Abstract
Background and Objective
Helicobacter pylori infects the mucus layer of the human stomach and causes peptic ulcers and adenocarcinoma. We have previously shown that H. pylori accumulates photoactive porphyrins making the organism susceptible to inactivation by light, and that small spot endoscopic illumination with violet light reduced bacterial load in human stomachs. This study assessed the feasibility and safety of whole-stomach intra-gastric violet phototherapy for the treatment of H. pylori infection.
Study Design/Materials and Methods
A controlled, prospective pilot trial was conducted using a novel light source consisting of laser diodes and diffusing fibers to deliver 408-nm illumination at escalating total fluences to the whole stomach. Eighteen adults (10 female) with H. pylori infection were treated at 3 U.S. academic endoscopy centers. Quantitative bacterial counts were obtained from biopsies taken from the antrum, body and fundus, and serial urea breath tests.
Results
The largest reduction in bacterial load was in the antrum (>97%), followed by body (>95%) and fundus (>86%). There was a correlation between log reduction and initial bacterial load in the antrum. There was no dose-response seen with increasing illumination times. The urea breath test results indicated that the bacteria repopulated in days following illumination.
Conclusion
Intragastric violet light phototherapy is feasible and safe and may represent a novel approach to eradication of H. pylori, particularly in patients who have failed standard antibiotic treatment. This was a pilot study involving a small number of patients. Further research is needed to determine if phototherapy can be effective for eradicating H. pylori.
Introduction
Helicobacter pylori (H. pylori) is a Gram-negative microaerophilic bacterium which selectively colonizes the mucus layer of the human stomach and duodenum; the organism is a major cause of chronic gastritis, gastric ulcer, duodenal ulcer, gastric lymphoma and gastric adenocarcinoma (1). H. pylori is a common pathogen with adult prevalence rates of approximately 80% in developing countries, and 20% to 50% in industrialized nations(2). Current treatment of H. pylori requires a combination of multiple antibiotics and proton pump inhibitors (PPI), administered over a 7 to 14 day period. The favored regimens employ metronidazole in combination with a PPI, tetracycline and bismuth, or clarithromycin in combination with a PPI and amoxicillin (3,4). These antibiotic regimens are complex, are often associated with unpleasant side effects and unsatisfactory patient compliance, and have eradication rates that vary between 72 and 95% (5). Acquired resistance of H. pylori to both metronidazole and clarithromycin is well-documented; clarithromycin resistance in the United States has been identified in 8% to 12% of isolates, and metronidazole resistance can be found in 13% to 39% of isolates (5). The impact of resistant isolates on treatment can be significant since resistant strains have a lower cure rate than susceptible strains. For the metronidazole-based regimens, resistant H. pylori strains can result in an excess failure rate of 20% compared to non-resistant strains; the overall treatment failure rate for clarithromycin resistant strains can be as high as 77% (6). The increasing occurrence of resistance necessitates a search for alternative treatment modalities to eradicate H. pylori, Many Gram-negative and Gram-positive bacteria, mycoplasma, fungi and viruses have been shown to be sensitive to the combination of photosensitizing dyes and different wavelengths of light (7). This combination of dyes and light is known as photodynamic therapy (PDT) and has been clinically approved for various malignant, premalignant and ophthalmologic conditions (8). PDT involves absorption of light by the dye leading to formation of a long-lived excited triplet state of the dye molecule that can transfer energy to molecular oxygen thus forming the reactive singlet oxygen (9). This oxidizing species can destroy proteins, lipids and nucleic acids causing cell death and tissue necrosis. PDT is being investigated as a treatment for infectious disease including H. pylori (7,10,11).
The Gram-positive bacterium that causes acne, Propionibacterium acnes, is killed by both blue and by red light in the absence of dyes (12,13). Recently the FDA approved a high-intensity narrow-band blue light therapy for the treatment of acne vulgaris (14). Sensitive autofluorescence spectroscopy demonstrates that P. acnes appears to naturally synthesize the fluorescent photosensitizer protoporphyrin IX (PPIX) which mediates the killing effect of visible light (15,16). Other bacteria including Actinomyces odontolyticus and Porphyromonas gingivalis, organisms that cause periodontitis, also naturally synthesize or accumulate PPIX and coproporphyrin (CP) and are also killed by blue or red light (13).
We previously showed that multiple strains of H. pylori are killed in vitro by exposure to modest levels of violet (405-nm) light (17). We found that, like P. acnes, H. pylori naturally accumulates significant quantities of the photoactive porphyrins, PPIX and CP (17). In a proof-of-principle clinical study we also showed that endoscopically delivered violet light to a focal region of the mucosa in the gastric antrum in chronically infected symptomatic patients could give greater than 90% killing of H. pylori as measured by quantitative cultures of before and after biopsies (18).
In the present report we describe a novel device that provides whole-stomach illumination with 12W of 408-nm (violet) light and a prospective, single-arm, dose-escalation, multi-center clinical trial on eighteen patients, which was conducted to provide data on safety and efficacy of whole stomach violet light therapy for H. pylori infection.
