Summary
Individual cells in their particular environments adhere to the extracellular matrix (ECM) and their neighbors via integrin- and cadherin-containing complexes, respectively. The dynamics of these interactions regulate the formation and maintenance of complex tissues. An expanding body of evidence accentuates the role of the small Rap1 GTPase and its associated signaling network in many of these processes. In this review we will discuss more recently revealed roles of Rap1 signaling by primarily focusing on functions of the Rap1 effectors RIAM, KRIT-1/CCM1 and AF-6/Afadin in junctional regulation of the vascular system and in epithelial cells. Furthermore, we will describe novel findings on the Rap activator PDZ-GEF in the regulation of cell-cell adhesion between epithelial cells and within a stem cell niche.
Introduction
Integrin-mediated adhesion to the ECM and cadherin-mediated adhesion between cells within developmental and physiological compartments are dynamically regulated. Consequently, failures in efficient adhesion control can result in pathogenic conditions. Interestingly, signaling through the small Rap1 GTPase has been implicated in both integrin- and cadherin-mediated adhesion events. As a member of the Ras superfamily of small GTPases, Rap1 is subject to a GTP/GDP exchange cycle in which dedicated Rap1 specific guanine nucleotide exchange factors (GEFs) promote the exchange of GDP to GTP, thereby converting Rap1 into an active signaling competent form. The latter in turn triggers spacio-temporally defined effector signaling that can stimulate a variety of cellular events. Rap1 GTPase activating proteins (GAPs), on the other hand, terminate the signal output through Rap1 by enhancing its intrinsic rate of GTP hydrolysis. Several Rap1 GEFs, such as C3G, and members of the Epac, CalDAG-GEF and PDZ-GEF subfamilies have been identified, which have been implicated in the regulation of adhesion complexes [1,2].Also, several Rap1 effectors have been ascribed to adhesion complexes[1,2]. In particular, recent light has been shed on the RIAM, KRIT-1/CCM1 and AF-6 proteins and the molecular machineries they impinge on. In this review we will exemplify these by illustrating them in the experimental model systems that enabled their discovery. These include genetically tractable organisms as well as specifically tailored cell culture systems.
Emerging mechanisms for Rap1 signaling in integrin activation
Rap1 has been implicated in a variety of integrin-mediated ‘inside-out’ signaling events. In particular, Rap1 regulates integrin β1, β2 and β3 subunits to affect both integrin affinity and avidity (clustering) [1]. A recent series of observations has unraveled a Rap1-dependent mechanism that could explain how agonist-induced stimulation of platelets modulates their interaction with extracellular ligands. The details of this mechanism likely will be applicable to a number of cell types. Due to their property to spread and aggregate, platelets protect the vascular system against damage. Agonists, like thrombin, are released or activated at sites of vascular injury and activate G-protein coupled receptors and protein kinase Cα (PKCα) to trigger the release of intracellular Ca2+ [3]. As a result αIIbβ3 integrins increase their affinity for extracellular ligands, such as Willebrand factor, fibrinogen or fibronectin to enable a clotting reaction and the formation of a thrombus. Han et al. were able to reconstitute the integrin activation process by engineering Chinese Hamster Ovary (CHO) cells that stably express αIIbβ3 integrin dimers, ectopic talin and PKCα and found them to respond to the PKC agonist Phorbol Myristate Acetate (PMA) [4••]. Talin gets recruited to integrin cytoplasmic tails in the activation process and establishes one of numerous integrin-cytoskeletal linkages [5]. The relevance of talin for cell-to-matrix adhesion is reflected by the inability of talin-1 deficient embryonic stem cells and fibroblasts to properly link integrins to the actin cytoskeleton, assemble focal adhesions, and spread on a permissive ECM substrate [6,7]. Moreover, conditional disruption of talin in platelets or megakaryocytes blocks αIIbβ3 activation and results in a bleeding diathesis in mice [8]. Talin is build of an N-terminal globular head and a C-terminal rod-like tail domain [5]. Within its head portion, talin harbors a FERM-domain that can associate with integrin β cytoplasmic tails. An additional integrin binding motif, as well as vinculin and actin binding sites, reside in the rod part of the protein. According to current models, the integrin interacting motifs in talin are functionally masked in cells with disengaged integrins, probably due to an intra- or inter-molecular interaction [9]. In its autoinhibited conformation, talin localizes to the cytoplasm and only after agonist stimulation translocates to integrin complexes [10]. Hence, the question how talin becomes ‘unlocked’ and stabilized in its open conformation, and thus rendered competent for productive integrin interaction is of central importance. Rap1 had been shown to be activated in platelets in response to thrombin stimulation [11], and, indeed, expression of Rap1GAP in reconstituted CHO cells inhibits αIIbβ3 integrin activation in response to PMA. Conversely, constitutively active Rap1 can bring about integrin activation independently of PKCα and in the absence of PMA. In this process, Rap1 strictly depends on talin, since Rap1-induced integrin activation is blocked by an integrin binding-deficient talin mutant and can be bypassed by an ‘activated’ talin fragment derived from its head domain. Rap1 causes talin to translocate from the cytoplasm into integrin complexes. Surprisingly, however, Rap1 does not stimulate talin phosphorylation or proteolytic cleavage between talin’s head and rod domains, which are two vividly discussed modes of talin activation [4••,9]. Thus, one explanation for Rap1’s effect on talin could be the induction of a conformational change that allows for translocation and integrin activation.
