Abstract
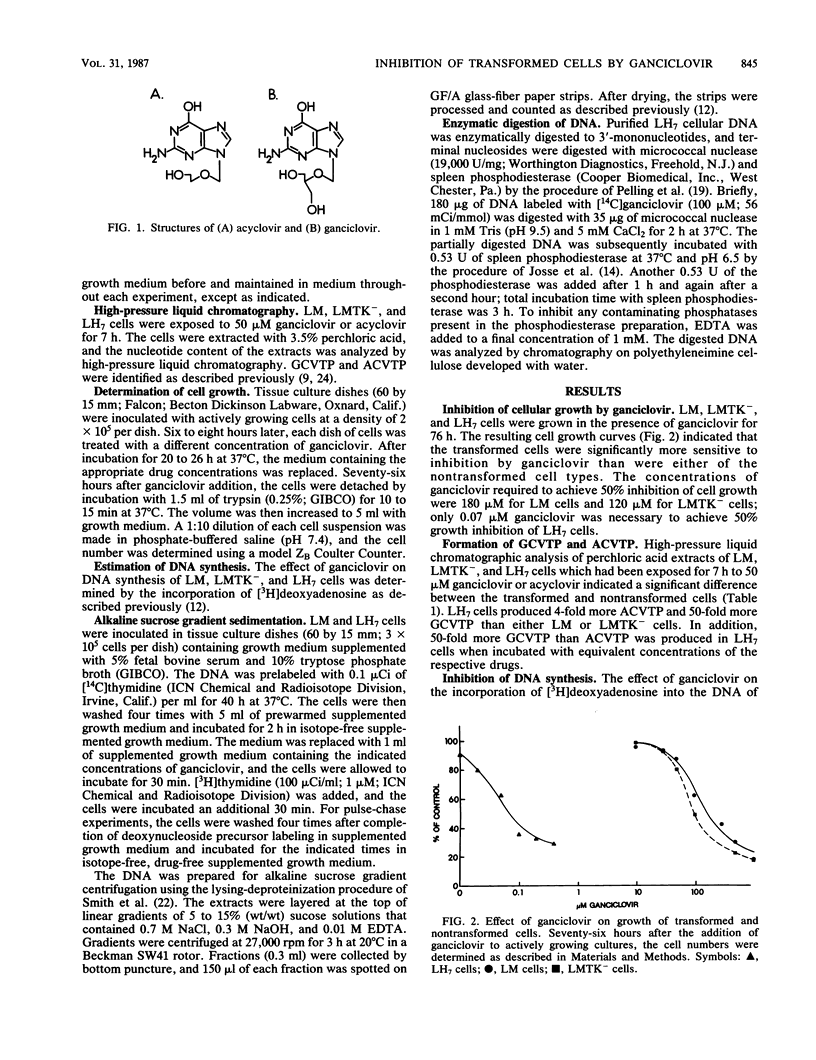
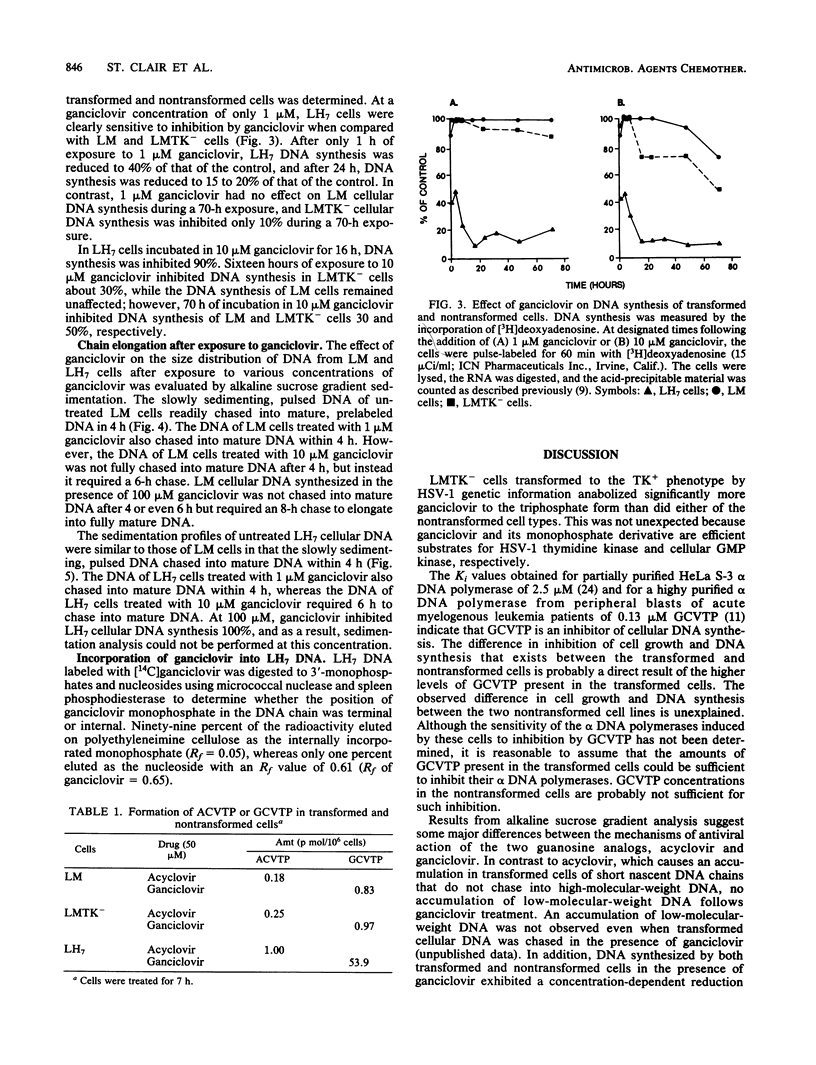
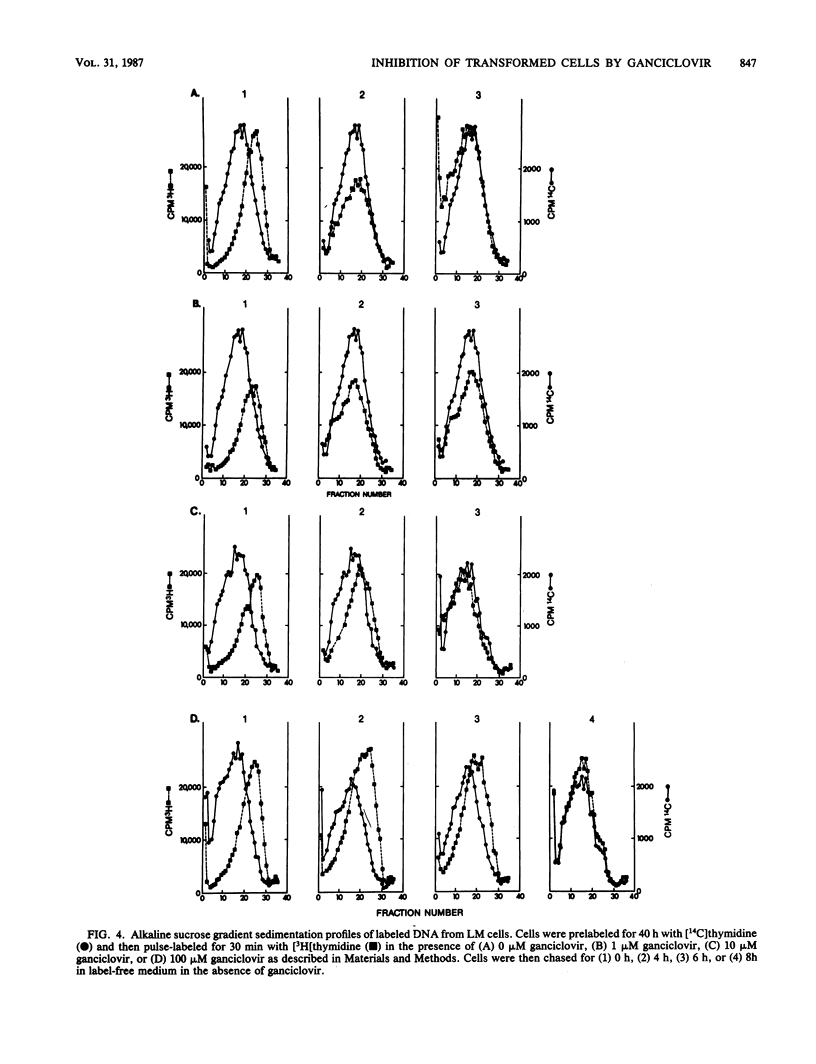
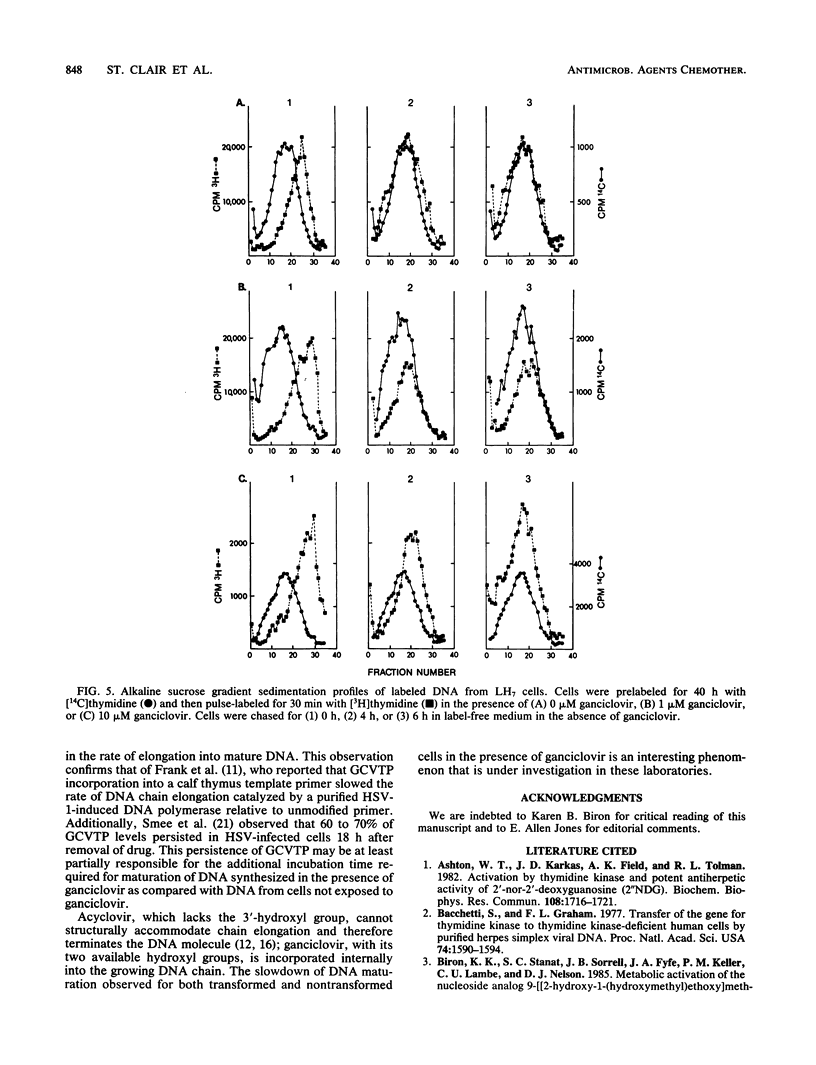
The ability of LM cells, thymidine kinase-deficient LM cells (LMTK-), and LMTK- cells transformed to the LMTK+ phenotype by herpes simplex virus type 1 genetic information (LH7 cells) to anabolize the acyclovir congener ganciclovir was examined. About 50-fold more ganciclovir triphosphate was produced by LH7 cells than by either LM or LMTK- cells. Growth inhibition studies indicated that 180 and 120 microM ganciclovir were required to achieve 50% growth inhibition of LM and LMTK- cells, respectively; only 0.07 microM ganciclovir was necessary to achieve 50% inhibition of LH7 cells. DNA synthesis in the transformed cells was significantly reduced by ganciclovir treatment, whereas ganciclovir had little effect on DNA synthesis in the nontransformed cells. Alkaline sucrose gradient sedimentation analysis of transformed cellular DNA indicated that LH7 DNA synthesized in the presence of ganciclovir chased into mature DNA. Both LM and LH7 DNA synthesized in the presence of ganciclovir exhibited a concentration-dependent reduction in the rate of elongation into mature DNA. Finally, [14C]ganciclovir was incorporated internally into the growing chains of LH7 cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton W. T., Karkas J. D., Field A. K., Tolman R. L. Activation by thymidine kinase and potent antiherpetic activity of 2'-nor-2'-deoxyguanosine (2'NDG). Biochem Biophys Res Commun. 1982 Oct 29;108(4):1716–1721. doi: 10.1016/s0006-291x(82)80109-5. [DOI] [PubMed] [Google Scholar]
