Abstract
Transepidermal inoculation of vaccinia virus (VV), or scarification, has been used effectively for the induction of specific and long-lasting immunity to smallpox and is superior to other routes of immunization. Scarification of individuals with atopic skin disease or immune deficiency, however, can lead to persistent viral replication and cause significant morbidity and mortality. These effects of scarification presumably reflect the unique immunologic properties of skin and the immune cells resident in, or recruited to, the site of inoculation. To explore these phenomena, we utilized transgenic mice engineered to over-express interleukin-1α, a critical mediator of cutaneous inflammation, in the epidermis. Following scarification with vaccinia virus, both transgenic and wild-type mice develop local pox. At high doses of vaccinia virus, IL-1α transgenic mice recruited immune cells to the inoculation site more rapidly and demonstrated enhanced T-cell and humoral immune responses. At limiting doses, however, IL-1α transgenic mice could effectively control virus replication without formation of pox lesions or activation of a memory response. This study suggests IL-1 may have use as an adjuvant to enhance antiviral immunity and promote safer vaccination strategies; however, understanding the balance of IL-1 effects on innate and adaptive immune functions will be critical to achieve optimal results.
Introduction
Smallpox, caused by variola virus, can be prevented by immunization with vaccinia virus (VV). Although most immunizations are delivered by subcutaneous or intramuscular injection, effective smallpox vaccination requires inoculation of live vaccinia virus into the skin- a process termed scarification - and the production of an epidermal pox reaction. Although vaccinia virus scarification in a normal host results in specific and long-lasting immunity, in atopic and immunodeficient patients viral replication can outstrip immune control, leading to devastating morbidity and mortality (Bray, 2003). Previous studies have shown that the balance and interaction of innate and acquired immune defense mechanisms at the cutaneous interface are crucial elements which determine the speed and character of the immune response (Kupper and Fuhlbrigge, 2004). Based on these observations, we hypothesize that the outcome of scarification is dependent on the state of immune-response elements at the site of inoculation and that manipulation of this environment can alter the immune response to viral challenge.
IL-1 is a pleiotropic cytokine and a primary mediator of cutaneous inflammation, serving as an important link between innate and acquired immune responses (Murphy et al., 2000). IL-1 activity is mediated by two homologous cytokines, IL-1α and IL-1β, that elicit essentially indistinguishable responses mediated by the signal adaptor protein MyD88. The agonist effects of IL-1α and IL-1β are balanced by co-expression in all tissues of IL-1 receptor antagonist (IL-1ra). Skin antigen presenting cells (APC: monocytes, macrophages, Langerhans cells, and dendritic cells), produce primarily IL-1β. Unstimulated keratinocytes, in contrast, contain large amounts of preformed and biologically active IL-1α, as well as significant quantities of immature IL-1β. Furthermore, keratinocytes also synthesize IL-1ra and express the two forms of IL-1 receptor (IL-1R1 and IL-1 R2). The epidermis, therefore, is unique in that all elements of the IL-1 axis are represented. Alterations of this complex regulatory network are associated with profound inflammatory disease (Dinarello, 1996).
Previously published studies have explored the biological consequences of disrupting the natural balance of the IL-1 network in skin by creating transgenic (Tg) mouse strains that overexpress IL-1 agonists or antagonists under control of the human keratin 14 (K14) promoter, which directs expression to the basal layer of the epidermis (Groves et al., 1995; Groves et al., 1996; Rauschmayr et al., 1997; Vassar et al., 1989). In this study, we have used K14/IL-1α transgenic mice to examine the influence of increased epidermal IL-1α on the immune response to vaccinia skin scarification.
Results
T cell immune response is enhanced and skewed toward Th1 in K14/IL-1α mice scarified with vaccinia virus
K14/IL-1α and WT mice were inoculated by scarification at the base of the tail with 2 × 106 PFU of WR-GFP virus and monitored by daily inspection. The vaccination site became erythematous by day 3 and developed a pustule near the end of the first week in both WT and transgenic mice. During the second week, pustules progressed to scab lesions, which fell off in the third week, leaving a small scar. The visual appearance and time course of the pox lesions were very similar in these two strains, as well as the C57BL/6 strain (Liu et al., 2006) and closely resembles local reactions of humans after scarification. None of these mice showed secondary pox lesions. Inguinal lymph node (ILN) and spleen T cells harvested from K14/IL-1α and WT mice and stimulated with vaccinia virus-infected APC showed no induction of IFNγ or IL-2 production at day 3 after scarification. By day 7 however, both IFNγ and IL-2 production were increased, with a substantial enhancement noted in K14/IL-1α mice compared with WT control mice (Figure 1A). On average, ILN T cells from K14/IL-1α mice produced three-fold more IL-2 (995.8 ± 40.4 pg/ml vs. 277.5 ± 4.3 pg/ml) and eight-fold more IFNγ (28816.7 ± 6840.5 pg/ml vs. 3273.3 ±80.8pg/ml) than WT mice on day 7 post-scarification. Splenic T cells from K14/IL-1α mice also showed increased cytokine production, but the differences were less dramatic. T-cell immune responses subsequently declined rapidly, with no significant difference in cytokine production between the two strains seen by 2 weeks after immunization. Four weeks after immunization, ILN T cells from both strains produced only trace amounts of cytokines when stimulated with vaccinia virus-infected APC, while splenic T cells still generated significant amounts of Th1-type cytokines. No significant increase in IL-4 or IL-10 production was detected from ILN or splenic T cells from either strain during the course of these experiments (data not shown).
Figure 1. K14/IL-1α mice develop stronger Th1 immune response following vaccinia scarification compared with WT mice.