Materials and Methods
Phototherapy System
A device was constructed with three functional subsystems: the power and control system (PCS), the light wand, and the catheter sheath. The PCS included a power source, the laser diodes together with user controls and indicators and a water cooling system (FIG 1A). InGaN 408 ± 2-nm laser diodes (Nichia Corp, Torrance, CA) were each coupled to a fiber optic bundle that comprised the proximal portion of the light wand. The PCS also contained a system for measuring the power output of the light wand before and after use. The light wand consisted of a 25-cm long cylindrical diffuser to provide substantially uniform illumination to the stomach along its entire length, and an over-tube to allow for the flow of coolant around the diffuser to maintain a catheter surface temperature of no more than 45 °C (Fig 1B). The catheter sheath consisted of a multi-segment balloon, which was inflated in the stomach to assist in the positioning of the light wand. The catheter sheath included a guide wire lumen to facilitate introduction of the catheter sheath and light wand into the stomach, a lumen for stomach insufflation, and a lumen to inflate and deflate the balloon. A thermocouple was embedded in the catheter sheath for monitoring of the temperature to ensure that the surface temperature did not rise above 45°C.
Figure 1. Phototherapy System.
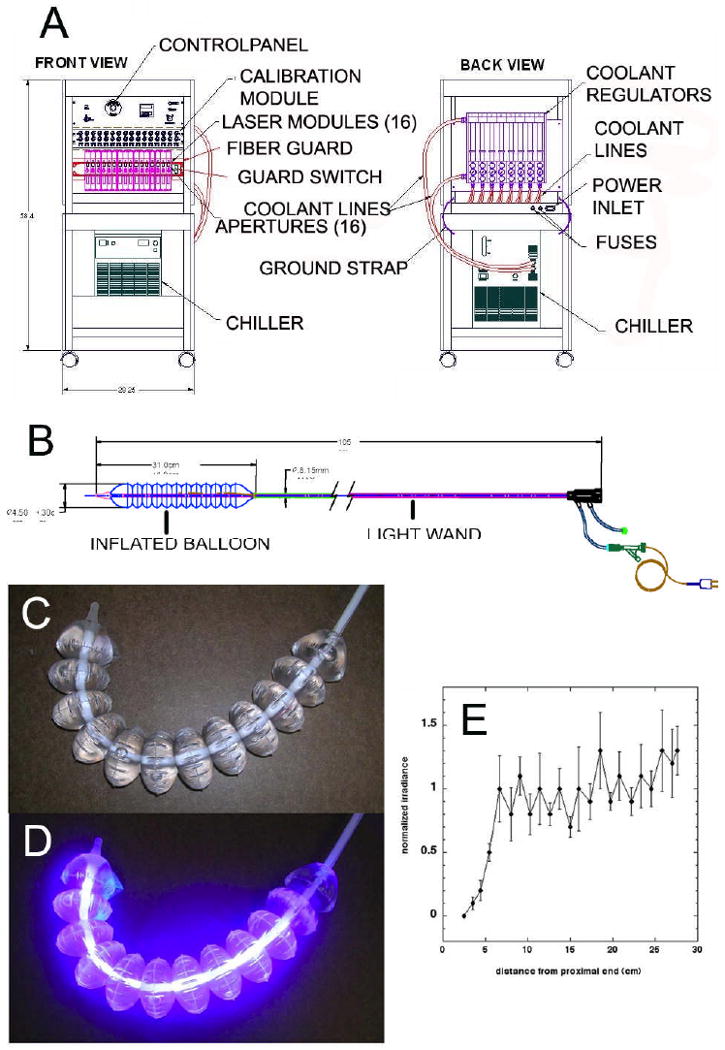
(A) The power and control system (front and back); (B) The light wand, catheter sheath and balloon; (C) Light wand balloon before illumination; (D) Light wand balloon illuminated; (E) Spatial distribution of irradiance along the length of the illuminated balloon.
Procedure
After sedation with a combination of midazolam and fentanyl in addition to topical benzocaine oral spray, a standard upper endoscope was used to inspect the stomach and obtain 3 biopsies from the antrum, body and fundus of the stomach. A stiff guidewire with a spring tip (Glidewire®, Boston Scientific, Inc, Natick, MA) was then passed through the endoscope into the pylorus, and the endoscope was removed, leaving the guide wire in place. The light wand was inserted into the catheter sheath, which was then passed into the stomach over the guide wire. Proper positioning of the balloon in the stomach was confirmed under direct visualization with an upper endoscope. The guide wire was then removed, the balloon unwrapped and inflated, and light therapy was performed. Upon completion of the light therapy, the balloon was deflated and the catheter sheath and light wand were removed from the patient. The upper endoscope was then used to assess for any damage to the mucosa of the upper gastrointestinal tract and obtain additional stomach biopsies.
