Summary
This study was initiated to investigate effects of the novel neuromodulator carisbamate (RWJ 333369) in the hippocampal neuronal culture model of status epilepticus and spontaneous epileptiform discharges. Whole-cell current clamp techniques were used to determine the effects of carisbamate on spontaneous recurrent epileptiform discharges (SREDs, in vitro epilepsy), depolarization-induced sustained repetitive firing (SRF) and low Mg2+-induced continuous high frequency spiking (in vitro status epilepticus). This in vitro model is an important tool to study the effects of anticonvulsant drugs (AEDs) on SREDs that occur for the life of the neurons in culture. Carisbamate dose dependently blocked the expression and reoccurrence of SREDs. The ED50 value for its antiepileptic effect was 58.75 ± 2.43 μM. Inhibition of SRF is considered a common attribute of many AEDs. Carisbamate (100 μM) significantly decreased SRF in hippocampal neurons. All these effects of carisbamate were reversed during a 5 to 30 min drug washout period. When exposed to low Mg2+ medium cultured hippocampal neurons exhibit high frequency spiking. This form of in vitro status epilepticus is not effectively blocked by conventional AEDs that are known to be effective in treating status epilepticus in humans. Carisbamate, like phenytoin and phenobarbital, had little or no effect on low Mg2+-induced continuous high frequency spiking. These results characterize the effects of carisbamate in the hippocampal neuronal culture model of epileptiform discharges and suggest that the ability of carisbamate to inhibit depolarization-induced SRF may account in part for some of it’s anticonvulsant effect.
Keywords: Carisbamate, Epilepsy, Status epilepticus, Neuronal cultures, Patch clamp
Introduction
Epilepsy is one of the most common neurological disorders affecting approximately 1–2% of population worldwide (Hauser and Hesdorffer, 1990; McNamara, 1999). It is characterized by the occurrence of spontaneous, recurrent unprovoked seizure discharges (Lothman et al., 1991). A seizure is the symptomatic, behavioral manifestation of abnormal, disordered, spontaneous and synchronized, high frequency firing of populations of neurons in the CNS (Delorenzo et al., 2005; Lothman et al., 1991; McNamara, 1999). Epilepsy also has severe socio-economic implications (Murray et al., 1996). Although great advances have been made in the development of newer antiepileptic drugs (AEDs) and surgical interventions for the treatment of epilepsy, approximately 40% of the patients are still refractory to treatment with conventional AED’s (Duncan et al., 2006; French, 2007). A sizeable population of epileptic patients deals with the issue of daily management of epilepsy that ultimately affects their quality of life (Cramer et al., 1999b). Thus, there is a growing demand for developing newer medications to treat epilepsy.
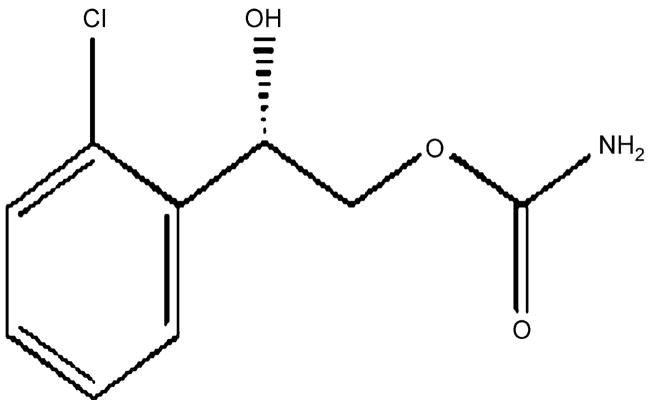
Carisbamate or RWJ-333369 (S-2-O-carbamoyl-1-o-chlorophenyl-ethanol) is a novel neuromodulator, initially developed by SK-Biopharmaceuticals and now under development by Johnson & Johnson, Pharmaceutical Research & Development L.L.C. (chemical structure shown in Fig. 1). It has completed phase II and initiated phase III clinical trials for treatment of epilepsy (Bialer et al., 2007). Carisbamate has been evaluated as a novel AED and has demonstrated antiepileptic activity in a variety of in vivo seizure models including hippocampal and corneal kindling (Bialer et al., 2007; Rogawski, 2006) and the GAERS model of absence epilepsy (Nehlig et al., 2005). It also inhibits spontaneous recurrent seizures after kainate induced epilepsy (Grabenstatter and Dudek, 2004) and delays or prevents Li-Pilocarpine (Francois et al., 2005) induced epilepsy. Carisbamate is also anticonvulsant against bicuculline and picrotoxin induced seizures and in pentylenetetrazole and maximal electroshock induced seizure models (Bialer et al., 2007; Rogawski, 2006; White et al., 2006). However, the exact mechanism(s) of action of carisbamate is still unknown. The dicarbamate felbamate has been reported to affect variety of ionic currents including the sodium (White et al., 1992), calcium (Stefani et al., 1996) and NMDA currents (Subramaniam et al., 1995). However, significance of this action for seizure protection is uncertain. Thus it is important to determine mechanisms underlying the antiepileptic effects of carisbamate.
Figure 1.