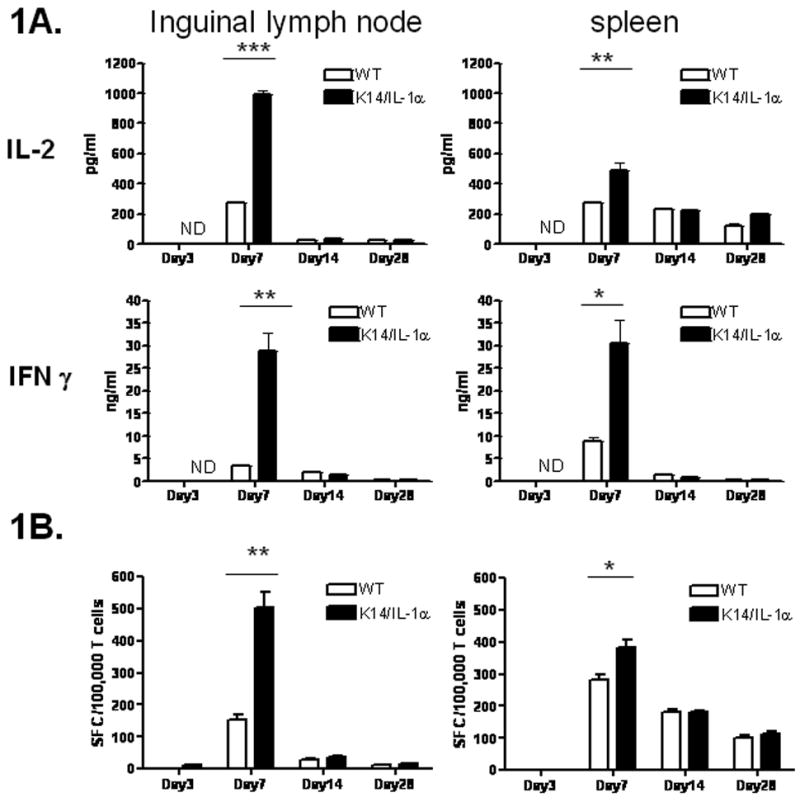
K14/IL-1α and WT mice were skin scarified at the base of their tails with 2 × 106 PFU of WR-GFP vaccinia virus. At indicated time points after immunization, purified inguinal LN or splenic T cells were cultured in vitro with infected syngeneic (APC). A. IL-2 and IFN-γ in the culture supernatant measured after 40 h. Bars represent means ± SE and are representative of four independent experiments. B. Frequency of vaccinia-specific T cells measured by IFN-γ ELISPOT assay. Data are expressed as spot-forming cells (SFC) per 106 T cells. Bars represent means ± SE and are representative of two independent experiments. * = p < 0.05, ** = p < 0.01, *** = p < 0.001, ND = not detected.
We further evaluated T-cell immunity by enumerating vaccinia virus-specific T cells in spleen and ILN following scarification. T cells were isolated on days 3, 7, 14, and 28 after scarification, and the frequency of T cells responding to vaccinia virus- infected APC was measured by IFNγ ELISPOT assay (Figure 1B). Consistent with the ELISA analysis of cytokine production, the number of vaccinia virus-specific IFNγ-producing T cells 7 days after immunization increased significantly in the ILN of K14/IL-1α mice relative to WT mice (K14/IL-1α 502 ± 85 vs. WT 154 ± 27) as well as in the spleens, although, again, the response at 7 days in spleen was not as striking as in ILN. The number of vaccinia virus-reactive T cells in the ILN was greatly reduced at week 2 in both strains, and very few IFNγ-producing T cells could be detected in the ILN at 4 weeks after vaccinia immunization. Although the number of vaccinia virus-specific T cells in the spleen also decreased at week 2, significant numbers of IFNγ-producing T cells were still present 4 weeks after immunization in both K14/IL-1α and WT mice. To summarize, both K14/IL-1α and WT mice demonstrated a Th1-dominated immune response following vaccinia virus scarification, with K14/IL-1α mice producing significantly greater numbers of IFNγ-producing T cells and more Th1 cytokines than WT control mice.
Production of antibody to vaccinia virus is enhanced in K14/IL-1α mice
To assess the humoral immune response of K14/IL-1α and WT mice to vaccinia virus, serum was collected weekly from day 0 to week 4 and then biweekly from week 6 to week 12 following scarification. Titer of vaccinia virus-specific antibody in individual mice was determined by ELISA. All mice scarified with vaccinia virus developed vaccinia virus-specific IgG after 2 weeks. Antibody responses reached their peak at about week 6 and remained stable for the next 6 weeks. K14/IL-1α mice produced vaccinia-specific IgG earlier and at higher titer in comparison with WT mice. Measurement of the distribution of IgG subclasses was used as a surrogate for the immunologic bias of the Th1 versus Th2 response. Although both vaccinia-specific IgG2a (Th1-associated) and IgG1 (Th2-associated) were produced after vaccinia scarification, the relatively higher expression of IgG2a for all mice indicates a bias toward a Th1 cellular response in response to vaccinia virus inoculation (Figure 2).
Figure 2. Kinetics of vaccinia-specific antibody response following scarification with vaccinia virus.
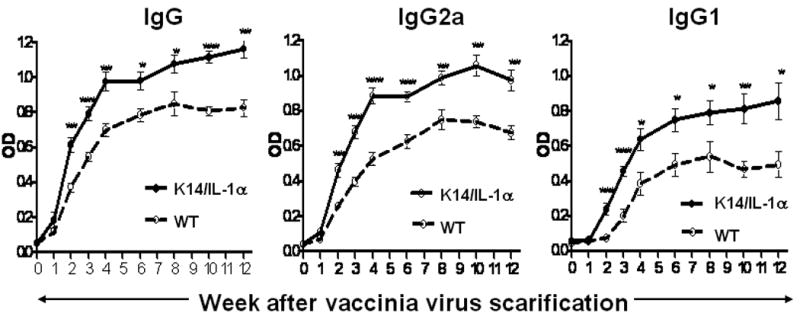
K14/IL-1α and WT mice were skin scarified at the base of their tails with 2 × 106 PFU of WR-GFP vaccinia virus. At various time points after immunization (weekly for weeks 1 to 4 and biweekly for weeks 6 to 12), mice from each group were bled and their sera were collected. Sera were also collected from control, uninfected (week 0) mice. The kinetics of the vaccinia-specific antibody response in the sera of individual mice at each time point was measured by ELISA. Data represent means ± SE using 6 mice per group and are representative of four independent experiments. * = p < 0.05, ** = p < 0.01, *** = p < 0.001.
