-Vaughan, 2008). From this work it has been suggested that LTD in CA1 contributes to encoding the spatial arrangement of novel objects. The current challenge for scientists in the field is to link what is known about the cellular mechanisms of mGluR-LTD from slice and culture preparations to experience dependent alterations in synaptic efficacy in the hippocampus and hippocampal learning. Gp 1 mGluRs and protein synthesis are required for stimulation-induced LTD in area CA1 and dentate gyrus in vivo and novelty exposure induces Arc transcription in CA1 neurons (Guzowski et al., 2006; Kelly and Deadwyler, 2002; Manahan-Vaughan, 1997; Manahan-Vaughan et al., 2000; Naie and Manahan-Vaughan, 2005; Ons et al., 2004). Future experiments are required to determine if mGluRs and Arc translation play a role in novelty mediated LTD or the encoding of novel object place.
In the perirhinal cortex, Bashir and colleagues have provided strong evidence for a role of Gq coupled receptor (mGluR and mAChR) dependent LTD and object recognition memory (Jo et al., 2006; Massey and Bashir, 2007; Massey et al., 2001; Warburton et al., 2003). Responses of perirhinal neurons respond strongly to novel visual stimuli, but more weakly when the same stimuli are presented later (within 24 hours), thus providing a mechanism to differentiate between a familiar or novel object (Brown and Aggleton, 2001; Brown and Xiang, 1998). LTD in the perirhinal cortex relies on group 1 mGluR, mAChRs and protein synthesis, and blockade of these Gq coupled receptors or AMPAR endocytosis in the perirhinal cortical region impairs object recognition memory (Griffiths et al., 2008; Jo et al., 2006; Massey and Bashir, 2007; Massey et al., 2001; Warburton et al., 2003).
Fragile X Syndrome Mental Retardation
A link between mGluR-LTD and cognitive disease was suggested by the finding that hippocampal and cerebellar mGluR-LTD are altered in a mouse model of mental retardation and autism, Fragile X Syndrome (FXS) which has lead to novel therapeutics for FXS acting at mGluR5. Fragile X Syndrome results from loss of function mutations in Fmr1, which encodes an RNA binding protein, Fragile X Mental retardation protein (FMRP) (Bassell and Warren, 2008). FMRP associates with dendritic mRNAs and RNA granules, as well as translating polyribosomes and is hypothesized to function as a translational regulator of dendritic mRNAs. Fmr1 mRNA is itself present in dendrites and was first shown to be translated in response to mGluR activation (Weiler et al., 1997) (reviewed in (Bassell and Warren, 2008)). Subsequently, many of the mRNAs that are translated in response to group 1 mGluRs also interact with FMRP, including Psd-95 (Todd et al., 2003), amyloid precursor protein (App) (Westmark and Malter, 2007), elongation factor 1a (Ef1a) (Huang et al., 2005), Map1b (Davidkova and Carroll, 2007; Hou et al., 2006), and Arc (Park et al., 2008; Waung et al., 2008). Consequently, FMRP may be a major regulator of mGluR-dependent protein synthesis and plasticity. Recent evidence supports a model whereby FMRP switches from a translational suppressor to an activator in response to mGluRs through either dephosphorylation or ubiquitination of FMRP or disassociation of FMRP from Cytoplasmic FMRP interacting protein (CYFIP1), a recently identified eIF4E binding protein (4EBP) (Hou et al., 2006; Napoli et al., 2008; Narayanan et al., 2007) (reviewed in (Bassell and Warren, 2008; Waung and Huber, 2009). According to this model, in the absence of FMRP, as in Fragile X Syndrome, there is a loss of steady state translational suppression which leads to increased protein levels of FMRP target mRNAs, as well as a deficit in mGluR-induced translation of these mRNAs for which there is experimental support (reviewed in (Bassell and Warren, 2008; Waung and Huber, 2009).