Bench testing of light source
The non-illuminated catheter is shown in Fig 1C and after illumination in Fig 1D. Catheter irradiance was measured using an International Light Radiometer (IL1700, Newburyport, MA) that was fitted with a 2 mm diffusing aperture over the standard SED 100 sensing head. For each of four orthogonal scans, the aperture was traversed over the length of the balloon catheter at a fixed radial distance of 2.3 cm. The results are shown in Fig 1E.
Animal testing
An animal safety study was conducted using a porcine model. The protocol was approved by the IACUC committee of Beth Israel Deaconess Medical Center. The catheter system was used to deliver a total of between 31 and 46 kJ of light at a peak wavelength of approximately 408 nm to the stomach of five anesthetized adult Yorkshire pigs. In each animal the illumination was split over the course of several illumination periods lasting 15 minutes each. During the study, a variety of system parameters were monitored, including catheter water temperature, catheter surface temperature and the stomach ambient temperature. The ambient temperatures measured by three thermocouples in the pig stomachs varied between 33-36 deg C before illumination, and between 37-40 deg C after 15 minutes of illumination. Physiological parameters were recorded, including heart rate and body core temperature. For each animal, the attending physician's (AJL, CK) assessment of ease of mechanical deployment, animal tolerance, endoscopic inspection of the living tissue and tissue condition at necropsy were recorded. Sections of stomach were then prepared as H&E stained tissue slides and evaluated microscopically by a board certified veterinary pathologist. Design and operating parameters were adjusted as necessary until the outcome was designated by attending physicians and pathologist to be safe and suitable for submission to the US FDA for an Investigational Device Exemption.
Clinical trial
The study protocol and consent form were approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (Boston MA), University of Massachusetts Worcester Medical Center (Worcester MA) and Abbott-Northwestern Hospital (Minneapolis, MN).
Inclusion Criteria
Patients between 18 and 90 years with documented H. pylori infection by urease breath test, stool antigen or histology within thirty days prior to procedure, who were willing to comply with study requirements, were eligible for the protocol.
Exclusion criteria
Patients were excluded if they had a history of gastric or duodenal carcinoma, prior gastric or duodenal surgery, active peptic ulcer disease (gastric or duodenal ulcer), esophagitis Grade II or higher, moderate or large hiatal hernia (i.e. gastroesophageal junction to diaphragm distance greater than or equal to 3 cm), macroscopic evidence of severe erosive or hemorrhagic gastritis at EGD, upper-gastrointestinal (esophageal, gastric or small intestine) stricture, diverticulum or other anatomic abnormality or anomaly that may have impeded device placement, balloon expansion or gastric insufflation. In addition, patients were not eligible if they reported antibiotic or bismuth subsalicylate (Pepto-Bismol) use within one month or proton pump inhibitory use within 2 weeks prior to entering the study. Patients with a known history of a bleeding disorder or anti-coagulant use that would prevent biopsy, positive pregnancy test, known porphyria, or weight < 100 lb or > 250 lb were also excluded.
Study Design
A dose escalation study design was employed, in which 5 patients were exposed to light therapy for 15 minutes; 5 patients were exposed for 30 minutes; 7 were exposed for 45 minutes, and 1 was exposed for 60 minutes. The original plan was to have 5 patients in each group, however, the 60 minute procedure was poorly tolerated in 2 patients and the procedure was discontinued after 45 minutes.(these 2 patients were added to the 45 minute group), and only 1 recruited patient completed the entire 60 minutes. For analysis purposes we chose to include this patient in the 45 minute group.
Patients were evaluated at enrollment, at 5 days post-treatment and again at 5 weeks (± 7 days) post treatment using a non-invasive urea breath test (13C UBT) (BreathTek UBT, Meretek Diagnostics Inc, Rockville, MD). Three patients received serial breath tests before treatment and at 8 hours, 24 hours, 3 days and 5 days post treatment.
Nine gastric biopsies were taken immediately pre-light treatment and at the conclusion of light treatment during the endoscopy procedure to allow quantitative microbiological culture and measure the level of H. pylori eradication achieved using the light therapy. Pre and post-treatment biopsies were obtained from the following sites during endoscopy: antrum at 2, 3, and 4 cm from the pylorus; greater curvature of the corpus at 3, 4 and 5 cm proximal to the angulus; fundus and cardia at 2, 3 and 4 cm from the GE junction.
Microbiological analysis
Biopsy samples were placed in Brucella broth containing 10% fetal calf serum and 20% glycerol and were snap frozen at minus 60 °C for transport to the Wellman Center for Photomedicine in Boston, MA. Each tissue sample (weighing from 2 – 20 mg) was thawed, blotted dry of liquid, weighed, and homogenized individually (using a PowerGen 35, Fisher Scientific, Pittsburgh, PA) in 2 mL of Brucella broth containing 10% fetal calf serum together with an antibiotic mixture of 10 μg/mL vancomycin, 5 μg/mL trimethoprim, 6 μg/mL nalidixic acid, and 5 μg/mL amphotericin B (19). The solid medium consisted of the former ingredients with 1.5% agar. The aliquots were serially diluted in PBS with vortexing to ensure disaggregation of bacterial clumps, and horizontally streaked on square agar plates according to the method of Jett et al (20). The plates were incubated at 37°C in microaerophilic gas jars (BBL GasPak with CampyPak Plus, Fisher Scientific) for 5-7 days until colonies were easily countable. Results were expressed as CFU per mg of biopsy tissue. Each biopsy was plated in duplicate. The microbiology lab personnel were blinded to the duration of light therapy and sample identities.