Chemical structure of carisbamate (RWJ 333369). Carisbamate (S-2-O-carbamoyl-1-o-chlorophenyl-ethanol) is a novel neuromodulator under development for the treatment of epilepsy. It has an excellent profile in preclinical models of epilepsy and has demonstrated efficacy and tolerability in a phase II clinical trial. Phase III clinical trials for carisbamate have been initiated for the treatment of epilepsy.
This study was initiated to investigate mechanisms underlying the antiepileptic effects of carisbamate using the hippocampal neuronal culture models of status epilepticus and spontaneous epileptiform discharges (SREDs) (Delorenzo et al., 2005; Deshpande et al., 2007c; Sombati and DeLorenzo, 1995). In addition, we also investigated effects of carisbamate on depolarization-induced sustained repetitive firing in cultured hippocampal neurons. The low Mg2+ model utilizes an episode of continuous seizure activity (in vitro status epilepticus) for 3 h. Upon return to normal Mg2+ solutions, networks of neurons manifest synchronized SREDs for their life in culture (in vitro model of SREDs). The low Mg2+ models of status epilepticus and SREDs in hippocampal neuronal cultures have been routinely used to characterize biochemical, electrophysiological and molecular mechanisms underlying epilepsy in an in vitro setting (Churn et al., 2000; Delorenzo et al., 2005; Deshpande et al., 2007c; Pal et al., 2001). These in vitro models also allow for careful control of the neuronal environment and are ideally suited to evaluate the effects of various investigational compounds on electrographic seizure activity.
Materials and methods
All the reagents were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise noted. Sodium pyruvate, minimum essential media containing Earle’s salts, fetal bovine serum and horse serum were obtained from Gibco-BRL (Invitrogen Corp., Carlsbad, CA). Carisbamate was provided by Johnson & Johnson, Pharmaceutical Research & Development L.L.C., Titusville, NJ, USA.
Hippocampal neuronal culture
All animal use procedures were in strict accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by Virginia Commonwealth University’s Institutional Animal Care and Use Committee. Studies were conducted on primary mixed hippocampal neuronal cultures prepared as described previously with slight modifications (Blair et al., 2006; Deshpande et al., 2007b; Sombati and DeLorenzo, 1995). In brief, hippocampal cells were obtained from 2-day postnatal Sprague–Dawley rats (Harlan, Frederick, MD) and plated at a density of 1 × 105 cells/cm2 onto 35-mm Falcon cell culture dishes (Becton Dickinson and Co., Franklin Lakes, NJ) previously coated with poly-L-lysine (0.05 mg/ml). Glial cultures were maintained at 37 °C in a 5% CO2/95% air atmosphere and fed thrice weekly with glial feed (minimal essential media with Earle’s Salts, 25 mM HEPES, 2 mM L-glutamine, 3 mM glucose, and 10% fetal bovine serum). When confluent, glial beds were treated with 5-μM cytosine arabinoside for 2 days to curtail cell division. On the 13th day in vitro, the media was fully replaced with a 5% horse serum supplemented neuronal feed (composition given below) in preparation for neuronal plating on the following day. At this time, these cultures predominantly consisted of glial cells with few, if any, neurons. On the 14th day in vitro, hippocampal cell suspension was plated on these confluent glial beds at a density of 2 × 105 cells/cm2. Twenty-four hours after plating, cultures were treated with 5-μM cytosine arabinoside to inhibit non-neuronal growth. Cultures were maintained at 37 °C in a 5% CO2/95% air atmosphere and fed twice weekly with neuronal feed (minimal essential media with Earle’s Salts, 25 mM HEPES, 2 mM L-glutamine, 3 mM glucose, 100 μg/ml transferrin, 5 μg/ml insulin, 100 μM putrescine, 3 nM sodium selenite, 200 nM progesterone, 1 mM sodium pyruvate, 0.1% ovalbumin, 0.2 ng/ml triiodothyroxine and 0.4 ng/ml corticosterone supplemented with 5% horse serum). These mixed cultures were used for experiments from 13 days in vitro following neuronal plating through the life of the cultures.
Whole-cell current clamp recordings
Whole-cell current clamp recordings were performed using previously established procedures (Blair et al., 2006; Deshpande et al., 2007b; Sombati and DeLorenzo, 1995). Briefly, a cell culture dish was mounted on the stage of an inverted microscope (Nikon Diaphot, Tokyo, Japan) continuously perfused with physiological basal recording solution (pBRS) containing (in mM): 145 NaCl, 2.5 KCl, 10 HEPES, 2 CaCl2, 10 glucose, and 0.002 glycine, pH 7.3, and osmolarity adjusted to 325 ± 5 mOsm with sucrose. Patch electrodes with a resistance of 2 to 4 MΩ were pulled on a Brown-Flaming P-80C electrode puller (Sutter Instruments, Novato, CA), fire-polished and filled with a solution containing (in mM): 140 K+ gluconate, 1.1 EGTA, 1 MgCl2, and 10 Na-HEPES, pH 7.2, osmolarity adjusted to 290 ± 10 mOsm with sucrose. Whole-cell recordings were carried out using an Axopatch 200B amplifier (Molecular Devices, Foster City, CA) in a current clamp mode. Data were digitized via Digi-data 1322A (Molecular Devices, Foster City, CA) and transferred to VHS tape using a PCM device (Neurocorder, New York, NY) and then played back on a DC-500 Hz chart recorder (Astro-Med Dash II, Warwick, RI). Carisbamate was included in the recording solution and applied to the whole-cell dish by using a multi-channel gravity-feed perfusion system (Warner Instruments, Hamden, CT).