Further illuminating the mechanism is the finding that Rap1’s ability to activate integrins in CHO cells hinges on the function of RIAM as an effector molecule [4••]. RIAM (for Rap1-GTP-interacting adaptor molecule), originally isolated by yeast-two-hybrid screening, is characterized by an RA-domain, which mediates its interaction with Rap1 [12•]. The protein sequence located N-terminally of RIAM’s RA domain harbors a proline-rich patch bracketed by two potential coiled-coil regions. Further C-terminal of the RA-domain reside a PH-domain and a second proline-rich region (Figure 1A). RIAM belongs to the MRL (Mig-10/RIAM/Lamellipodin) family of adaptor proteins, with members being present in organisms ranging from C. elegans and Drosophila to humans. When overexpressed, RIAM can induce cells to spread and form lamellipodia as well as activate β1 and β2-containing integrin complexes [12•]. Interestingly, RIAM co-immunoprecipitates with talin and both proteins co-localize to focal adhesions (FA) early in their formation. Importantly, ectopic expression of RIAM actively triggers talin-dependent integrin activation [4••]. Conversely, RIAM depletion in reconstituted CHO cells effectively decreases both Rap1V12 and thrombin receptor/Protease Activated Receptor (PAR)-induced integrin activation. Also RNAi against RIAM in Jurkat T-cells inhibits integrin adhesion to extracellular fibronectin [4••,12•,13•]. The nature of the Rap1/RIAM interaction, however, suggests some level of complexity, since RIAM - in at least one setting - was able to modulate the distribution of Rap1 itself. RIAM’s down-regulation by RNAi coincided with a displacement of endogenous Rap1 from the plasma membrane [12•]. In a different set of experiments, expression of a fusion between a small motif in RIAM’s N-terminus predicted to form an amphopathic helix and the C-terminal membrane-targeting CAAX box of Rap1, is sufficient to translocate talin to integrin complexes [14]. While the intermolecular dependencies are not completely understood, it is likely that RIAM provides a scaffolding function by binding to Rap1, which enables subsequent talin tethering and integrin activation (Figure 1B). Watanabe and colleagues corroborated this model by live cell experiments, in which they used elegant bimolecular fluorescence complementation (BiFC) microscopy with split moieties of the Venus protein. Expression of the N-terminal half of Venus fused to the N-terminus of talin and the C-terminal half of Venus fused to the αIIb integrin subunit reconstitutes Venus-specific fluorescence when an integrin-activating signal is relayed via talin. This bimolecular complementation signal is enhanced by the presence of constitutively active Rap1V12 or over-expressed RIAM. Conversely, the signal can be dampened by RNAi-mediated down-regulation of RIAM in cells whose PAR receptors are activated by the addition of thrombin [13•]. The above assay represents a sophisticated experimental tool that could be employed to examine the contribution of individual RapGEF proteins, and varied to further decipher the role of Rap1 signaling networks in various integrin activation events. Although not investigated yet in Rap1/RIAM/talin-dependent αIIbβ3 integrin activation in thrombin-responsive CHO cells or platelets, a relevant RapGEF required for Rap1 activation could be CalDAG-GEF1. CalDAG-GEF1 is responsive to Ca2+ and diacylglycerol (DAG), and knock-down or knock-out of CalDAG-GEF1 interferes with αIIbβ3 activation [15,16].