K14/IL-1α mice recruit T cells and DC to the site of inoculation more rapidly than WT mice
To assess lesion development and the kinetics of infiltration with T cells, we performed immunohistochemical staining of skin on days 0, 3, 7, and 10 following scarification with vaccinia virus. Skin from unimmunized K14/IL-1α and WT mice (day 0) did not appear significantly different by H&E or anti-CD3 staining at baseline (data not shown). Although the pox lesions induced by vaccinia virus scarification on wild-type and K14/IL-1α mice did not appear different by gross inspection (as noted above), there was a substantial difference in the histologic appearance. At day 3 post-scarification, scattered infiltrating cells were seen by H&E stain in the dermis and hypodermis of wild-type mice (Figure 3). In contrast, a dense cellular infiltrate was observed in the skin of K14/IL-1α Tg mice, particularly in the perifollicular regions. The infiltrates were composed primarily of mononuclear cells. Staining for CD3 showed very few T cells at the site of inoculation in wild-type mice, while strong CD3+ staining was evident at the site of innoculation in K14/IL-1α mice. Similarly, while Class II MHC+ stained cells are seen in WT skin at d3 post-innoculation, they are significantly increased (>4 fold) in the skin of K14/IL-1α mice. These cells are in the same perifollicular distribution as the CD3+ cells. Although some likely represent activated T cells, many are dendritic in shape and thus most likely APC. While these findings hold for all time points examined (d3, d7, and d10), they were first noted and were most striking on day 3 (day 7 and day 10 data not shown). These combined data support the impression that there is a robust cutaneous immune response at the site of scarification with vaccinia virus that consists primarily of T cells and Class II MHC-positive APC, and that this response is augmented in K14/IL-1α mice compared with WT controls. Furthermore, these data highlight the lack of sensitivity of the gross appearance of lesions in assessment of the degree and character of the cutaneous immune response. Although WT and K14/IL-1α mice developed quite similar appearing pox lesions following scarification (size, character, duration), functional and phenotypic analyses of the immune response as detailed here show a substantial difference between these strains.
Figure 3. Histology of skin lesions from vaccinia-scarified WT and K14/IL-1α mice.
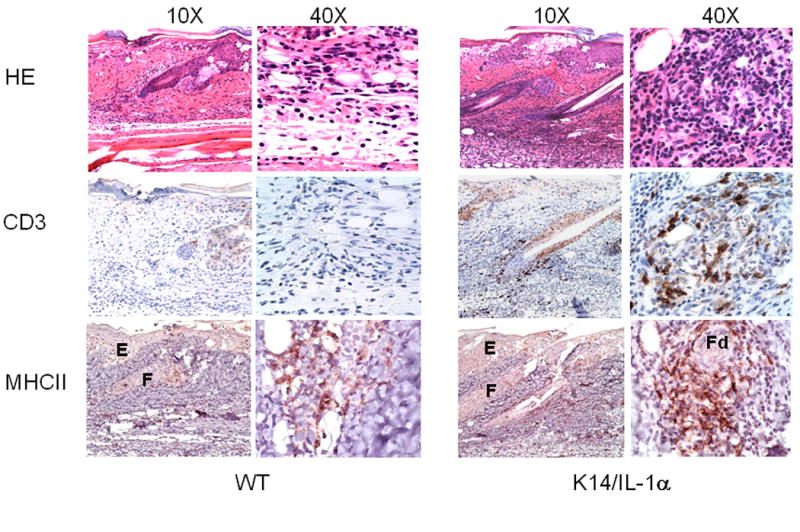
Skin samples from the site of inoculation were obtained from WT and K14/IL-1α mice 3 days after scarification with 2 × 106 PFU of WR-GFP vaccinia virus. Formalin-fixed paraffin-embedded tail-skin samples were stained with H&E as well as anti-CD3 and anti-Ia (MHC Class II) mAb. Labels indicate epidermis (E), proximal follicle (F), and distal follicle (Fd) regions. The data shown are representative of three to four individual mice per experimental group.
Phenotypic maturation of DC in the draining lymph node is accelerated in K14/IL-1α mice after vaccinia virus scarification
To explore the mechanism of enhanced immune response in K14/IL-1α mice, we examined DC from draining LN for expression of surface markers related to DC activation and maturation. CD11c+ cells from lymph nodes of vaccinia virus-naïve K14/IL-1α mice expressed similar levels of MHC Class II, CD86, and slightly higher CD80 compared with WT mice, as assessed by flow cytometry (Figure 4). Scarification with vaccinia virus resulted in increased expression of these markers at 24 h on CD11c+ cells from K14/IL-1α, but not in wild-type mice. At 48 h post-infection, CD11c+ cells from WT mice began to show upregulation of these maturation markers, but still expressed significantly lower levels than K14/IL-1α mice. These results suggest that excess IL-1α in the epidermis promotes the maturation of DC draining to the local lymph nodes, which, in turn, enhances the T cell immune response to vaccinia scarification.
Figure 4. Phenotypic maturation of inguinal LN DC after vaccinia scarification.
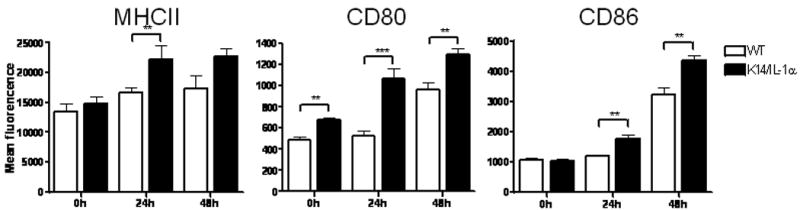
Cells recovered from inguinal lymph nodes harvested at the time points indicated were stained for CD11c and MHC Class II, CD80 or CD86. Bars represent mean fluorescence intensity of MHC Class II, CD80 and CD86 on CD11c+ gated cells. Data represent the means ± SE of four to five individual mice in one experiment. * = p < 0.05, ** = p < 0.01, *** = p < 0.001, n.s. = not significant.
K14/IL-1α mice show a threshold adaptive immune response to scarification
Since K14/IL-1α mice demonstrated enhanced T cell and humoral immune responses to vaccinia virus scarification, we explored whether a suboptimal viral inoculation dose could protect these mice from lethal challenge. 2 × 106 or 5 × 103 PFU of WR-GFP virus was applied by scarification on the tails of K14/IL-1α mice and WT mice in preparation for subsequent intranasal challange. Somewhat surprisingly, while a viral dose of 5 × 103 PFU/mouse inoculated via scarification still resulted in pox lesions at the inoculation site in WT mice, only ∼50% of K14/IL-1α mice developed pox reactions at this dose. This observation held through multiple repetitions of the experiment with careful attention to preparation and dilution of the viral stock used. Quantitative PCR (qPCR) was used to assess viral load at the inoculation site. At day 7 post scarification, the number of vaccinia virus copies present in the skin of K14/IL-1α mice that developed pox lesions following scarification with high or low doses of vaccinia virus were comparable with that of WT control mice, while samples from K14/IL-1α mice that did not form pox lesions showed significantly diminished levels of vaccinia virus DNA (Figure 5A).