Hippocampal neuronal culture model of SREDs
SREDs were induced into neuronal cultures by exposing them for 3-h to a solution containing no added MgCl2 (low Mg2+) using procedures described previously (Blair et al., 2006; Deshpande et al., 2007b; Sombati and DeLorenzo, 1995). Briefly, after the removal of maintenance media, neurons were gently washed with 3 × 1.5 ml of pBRS (±1 mM MgCl2) and then allowed to incubate in this solution at 37 °C under 5% CO2/95% air atmosphere. At the end of the 3-h period, cultures were restored to the physiological concentration (1 mM) of MgCl2 by gently washing with 3 × 1.5 ml of minimum essential medium, returned to the maintenance medium and incubated at 37 °C under 5% CO2/95% air atmosphere. Thus, low Mg2+ treatment was carried out with pBRS without added MgCl2, whereas sham controls were treated with pBRS containing 1 mM MgCl2.
Sustained repetitive firing in cultured hippocampal neurons
Sustained repetitive firing (SRF) was induced by S-90 Tri-level Stimulator (Medical Systems Corp., Greenvale, NY) by depolarizing pulses of 500 ms duration, 0.3 Hz and increasing current strength from 0.1 to 0.5 nA through the micropipette. The duration and frequency of depolarizing pulses were kept constant for all experiments. Current strength was increased until the cell under study exhibited a suitable number of action potentials throughout the duration of the depolarizing pulse.
Hippocampal neuronal culture model of status epilepticus
After 2-weeks in cultures, neurons were utilized for experimentation. Status epilepticus-like activity was induced in vitro as described previously (Sombati and DeLorenzo, 1995). Maintenance medium was replaced with pBRS with or without MgCl2. Continuous high frequency epileptiform bursts (in vitro status epilepticus) were induced by exposing neuronal cultures to pBRS without added MgCl2 (low Mg2+). The high frequency spiking continued until pBRS containing 1 mM MgCl2 was added back to the cultures. Unless indicated as low Mg2+ treatment, experimental protocols utilized pBRS containing 1 mM MgCl2.
Data analyses
Data are reported as mean ± S.E.M. For concentration-response analysis, suppression of status epilepticus or SREDs was determined as a percentage decrease in frequency over increasing concentrations of carisbamate. Status epilepticus frequency was determined by counting individual epileptiform bursts over a recording duration of 5 min for each neuron analyzed before and after application of the drug. SRED frequency was determined by counting individual SREDs over a recording duration of 30 min for each neuron analyzed before and after application of the drug. Frequency analysis of SREDs and status epilepticus-like firing were carried out on multiple neurons at each concentration before and after application of drug to measure effectiveness of carisbamate on these parameters. Student t-test was then performed to determine significance or lack of thereof. Least-square linear regression analysis was then used to calculate the ED50 (effective dose that produced 50% of maximal effect) of carisbamate for suppression of SREDs or status epilepticus burst discharges. SRF data were examined using one-way analysis of variance (ANOVA) followed by a post hoc Tukey test. Data were analyzed using SigmaStat 2.0 and graphs were generated using SigmaPlot analysis software 8.02 (SPSS Inc., Chicago, IL).
Results
Effects of carisbamate on SREDs
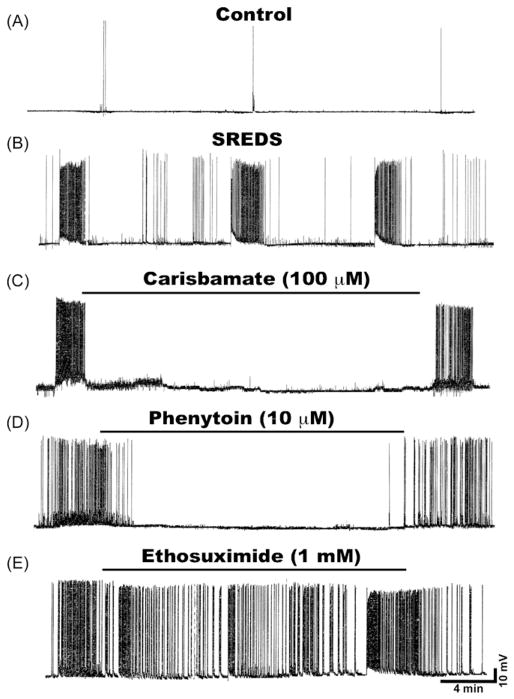
Whole-cell current clamp recordings from neurons in cultures 1-day after a 3-h, low-Mg2+ treatment demonstrated SREDs or in vitro seizure events. Fig. 2B shows recording from a neuron that exhibited three SREDs that each lasted between 1 and 2 min in duration. These SREDs occurred for the life of the neurons and manifested paroxysmal depolarization shifts, a pathophysiological characteristic of acquired epilepsy. SREDs started in an unprovoked manner and stopped spontaneously. Multiple recordings from adjacent neurons demonstrate that these SREDs were synchronous events occurring throughout the neuronal network in populations of neurons (data not shown, (Sombati and DeLorenzo, 1995). Thus, SREDs are the in vitro analog of spontaneous electographic seizure discharges. Age-matched control neurons revealed “normal” baseline activity consisting of intermittent action potentials (Fig. 2A). There were no significant differences in membrane potential and input resistance between control and epileptic neurons.