Although FMRP may acutely regulate mGluR-stimulated protein synthesis, mGluR-LTD in both the cerebellum and hippocampal CA1 is enhanced in Fmr1 KO mice. Enhanced mGluR-LTD at the PF-PC synapse is due to loss of FMRP in postsynaptic Purkinje neurons and is associated with deficits in eye-blink conditioning, a cerebellar mediated form of learning, in both Fmr1 KO mice and Fragile X patients (Koekkoek et al., 2005). These results suggest that excess PF-PC LTD can disrupt cerebellar-mediated learning. Work in hippocampal CA1 neurons has provided some insight into the cellular alterations that lead to enhanced LTD in Fragile X. In CA1, mGluR-LTD in the Fmr1 KO mice is associated with decreases in AMPAR surface expression and also requires Arc, similar to WT mice. However, mGluR-LTD and AMPAR surface decreases persist independently of new protein synthesis as well as upstream activators of protein synthesis (Hou et al., 2006; Nosyreva and Huber, 2006; Park et al., 2008; Ronesi and Huber, 2008b). Based on these results and the known molecular functions of FMRP, FMRP may translationally suppress mRNAs encoding proteins whose synthesis is necessary for the persistence of LTD and AMPAR surface decreases, such as Arc, MAP1b and STEP (Bassell and Warren, 2008; Ronesi and Huber, 2008b; Waung and Huber, 2009). Therefore, in the absence of FMRP mediated translational suppression, Arc, MAP1b or STEP proteins may be available or even enhanced in the dendrite. While mGluR activation and Arc are required to trigger AMPAR endocytosis and LTD in Fmr1 KO mice, the availability of “LTD proteins” may enhance the magnitude of LTD at KO synapses and relieve the requirement for de novo synthesis (Fig. 4). Understanding how Arc, MAP1b and STEP translation is regulated in dendrites of normal and Fmr1 KO neurons will help to better understand the cellular underpinnings of altered LTD in Fragile X.
FMRP also suppresses other forms of mGluR- and protein synthesis- dependent plasticity, such as that leading to long-term increases in neuronal excitability. In area CA3 of wildtype animals, co-application of GABAa blockers with the mGluR agonist, DHPG, induces a long-term and protein synthesis dependent increase in neuronal excitability, observed as prolonged bursts of action potentials of CA3 neurons that resemble interictal bursts in epilepsy (Merlin et al., 1998). Recent work suggests that one of the proteins that is synthesized and required for these epileptiform bursts, mediates a voltage-dependent cation current, termed ImGluR(V) (Bianchi et al., 2009) ImGluR(V) is induced in CA3 neurons with mGluR activation and the persistence of ImGluR(V) requires new protein synthesis (Bianchi et al., 2009). In Fmr1 KO mice the prolonged epileptiform bursts, as well as ImGluR(V), develop with GABAa blockade alone, but are blocked by the mGluR5 antagonist, MPEP (Bianchi et al., 2009; Chuang et al., 2005). Interestingly, unlike mGluR-LTD in Fmr1 KO mice, mGluR-induced bursts and ImGluR(V) in CA3 neurons are blocked by protein synthesis inhibitors. These results suggest that FMRP suppresses translation of proteins required for the generation of epileptiform bursts, such as those mediating ImGluR(V). Therefore strong activation of mGluR5 with an agonist is required in wildtype animals to generate the bursts. In the absence of FMRP, weaker, synaptic activation of mGluR5 is sufficient to trigger the epileptiform bursts and translation of proteins that lead to the prolonged epileptiform bursts (Chuang et al., 2005). The identification of the channels and/or interacting proteins that mediate ImGluR(V) is important to further develop this model. Enhanced mGluR-dependent epileptiform bursts in Fmr1 KO mice likely contribute to the audiogenic seizures observed in these mice as well as the epilepsy observed in some Fragile X patients. These findings suggest that FMRP may serve a general function to suppress translation of mRNAs that are normally translated in response to mGluR activation. Therefore, in the absence of FMRP, as in Fragile X Syndrome, there is a loss of translational suppression which leads to enhanced and dysregulated mGluR-dependent plasticity.
The observation of enhanced mGluR5-dependent plasticity in the Fragile X mouse model motivated the hypothesis that overactive mGluR5 function mediates many of the symptoms of Fragile X and mGluR5 antagonism may be a viable therapeutic strategy for the disease. Together, these postulates comprise the “mGluR-theory of Fragile X Syndrome” (Bear et al., 2004). Remarkably, data from animal models of Fragile X support the mGluR theory and have been recently reviewed (Bassell and Warren, 2008; Bear, 2005; Dolen and Bear, 2008). Briefly, mGluR5 antagonists or genetic reduction of mGluR5 (mice that are heterozygous for mGluR5) reverse multiple phenotypes in the Fmr1 KO mice, including audiogenic seizures, consistent with observations of enhanced mGluR-induced epileptiform bursts in slices of hippocampal CA3 (Dolen et al., 2007; Yan et al., 2005). Genetic reduction of mGluR5 reverses other phenotypes in Fmr1 KO mice such as deficits in experience-dependent plasticity in the visual cortex, increased dendritic spine density and hippocampal dependent learning. In support of a role of mGluR-LTD in hippocampal extinction learning, Fmr1 KO mice display accelerated extinction of a hippocampal-dependent, inhibitory avoidance memory, and this is normalized by genetic reduction of mGluR5 (Dolen et al., 2007). In the Fragile X Syndrome fly model, (dFXR null fly) treatment of adults with antagonists of mGluRs or the mGluR signaling pathway (i.e. Lithium) reverse cognitive or behavioral deficits in the fly model (McBride et al., 2005). The remarkable effectiveness of mGluR antagonism in the animal models of Fragile X Syndrome has motivated trial studies in human Fragile X patients using Lithium and fenobam, an mGluR5 antagonist, with encouraging results (Berry-Kravis et al., 2009; Berry-Kravis et al., 2008; Hagerman et al., 2009).