Statistical Analysis
Statistical analysis was performed using one-way ANOVA in Microsoft Excel. Correlation and linear regression analysis was performed with VassarStats (http://faculty.vassar.edu/lowry/VassarStats.html).
Results
Demographics
Eighteen patients underwent light therapy. Patient demographics and procedure location are shown in Table 1.
Table 1. Patient demographics.
| Patient | gender | Age (years) | Site |
|---|---|---|---|
| 1 | female | 45 | BIDMC |
| 2 | female | 21 | BIDMC |
| 3 | male | 47 | BIDMC |
| 4 | male | 55 | BIDMC |
| 5 | male | 34 | BIDMC |
| 6 | female | 61 | BIDMC |
| 7 | female | 66. | MNGI |
| 8 | male | 45 | MNGI |
| 9 | female | 52 | MNGI |
| 10 | male | 55 | MNGI |
| 11 | male | 28 | BIDMC |
| 12 | female | 49 | BIDMC |
| 13 | female | 63 | MNGI |
| 14 | female | 49 | MNGI |
| 15 | female | 54 | MNGI |
| 16 | male | 33 | MNGI |
| 17 | female | 39 | BIDMC |
| 18 | male | 40 | UMASS |
BIDMC, Beth Israel Deaconess Medical Center, Boston, MA,
MNGI, Minnesota Gastroenterology, P.A., Abbott-Northwestern Hospital, Minneapolis, MN,
UMASS, UMASS Medical Center, Worcester, MA
Safety
Post-procedure recovery was routine and patients were discharged 30 minutes after the completed treatments and resumed their usual diets. All patients tolerated the procedure well. The following self-limited adverse events were reported: sore throat (n= 8), dull ache in stomach (n=2), runny nose (n=2), sore neck (n=1), and throat irritation (n=1). All events resolved within 3 days after the procedure. Endoscopic appearance of the stomach post-procedure appeared normal in all patients with the exception of one patient who was noted have a small Mallory-Weiss tear just proximal to the gastroesophageal junction. No active bleeding was noted or no intervention necessary. One patient had persistent oozing from a pretreatment biopsy site requiring <1 cc of epinephrine injection.
Urease Breath Test (13C UBT) Results
The 13C UBT results were similar between pre-treatment and post-treatment (day 5 and week 5) (Table 2A) indicating persistent H. pylori infection. 4 patients who received greater than 30 minutes of light therapy had lower readings at day 5 and even lower values at week 5 (patients 9, 13, 15 and 16). 3 patients (patients 11, 17 and 18) were also given 13C UBT 8 hours post-treatment as well as 1,3, and 5 days after light. All 3 patients had large decreases (>75%) in the reading at 8 hours (Table 2B). In patient 18 the 13C UBT result was negative at 8 hours post-procedure, however the succeeding readings in this patient as well as in patients 11 and 17 were positive and increasing.
Table 2A. 13C UBT results.
| Patient | Time | Breath-Tek values | ||
|---|---|---|---|---|
| Pre Tx | 5 day post Tx | 5 week post Tx | ||
| 1 | 15 | na | na | na |
| 2 | 15 | 0.193 | 0.188 | 0.169 |
| 3 | 15 | 0.35 | 0.368 | 0.256 |
| 4 | 15 | 0.226 | 0.269 | 0.133 |
| 5 | 15 | 0.263 | 0.244 | 0.139 |
| 6 | 30 | 0.16 | 0.198 | 0.133 |
| 7 | 30 | 0.035 | 0.156 | 0.227 |
| 8 | 30 | 0.113 | 0.139 | 0.112 |
| 9 | 30 | 0.513 | 0.378 | 0.158 |
| 10 | 30 | 0.129 | 0.186 | 0.21 |
| 11 | 45 | 0.29 | 0.282 | na |
| 12 | 45 | na | na | na |
| 13 | 45 | 0.398 | 0.38 | 0.247 |
| 14 | 45 | 0.157 | na | na |
| 15 | 45 | 0.675 | 0.443 | 0.277 |
| 16 | 45 | 0.153 | 0.108 | 0.058 |
| 17 | 45 | 0.191 | 0.19 | 0.197 |
| 18 | 45* | 0.278 | 0.285 | na |
na, not available * received 60 minutes of illumination tx: treatment
Table 2B. Urease breath test results at early time points for selected patients.