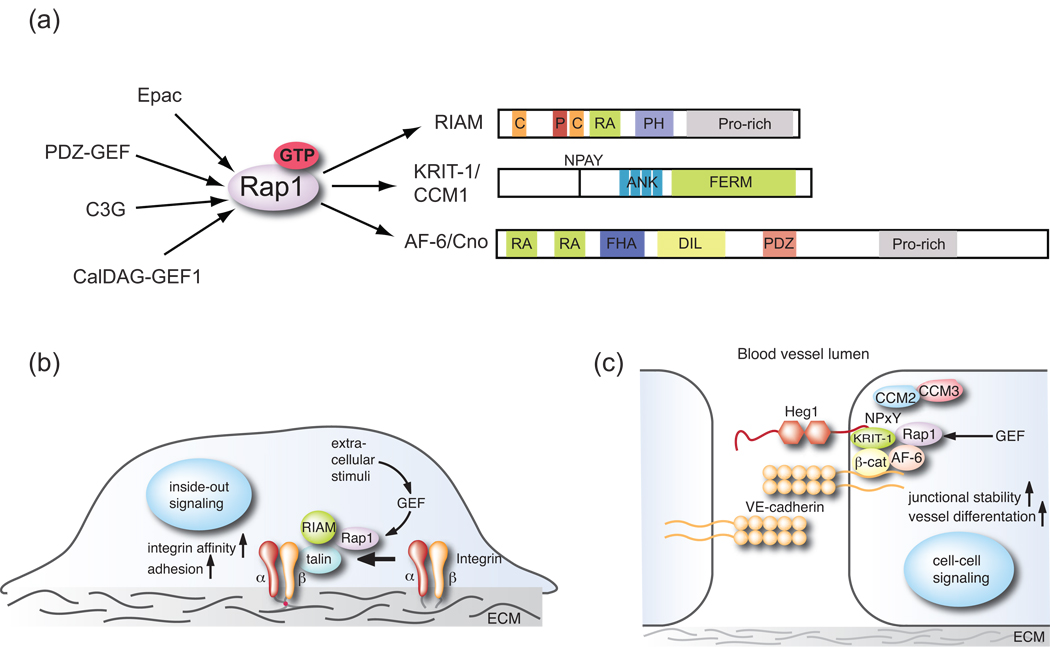
Figure 1.
(A) Rap1 is activated by a group of highly specific GEFs that perform a GDP/GTP nucleotide exchange reaction. Loaded with GTP, Rap1 is competent to associate with effector proteins to initiate downstream signaling. The RIAM, KRIT/CCM1 and AF-6 protein are distinguished by various scaffolding domains: C, coiled-coil; P, proline-rich; RA, Ras associating; PH, Pleckstrin homology; ANK, Ankyrin repeat; FERM, band 4.1/Ezrin/Radixin/Moesin homology; FHA, fork head associated; DIL, dilute/Myo5 homology and PDZ, PSD-95/Discs large/ZO-1 domains. (B) Schematic diagram of the RIAM and talin-dependent effect of Rap1 on integrin complexes and their increased affinity to ECM molecules. (C) Schematic view of the proteins that act downstream of Rap1 in endothelial junctions. All diagrams are incomplete depictions of Rap1-interacting proteins and complexes and restricted to the molecules referred to in the text.
Comparative experiments showed that the closely related RIAM homologue Lamellipodin (Lpd) has no effect on Rap1’s potential to activate integrins, and an interaction between Rap1 and Lpd in vivo so far has not been demonstrated [13•,17]. Since also Lamellipodin contains a well-conserved RA-domain it is possible that it enters into a complex with another Ras family GTPase. Such an interaction might either regulate adhesion events or be otherwise required. It is interesting to note that many integrin-mediated events that induce gross morphological changes in cells, like e.g. motility in migration processes, are associated with an expansion and/or treadmilling of the integrin linked actin cytoskeleton. Both, RIAM and Lpd stimulate actin polymerization through their affinity to members of the Ena/VASP family, which catalyze actin assembly and branching [12•,17]. Also, the C. elegans MRL protein Mig-10 uses its affinity to Ena/VASP to promote axon guidance and outgrowth [18]. Depletion of RIAM can markedly increase the cellular ratio between monomeric G-actin and filamentous F-actin [12•,17]. It is thus tempting to speculate that the talin-activating role of RIAM is coupled to local changes in actin assembly. Many of the constituents in integrin complexes actively associate with actin to stabilize the interaction with ECM ligands. Noteworthy, the only MRL representative in Drosophila, Pico, also has the ability to shift G:F-actin ratios toward the polymerized state due to a direct effect on Ena [19•]. Importantly, this in turn stimulates the serum response factor (SRF) to provoke tissue growth. In agreement with this, loss of Pico inhibits growth of the entire organism, while ectopic activity of Pico can produce over-growth phenotypes [19•]. These findings taken together demonstrate an unanticipated versatility within the MRL family and imply that actin-related activities proximal to adhesion complexes could be coupled to growth regulation of the whole organism.