Alzheimer’s disease
Alzheimer’s disease is a progressive neurodegenerative disease that is associated with early learning and memory impairments and ultimately other higher cognitive functions. Evidence indicates that altered synaptic structure, function and plasticity precede the neurodegeneration and contribute to the early learning and memory deficits. Alzheimer’s disease is linked with mutations in amyloid precursor protein (APP) that result in abnormal proteolysis and cleavage which generates amyloid β protein (Aβ) that ultimately aggregate into the hallmark senile plaques (Selkoe and Schenk, 2003). Although Aβ aggregates (i.e. insoluble Aβ) is ultimately observed in the brains of Alzheimer’s patients, it has become evident that cognitive and memory impairments precede the occurrence of amyloid plaques. Therefore, researchers turned to investigating the role of soluble Aβ peptides on neuronal function and structure (reviewed in (Selkoe, 2008). Accumulating evidence indicates that soluble Aβ oligomers lead to a depression synaptic transmission through an LTD-like mechanism. Exogenous application of soluble Aβ peptides from either human patients or cell lines inhibits LTP as well as enhances LTD induction (Li et al., 2009; Shankar et al., 2008). Exogenous expression of Aβ in neurons for several days leads to a removal of AMPARs that mimics and occludes mGluR-dependent LTD (Hsieh et al., 2006; Kamenetz et al., 2003). Recent work finds that Aβ facilitates both mGluR and NMDAR-dependent LTD and does so, in part, through inhibition of glutamate transporters (Li et al., 2009). These results suggest that Aβ mediated inhibition of glutamate uptake leads to an accumulation of extracellular glutamate levels and activation of mGluRs and NMDARs to levels that result in AMPAR endocytosis and LTD and inhibition of LTP. The depressive effect of Aβ on synaptic transmission is thought to ultimately lead to loss of structural and functional synapses or spines. Consistent with this interpretation, both NMDAR- and mGluR-dependent LTD are associated with shrinkage and/or loss of dendritic spines and Aβ peptides cause spine loss that relies and NMDARs and pathways implicated in NMDAR-LTD (Kamikubo et al., 2006; Nagerl et al., 2004; Shankar et al., 2008; Zhou et al., 2004). The loss of functional synapses together with the inhibition of LTP and occlusion of LTD, may underlie the early learning and memory deficits in Alzheimer’s disease. Whether APP or its cleavage products contribute to LTD under normal physiological conditions is not known. Clues to this issue come from Westmark and Malter (2007) who discovered that APP mRNA interacts with FMRP and is rapidly translated in response to mGluR activation. Interestingly, APP levels are elevated in synaptoneurosomes of Fmr1 KO mice and may contribute to the altered mGluR-LTD and cognitive deficits in Fragile X Syndrome (Westmark and Malter, 2007).
Concluding Remarks
In the last decade the development of specific pharmacological tools and transgenic mice have advanced our understanding of mGluR1 and mGluR5 function in the central nervous system. Here we focused on their function in mGluR-LTD, which plays a role in learning and cognitive function, as well as in several neurological and neuropsychiatric diseases. As highlighted here, the discovery of mGluR-LTD in the striatum has revealed the synaptic mechanisms underlying goal-directed learning and has helped to understand how addictive drugs alter the striatal circuitry, ultimately hijacking behavior. Unraveling the novel cellular mechanisms of mGluR-dependent plasticity in the hippocampus has revealed unexpected links with diseases of cognition such as Fragile X Syndrome which, in turn, has lead to very exciting and encouraging therapeutic strategies for this disease.