| Patient | Breath Tek values | |||
|---|---|---|---|---|
| Pre Tx | 8 hour post Tx | 1 day post Tx | 3 day post Tx | |
| 11 | 0.29 | 0.077 | 0.183 | 0.447 |
| 17 | 0.191 | 0.035 | 0.058 | 0.151 |
| 18 | 0.278 | 0.00 | 0.101 | 0.123 |
Tx: Treatment
Microbiology results
The mean values from the gastric biopsy quantitative cultures are shown in Table 3. Patients 7, 10 and 11 failed to grow any colonies from any of the biopsies (pre and post treatment) most likely due to mishandling of the frozen specimens in transit thus inactivating the microaerophilic H. pylori. In 7 cases (patients 6, 12, 13 and 15 antrum biopsies, patient 8 body and fundus biopsies, and patient 9 fundus biopsy) all the 6 post-treatment cultures from a particular patient's stomach region failed to grow colonies and this was taken as complete killing of the H. pylori.
Table 3. Microbiological Culture of H. pylori.
| Patient | time | Antrum pre | Antrum post | Antrum log reduction | Body pre | Body post | Body log reduction | Fundus pre | Fundus post | Fundus log reduction | Mean pre | Mean log reduction |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | 327±331 | 4±4 | 2.16±0.38 | 328±316 | 1±1 | 2.64±1.2 | 906±648 | 772±727 | 0.2±0.4 | 582±598 | 1.67±1.3 |
| 2 | 15 | 25±20 | 1±1 | 1.43±0.4 | 12±7 | 1±1 | 1.42±0.63 | 15±7 | 5±5 | 0.76±0.43 | 17±13 | 1.28±0.56 |
| 3 | 15 | 399±343 | 6±6 | 1.95±0.6 | 67±37 | 30±28 | 0.41±0.23 | 59±61 | 33±37 | 0.7±0.7 | 176±292 | 1.02±0.85 |
| 4 | 15 | 116±47 | 1±1 | 2.03±0.51 | 52±44 | 6±6 | 1.07±0.16 | 20±3 | 6±10 | 0.62±0.9 | 74±56 | 1.44±0.82 |
| 5 | 15 | 57±37 | 1±1 | 1.81±0.6 | 3±1 | 1±1 | 0.42±0.1 | 16±10 | 8±5 | 0.27±0.39 | 25±31 | 0.83±0.81 |
| 6 | 30 | 137±93 | 0.5* | 2.35±0.3 | 258±158 | 37±56 | 1.24±0.96 | 306±160 | 20±9 | 1.16±0.43 | 239±151 | 1.53±0.81 |
| 7 | 30 | n.g. | n.g. | n.g. | n.g. | n.g. | n.g. | |||||
| 8 | 30 | 75±38 | 14±12 | 0.89±0.38 | 4±4 | 0.5* | 0.88±0.2 | 3±1 | 0.5* | 0.83±0.11 | 26±44 | 0.86±0.28 |
| 9 | 30 | 38±10 | 1±1 | 1.72±0.25 | 10±8 | 4±4 | 0.55±0.27 | 8±4 | 0.5* | 1.17±0.2 | 43±94 | 1.15±0.54 |
| 10 | 30 | n.g. | n.g. | n.g. | n.g. | n.g. | n.g. | |||||
| 11 | 30 | n.g. | n.g. | n.g. | n.g. | n.g. | n.g. | |||||
| 12 | 30 | 880±822 | 1±1 | 2.88±0.6 | 453±603 | 113±117 | 0.28±0.4 | 27±20 | 21±10 | 0.05±0.19 | 473±650 | 1.07±1.38 |
| 13 | 45 | 12±8 | 0.5* | 1.32±0.27 | 11±10 | 0.5* | 1.18±0.45 | 7±5 | 3±3 | 0.41±0.3 | 10±7 | 0.97±0.53 |
| 13 | 45 | 27±23 | 0.5* | 1.6±0.37 | 58±23 | 30±17 | 0.32±0.39 | 38±12 | 36±18 | 0.09±0.28 | 43±25 | 0.67±0.75 |
| 14 | 45 | 36±17 | 17±3 | 0.71±0.37 | 43±23 | 19±8 | 0.33±0.41 | 44±41 | 4±3 | 0.97±0.94 | 43±28 | 0.67±0.65 |
| 15 | 60 | 3±2 | 0.5* | 0.64±0.3 | 33±20 | 1±1 | 1.52±0.39 | 107±101 | 1±1 | 1.85±0.26 | 47±72 | 1.33±0.61 |
| 16 | 60 | 534±639 | 12±14 | 1.81±0.66 | 32±19 | 28±23 | 0.12±0.48 | 56±44 | 13±6 | 0.68±0.37 | 207±425 | 0.87±0.87 |
| 18 | 60 | 72±28 | 1±1 | 1.26±0.25 | 132±73 | 21±19 | 1.64±1.31 | 233±237 | 37±45 | 1.21±1.04 | 183±170 | 1.57±0.96 |
| Mean | 182±250 | 4±5 | 1.64±0.62 | 99±136 | 19±29 | 0.93±0.69 | 133±233 | 64±196 | 0.73±0.49 | 145±173 | 1.13±0.33 | |
Values for pre and post columns are in CFU/mg tissue; means +/- SD of duplicate cultures of 3 biopsies per stomach region. Log reductions are means of logs of ratios for paired pre and post biopsies +/- SD. A notional value of 0.5 CFU/mg was assigned to these measured zero values to allow log reduction values to be calculated. Mean log reduction values were calculated from the individual post-illumination CFU/mg values divided by the paired (i.e. adjacent areas of the stomach) pre-illumination CFU/mg values. Note that the mean log reduction value (mean of ratios) is not the same as the log reduction calculated from the mean post and pre CFU/mg values (ratio of means). n.g.= no growth in any culture.