- Bacchetti S., Graham F. L. Transfer of the gene for thymidine kinase to thymidine kinase-deficient human cells by purified herpes simplex viral DNA. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1590–1594. doi: 10.1073/pnas.74.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron K. K., Stanat S. C., Sorrell J. B., Fyfe J. A., Keller P. M., Lambe C. U., Nelson D. J. Metabolic activation of the nucleoside analog 9-[( 2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine in human diploid fibroblasts infected with human cytomegalovirus. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2473–2477. doi: 10.1073/pnas.82.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme R. E. Phosphorylation of the antiviral precursor 9-(1,3-dihydroxy-2-propoxymethyl)guanine monophosphate by guanylate kinase isozymes. J Biol Chem. 1984 Oct 25;259(20):12346–12349. [PubMed] [Google Scholar]
- Cheng Y. C., Grill S. P., Dutschman G. E., Nakayama K., Bastow K. F. Metabolism of 9-(1,3-dihydroxy-2-propoxymethyl)guanine, a new anti-herpes virus compound, in herpes simplex virus-infected cells. J Biol Chem. 1983 Oct 25;258(20):12460–12464. [PubMed] [Google Scholar]
- Cheng Y. C., Huang E. S., Lin J. C., Mar E. C., Pagano J. S., Dutschman G. E., Grill S. P. Unique spectrum of activity of 9-[(1,3-dihydroxy-2-propoxy)methyl]-guanine against herpesviruses in vitro and its mode of action against herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1983 May;80(9):2767–2770. doi: 10.1073/pnas.80.9.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpacker C. S., Kowalsky P. N., Oliver S. A., Schnipper L. E., Field A. K. Resistance of herpes simplex virus to 9-[[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl]guanine: physical mapping of drug synergism within the viral DNA polymerase locus. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1556–1560. doi: 10.1073/pnas.81.5.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. L., Kaufman E. R., Crumpacker C. S., Schnipper L. E. Inhibition of herpes simplex virus transformed and nontransformed cells by acycloguanosine: mechanisms of uptake and toxicity. Virology. 1981 Aug;113(1):9–19. doi: 10.1016/0042-6822(81)90132-x. [DOI] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. K., Davies M. E., DeWitt C., Perry H. C., Liou R., Germershausen J., Karkas J. D., Ashton W. T., Johnston D. B., Tolman R. L. 9-([2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine: a selective inhibitor of herpes group virus replication. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4139–4143. doi: 10.1073/pnas.80.13.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank K. B., Chiou J. F., Cheng Y. C. Interaction of herpes simplex virus-induced DNA polymerase with 9-(1,3-dihydroxy-2-propoxymethyl)guanine triphosphate. J Biol Chem. 1984 Feb 10;259(3):1566–1569. [PubMed] [Google Scholar]
- Furman P. A., McGuirt P. V., Keller P. M., Fyfe J. A., Elion G. B. Inhibition by acyclovir of cell growth and DNA synthesis of cells biochemically transformed with herpesvirus genetic information. Virology. 1980 Apr 30;102(2):420–430. doi: 10.1016/0042-6822(80)90109-9. [DOI] [PubMed] [Google Scholar]
- Germershausen J., Bostedor R., Field A. K., Perry H., Liou R., Bull H., Tolman R. L., Karkas J. D. A comparison of the antiviral agents 2'-nor-2'-deoxyguanosine and acyclovir: uptake and phosphorylation in tissue culture and kinetics of in vitro inhibition of viral and cellular DNA polymerases by their respective triphosphates. Biochem Biophys Res Commun. 1983 Oct 31;116(2):360–367. doi: 10.1016/0006-291x(83)90530-2. [DOI] [PubMed] [Google Scholar]