Many questions remain concerning how mGluRs and the resulting synaptic plasticity contribute to complex behaviors and diseases of the brain. For example, what is the specific role of mGluR-LTD in hippocampal dependent learning and does its altered regulation in Fragile X Syndrome contribute to the cognitive deficits? Is there altered mGluR function and plasticity in other related diseases of cognition such as autism? In regard to addiction; does individual variability of mGluR1 function exist in humans that may explain individual vulnerability for the disease? To address these and many other questions, we have an arsenal of approaches at hand that combines classical pharmacology with sophisticated third generation genetic manipulations in mice and novel optogenetic tools to manipulate neuronal circuits and to control signaling pathways in a cell type-specific manner. Put at work properly, researchers in the field are in for unprecedented insights into mGluR-LTD function (and certainly some surprises) in the not too distant future.
Acknowledgements
K.M.H. is supported by grants from the NIH (NS045711, HD052731), Autism Speaks and Simons Foundation. C.L. is supported by grants from the Swiss National Science Foundation and the Swiss initiative in system biology (SystemsX: neurochoice). K.M.H. is a shareholder and paid SAB member of Seaside Therapeutics. We would like to thank Carlos Gonzalez for assistance with the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christian Lüscher, Department of Basic Neurosciences, Medical Faculty, and Department of Clinical Neurosciences, Geneva University Hospital, Geneva, Switzerland.
Kimberly M. Huber, Department of Neuroscience, University of Texas Southwestern Medical Center, Dallas, Texas, USA
References
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- Antion MD, Hou L, Wong H, Hoeffer CA, Klann E. mGluR-dependent long-term depression is associated with increased phosphorylation of S6 and synthesis of elongation factor 1A but remains expressed in S6K–deficient mice. Mol Cell Biol. 2008;28:2996–3007. doi: 10.1128/MCB.00201-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Banko JL, Hou L, Poulin F, Sonenberg N, Klann E. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2006;26:2167–2173. doi: 10.1523/JNEUROSCI.5196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastrikova N, Gardner GA, Reece JM, Jeromin A, Dudek SM. Synapse elimination accompanies functional plasticity in hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105:3123–3127. doi: 10.1073/pnas.0800027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF. Therapeutic implications of the mGluR theory of fragile X mental retardation. Genes Brain Behav. 2005;4:393–398. doi: 10.1111/j.1601-183X.2005.00135.x. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bear MF, Linden DJ. In: The mechanisms and meaning of long-term synaptic depression in the mammalian brain. Synapses WM, Cowan WM, Sudhof TC, Stevens CF, editors. Baltimore: Johns Hopkins University Press; 2001. pp. 455–517. [Google Scholar]
- Becker N, Wierenga CJ, Fonseca R, Bonhoeffer T, Nagerl UV. LTD induction causes morphological changes of presynaptic boutons and reduces their contacts with spines. Neuron. 2008;60:590–597. doi: 10.1016/j.neuron.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Bellone C, Lüscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci. 2005;21:1280–1288. doi: 10.1111/j.1460-9568.2005.03979.x. [DOI] [PubMed] [Google Scholar]
- Bellone C, Lüscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Bellone C, Lüscher C, Mameli M. Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cell Mol Life Sci. 2008;65:2913–2923. doi: 10.1007/s00018-008-8263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, Hutchison J, Snape M, Tranfaglia M, Nguyen DV, Hagerman R. A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J Med Genet. 2009;46:266–271. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E, Sumis A, Hervey C, Nelson M, Porges SW, Weng N, Weiler IJ, Greenough WT. Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. J Dev Behav Pediatr. 2008;29:293–302. doi: 10.1097/DBP.0b013e31817dc447. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Chuang SC, Zhao W, Young SR, Wong RK. Cellular plasticity for group I mGluR-mediated epileptogenesis. J Neurosci. 2009;29:3497–3507. doi: 10.1523/JNEUROSCI.5447-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black RA. Tumor necrosis factor-alpha converting enzyme. Int J Biochem Cell Biol. 2002;34:1–5. doi: 10.1016/s1357-2725(01)00097-8. [DOI] [PubMed] [Google Scholar]
- Blobel CP. Remarkable roles of proteolysis on and beyond the cell surface. Curr Opin Cell Biol. 2000;12:606–612. doi: 10.1016/s0955-0674(00)00139-3. [DOI] [PubMed] [Google Scholar]