Rap1 signaling can engage different mechanisms of integrin activation depending on the specific complex and cell type under consideration. Besides RIAM, also the Rap1 effector RapL has been implicated in integrin activation. In lymphoid cells, Rap1 promotes the translocation of RapL to αLβ2-type LFA-1 integrin receptors, which consequently become activated [20,21]. In RapL deficient mice, lymphocyte adhesion and migration as well as lymphocyte homing are drastically impaired [22]. Another level of complexity is given by the fact that different RapGEFs can activate Rap1 to promote integrin-mediated adhesion. As indicated above, CalDAG-GEF1 affects integrin adhesion in platelets and megakaryocytes [15,16]. However, in different biological contexts other GEFs mediate Rap1 activation or even work in concert with each other. For example, in fibroblasts that lack C3G, integrin-mediated adhesion is ineffective and cells show an ectopic migration behavior. CalDAG-GEF1 and Epac can both compensate for C3G in these circumstances [23]. Expression of a dominant-negative C3G protein also interferes with cell scattering and adhesion induced by hepatocyte growth factor (HGF) [24]. Interestingly, also the Drosophila paralogue of the PDZ-GEF proteins mediates integrin-dependent cell shape changes of hemocytes in the fly embryo [25]. Thus, different GEFs may control Rap1 in its capacity to activate the integrin-ECM adhesion switch and the precise requirements and contributions will have to be further dissected.
Diverse roles for Rap1 in the endothelium
Research in different arenas has revealed functions for Rap1 in the endothelium and in cells that interact with it. For example, Rap1 regulates adhesion among endothelial cells and also has functions in lymphoid cells that are involved in transendothelial migration [1,26]. Tight regulation of angiogenesis and permeability of differentiated blood vessels is essential to the homeostasis of any higher organism. Consequently, failures in establishing a normal vasculature, in adjusting its architecture to varying oxygen requirements and in maintaining its integrity can have detrimental consequences [27]. Also, the microvasculature in developing tumors appears to be especially refractile to mechanical stress with a compounding effect on overall tumor etiology [28]. Central for endothelial function are VE-cadherin-based adherens junctions (AJ), and Rap1 signaling has been implicated in their differentiation and maintenance [2]. Among the Rap1GEFs, Epac promotes endothelial barrier formation in response to cAMP. The Epac-specific cAMP analog 8CPT-2'O-Me-cAMP (007) is able to activate Rap1 to increase permeability of cultured endothelial monolayers in a VE-cadherin-dependent manner [29–32]. The structural basis for Epac activation, its translocation to the membrane and activation of Rap1 has recently been revealed by crystallographic and detailed microcopic studies [33,34]. In human umbilical vein endothelial cells (HUVECs) and human arterial endothelial cells (HAECs), VE-cadherin also recruits the scaffolding protein MAGI-1 to AJs. The MAGI-1 protein is linked to β-catenin and PDZ-GEF1, another RapGEF [35]. These interactions are relevant for VE-cadherin mediated adhesion since depletion of MAGI-1 in HUVECs reduces their adhesive potential [35]. Moreover, vascular development within the yolk sac and embryo is disrupted in PDZ-GEF1 mutant mice [36]. Thus, different aspects of AJ maturation and stablility in the endothelium may be regulated by different Rap1 activating mechanisms.