Figure 5. Influence of low-dose vaccinia virus on protection in mouse challenge models.
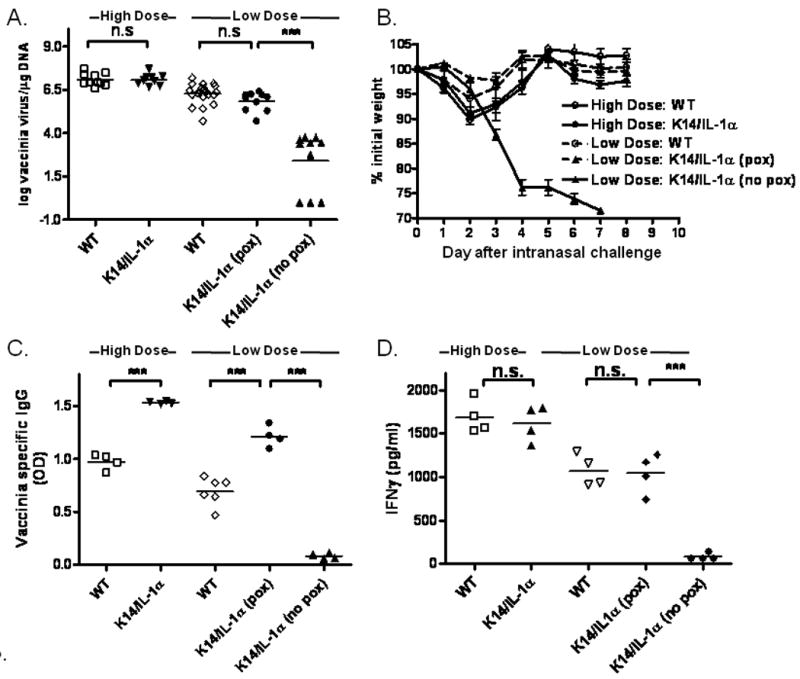
K14/IL-1α and WT mice were skin scarified at the base of their tails with 2 × 106 PFU (high dose) or 5 × 103 PFU (low dose) of WR-GFP vaccinia virus. All of the WT mice, immunized with either high dose (n= 10) or the low dose (n= 20) vaccinia virus, developed pox lesions. Of K14/IL-1α mice immunized with low-dose virus (n=20), half did not form pox lesions (n=10). A. Inoculated skin was removed at 7 days post-immunization and vaccinia viral DNA content determined by quantitative PCR. This method can detect levels as low as 1 virus copy/μg DNA. Data shown represent the individual values (symbols), and means (bars) of 9 to 20 mice per group in two experiments. *** = p < 0.001, n.s. = not significant. B. Five weeks after vaccination, mice received an intranasal challenge with 1 × 107 PFU of vaccinia virus WR and were weighed daily. The data plotted represent the means ± SE for each group. C. Vaccinia-specific IgG in the sera of individual mice measured by ELISA one month after immunization. Data represent the means ± SE of 4 to 6 mice per group. *** = p < 0.001. D. Thirty-five days post-infection, whole splenocytes were prepared from each mouse and stimulated with in vitro–infected syngeneic APC. IFN-γ in the culture supernatant was measured after 40 h. Bars represent means ± SE for mice in each group. *** = p < 0.001, n.s. = not significant.
This finding was important, as development of a pox lesion after vaccinia scarification has historically been accepted as evidence of successful immunization against variola. To assess the development of protective immune responses, mice inoculated by scarification with high- and low-dose vaccinia received an intranasal challenge of 107 PFU of Western Reserve (WR) strain vaccinia virus, a dose that is lethal to nonimmune mice, 5 weeks after initial immunization. Sham-immunized K14/IL-1α and WT mice lost weight rapidly after intranasal challenge and succumbed to infection or were euthanized (because of ≥ 25% body weight loss) by 7 days post-challenge (data not shown). In contrast, K14/IL-1α and WT mice immunized with high-dose (2 × 106 PFU) vaccinia virus WR-GFP showed only transient weight loss and all survived (Figure 5B). Low-dose immunization (5 × 103 PFU) also led to protective immunity in WT mice and in those K14/IL-1α mice that formed pox lesions, whereas the K14/IL-1α mice that did not develop pox lesions succumbed to infection with a time course similar to non-immunized mice. Thus, development of a pox lesion at the site of inoculation appeared indicative of an immune response that resulted in protective immunity. To test this hypothesis, we measured vaccinia virus-specific antibody production and T cell IFNγ production by WT and K14/IL-1α mice scarified with high- vs. low-dose vaccinia virus at one month post inoculation. Consistent with the responses seen with high-dose (2 × 106 PFU) inoculation, K14/IL-1α mice that developed cutaneous pox lesions when scarified with low-dose (5 × 103 PFU) vaccinia virus had significantly higher vaccinia-specific antibody titers than did WT controls treated in the same fashion. However, K14/IL-1α mice that did not form pox lesions when scarified with low-dose (5 × 103 PFU) vaccinia virus did not show detectable vaccinia virus-specific antibody at 30 days post inoculation (Figure 5C). Similarly, production of IFNγ by splenocytes stimulated with vaccinia virus-infected APC at one month post-inoculation was comparable for K14/IL-1α mice that developed pox lesions and WT control mice following scarification with high or low doses of vaccinia virus, while splenocytes of K14/IL-1α mice that did not form pox lesions did not produce significant IFNγ in response to vaccinia virus-infected APC (Figure 5D).