- Bolshakov VY, Siegelbaum SA. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science. 1994;264:1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Brown MW, Xiang JZ. Recognition memory: neuronal substrates of the judgement of prior occurrence. Prog Neurobiol. 1998;55:149–189. doi: 10.1016/s0301-0082(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Ascone CM, Centonze D, Pisani A, Sancesario G, D'Angelo V, Bernardi G. Opposite membrane potential changes induced by glucose deprivation in striatal spiny neurons and in large aspiny interneurons. J Neurosci. 1997;17:1940–1949. doi: 10.1523/JNEUROSCI.17-06-01940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RW, Park JM, Wolff SB, Xu D, Hopf C, Kim JA, Reddy RC, Petralia RS, Perin MS, Linden DJ, Worley PF. mGluR1/5-dependent long-term depression requires the regulated ectodomain cleavage of neuronal pentraxin NPR by TACE. Neuron. 2008;57:858–871. doi: 10.1016/j.neuron.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Decreased frequency but not amplitude of quantal synaptic responses associated with expression of corticostriatal long-term depression. J Neurosci. 1997;17:8613–8620. doi: 10.1523/JNEUROSCI.17-21-08613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SC, Zhao W, Bauchwitz R, Yan Q, Bianchi R, Wong RK. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. J Neurosci. 2005;25:8048–8055. doi: 10.1523/JNEUROSCI.1777-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science. 2003;300:1751–1755. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- Cole AE, Nicoll RA. Acetylcholine mediates a slow synaptic potential in hippocampal pyramidal cells. Science. 1983;221:1299–1301. doi: 10.1126/science.6612345. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidkova G, Carroll RC. Characterization of the role of microtubule-associated protein 1B in metabotropic glutamate receptor-mediated endocytosis of AMPA receptors in hippocampus. J Neurosci. 2007;27:13273–13278. doi: 10.1523/JNEUROSCI.3334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol. 2008;586:1503–1508. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of Fragile X Syndrome in Mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, Parkitna JR, Lujan R, Halbout B, Mameli M, et al. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Kingston AE, Lodge D, Collingridge GL. DHPG-induced LTD in area CA1 of juvenile rat hippocampus; characterisation and sensitivity to novel mGlu receptor antagonists. Neuropharmacology. 1999;38:1577–1583. doi: 10.1016/s0028-3908(99)00123-9. [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Palmer MJ, May JE, Neeson A, Morris SA, Collingridge GL. A characterisation of long-term depression induced by metabotropic glutamate receptor activation in the rat hippocampus in vitro. J Physiol. 2001;537:421–430. doi: 10.1111/j.1469-7793.2001.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher SM, Daly CA, Bear MF, Huber KM. Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J Neurosci. 2004;24:4859–4864. doi: 10.1523/JNEUROSCI.5407-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Indirect-pathway neurons lose their spines in Parkinson disease. Nat Neurosci. 2006;9:157–158. doi: 10.1038/nn0206-157. [DOI] [PubMed] [Google Scholar]
- Gladding CM, Fitzjohn SM, Molnar E. Metabotropic Glutamate Receptor-Mediated Long-Term Depression: Molecular Mechanisms. Pharmacol Rev. 2009 doi: 10.1124/pr.109.001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci. 2008;31:552–558. doi: 10.1016/j.tins.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths S, Scott H, Glover C, Bienemann A, Ghorbel MT, Uney J, Brown MW, Warburton EC, Bashir ZI. Expression of long-term depression underlies visual recognition memory. Neuron. 2008;58:186–194. doi: 10.1016/j.neuron.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Gosnell HB, Olsen CM, Schramm-Sapyta NL, Nekrasova T, Landreth GE, Winder DG. Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J Neurosci. 2006;26:3210–3219. doi: 10.1523/JNEUROSCI.0170-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubellini P, Saulle E, Centonze D, Bonsi P, Pisani A, Bernardi G, Conquet F, Calabresi P. Selective involvement of mGlu1 receptors in corticostriatal LTD. Neuropharmacology. 2001;40:839–846. doi: 10.1016/s0028-3908(01)00021-1. [DOI] [PubMed] [Google Scholar]