Figure 2.
Effects of various AEDs on SREDs in cultured hippocampal neurons. (A) Current clamp recording from a representative control neuron displaying baseline activity consisting of intermittent action potentials. (B) Recording from an “epileptic” neuron 1-day following a 3-h exposure to low Mg2+ solution demonstrates occurrence of characteristic SREDs. Three SREDs or spontaneous seizure episodes lasting ~60–90 s can be seen in this recording frame. These SREDs occurred throughout the life of the cultures and are indicative of the pathophysiological “epileptic” phenotype. Effects of various AEDs on SREDs in cultured hippocampal neurons. Application of (C) Carisbamate (100 μM) or (D) Phenytoin (10 μM) to the epileptic neurons abolished the expression of SRED activity. However application of (E) Ethosuximide (1 mM) failed to stop SRED activity. The effects of AEDs on SREDs were reversible and seizures reappeared following drug washout.
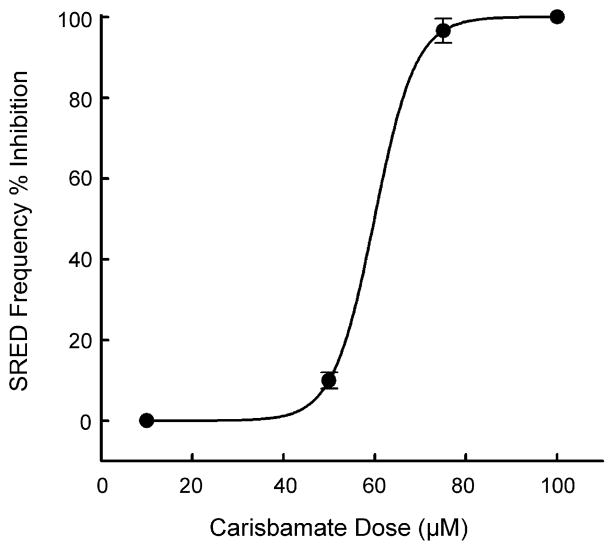
To evaluate the antiepileptic effects of carisbamate we used the in vitro hippocampal neuronal culture model of SREDs. As shown in Fig. 1C, application of carisbamate (100 μM) fully inhibited the expression and reoccurrence of SREDs. The effects of carisbamate were dose dependent and concentrations of 10–100 μM caused a progressive decrease in the occurrence of SREDs. The graph in Fig. 3 demonstrates the effects of increasing carisbamate concentration on the frequency of SREDs. Carisbamate displayed a steep dose–response curve. The lower doses were less effective in stopping SREDs while higher doses produced almost complete inhibition of SREDs. The ED50 value was computed to be 58.75 ± 2.43 μM. Carisbamate was applied to neurons during SREDs and it took approximately 10–15 min for its antiepileptic effect to be fully manifested. Upon washout of carisbamate, SREDs returned after approximately 15–20 min (Fig. 2C). Carisbamate produced identical effects to well known anticonvulsants in this in vitro model of acquired epilepsy (Sombati and DeLorenzo, 1995). Phenytoin, a clinically used AED, is a use dependent Na+ channel blocker that is used for the treatment of generalized tonic–clonic and partial complex seizures (Kwan et al., 2001; Rogawski and Loscher, 2004). As shown in Fig. 2D, phenytoin (10 μM) at therapeutically relevant concentrations reversibly blocked SREDs. Phenobarbital, a GABAergic agonist, is another anticonvulsant that effectively blocked SREDs in our model (data not shown). Ethosuximide, a T-type Ca2+ channel blocker used to treat absence seizures, failed to block SREDs at concentrations up to 1 mM in this model (Fig. 2E). These results are consistent with clinical effects of these drugs for the treatment of temporal lobe epilepsy.
Figure 3.
Carisbamate inhibits low Mg2+-induced SRED activity in a concentration dependent manner. Frequency of SREDs in the presence of varying concentrations of carisbamate was measured over a 30-min period and then plotted as a percentage change (inhibition) from control (absence of carisbamate) SRED frequencies. Carisbamate blocked low Mg2+-induced SREDs with an EC50 = 58.75 ± 2.43 μM. Each data point represents percentage change from control ± S.E.M. (n = 5–7 neurons/concentration).