Discussion
In humans, induction of protective immunity to smallpox by immunization with vaccinia virus depends on the delivery of virus to the epidermis by scarification and is characterized by development of an epidermal “pox” reaction. Indeed, vaccination by intradermal, subcutaneous and intramuscular strategies have all been shown to result in lower neutralizing antibody titers and a reduced vaccinia-specific cytotoxic T lymphocyte response than vaccination by scarification (McClain et al., 1997). From early in the history of smallpox vaccination, it has been recognized that certain patient groups are at risk from complications of the scarification technique (Lofquist et al., 2003). Vaccinia necrosum, or progressive vaccinia, is seen in severely immunocompromised patients and leads to severe morbidity and death from overwhelming infection. Eczema vaccinatum, which occurs in patients with active or quiescent atopic dermatitis, reflects an inability of the atopic host to control the spread of virus from the inoculation site and can result in substantial tissue injury or death. Generalized vaccinia infection, which occurs in patients without atopic dermatitis, is not typically lethal but can also cause significant injury. Due to concern regarding these complications of vaccine use, and the apparent elimination of smallpox infection worldwide, widespread vaccination with vaccinia virus was halted in the United States in 1972 and worldwide in the years to follow. Recent concerns about the possible reemergence of smallpox, or its deliberate release as a bioterror agent, has led to renewed interest in vaccination for the armed forces, front-line health care personnel, and, perhaps, the general population. At the same time, a relative increase over the past 30 years in the percent of the world population with atopic dermatitis, and immune deficiency related to organ transplantation, chemotherapy treatments, and HIV disease has spurred interest in understanding the biology of vaccinia immune responses and driven efforts to design safer vaccination protocols.
The basis for the severe adverse effects of vaccinia scarification seen in atopic patients has not been defined. Vaccination with live virus inherently involves a “race” between virus growth and effective immune response. In the case of inoculation by scarification, the balance and interaction of innate and acquired immune mechanisms in the skin are crucial. Complications can result from an inability to respond to a specific infection or an imbalance that delays control of viral growth. Large DNA viruses, including poxviruses and herpesviruses, commonly encode homologues of cytokines, chemokines, and their receptors as a strategy to evade the host immune response (Alcami, 2003). Genes expressed by vaccinia virus encode proteins that inhibit complement, bind IL-1 and IL-18, inhibit CC chemokines, and block IFN function, among others (Alcami and Smith, 1992; Alcami et al., 1998; Kotwal et al., 1990; Moss and Shisler, 2001; Smith et al., 2000; Symons et al., 1995). Together, these viral products alter the evolution of inflammatory signals and limit the recruitment of leukocytes to the site of infection, providing the virus with a crucial advantage in the race with the immune response. In the normal host, APCs carrying viral antigen migrate from the site of infection through afferent lymphatics to the draining lymph nodes where the act to prime antigen-specific T cells. Vaccinia-specific CD8+ T cells activated in skin draining lymph nodes become imprinted with a skin-homing phenotype and will home preferentially to the inoculation site (Liu et al., 2006), where they can lyse infected target cells promote resolution of the infection (Robert and Kupper, 1999). The dose of vaccinia virus used for scarification has been empirically optimized such that the immune system of a normal host usually “wins,” leading to control of virus growth and long-term immunity. In AD patients, however, the immune response is altered or inhibited such that vaccinia virus replicates faster than the immune system responds, leading to greater local tissue injury and a risk for dissemination.
Atopic skin, therefore, appears to provide a specialized environment that promotes vaccinia virus growth and/ or inhibits anti-viral immunity. Evidence for both functions has been described. Activation and growth of cytotoxic anti-viral T cells, for example, are associated with Th1-type cytokines (e.g., IL-12 and IFNγ). Atopic skin is characterized, however, by reduced expression of Th1 cytokines and increased expression of Th2 cytokines (e.g., IL-4 and IL-13) (Hamid et al., 1994). Vaccinia virus infection of human keratinocytes has also been shown to induce expression of Th2 cytokines and other immunoregulatory factors, including transforming growth factor beta, interleukin-10 (IL-10), and IL-13, suggesting that vaccinia may skew local cytokine production against the generation of a protective Th1 and cytotoxic T cell responses (Liu et al., 2005). Innate immune mechanisms are also altered in atopic hosts. LL-37, as an example, is a cathelicidin antimicrobial peptide produced by mammalian skin that exhibits antiviral activity against purified vaccinia virus. Recent studies have demonstrated that atopic skin has decreased LL-37 expression and supports increased replication of vaccinia virus compared with normal or psoriasis skin (Howell et al., 2006). IL-4 and IL-13 treatment of vaccinia-infected keratinocytes, in turn, enhanced replication of vaccinia virus while down-regulating LL-37 expression. These and other alterations in innate and adaptive immune responses seen in the skin of atopic individuals may promote local growth of vaccinia virus and restrict or delay the expansion or function of Th1 and Tc1 cells that form the basis for the anti-viral response.
As noted previously, the balance of IL-1 agonists and antagonists plays a central role in the regulation of cutaneous immune responses and the development of inflammatory skin diseases (Groves et al., 1995; Groves et al., 1996). In patients with atopic skin disease (AD), for example, the ratio of IL-1ra to IL-1α in stratum corneum samples from uninvolved skin of the face, trunk and extremities is significantly increased due to a decrease in IL-1α and an increase in IL-1ra production (Terui et al., 1998). IL-1β mRNA is increased at baseline in lesional skin from patients with AD, and has been shown to rise in response to patch testing with house dust mite antigen (Jeong et al., 2003; Junghans et al., 1998). It is therefore, perhaps, not surprising to find inhibitors of IL-1 function among the immune modulators produced by vaccinia. The vaccinia virus gene B15R, for example, encodes a soluble IL-1 receptor, which binds soluble IL-1β and inhibits functional responses. Experimental deletion of B15R from vaccinia virus accelerates the appearance of symptoms of illness and mortality in mice infected intranasally (Alcami and Smith, 1992). Myxoma virus, a poxvirus that infects rabbits, encodes a protein named M13L-PYD that blocks production of the interleukin-1 family cytokines by inhibiting the activation of the cytoplasmic “inflammasome.” Knockout viruses that do not express this protein are markedly attenuated, showing decreased viral dissemination and enhanced inflammatory responses at sites of infection (Johnston et al., 2005). These studies support the impression that over-expression of IL-1 could enhance the innate immune response, inhibit vaccinia replication, and thus diminish the adaptive immune response (Alcami and Smith, 1992; Johnston et al., 2005; Spriggs et al., 1992). A positive protective role for IL-1 in vaccinia virus infection is also reported by several groups. Modified vaccinia virus Ankara (MVA), which is a isolate that lacks the soluble IL-1β receptor gene as well as other immune modulating genes, induces a better CD8+ T cell memory response and confers higher levels of protection against subsequent lethal respiratory with wild-type vaccinia virus (Staib et al., 2005). Similarly, the viral gene B13R (SPI-2) encodes a serpin homolog that inhibits caspase-1, and thus prevents the maturation of IL-1 β from inactive precursor to active cytokine. Although deletion of B13R diminishes virulence, this recombinant virus still elicited potent humoral, T cell helper, and cytotoxic T cell immune response in the mice, revealing that attenuation did not implicitly reduce immunogenicity (Legrand et al., 2004).