- Gutlerner JL, Penick EC, Snyder EM, Kauer JA. Novel protein kinase a-dependent long-term depression of excitatory synapses. Neuron. 2002;36:921–931. doi: 10.1016/s0896-6273(02)01051-6. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Miyashita T, Chawla MK, Sanderson J, Maes LI, Houston FP, Lipa P, McNaughton BL, Worley PF, Barnes CA. Recent behavioral history modifies coupling between cell activity and Arc gene transcription in hippocampal CA1 neurons. Proc Natl Acad Sci U S A. 2006;103:1077–1082. doi: 10.1073/pnas.0505519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Kronk R, Delahunty C, Hessl D, Visootsak J, et al. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123:378–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. J Neurosci. 2000;20:8987–8995. doi: 10.1523/JNEUROSCI.20-24-08987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbro N, Grunditz A, Oertner TG. Differential distribution of endoplasmic reticulum controls metabotropic signaling and plasticity at hippocampal synapses. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0905110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Hou L, Klann E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2004;24:6352–6361. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Chotiner JK, Steward O. The mRNA for elongation factor 1alpha is localized in dendrites and translated in response to treatments that induce long-term depression. J Neurosci. 2005;25:7199–7209. doi: 10.1523/JNEUROSCI.1779-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent LTD. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Ito-Ishida A, Kakegawa W, Yuzaki M. ERK1/2 but not p38 MAP kinase is essential for the long-term depression in mouse cerebellar slices. Eur J Neurosci. 2006;24:1617–1622. doi: 10.1111/j.1460-9568.2006.05055.x. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar control of the vestibulo-ocular reflex--around the flocculus hypothesis. Annu Rev Neurosci. 1982;5:275–296. doi: 10.1146/annurev.ne.05.030182.001423. [DOI] [PubMed] [Google Scholar]
- Jo J, Ball SM, Seok H, Oh SB, Massey PV, Molnar E, Bashir ZI, Cho K. Experience-dependent modification of mechanisms of long-term depression. Nat Neurosci. 2006;9:170–172. doi: 10.1038/nn1637. [DOI] [PubMed] [Google Scholar]
- Jorntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron. 2006;52:227–238. doi: 10.1016/j.neuron.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Kamikubo Y, Egashira Y, Tanaka T, Shinoda Y, Tominaga-Yoshino K, Ogura A. Long-lasting synaptic loss after repeated induction of LTD: independence to the means of LTD induction. Eur J Neurosci. 2006;24:1606–1616. doi: 10.1111/j.1460-9568.2006.05032.x. [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Tabata T. Type-1 metabotropic glutamate receptor in cerebellar Purkinje cells: a key molecule responsible for long-term depression, endocannabinoid signalling and synapse elimination. Philos Trans R Soc Lond B Biol Sci. 2008;363:2173–2186. doi: 10.1098/rstb.2008.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karachot L, Shirai Y, Vigot R, Yamamori T, Ito M. Induction of long-term depression in cerebellar Purkinje cells requires a rapidly turned over protein. J Neurophysiol. 2001;86:280–289. doi: 10.1152/jn.2001.86.1.280. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kelly MP, Deadwyler SA. Acquisition of a novel behavior induces higher levels of Arc mRNA than does overtrained performance. Neuroscience. 2002;110:617–626. doi: 10.1016/s0306-4522(01)00605-4. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci U S A. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb Cortex. 2008;18:968–977. doi: 10.1093/cercor/bhm136. [DOI] [PubMed] [Google Scholar]
- Kemp N, Bashir ZI. Induction of LTD in the adult hippocampus by the synaptic activation of AMPA/kainate and metabotropic glutamate receptors. Neuropharmacology. 1999;38:495–504. doi: 10.1016/s0028-3908(98)00222-6. [DOI] [PubMed] [Google Scholar]
- Kleppisch T, Voigt V, Allmann R, Offermanns S. G(alpha)q-deficient mice lack metabotropic glutamate receptor-dependent long-term depression but show normal long-term potentiation in the hippocampal CA1 region. J Neurosci. 2001;21:4943–4948. doi: 10.1523/JNEUROSCI.21-14-04943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoflach F, Mutel V, Jolidon S, Kew JN, Malherbe P, Vieira E, Wichmann J, Kemp JA. Positive allosteric modulators of metabotropic glutamate 1 receptor: characterization, mechanism of action, and binding site. Proc Natl Acad Sci U S A. 2001;98:13402–13407. doi: 10.1073/pnas.231358298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koekkoek SK, Yamaguchi K, Milojkovic BA, Dortland BR, Ruigrok TJ, Maex R, De Graaf W, Smit AE, VanderWerf F, Bakker CE, et al. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron. 2005;47:339–352. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Foster TC. Shift in induction mechanisms underlies an age-dependent increase in DHPG-induced synaptic depression at CA3 CA1 synapses. J Neurophysiol. 2007;98:2729–2736. doi: 10.1152/jn.00514.2007. [DOI] [PubMed] [Google Scholar]
- Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci U S A. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Mameli M, Balland B, Lujan R, Lüscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Lüscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Group 1 and 2 metabotropic glutamate receptors play differential roles in hippocampal long-term depression and long-term potentiation in freely moving rats. J Neurosci. 1997;17:3303–3311. doi: 10.1523/JNEUROSCI.17-09-03303.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci U S A. 1999;96:8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. The metabotropic glutamate receptor, mGluR5, is a key determinant of good and bad spatial learning performance and hippocampal synaptic plasticity. Cereb Cortex. 2005;15:1703–1713. doi: 10.1093/cercor/bhi047. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Kulla A, Frey JU. Requirement of translation but not transcription for the maintenance of long-term depression in the CA1 region of freely moving rats. J Neurosci. 2000;20:8572–8576. doi: 10.1523/JNEUROSCI.20-22-08572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey PV, Bashir ZI. Long-term depression: multiple forms and implications for brain function. Trends Neurosci. 2007;30:176–184. doi: 10.1016/j.tins.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Massey PV, Bhabra G, Cho K, Brown MW, Bashir ZI. Activation of muscarinic receptors induces protein synthesis-dependent long-lasting depression in the perirhinal cortex. Eur J Neurosci. 2001;14:145–152. doi: 10.1046/j.0953-816x.2001.01631.x. [DOI] [PubMed] [Google Scholar]
- McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, Sehgal A, Siwicki KK, Dockendorff TC, Nguyen HT, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Merlin LR, Bergold PJ, Wong RK. Requirement of protein synthesis for group I mGluR-mediated induction of epileptiform discharges. J Neurophysiol. 1998;80:989–993. doi: 10.1152/jn.1998.80.2.989. [DOI] [PubMed] [Google Scholar]
- Moult PR, Correa SA, Collingridge GL, Fitzjohn SM, Bashir ZI. Co-activation of p38 mitogen-activated protein kinase and protein tyrosine phosphatase underlies metabotropic glutamate receptor-dependent long-term depression. J Physiol. 2008;586:2499–2510. doi: 10.1113/jphysiol.2008.153122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moult PR, Gladding CM, Sanderson TM, Fitzjohn SM, Bashir ZI, Molnar E, Collingridge GL. Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptor-mediated long-term depression. J Neurosci. 2006;26:2544–2554. doi: 10.1523/JNEUROSCI.4322-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moult PR, Schnabel R, Kilpatrick IC, Bashir ZI, Collingridge GL. Tyrosine dephosphorylation underlies DHPG-induced LTD. Neuropharmacology. 2002;43:175–180. doi: 10.1016/s0028-3908(02)00110-7. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Naie K, Manahan-Vaughan D. Regulation by metabotropic glutamate receptor 5 of LTP in the dentate gyrus of freely moving rats: relevance for learning and memory formation. Cereb Cortex. 2004;14:189–198. doi: 10.1093/cercor/bhg118. [DOI] [PubMed] [Google Scholar]
- Naie K, Manahan-Vaughan D. Investigations of the protein synthesis dependency of mGluR-induced long-term depression in the dentate gyrus of freely moving rats. Neuropharmacology. 2005;(49 Suppl 1):35–44. doi: 10.1016/j.neuropharm.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Napoli I, Mercaldo V, Boyl PP, Eleuteri B, Zalfa F, De Rubeis S, Di Marino D, Mohr E, Massimi M, Falconi M, et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E–BP. Cell. 2008;134:1042–1054. doi: 10.1016/j.cell.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Narayanan U, Nalavadi V, Nakamoto M, Pallas DC, Ceman S, Bassell GJ, Warren ST. FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGluR and mediated by PP2A. J Neurosci. 2007;27:14349–14357. doi: 10.1523/JNEUROSCI.2969-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Developmental switch in synaptic mechanisms of hippocampal metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2005;25:2992–3001. doi: 10.1523/JNEUROSCI.3652-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile x syndrome. J Neurophysiol. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- Ons S, Marti O, Armario A. Stress-induced activation of the immediate early gene Arc (activity-regulated cytoskeleton-associated protein) is restricted to telencephalic areas in the rat brain: relationship to c-fos mRNA. J Neurochem. 2004;89:1111–1118. doi: 10.1111/j.1471-4159.2004.02396.x. [DOI] [PubMed] [Google Scholar]
- Palmer MJ, Irving AJ, Seabrook GR, Jane DE, Collingridge GL. The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology. 1997;36:1517–1532. doi: 10.1016/s0028-3908(97)00181-0. [DOI] [PubMed] [Google Scholar]