Effect of carisbamate on SRF
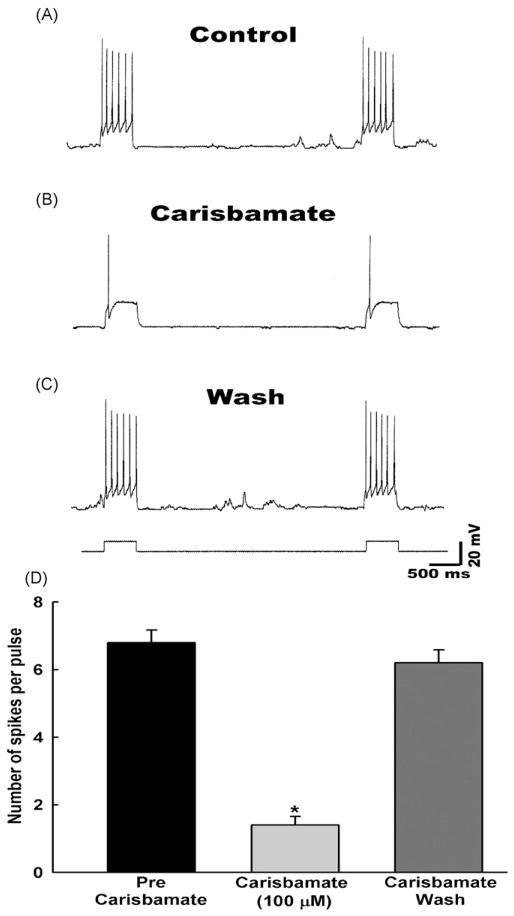
Several AEDs including phenytoin, carbamazepine, valproic acid and lamotrigine are known to block SRF in neurons (Kwan et al., 2001; Rogawski and Loscher, 2004). This effect of AEDs is believed to be due to their ability to block voltage gated Na+ channels. We investigated if carisbamate shares this property with other AEDs. Cultured hippocampal neurons respond to a sustained depolarizing current by generating a series of action potentials. Under control conditions, a depolarizing current (0.5 nA, 0.3 Hz) elicited an average of 4–10 action potentials (spikes) (Fig. 4A). Carisbamate (100 μM) caused a significant (p < 0.001) decrease in the frequency of spikes elicited by the depolarizing current and abolished the late sustained phase of firing without blocking the initial spike (Fig. 4B and D). While the effects of carisbamate were reversible, it took between 10 and 15 min for the restoration of SRF following washout (Fig. 4C). Spike frequency following carisbamate washout was not significantly different from pre-carisbamate spike frequency (Fig. 4D).
Figure 4.
Carisbamate inhibition of sustained repetitive firing (SRF) in cultured hippocampal neurons. Depolarizing pulses (0.5 nA, 0.3 Hz) were applied during each set of recordings obtained as follows: (A) control period before application of carisbamate, (B) during the application of carisbamate (100 μM) and (C) following carisbamate washout. (D) A bar graph representation of average inhibitory effect of carisbamate on SRF in hippocampal neurons. Data represented as mean spike frequency ± S.E.M. (n = 6, *p < 0.001).
Effects of carisbamate on low Mg2+-induced high frequency spiking
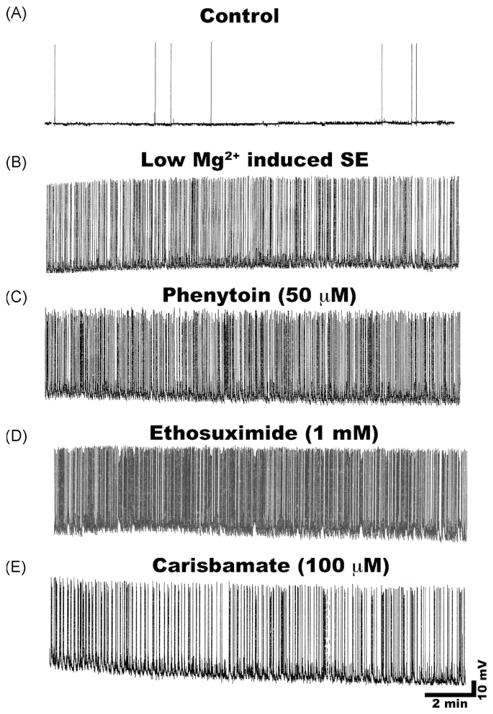
In normal condition medium, cultured hippocampal neurons exhibited spontaneous action potentials that were comparatively infrequent and irregular (Fig. 5A). However, upon removal of Mg2+ from extracellular medium, neurons generated bursts of action potentials resulting in the development of continuous high frequency epileptiform discharges (Fig. 5B). This epileptiform activity consisted of repetitive burst discharges with each burst being comprised of multiple spikes overlaying a depolarization shift. Spike frequency upon removal of Mg2+ was greater than 3 Hz, meeting the criteria of status epilepticus (Sombati and DeLorenzo, 1995). This technique has been widely used to model electrographic status epilepticus-like condition in vitro.
Figure 5.
Effect of carisbamate and other AEDs on low Mg2+-induced status epilepticus in cultured hippocampal neurons. (A) Representative control neuron displaying intrinsic baseline activity consisting of spontaneous action potentials. (B) Induction of continuous epileptiform status epilepticus-like activity in a neuron upon low Mg2+ treatment. Effects of clinically used AEDs on low Mg2+-induced high frequency spiking. (C) Phenytoin (50 μM) or (D) Ethosuximide (1 mM) failed to block high frequency spiking. (E) Carisbamate (100 μM) appeared to slow down spike frequency compared to phenytoin or ethosuximide but this effect was not different from neurons exposed to low Mg2+ alone.