In this study, we used a K14/IL-1α transgenic mouse model to examine the role of cutaneous IL-1α as a modulator of immune response to vaccinia virus inoculated by scarification. As shown, K14/IL-1α mice scarified with 2 × 106 PFU of vaccinia mounted an earlier and stronger T-cell immune response and an enhanced humoral immune response compared with WT control animals. Furthermore, K14/IL-1α mice recruited T cells and APC more rapidly to the site of inoculation, and displayed more rapid maturation of DC arriving at the draining lymph nodes. In addition to the effects on adaptive immunity, K14/IL-1α mice also displayed a stronger innate immune response than WT mice. When the dose used for scarification was decreased to 5,000 PFU, WT mice still developed pox lesions and produced significant vaccinia-specific antibody and cell-mediated responses. K14/IL-1α transgenic mice, however, showed much lower levels of virus present in the site of inoculation at 7 days and more than half did not develop a local pox reaction or subsequently show evidence of a memory immune response. We hypothesize that low dose vaccinia virus inoculated by scarification in WT mice is able to suppress the local innate immune response sufficiently to allow local growth of virus, leading ultimately to strong activation of the adaptive immune response and eventual control of the infection. Innate immune function is sufficiently enhanced in K14/ IL-1α mice to clear the virus before there can be growth sufficient to create a pox and/or activate an adaptive immune response. High-dose innoculation, in contrast, overwhelms the protective effect of excess IL-1 in the transgenic animals, resulting in pox lesion formation and strong immune responses in both WT and IL-1 transgenic mice.
In summary, the observation that epidermal IL-1α enhances T cell and antibody responses to vaccinia virus suggests that IL-1α could be used as an adjuvant in humans, increasing the effectiveness of vaccinia virus inoculation in at-risk populations and potentially supporting the use of lower inoculation doses or attenuated virus strains. It is clear, however, that there is an optimal balance between innate and adaptive immune function that must be considered in any such strategy, and that development of a pox lesion may still be the most suitable indicator of successful, protective immunization.
Materials and Methods
Virus
Recombinant thymidine kinase-negative (TK-)vaccinia virus VV.NP-S-EGFP (WR-GFP, a kind gift of Dr Bernard Moss, NIH) and TK+ vaccinia virus Western Reserve strain (WR) were expanded in HeLa cells and titered in CV-1 cells (American Type Culture Collection) using standard procedures (Earl et al., 1998).
Mice
Wild-type (WT) FVB/N mice were purchased from the Jackson Laboratories (Bar Harbor, Maine). The K14/ IL-1α 1.2 line was generated in inbred FVB/N mice (Groves et al., 1995). Heterozygous transgenic mice from the K14/IL-1α strain were bred to nontransgenic siblings or to FVB/N wild-type mice to produce synchronized delivery of sufficient numbers of animals for scarification experiments. F1 progeny were screened by PCR with primers for the transgenic K14 promoter. Mice lacking transgene were designated as WT littermate controls. As has been described previously, unstimulated transgenic K14/IL-1α mice display only subtle signs of skin inflammation, such as minor erythema around the snout and mild alopecia as they age, relative to WT mice. All mice were handled in accordance with guidelines set out by the Center for Animal Resources and Comparative Medicine at Harvard Medical School.
Immunizations and challenges
Mice were immunized with vaccinia virus WR-GFP by scarification as described (Liu et al., 2006). Briefly, mice were anesthetized with 2,2,2 tribromoethanol (250 mg/kg, Sigma) by intraperitoneal injection (i.p.) with a target of 25-30 min of immobility. Five microliters of trypsinized virus at varying titer were placed on the base of the tail. The inoculation site was scarified with a 28-g needle (500-μl insulin syringe) by poking 25 times and scratching 25 times, endeavoring to stay within the superficial epidermis and to minimize bleeding. For the high dose/low dose studies, a single aliquot of virus was prepared for use with all mice at each dose (WT and K14/IL-1α) to minimize the risk of errors in dilution and handling. For challenge experiments, mice were anesthetized with isoflurane and inoculated intranasally with 107 PFU of vaccinia virus WR (10 μl of 5 × 108 PFU/ml stock was administered into each nostril). The individual mice were weighed and checked for survival daily (Snyder et al., 2004). Mice that lost more than 25% of their body weight were euthanized.
In vitro restimulation assay
Single-cell suspensions were prepared from inguinal lymph nodes (ILN) and spleens of immunized mice. Red blood cells were lysed with lysis buffer (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM Na EDTA, pH 7.3). T cells were purified from infected ILN or spleens using a Pan-T cell isolation kit (Miltenyi Biotec). In all experiments using T cells, the purity of the cells was ≥95% as determined by flow cytometry. Splenocytes from naïve syngeneic mice were infected with vaccinia virus WR-GFP at a MOI of 20 for 8 h and irradiated with 3300 Rad for use as antigen presentation cells (APC). For stimulation assays, a total of 3 × 105 purified T cells or whole splenocytes were stimulated with 3 × 105 infected splenocytes in 96-well flat-bottom plates. After 40 h, cytokines in the culture were measured by ELISA (BD Pharmingen).
Vaccinia virus–specific IFNγ ELISPOT
IFNγ ELISPOT assay was performed using the BD ELISPOT kit (BD Biosciences) according to the manufacturer's instructions. Briefly, ELISPOT plates were coated with an anti-IFNγ monoclonal antibody (capture mAb) overnight at 4°C. The plates were washed and blocked with complete RPMI 1640 with 10% fetal calf serum (FCS). Purified T cells and vaccinia virus-infected APC prepared as in the in vitro restimulation assay were added to the wells and cultured at 37°C for 40 h. The plates were washed and a biotinylated anti-IFNγ mAb was added for 2 h, followed by washing. Streptavidin-alkaline phosphatase was added for 1 h, followed by washing and development of a color reaction using the 3-amino-9-ethylcarbazole substrate reagent provided. The reaction was stopped with water and an immunospot analyzer (Series 3A; Cellular Technology) was used to count the spots (Tian et al., 2005).