The difference in the mean CFU/mg pre and post illumination was significant for the antrum and for the body of the stomach but not for the fundus (Fig 2A). The phototherapy had the largest antibacterial effect in the antrum region of the stomach (Fig 2A). The overall mean log reduction for all illumination times in the antrum was 1.637, equivalent to killing of 97.7% of bacteria. This value was significantly higher than the log reduction for the body (p=0.007) and for the fundus (P=0.0002) (Fig 2B). The range of log reductions found in the antrum was 0.64 to 2.88, equivalent to bacterial killing between 77% and 99.9%. The body of the stomach had the next most bacterial killing after the antrum with a mean log reduction for all illumination times of 0.947 (>88% bacterial killing) but this value was not significantly different from the log reduction for the fundus, which exhibited least killing with a mean log reduction of 0.737 (>81%).
Figure 2.
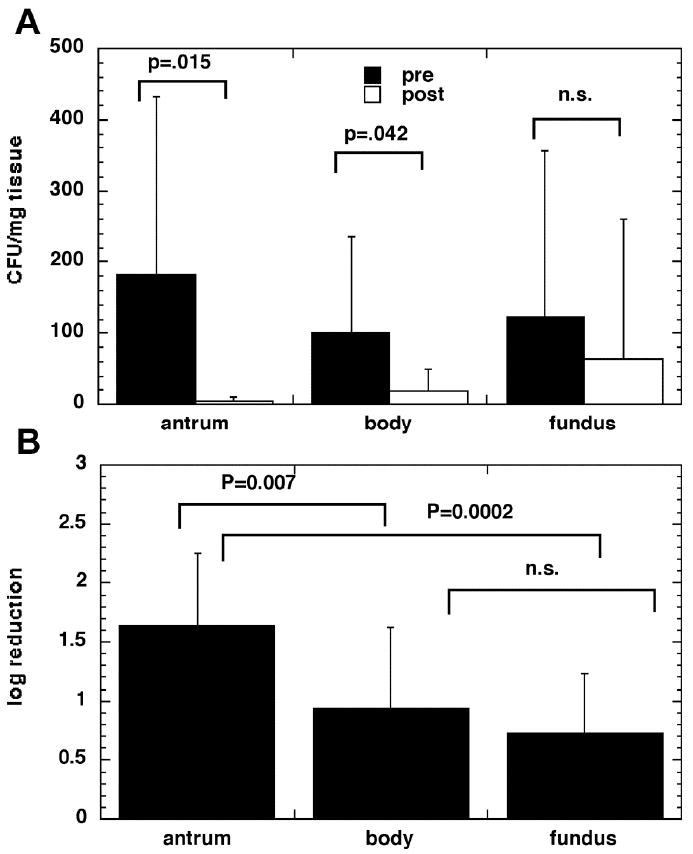
(A) H. Pylori Culture Results Pre and Post Phototherapy. There was a significant difference in the mean CFU/mg cultured from biopsies removed pre and immediately post illumination for the antrum and body but not for the fundus. (B) Mean log reduction of H. Pylori in three regions of the stomach. The reduction was significantly higher in the antrum than the body and fundus.
Log reduction in the antrum for each patient correlated with the initial bacterial load in CFU/mg (linear correlation coefficient r2 of 0.565, p=0.0012) (Fig 3) but not the body of the stomach (r2 = 0.066, p=0.3538), fundus (r2 = 0.01, p=0.7196) or whole stomach (r2 = 0.218, p=0.0792). Surprisingly there was no evidence of a dose response when log reductions were compared for increasing illumination times. Pre-illumination H. pylori concentrations in the antrum were 184, 83 and 258 CFU/mg for the 15, 30 and 45-minute patient groups respectively and therefore initial bacterial load did not appear to explain the lack of a dose response with increasing illumination times.
Figure 3. Initial H. Pylori Load (CFU/mg) in the Antrum Correlates with Log Reduction Following Phototherapy.