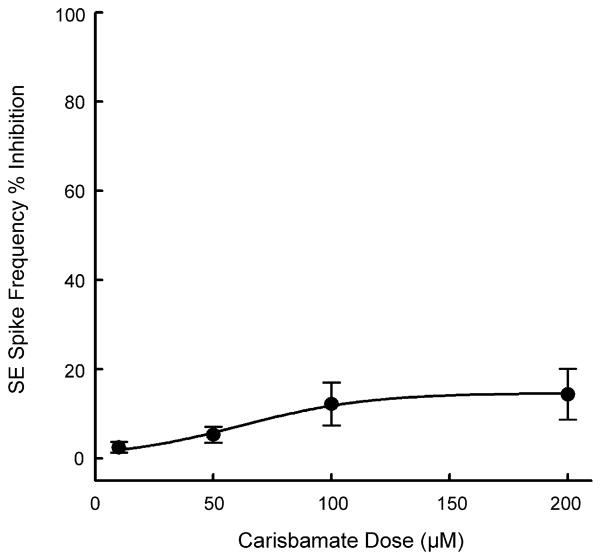
As shown in Fig. 5, clinically used AEDs including phenytoin (50 μM) and ethosuximide (1 mM) at therapeutic concentrations or higher failed to stop low Mg2+-induced in vitro status epilepticus-like activity in this model (Sombati and DeLorenzo, 1995). This type of in vitro continuous seizure discharge has been extremely refractory to anticonvulsant treatment (Blair et al., 2006; Deshpande et al., 2007b; Sombati and DeLorenzo, 1995). We investigated if carisbamate could act differently and block this high frequency spike activity. Treatment with carisbamate (100 μM) appeared to marginally slow down the low Mg2+-induced high frequency spiking (Fig. 5E). However, this effect was not significantly different from low Mg2+ treated neurons. The graph in Fig. 6 shows the effects of increasing concentrations of carisbamate on the inhibition of low Mg2+-induced high frequency spike activity. Even at concentrations of up to 200 μM, carisbamate inhibited spike frequency only by 10–15%. The ED50 value for effects of carisbamate on inhibition of status epilepticus-like activity could not be calculated since the effects never reached 50% inhibition. High frequency firing was restored within 10–15 min following washout of carisbamate with low Mg2+ solution.
Figure 6.
Concentration–response analysis of effects of carisbamate on low Mg2+-induced high frequency spiking in cultured hippocampal neurons. Increasing concentrations of carisbamate had little effect on low Mg2+-induced high frequency spiking. Even at concentrations of up to 200 μM only a 10–12% inhibition was achieved. Spike frequencies in the presence of varying concentrations of carisbamate were measured over a 15-min period and then plotted as a percentage change (inhibition) from control (absence of carisbamate) spike frequencies. An EC50 value could not computed since 50% inhibition was not achieved. Each data point represents percentage change from control ± S.E.M. (n = 5–7 neurons/concentration).
Discussion
This study demonstrates that carisbamate blocks the expression of SREDs in the hippocampal neuronal culture model. In addition, carisbamate reduced the frequency of action potentials generated during depolarization-induced SRF. Our results are consistent with other studies that have demonstrated antiepileptic properties of carisbamate in various in vivo models of seizures (Bialer et al., 2007; Francois et al., 2005; Grabenstatter and Dudek, 2004; Nehlig et al., 2005; Rogawski, 2006; White et al., 2006). While the exact mechanism of action of carisbamate is not known and is being actively investigated, the ability of carisbamate to inhibit depolarization-induced SRF may account in part for some of the anticonvulsant effects of carisbamate.
The majority of patients with epilepsy have their seizures well controlled with conventional AEDs; however, approximately 40% of patients manifest refractory epilepsy (Duncan et al., 2006; French, 2007). Thus, there is a significant need to develop new AEDs to attempt to treat refractory patients. Novel AEDs are being developed to address this need of drug resistant epilepsy (Bialer et al., 2007; Rogawski, 2006). In fact, out of the currently available 20 AED’s, 8 were introduced in the past decade (LaRoche and Helmers, 2004). However, despite introduction of newer AEDs, the prognosis still remains poor (Cramer et al., 1999a; French, 2007). Thus there are continual efforts to develop more efficacious drugs that have fewer side effects and a wider margin of safety (Bialer et al., 2007; Rogawski, 2006).
The novel neuromodulator carisbamate displays high potency in a variety of in vivo and in vitro seizure models at doses well below those that produce CNS toxicity (Novak et al., 2007). Dose-related CNS toxicities such as decreased activity, ataxia, sedation, and prostration were observed at doses well above 350 mg/kg orally, whereas carisbamate afforded protection in various seizure models is observed below 20 mg/kg, i.p. In addition, no significant organ toxicity was observed in repeated-dose oral toxicity studies in adult rats up to 6 months (Novak et al., 2007). However, carisbamate’s mechanism(s) of action remains to be elucidated. As a phenyl monocarbamate, carisbamate has some structural features in common with the phenyl dicarbamate felbamate. Felbamate-related dicarbamates have been shown to affect Na+, Ca2+, and NMDA currents (White et al., 1992; Stefani et al., 1996; Subramaniam et al., 1995). Flurofelbamate at 100 μM concentrations has been reported to cause a slight decrease in GABA-mediated responses (Roecklein et al., 2007). All of these effects could underlie the antiepileptic effects of dicarbamates. While the effects of carisbamate on these ionic currents and contribution of these actions towards its antiepileptic profile remains to be investigated, our studies on the effects of carisbamate on depolarization-induced SRF indicate that a possible use-or state-dependent block of Na+ channels could underlie some of its antiepileptic effects. Alterations in intracellular Ca2+ dynamics have been demonstrated to underlie the expression of SREDs in this in vitro model of acquired epilepsy (Delorenzo et al., 2005). The ability of carisbamate to inhibit SREDs could also be due to its effects on restoration of altered Ca2+ homeostasis in epileptic neurons. It will be interesting to investigate this possibility in future studies.