- JOSSE J., KAISER A. D., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. VIII. Frequencies of nearest neighbor base sequences in deoxyribonucleic acid. J Biol Chem. 1961 Mar;236:864–875. [PubMed] [Google Scholar]
- Maitland N. J., McDougall J. K. Biochemical transformation of mouse cells by fragments of herpes simplex virus DNA. Cell. 1977 May;11(1):233–241. doi: 10.1016/0092-8674(77)90334-8. [DOI] [PubMed] [Google Scholar]
- McGuirt P. V., Shaw J. E., Elion G. B., Furman P. A. Identification of small DNA fragments synthesized in herpes simplex virus-infected cells in the presence of acyclovir. Antimicrob Agents Chemother. 1984 Apr;25(4):507–509. doi: 10.1128/aac.25.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y., Rapp F. Anticellular effects of 9-(2-hydroxyethoxymethyl) guanine against herpes simplex virus-transformed cells. J Gen Virol. 1979 Oct;45(1):227–230. doi: 10.1099/0022-1317-45-1-227. [DOI] [PubMed] [Google Scholar]
- Oliver S., Bubley G., Crumpacker C. Inhibition of HSV-transformed murine cells by nucleoside analogs, 2'-NDG and 2'-nor-cGMP: mechanisms of inhibition and reversal by exogenous nucleosides. Virology. 1985 Aug;145(1):84–93. doi: 10.1016/0042-6822(85)90203-x. [DOI] [PubMed] [Google Scholar]
- Pelling J. C., Drach J. C., Shipman C., Jr Internucleotide incorporation of arabinosyladenine into herpes simplex virus and mammalian cell DNA. Virology. 1981 Mar;109(2):323–335. doi: 10.1016/0042-6822(81)90503-1. [DOI] [PubMed] [Google Scholar]