Measurement of vaccinia virus-specific antibody
Vaccinia virus-specific antibody was determined at the indicated time points by ELISA. A 96-well EIA/RIA plate (Cat# 3369, Costar) was coated with UV-inactivated vaccinia virus suspension containing 105 PFU/well vaccinia virus WR-GFP in PBS at 4°C overnight. The plate was washed and blocked with PBS with 10% FCS and 0.05% Tween-20 (PBST) for 2 h at room temperature (RT). Serum samples were diluted at 1:200 with PBST and incubated at 4°C for 1 h, washed 3 times, and incubated an additional 1 h at RT with HRP conjugated goat anti-mouse IgG, IgG1 or IgG2a (Southern). The plate was again washed and developed using 3,3′, 5,5′ tetramethylbenzidine (TMB, BD Biosciences) as a substrate. Color development was stopped by adding 2% H2SO4 to each well. Relative ELISA units were calculated from the absorbance at 450nm wavelength (OD450) on a Kinetic Microplate Reader (Molecular Devices).
Quantitative PCR for determination of viral load
Vaccinia viral load was evaluated by quantitative real-time PCR as described (Freyschmidt et al., 2007). Briefly, inoculated skin and various organs were harvested at defined time points after scarification and DNA was purified with the DNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. PCR was performed with the Bio-Rad iCycler iQTM Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). The primers and TaqMan probe used in the quantitative PCR assay are specific for the vaccinia ribonucleotide reductase Vvl4L. The sequences of the primers are: forward, 5′-GAC ACT CTG GCA GCC GAA AT-3′; and reverse, 5′-CTG GCG GCT AGA ATG GCA TA-3′. The TaqMan probe was synthesized by Applied Biosystems (Foster City, CA) with 5′-labeled with FAM and 3′-labeled with TAMRA. The sequence of the probe is: 5′-AGC AGC CAC TTG TAC TAC ACA ACA TCC GGA-3′. Amplification reactions were performed in a 96-well PCR plate (Bio-Rad) in a 20-μl volume containing 2× TaqMan Master Mix (Applied Biosystems), 500 nM forward primer, 500 nM reverse primer, 150 nM probe, and the template DNA. Thermal cycling conditions were 50°C for 2 min and 95°C for 10 min for one cycle. Subsequently, 45 cycles of amplification were performed at 94°C for 15 s and 60°C for 1 min. Viral load was determined by establishing a standard curve from DNA of a vaccinia virus stock with a previously calculated PFU determined by plaque assay. Corresponding CT values obtained by the real-time PCR methods were plotted on the standard curve to estimate sample viral load.
Histologic analysis
Inoculated skin was harvested from three to four mice per group at each time point. Skin was preserved in formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E), anti-CD3 or anti-Ia antibody. Images were obtained with a Nikon E600 microscope and a Nikon EDX-35 digital camera. The images were assembled in Adobe Photoshop 7.0.
Flow cytometric analysis
Inguinal lymph nodes (ILN) were excised from immunized mice and a single-cell suspension prepared by digestion with 250μg/ml Liberase CI (Roche Applied Science) in RPMI for 40 min at 37°C. Cells were stained with APC-anti-CD11c and either PE-anti -CD80, -CD86, or IA/IE (BD Pharmingen). The incidence of positive cells and geometric mean fluorescence intensity (GMFI) were determined by flow cytometry (BD FACSCanto) and analyzed using FACSAria software (BD Biosciences).
Acknowledgments
This work was supported by the NIH Atopic Dermatitis Vaccinia Network (NIH/ NIAID contract HHSN266200400030C) and the Harvard Skin Disease Research Center (NIH/NIAMS grant P30 AR42689). We would also like to thank Mr. Stanley Wong for his technical assistance and Dr. Igor Belyakov for his valuable advice.
Abbreviations
- AD
atopic dermatitis
- APC
antigen presentation cells
- DC
dendritic cells
- ELISPOT
enzyme-linked immune spot
- HE
hematoxylin and eosin
- IL-1
Interleukin-1
- IL-1ra
IL-1 receptor antagonist
- ILN
inguinal lymph node
- K14
keratin 14
- MVA
modified vaccinia virus Ankara
- qPCR
Quantitative PCR
- SFC
spot-forming cell
- TMB
tetramethylbenzidine
- VV
vaccinia virus
- WR
Western Reserve
- WT
wild-type
Footnotes
Conflict of Interest: The authors state no conflicts of interest.