So far, the selectivity of RapGEFs towards specific Rap1 effectors has been insufficiently resolved. However, two effectors that reside in AJs are the KRIT-1/CCM1 and AF-6/Afadin proteins. Recent evidence has clearly implicated KRIT-1/CCM1 in the morphogenesis and homeostasis of the vascular system. Mutations in the human KRIT-1 gene (KRIT-1 stands for Rap1/Krev1 interaction trapped 1; [37]) are a cause of cerebral cavernous malformations (CCMs) [38]. CCMs are vascular dysplasias arising spontaneously or due to familial, autosomal, dominant mutations in 3 genes that so far have been found causative for the disease, KRIT-1/CCM1, MGC4607/CCM2 and PDCD10/CCM3 [38–41]. Hallmarks of CCMs are densely packed conglomerates of thin-walled dilated vessels that are enveloped by a collagen layer and often leak blood. CCMs develop mainly in the central nervous system [42,43], however, they also occur systemically in other parts of the body [44]. Animal studies in mice and zebrafish demonstrated that exclusive loss of CCM function in the endothelial compartment of mice and zebrafish results in heart, arterial and venous malformations [27]. However, in light of the low frequency with which hemorrhagic lesions occur in mice heterozygous for KRIT-1, it has been suggested that additional mutations are necessary for a phenotypic onset. Indeed, in a recently developed mouse model the incidence rate of vascular lesions evoked by KRIT-1 heterozygosity is significantly elevated in a p53 −/− background. Because the remaining wild type allele of KRIT-1 remained unaffected in the destabilized genome, either a p53-dependent mechanism or yet unidentified mutations must interact with mutant KRIT-1 in mice [45]. This model may empower the search for cooperating players by genetic means.
The KRIT-1 protein is present at low amounts in AJs within the human endothelium [46] and in cultured arterial (BAECs) and venous (UVECs) endothelial cells [47••]. Importantly, the level of KRIT-1 in endothelial AJs correlates with the permeability of endothelial cell monolayers. KRIT-1 immunoprecipitates in a complex with VE-cadherin, β-catenin, p120catenin and AF-6, and its concentration in AJs is augmented in the presence of constitutively active Rap1V12 [47••]. BAECs treated with thrombin loose KRIT-1 from their AJs, which is reversed by addition of the cAMP analog 8CPT-2'O-Me-cAMP, supporting the notion that Epac and Rap1 stimulate KRIT’s translocation to cell-cell contacts. Structurally, KRIT-1 is lacking a consensus RA-domain that characterizes most Ras/Rap effectors. Instead, it features 4 centrally located ankyrin repeats and a C-terminal FERM-domain (Figure 1A), the latter of which has been demonstrated to mediate KRIT-1’s localization to AJs as well as its interaction with Rap1. These binding characteristics evoke a possible mechanism in which Rap1 binding increases the accessibility of KRIT-1’s FERM domain to adherens junctional partners which in turn may promote junctional maturation and/or integrity [47••].
Endothelial junctions, like epithelial junctions, are strengthened by a prominent actin cytoskeleton and the extent of actin polymerization in the proximity of junctions governs the permeability of the endothelium [32,48,49]. In fact, over-expression of KRIT-1 elevates the levels of junction-associated F-actin whereas depletion of KRIT-1 provokes a loss of F-actin at AJs [47••]. How KRIT-1 interacts with junctional partners and triggers actin polymerization are pertinent questions. First clues are provided by observations that KRIT-1 is found in a complex with other CCM proteins [47••,50–52]. In particular, a phospho-tyrosine binding (PTB) domain in CCM2 couples to a consensus NPxY motif in KRIT-1, the relevance of which is testified by a familial missense mutation in CCM2 that specifically disrupts this interaction [51,53]. It should be noted that CCM2 binds in an analogous way to ICAP1, a β1 integrin interacting protein [54], suggesting that CCM proteins might have distinct functions in VE-cadherin and integrin-dependent adhesion complexes [53,55]. Like in KRIT-1 depleted cells, the AJ-associated cortical actin cytoskeleton cannot be maintained in CCM2-depleted cells, and instead stress fibers are formed, with the result of an impaired barrier function [56]•. The CCM2 mutant cytoskeletal phenotype correlates with an increase in RhoA activity and elevated stress signaling via JNK. RhoA and Rac1 both coimmunoprecipitate with CCM2 from endothelial cell extracts, and, importantly, inhibition of RhoA activity with simvastatin, a compound which blocks RhoA isoprenylation, partially eliminates the amount of stress fibers and suppresses permeability defects in cells with depleted CCM2. Interestingly, in CCM2−/− mice treated with VEGF, simvastatin normalizes the vascular permeability response [56]. Thus, it is tempting to speculate that the CCM protein complex is activated by Rap1 to locally dampen RhoA signaling during vascular modeling.