- Pan B, Hillard CJ, Liu QS. Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. J Neurosci. 2008;28:1385–1397. doi: 10.1523/JNEUROSCI.4033-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD. Addiction as a computational process gone awry. Science. 2004;306:1944–1947. doi: 10.1126/science.1102384. [DOI] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A. A unified framework for addiction: vulnerabilities in the decision process. Behav Brain Sci. 2008;31:415–437. doi: 10.1017/S0140525X0800472X. discussion 437–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O'Malley K, Van d.P.A, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci. 2008a;28:543–547. doi: 10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM. Metabotropic glutamate receptors and fragile x mental retardation protein: partners in translational regulation at the synapse. Sci Signal. 2008b;1:pe6. doi: 10.1126/stke.15pe6. [DOI] [PubMed] [Google Scholar]
- Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, Tu J, Worley PF, Snyder SH, Ye K. PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci. 2003;6:1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Schnabel R, Kilpatrick IC, Collingridge GL. An investigation into signal transduction mechanisms involved in DHPG-induced LTD in the CA1 region of the hippocampus. Neuropharmacology. 1999;38:1585–1596. doi: 10.1016/s0028-3908(99)00062-3. [DOI] [PubMed] [Google Scholar]
- Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Sudhof TC. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, Schenk D. Alzheimer's disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43:545–584. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- Seog DH. Glutamate receptor-interacting protein 1 protein binds to the microtubule-associated protein. Biosci Biotechnol Biochem. 2004;68:1808–1810. doi: 10.1271/bbb.68.1808. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol. 1992;322:121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- Shinoe T, Matsui M, Taketo MM, Manabe T. Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J Neurosci. 2005;25:11194–11200. doi: 10.1523/JNEUROSCI.2338-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla S, Kreitzer AC, Malenka RC. Mechanisms for synapse specificity during striatal long-term depression. J Neurosci. 2007;27:5260–5264. doi: 10.1523/JNEUROSCI.0018-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Steinberg JP, Huganir RL, Linden DJ. N-ethylmaleimide-sensitive factor is required for the synaptic incorporation and removal of AMPA receptors during cerebellar long-term depression. Proc Natl Acad Sci U S A. 2004;101:18212–18216. doi: 10.1073/pnas.0408278102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg JP, Takamiya K, Shen Y, Xia J, Rubio ME, Yu S, Jin W, Thomas GM, Linden DJ, Huganir RL. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron. 2006;49:845–860. doi: 10.1016/j.neuron.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd PK, Mack KJ, Malter JS. The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95. Proc Natl Acad Sci U S A. 2003;100:14374–14378. doi: 10.1073/pnas.2336265100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Volk LJ, Daly CA, Huber KM. Differential roles for group 1 mGluR subtypes in induction and expression of chemically induced hippocampal long-term depression. J Neurophysiol. 2006;95:2427–2438. doi: 10.1152/jn.00383.2005. [DOI] [PubMed] [Google Scholar]
- Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J Neurosci. 2007;27:11624–11634. doi: 10.1523/JNEUROSCI.2266-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25:635–647. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Koder T, Cho K, Massey PV, Duguid G, Barker GR, Aggleton JP, Bashir ZI, Brown MW. Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron. 2003;38:987–996. doi: 10.1016/s0896-6273(03)00358-1. [DOI] [PubMed] [Google Scholar]
- Waung MW, Huber KM. Protein translation in synaptic plasticity: mGluR-LTD, Fragile X. Curr Opin Neurobiol. 2009;19:319–326. doi: 10.1016/j.conb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K, Comery TA, Patel B, Eberwine J, Greenough WT. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci U S A. 1997;94:5395–5400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 2007;5:pe52. doi: 10.1371/journal.pbio.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RK, Chuang SC, Bianchi R. Plasticity mechanisms underlying mGluR-induced epileptogenesis. Adv Exp Med Biol. 2004;548:69–75. doi: 10.1007/978-1-4757-6376-8_5. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J Neurosci. 2009;29:3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Yin HH, Davis MI, Ronesi JA, Lovinger DM. The role of protein synthesis in striatal long-term depression. J Neurosci. 2006;26:11811–11820. doi: 10.1523/JNEUROSCI.3196-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Venkitaramani DV, Gladding CM, Kurup P, Molnar E, Collingridge GL, Lombroso PJ. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28:10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]