- Reichert C. M., O'Leary T. J., Levens D. L., Simrell C. R., Macher A. M. Autopsy pathology in the acquired immune deficiency syndrome. Am J Pathol. 1983 Sep;112(3):357–382. [PMC free article] [PubMed] [Google Scholar]
- Smee D. F., Boehme R., Chernow M., Binko B. P., Matthews T. R. Intracellular metabolism and enzymatic phosphorylation of 9-(1,3-dihydroxy-2-propoxymethyl)guanine and acyclovir in herpes simplex virus-infected and uninfected cells. Biochem Pharmacol. 1985 Apr 1;34(7):1049–1056. doi: 10.1016/0006-2952(85)90608-2. [DOI] [PubMed] [Google Scholar]
- Smith G. J., Charlton R. K., Grisham J. W., Kaufman D. G. Increased sensitivity for detection of carcinogen-induced DNA repair with the chain terminator dideoxythymidine. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1538–1544. doi: 10.1016/0006-291x(78)91396-7. [DOI] [PubMed] [Google Scholar]
- Smith K. O., Galloway K. S., Kennell W. L., Ogilvie K. K., Radatus B. K. A new nucleoside analog, 9-[[2-hydroxy-1-(hydroxymethyl)ethoxyl]methyl]guanine, highly active in vitro against herpes simplex virus types 1 and 2. Antimicrob Agents Chemother. 1982 Jul;22(1):55–61. doi: 10.1128/aac.22.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair M. H., Miller W. H., Miller R. L., Lambe C. U., Furman P. A. Inhibition of cellular alpha DNA polymerase and herpes simplex virus-induced DNA polymerases by the triphosphate of BW759U. Antimicrob Agents Chemother. 1984 Feb;25(2):191–194. doi: 10.1128/aac.25.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocci M. J., Livelli T. J., Perry H. C., Crumpacker C. S., Field A. K. Effects of the nucleoside analog 2'-nor-2'-deoxyguanosine on human cytomegalovirus replication. Antimicrob Agents Chemother. 1984 Feb;25(2):247–252. doi: 10.1128/aac.25.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]