Expression of recurrent epileptiform discharges after low Mg2+-induced status epilepticus-like injury in vitro is a commonly used model to study the mechanisms underlying seizures, seizure-induced plasticity and development of pharmacoresistant seizures (Delorenzo et al., 2005; Deshpande et al., 2007a; Engel et al., 2000; Goodkin et al., 2005). The hippocampal neuronal culture model of SREDs mimics the electrophysiological and molecular changes observed during the induction, maintenance and expression phases of epilepsy (Delorenzo et al., 2005). Moreover, the SREDs exhibited in vitro in cultured neurons manifested essentially identical electrographic properties to seizures recorded from in vivo models of epilepsy and these in vitro SREDs also demonstrated neuronal population synchronicity and anticonvulsant sensitivity in a manner that reflects the characteristics of seizures in intact animal models (Delorenzo et al., 2005; Mangan and Kapur, 2004; Sombati and DeLorenzo, 1995). Although the neuronal cultures do not have true anatomical connections and do not display in vivo seizures, the hippocampal neuronal culture model of epileptiform discharges has the unique advantage of applying various in vitro techniques to study molecular mechanisms underlying epilepsy and mechanisms of actions of investigational drugs in a more controlled environment.
Acknowledgments
This research was funded by support from Johnson & Johnson Pharmaceutical Research and Development and from the National Institute of Neurological Disorders and Stroke grants RO1NS051505, RO1NS052529 and UO1NS058213 and from the Milton L. Markel Alzheimer’s Disease and the Sophie and Nathan Gumenick Neuroscience Research Funds to R.J.D. We thank Elisa Attkisson for her help with preparation and maintenance of neuronal cultures.
References
- Bialer M, Johannessen SI, Kupferberg HJ, Levy RH, Perucca E, Tomson T. Progress report on new antiepileptic drugs: A summary of the Eigth Eilat Conference (EILAT VIII) Epilepsy Res. 2007;73:1–52. doi: 10.1016/j.eplepsyres.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Blair RE, Deshpande LS, Sombati S, Falenski KW, Martin BR, DeLorenzo RJ. Activation of the cannabinoid type-1 receptor mediates the anticonvulsant properties of cannabinoids in the hippocampal neuronal culture models of acquired epilepsy and status epilepticus. J Pharmacol Exp Ther. 2006;317:1072–1078. doi: 10.1124/jpet.105.100354. [DOI] [PubMed] [Google Scholar]
- Churn SB, Sombati S, Jakoi ER, Sievert L, DeLorenzo RJ. Inhibition of calcium/calmodulin kinase II alpha subunit expression results in epileptiform activity in cultured hippocampal neurons. Proc Natl Acad Sci USA. 2000;97:5604–5609. doi: 10.1073/pnas.080071697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer JA, Fisher R, Ben-Menachem E, French J, Mattson RH. New antiepileptic drugs: Comparison of key clinical trials. Epilepsia. 1999a;40:590–600. doi: 10.1111/j.1528-1157.1999.tb05561.x. [DOI] [PubMed] [Google Scholar]
- Cramer JA, Westbrook LE, Devinsky O, Perrine K, Glassman MB, Camfield C. Development of the quality of life in epilepsy inventory for adolescents: The QOLIE-AD-48. Epilepsia. 1999b;40:1114–1121. doi: 10.1111/j.1528-1157.1999.tb00828.x. [DOI] [PubMed] [Google Scholar]
- Delorenzo RJ, Sun DA, Deshpande LS. Cellular mechanisms underlying acquired epilepsy: the calcium hypothesis of the induction and maintainance of epilepsy. Pharmacol Ther. 2005;105:229–266. doi: 10.1016/j.pharmthera.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande LS, Blair RE, Nagarkatti N, Sombati S, Martin BR, DeLorenzo RJ. Development of pharmacoresistance to benzodiazepines but not cannabinoids in the hippocampal neuronal culture model of status epilepticus. Exp Neurol. 2007a;204:705–713. doi: 10.1016/j.expneurol.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande LS, Blair RE, Ziobro JM, Sombati S, Martin BR, DeLorenzo RJ. Endocannabinoids block status epilepticus in cultured hippocampal neurons. Eur J Pharmacol. 2007b;558:52–59. doi: 10.1016/j.ejphar.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande LS, Sombati S, Blair RE, Carter DS, Martin BR, DeLorenzo RJ. Cannabinoid CB1 receptor antagonists cause status epilepticus-like activity in the hippocampal neuronal culture model of acquired epilepsy. Neurosci Lett. 2007c;411:11–16. doi: 10.1016/j.neulet.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. The Lancet. 2006;367:1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- Engel D, Endermann U, Frahm C, Heinemann U, Draguhn A. Acute effects of [gamma]-vinyl-GABA on low-magnesium evoked epileptiform activity in vitro. Epilepsy Res. 2000;40:99–107. doi: 10.1016/s0920-1211(00)00112-1. [DOI] [PubMed] [Google Scholar]