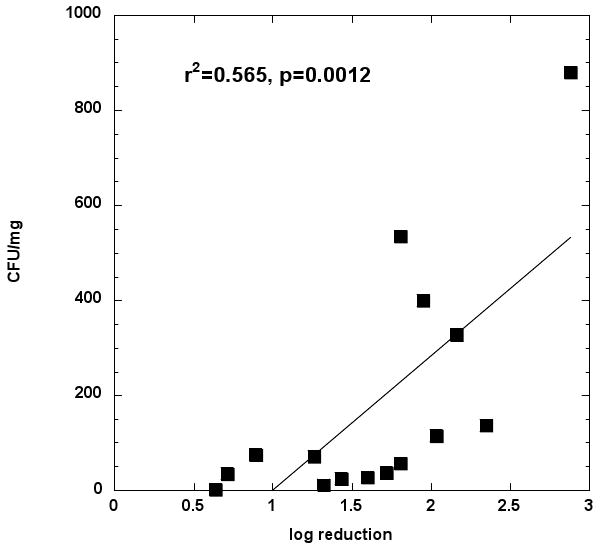
Shown is the log reduction in the antrum and the initial bacterial load in CFU/mg for each patient. The linear correlation coefficient r2 was 0.565, p=0.0012. Correlation coefficients were not significant for the stomach or fundus.
Discussion
This is the first report to show that in patients infected with H. pylori, the whole stomach can be safely illuminated with violet light, and that this treatment results in a significant antibacterial effect especially with regard to those bacteria infecting the antrum. Although none of the patients achieved complete and sustained eradication of H. pylori with this therapy, the results of this study are promising.
We found the greatest violet light antibacterial effect in the antrum compared to the body and fundus regions of the stomach. The most likely explanation for the greater killing in the antrum is that the light source employed in this study delivered more light to the distal region of the stomach compared to the more proximal regions of the stomach, i.e. the body and the fundus (see Fig 1E). It is quite likely that the irradiance of the light wand was lowest in these proximal regions. Alternatively, it is possible that the bacteria in the antrum are more accessible to light due to the anatomical differences between the antrum, body and fundus. Finally, it is possible that the bacteria in the antrum are more susceptible to light therapy due to potential morphological differences (e.g., increased amount of porphyrins) in the bacteria between the antrum and other parts of the stomach. The finding that there was a good linear correlation between log reduction and initial bacterial load in the antrum suggests that when the bacteria are dense in the gastric mucosa they are either more accessible to the light or more susceptible to the light, than when they are sparse.
Surprisingly, we did not find a dose response between increasing illumination time and reduction in H. pylori. In our previous studies, good dose response killing curves were found for blue-light inactivation of several strains of H. pylori in vitro (17,18). Furthermore the relative lack of bacterial killing in the fundus was attributed to the lack of illumination at the very proximal part of the light wand (Fig 1E). This unexpected finding (no dose response) in vivo could potentially be due to the existence of 2 (or more) populations of H. pylori. One population, potentially more likely to occur when the overall density of bacteria is high, may be more superficial and therefore easier to kill with violet light. Another population may be located deeper in the mucosal ruggae or crypts, and may possibly represent organisms adherent to gastric epithelial cells, and may therefore be harder to kill due to greater concealment from violet light even with a relatively long illumination time.
The breath test results did not show any significant differences when measured at 5 days post-light or at 5 weeks post-light. In other words the treatment failed to produce eradication in any patient. However the breath-test measurements carried out at the early time point of 8 hours post-light did show a major decrease and in one case a negative result. We interpret these data to suggest that H. pylori undergoes a relatively rapid regrowth in the stomach after being substantially killed by the violet light, and that the survival of even a small population of organisms is enough to reestablish colonization. Although antibiotic therapy is intended and usually does produce permanent eradication, it has been shown that recurrence of the infection in failures is due to recrudescence or bacterial regrowth of the same strain, rather than re-infection with a new strain. (21).
In order to answer the question of whether any therapeutic benefit might be gained by violet light phototherapy in the human stomach for H. pylori infection, there are 3 problems with the current system that need to be addressed. The first is the potential problem of uneven light distribution within the stomach. Due to the configuration of the stomach, the distance from the light wand to the mucosa varies considerably. This can be presumably solved by a different construction that allows for more light to be emitted in the fundus and body and less in the antrum. The second problem is that of bacterial regrowth in the days after treatment and whether it can be prevented. It may be possible to employ violet light to kill approximately 99% of the initial bacterial load, and then follow with lower dose, or less noxious, antibiotic combinations to prevent regrowth. It is also possible that light could give a “kick start” to eradication in the case of antibiotic resistant strains of H. pylori. The third potential problem is the possible presence of a light-resistant sub-population of bacteria, especially in patients with low overall bacterial loads. This problem, if proven to be the case, might be overcome by the addition of a suitable “adjuvant” to the light therapy. For instance a mucolytic agent may allow deep-seated bacteria to become more accessible to light mediated killing (22). Nonetheless, despite the above limitations, the present study lends credence to the possibility of employing violet light phototherapy, either alone or in conjunction with antibiotics, for the treatment of H. pylori. Further studies using this modality are warranted.
Acknowledgments
This work was supported by LumeRx Inc, Marlborough MA. MRH was supported by NIH R01AI050875. The authors have declared competing financial interests.