An interesting functional link has recently been revealed by the analysis of the heart of glass (Heg1) gene in mice and zebrafish. Similar to CCM mutants, Heg1 −/− animals experience serious defects in heart and blood vessel development and interestingly Heg1 knockdown in the zebrafish synergizes with that of KRIT-1 and CCM2 [57]. Although no vascular defects in Heg1−/− mice were observed before midgestation, Heg1 −/−; CCM2 +/− compound mutant zebrafish die earlier with an abnormal heart and vasculature [58••]. Transmission electron microscopy revealed that Heg1 −/−; CCM2 +/− mutant endothelia display significantly shortened AJs, suggesting a direct role for Heg1/CCM signaling in junctional formation and maintenance. In addition, depletion of CCM2 in cultured HUVECs interferes with spontaneous lumen formation of single cells [56,58••]. In zebrafish embryos, Heg1 loss of function, in combination with a reduced dosage at the CCM2 locus, inhibits endothelial cells to form lumenized tubes [58••].
Interestingly, biochemical experiments showed that CCM2 is recruited into a complex with Heg1 in a KRIT-1-dependent manner. Owing to its ability to form a complex with AJ proteins like β-catenin and AF-6, KRIT-1 may function to bridge the intracellular complexes associated with the AJ and the Heg1 membrane protein. (Figure 1C). Based on the above phenotypical and biochemical findings, it is tempting to speculate that a Rap1 regulated CCM/Heg1 complex affects AJ maturation and lumen formation in the differentiating vasculature. Rap1 in endothelial junctions might even undergo consecutive interactions with AF-6 and KRIT-1 to regulate VE-cadherin function and mediate crosstalk between Heg1 and VE-cadherin. It also will be interesting to see whether a human Heg1 orthologue will play a role in the etiology of CCMs.
Lessons from invertebrates
Rap1 functions in cell shape determination
Many developmental episodes in embryogenesis and organogenesis rely on the precise orchestration of cell shape changes, among which gastrulation offers often striking examples. To accomplish gastrulation processes, cells need to invaginate, intercalate, elongate or migrate, all of which likely require AJs to be modeled through changes in their associated proteins, including cytoskeletal components [59,60]. Specifically, work in Drosophila embryogenesis indicated that Rap1 participates in developmental processes that are distinguished by diverse shape alterations. These include the ventral invagination of the presumptive mesoderm, dorsal closure (DC) of the bilateral ectoderm, and the migration of primordial germ cells [61]. Although direct mechanistic proof is still lacking, it seems likely that signal transduction through Rap1 at least in part impinges on E-cadherin function to modulate these processes. Significantly, observations in proliferating epithelia indicate that Rap1 activity promotes AJ continuity. Rap1 accumulates in the cytokinetic furrow to specifically remodel the invaginating AJs on the mother cell’s membrane into separated junctional rings surrounding both sister cell’s apical membrane domains, thereby guarding AJ continuity over the entire tissue [62••].
Evidence from Drosophila DC has connected Rap1 to the Canoe (Cno) protein, the fly orthologue of AF-6 [63•]. Both genes genetically cooperate in embryos proceeding through DC. cno loss of function and expression of dominant negative Rap1N17 both provoke penetrant ‘dorsal open’ phenotypes in which the dorsalward migration of the bilateral ectoderm is blocked. Importantly, over-expression of a wild-type cno transgene can rescue all ectodermal defects produced by Rap1N17, while an N-terminal deletion mutant of Cno, which lacks the two RA-domains (Figure 1A) required for Rap1 interaction, can not. These data indicate that Cno operates downstream of Rap1 in DC by serving a bona fide effector function [63•]. More recently, in genetic interaction and epistasis experiments, DPDZ-GEF, the fly PDZ-GEF counterpart, has been placed upstream of Rap1 in DC. In agreement with this, DPDZ-GEF loss-of-function is associated with abnormalities in DC, which can be overcome by ectopic expression of both Rap1 and Cno. Interestingly, this newly defined DPDZ-GEF/Rap1/Cno module regulates MyosinII function(s) in DC. MyosinII-dependent cell stretching and the assembly of a force-generating MyosinII purse-string cable in the dorsal most rows of cells within the bilateral ectoderm are disrupted by a dysfunction of the module. This relationship is corroborated by strong genetic interactions between DPDZ-GEF and cno with the MyosinII encoding zipper gene [64•]. MyosinII in other morphogenetic processes, such as