- Francois J, Ferrandon A, Koning E, Nehlig A. A new drug RWJ 333369 protects limbic areas in the lithium-pilocarpine model (lipilo) of epilepsy and delays or prevents the occurrence of spontaneous seizures (abstract) Epilepsia. 2005;46(C204):269. [Google Scholar]
- French JA. Refractory epilepsy: clinical overview. Epilepsia. 2007;48:3–7. doi: 10.1111/j.1528-1167.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci. 2005;25:5511–5520. doi: 10.1523/JNEUROSCI.0900-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenstatter HL, Dudek FE. The use of chronic models in antiepileptic drug discovery: the effect of RWJ-333369 on spontaneous motor seizures in rats with kainate-induced epilepsy (abstract) Epilepsia. 2004;45(192016):197. doi: 10.1111/j.0013-9580.2005.13404.x. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Hesdorffer DC. Epilepsy: Frequency, Causes and Consequences. Demos; New York: 1990. [Google Scholar]
- Kwan P, Sills GJ, Brodie MJ. The mechanisms of action of commonly used antiepileptic drugs. Pharmacol Therapeut. 2001;90:21–34. doi: 10.1016/s0163-7258(01)00122-x. [DOI] [PubMed] [Google Scholar]
- LaRoche SM, Helmers SL. The new antiepileptic drugs: scientific review. JAMA. 2004;291:605–614. doi: 10.1001/jama.291.5.605. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Bertram EH, 3rd, Stringer JL. Functional anatomy of hippocampal seizures. Prog Neurobiol. 1991;37:1–82. doi: 10.1016/0301-0082(91)90011-o. [DOI] [PubMed] [Google Scholar]
- Mangan PS, Kapur J. Factors underlying bursting behavior in a network of cultured hippocampal neurons exposed to zero magnesium. J Neurophysiol. 2004;91:946–957. doi: 10.1152/jn.00547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara JO. Emerging insights into the genesis of epilepsy. Nature. 1999;399:A15–A22. doi: 10.1038/399a015. [DOI] [PubMed] [Google Scholar]
- Murray MI, Halpern MT, Leppik IE. Cost of refractory epilepsy in adults in the USA. Epilepsy Res. 1996;23:139–148. doi: 10.1016/0920-1211(95)00090-9. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Rigoulot MA, Boehrer A. A new drug, RWJ 333369 displays potent antiepileptic properties in genetic models of absence and audiogenic epilepsy (abstract) Epilepsia. 2005;46(212368):215. [Google Scholar]
- Novak GP, Kelley M, Zannikos P, Klein B. Carisbamate (RWJ-333369) Neurotherapeutics. 2007;4:106–109. doi: 10.1016/j.nurt.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Sun D, Limbrick D, Rafiq A, DeLorenzo RJ. Epileptogenesis induces long-term alterations in intracellular calcium release and sequestration mechanisms in the hippocampal neuronal culture model of epilepsy. Cell Calcium. 2001;30:285–296. doi: 10.1054/ceca.2001.0236. [DOI] [PubMed] [Google Scholar]
- Roecklein BA, Sacks HJ, Mortko H, Stables J. Fluoro-felbamate. Neurotherapeutics. 2007;4:97–101. doi: 10.1016/j.nurt.2006.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA. Diverse mechanisms of antiepileptic drugs in the development pipeline. Epilepsy Res. 2006;69:273–294. doi: 10.1016/j.eplepsyres.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- Sombati S, DeLorenzo RJ. Recurrent spontaneous seizure activity in hippocampal neuronal networks in culture. J Neurophysiol. 1995;73:1706–1711. doi: 10.1152/jn.1995.73.4.1706. [DOI] [PubMed] [Google Scholar]
- Stefani A, Calabresi P, Pisani A, Mercuri N, Siniscalchi A, Bernardi G. Felbamate inhibits dihydropyridine-sensitive calcium channels in central neurons. J Pharmacol Exp Ther. 1996;277:121–127. [PubMed] [Google Scholar]
- Subramaniam S, Rho J, Penix L, Donevan S, Fielding R, Rogawski M. Felbamate block of the N-methyl-D-aspartate receptor. J Pharmacol Exp Ther. 1995;273:878–886. [PubMed] [Google Scholar]
- White HS, Srivastava A, Klein B, Zhao B, Choi YM, Gordon R, Lee SJ. The novel investigational neuromodulator RWJ 333369 displays a broad-spectrum anticonvulsant profile in rodent seizure and epilepsy models (abstract) Epilepsia. 2006;47:200–201. [Google Scholar]
- White HS, Wolf HH, Swinyard EA, Skeen GA, Sofia RD. A neuropharmacological evaluation of felbamate as a novel anticonvulsant. Epilepsia. 1992;33:564–572. doi: 10.1111/j.1528-1157.1992.tb01711.x. [DOI] [PubMed] [Google Scholar]