References
- 1.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347(15):1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 2.Graham DY, Malaty HM, Evans DG, Evans DJ, Jr, Klein PD, Adam E. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology. 1991;100(6):1495–1501. doi: 10.1016/0016-5085(91)90644-z. [DOI] [PubMed] [Google Scholar]
- 3.Katelaris PH, Forbes GM, Talley NJ, Crotty B. A randomized comparison of quadruple and triple therapies for Helicobacter pylori eradication: The QUADRATE Study. Gastroenterology. 2002;123(6):1763–1769. doi: 10.1053/gast.2002.37051. [DOI] [PubMed] [Google Scholar]
- 4.Laine L, Hunt R, El-Zimaity H, Nguyen B, Osato M, Spenard J. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American trial. Am J Gastroenterol. 2003;98(3):562–567. doi: 10.1111/j.1572-0241.2003.t01-1-07288.x. [DOI] [PubMed] [Google Scholar]
- 5.Megraud F, Marshall BJ. How to treat Helicobacter pylori. First-line, second-line, and future therapies. Gastroenterol Clin North Am. 2000;29(4):759–773. doi: 10.1016/s0889-8553(05)70145-x. [DOI] [PubMed] [Google Scholar]
- 6.McMahon BJ, Hennessy TW, Bensler JM, Bruden DL, Parkinson AJ, Morris JM, Reasonover AL, Hurlburt DA, Bruce MG, Sacco F, Butler JC. The relationship among previous antimicrobial use, antimicrobial resistance, and treatment outcomes for Helicobacter pylori infections. Ann Intern Med. 2003;139(6):463–469. doi: 10.7326/0003-4819-139-6-200309160-00008. [DOI] [PubMed] [Google Scholar]
- 7.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3(5):436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dougherty TJ. An update on photodynamic therapy applications. J Clin Laser Med Surg. 2002;20(1):3–7. doi: 10.1089/104454702753474931. [DOI] [PubMed] [Google Scholar]
- 9.Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J Photochem Photobiol B. 1997;39(1):1–18. doi: 10.1016/s1011-1344(96)07428-3. [DOI] [PubMed] [Google Scholar]
- 10.Demidova TN, Hamblin MR. Photodynamic therapy targeted to pathogens. IntJImmunopatholPharmacol. 2004;17(3):245–254. doi: 10.1177/039463200401700304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bown SG, Millson CE. Photodynamic therapy in gastroenterology. Gut. 1997;41(1):5–7. doi: 10.1136/gut.41.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashkenazi H, Malik Z, Harth Y, Nitzan Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol Med Microbiol. 2003;35(1):17–24. doi: 10.1111/j.1574-695X.2003.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 13.Konig K, Teschke M, Sigusch B, Glockmann E, Eick S, Pfister W. Red light kills bacteria via photodynamic action. Cell Mol Biol (Noisy-le-grand) 2000;46(7):1297–1303. [PubMed] [Google Scholar]
- 14.Elman M, Slatkine M, Harth Y. The effective treatment of acne vulgaris by a high-intensity, narrow band 405-420 nm light source. J Cosmet Laser Ther. 2003;5(2):111–117. [PubMed] [Google Scholar]
- 15.Melo TB, Johnsson M. In vivo porphyrin fluorescence for Propionibacterium acnes. A characterization of the fluorescing pigments. Dermatologica. 1982;164(3):167–174. [PubMed] [Google Scholar]
- 16.Romiti R, Schaller M, Jacob K, Plewig G. High-performance liquid chromatography analysis of porphyrins in Propionibacterium acnes. Arch Dermatol Res. 2000;292(6):320–322. doi: 10.1007/s004030000122. [DOI] [PubMed] [Google Scholar]
- 17.Hamblin MR, Viveiros J, Yang C, Ahmadi A, Ganz RA, Tolkoff MJ. Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrobial Agents and Chemotherapy. 2005;49(7):2822–2827. doi: 10.1128/AAC.49.7.2822-2827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganz RA, Viveiros J, Ahmad A, Ahmadi A, Khalil A, Tolkoff MJ, Nishioka NS, Hamblin MR. Helicobacter pylori in patients can be killed by visible light. Lasers Surg Med. 2005;36(4):260–265. doi: 10.1002/lsm.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piccolomini R, Di Bonaventura G, Festi D, Catamo G, Laterza F, Neri M. Optimal combination of media for primary isolation of Helicobacter pylori from gastric biopsy specimens. J Clin Microbiol. 1997;35(6):1541–1544. doi: 10.1128/jcm.35.6.1541-1544.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23(4):648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 21.Niv Y. H pylori recurrence after successful eradication. World J Gastroenterol. 2008;14(10):1477–1478. doi: 10.3748/wjg.14.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huynh HQ, Couper RT, Tran CD, Moore L, Kelso R, Butler RN. N-acetylcysteine, a novel treatment for Helicobacter pylori infection. Dig Dis Sci. 2004;49(1112):1853–1861. doi: 10.1007/s10620-004-9583-2. [DOI] [PubMed] [Google Scholar]