References
- Alcami A. Viral mimicry of cytokines, chemokines and their receptors. Nat Rev Immunol. 2003;3:36–50. doi: 10.1038/nri980. [DOI] [PubMed] [Google Scholar]
- Alcami A, Smith GL. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- Alcami A, Symons JA, Collins PD, Williams TJ, Smith GL. Blockade of chemokine activity by a soluble chemokine binding protein from vaccinia virus. J Immunol. 1998;160:624–633. [PubMed] [Google Scholar]
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral research. 2003;58:101–114. doi: 10.1016/s0166-3542(03)00008-1. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Earl PL, Cooper N, Wyatt LS, Moss B, Carroll MW. Preparation of Cell Cultures and Vaccinia Virus Stocks. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, et al., editors. Current Protocols in Molecular Biology. John Wiley & Sons, Inc.; Mississauga: 1998. pp. 16.16.14–16. [Google Scholar]
- Freyschmidt EJ, Mathias CB, MacArthur DH, Laouar A, Narasimhaswamy M, Weih F, et al. Skin inflammation in RelB(-/-) mice leads to defective immunity and impaired clearance of vaccinia virus. J Allergy Clin Immunol. 2007;119:671–679. doi: 10.1016/j.jaci.2006.12.645. [DOI] [PubMed] [Google Scholar]
- Groves RW, Mizutani H, Kieffer JD, Kupper TS. Inflammatory skin disease in transgenic mice that express high levels of interleukin 1 alpha in basal epidermis. Proc Natl Acad Sci U S A. 1995;92:11874–11878. doi: 10.1073/pnas.92.25.11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves RW, Rauschmayr T, Nakamura K, Sarkar S, Williams IR, Kupper TS. Inflammatory and hyperproliferative skin disease in mice that express elevated levels of the IL-1 receptor (type I) on epidermal keratinocytes. Evidence that IL-1-inducible secondary cytokines produced by keratinocytes in vivo can cause skin disease. J Clin Invest. 1996;98:336–344. doi: 10.1172/JCI118797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94:870–876. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–348. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Jeong CW, Ahn KS, Rho NK, Park YD, Lee DY, Lee JH, et al. Differential in vivo cytokine mRNA expression in lesional skin of intrinsic vs. extrinsic atopic dermatitis patients using semiquantitative RT-PCR. Clin Exp Allergy. 2003;33:1717–1724. doi: 10.1111/j.1365-2222.2003.01782.x. [DOI] [PubMed] [Google Scholar]
- Johnston JB, Barrett JW, Nazarian SH, Goodwin M, Ricciuto D, Wang G, et al. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity. 2005;23:587–598. doi: 10.1016/j.immuni.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Junghans V, Gutgesell C, Jung T, Neumann C. Epidermal cytokines IL-1beta, TNF-alpha, and IL-12 in patients with atopic dermatitis: response to application of house dust mite antigens. J Invest Dermatol. 1998;111:1184–1188. doi: 10.1046/j.1523-1747.1998.00409.x. [DOI] [PubMed] [Google Scholar]
- Kotwal GJ, Isaacs SN, McKenzie R, Frank MM, Moss B. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science. 1990;250:827–830. doi: 10.1126/science.2237434. [DOI] [PubMed] [Google Scholar]
- Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4:211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand FA, Verardi PH, Jones LA, Chan KS, Peng Y, Yilma TD. Induction of potent humoral and cell-mediated immune responses by attenuated vaccinia virus vectors with deleted serpin genes. J Virol. 2004;78:2770–2779. doi: 10.1128/JVI.78.6.2770-2779.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity. 2006;25:511–520. doi: 10.1016/j.immuni.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Liu L, Xu Z, Fuhlbrigge RC, Pena-Cruz V, Lieberman J, Kupper TS. Vaccinia virus induces strong immunoregulatory cytokine production in healthy human epidermal keratinocytes: a novel strategy for immune evasion. J Virol. 2005;79:7363–7370. doi: 10.1128/JVI.79.12.7363-7370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofquist JM, Weimert NA, Hayney MS. Smallpox: a review of clinical disease and vaccination. Am J Health Syst Pharm. 2003;60:749–756. quiz 757-748. [PubMed] [Google Scholar]
- McClain DJ, Harrison S, Yeager CL, Cruz J, Ennis FA, Gibbs P, et al. Immunologic responses to vaccinia vaccines administered by different parenteral routes. J Infect Dis. 1997;175:756–763. doi: 10.1086/513968. [DOI] [PubMed] [Google Scholar]
- Moss B, Shisler JL. Immunology 101 at poxvirus U: immune evasion genes. Semin Immunol. 2001;13:59–66. doi: 10.1006/smim.2000.0296. [DOI] [PubMed] [Google Scholar]
- Murphy JE, Robert C, Kupper TS. Interleukin-1 and cutaneous inflammation: a crucial link between innate and acquired immunity. J Invest Dermatol. 2000;114:602–608. doi: 10.1046/j.1523-1747.2000.00917.x. [DOI] [PubMed] [Google Scholar]
- Rauschmayr T, Groves RW, Kupper TS. Keratinocyte expression of the type 2 interleukin 1 receptor mediates local and specific inhibition of interleukin 1-mediated inflammation. Proc Natl Acad Sci U S A. 1997;94:5814–5819. doi: 10.1073/pnas.94.11.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Kupper TS. Inflammatory skin diseases, T cells, and immune surveillance. N Engl J Med. 1999;341:1817–1828. doi: 10.1056/NEJM199912093412407. [DOI] [PubMed] [Google Scholar]
- Smith VP, Bryant NA, Alcami A. Ectromelia, vaccinia and cowpox viruses encode secreted interleukin-18-binding proteins. J Gen Virol. 2000;81:1223–1230. doi: 10.1099/0022-1317-81-5-1223. [DOI] [PubMed] [Google Scholar]
- Snyder JT, Belyakov IM, Dzutsev A, Lemonnier F, Berzofsky JA. Protection against lethal vaccinia virus challenge in HLA-A2 transgenic mice by immunization with a single CD8+ T-cell peptide epitope of vaccinia and variola viruses. J Virol. 2004;78:7052–7060. doi: 10.1128/JVI.78.13.7052-7060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs MK, Hruby DE, Maliszewski CR, Pickup DJ, Sims JE, Buller RM, et al. Vaccinia and cowpox viruses encode a novel secreted interleukin-1-binding protein. Cell. 1992;71:145–152. doi: 10.1016/0092-8674(92)90273-f. [DOI] [PubMed] [Google Scholar]
- Staib C, Kisling S, Erfle V, Sutter G. Inactivation of the viral interleukin 1beta receptor improves CD8+ T-cell memory responses elicited upon immunization with modified vaccinia virus Ankara. J Gen Virol. 2005;86:1997–2006. doi: 10.1099/vir.0.80646-0. [DOI] [PubMed] [Google Scholar]
- Symons JA, Alcami A, Smith GL. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81:551–560. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Terui T, Hirao T, Sato Y, Uesugi T, Honda M, Iguchi M, et al. An increased ratio of interleukin-1 receptor antagonist to interleukin-1alpha in inflammatory skin diseases. Exp Dermatol. 1998;7:327–334. doi: 10.1111/j.1600-0625.1998.tb00332.x. [DOI] [PubMed] [Google Scholar]
- Tian T, Woodworth J, Skold M, Behar SM. In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates the outcome of infection. J Immunol. 2005;175:3268–3272. doi: 10.4049/jimmunol.175.5.3268. [DOI] [PubMed] [Google Scholar]
- Vassar R, Rosenberg M, Ross S, Tyner A, Fuchs E. Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. ProcNatlAcadSciUSA. 1989;86:1563–1567. doi: 10.1073/pnas.86.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]