extension of the germband in Drosophila embryogenesis, modulates E-cadherin plasticity directly [65]. Therefore, while Rap1 signaling during DC, and maybe also at earlier stages does not significantly affect the levels of AJ proteins, it may modulate AJ plasticity via MyosinII. How Rap1 signaling affects MyosinII function in DC and whether MyosinII is indeed involved in AJ remodeling during embryonic closure processes are important issues, especially in light of the remarkable resemblances between DC and certain wound healing processes in mammals. Intriguingly, the mammalian PDZ-GEF2 protein recently has been implicated in the maturation of AJs of A549 lung carcinoma and primary endothelial cells [66•]. Depletion of PDZ-GEF2 results in a zipper-like morphology of AJs with frequent gaps between the AJ strands on juxtaposed cells. Compared to wild type cells, PDZ-GEF2 deficient cells have lowered levels of surface E-cadherin. This phenotype is reminiscent of immature AJs that form after initial cell-cell contact. In PDZ-GEF2-depleted cells, however, the AJs are not transitioning to a mature, wild type morphology with opposing strands running in parallel and forming a tightly sealed apical belt [66•]. The differences in effects on E-cadherin levels between the PDZ-GEF2 knock-down study in human lung carcinoma cells and those observed in DPDZ-GEF mutant Drosophila may be related to the fundamentally different mechanisms that regulate AJ organization in mammalian cells grown in culture and Drosophila embryogenesis.
Another paradigm that allowed recent insight into Rap1’s functions in epithelial morphogenesis is the ventral closure (VC) process in the C. elegans embryo. Surprisingly, in the nematode, zygotic elimination of Rap1 produces a significant fraction of viable and fertile animals that proceed through all developmental stages whereas the remaining lethal fraction of embryos displays a defective hypodermis [67••,68]. Frische and co-workers performed an RNAi screen to identify factors that cooperate with Rap1 and that when markedly reduced give rise to synthetic lethality. This approach identified several components of the Ral-1/exocyst pathway, including RalGDS (encoding a RalGEF), RalA, Exo84 and sec5. RNAi against these exocytosis-relevant molecules in Rap1 null embryos provokes a synthetic block in hypodermal migration. Interestingly, in Rap1 mutant embryos with reduced RalA, Sec5 or Exo84 expression, α-catenin was not detectible in defined adherens junctional structures. Thus, in C.elegans the exocyst in conjunction with Rap1 is required for delivery of AJ components to the basolateral membrane [67••]. The question of whether and how Rap1 could be biochemically linked to RalA/exocyst function still needs to be answered. The exocyst certainly has additional functions separate from Rap1, raising the possibility that Rap1 signaling could either activate a specific subcomplex of the exocyst or converge with it further downstream to control AJ levels and hypodermal migration. A candidate for a relevant Rap1 activator in C. elegans VC is Pxf-1, the worm PDZ-GEF orthologue. Pxf-1 is strongly expressed in the hypodermis and pxf-1 mutants display a highly abnormal hypodermis that resembles that of defective Rap1; Rap2 double mutant embryos. Importantly, this phenotype can be rescued with a constitutively active Rap1 transgene [68]. Until now, however, interactions between pxf-1 and the RalA/exocyst pathway have not been reported. Polarized secretion is an absolute requirement for the generation of the embryonic epithelium in Drosophila, and vice versa, VC of the C. elegans embryo also relies on a purse-string-like structure in a row of 6 cells that follows the two pairs of leading cells [60]. Future work should reveal whether DC in Drosophila and VC in C. elegans have more in common than is currently known or whether both processes evolved on the basis of separate strategies.
Adherens junction formation in a stem cell niche
Adherens junctional control by Rap1 not only establishes cohesion between identical cell types in a single tissue, but also provides for adhesion between cells of different developmental lineages. An example is that of stem cell adhesion in their somatic niche. In both Drosophila males and females, homozygous loss of the Rap1 activator DPDZ-GEF causes sterility in rarely surviving adults [69,70••]. While DPDZ-GEF needs to be better analyzed in mutant ovaries, the investigation of DPDZ-GEF in the male germ line has allowed for a more comprehensive view. Here, both germ line stem cells (GSCs), and their encysting somatic stem cells (SSCs) adhere to the hub, a cluster of postmitotic somatic cells [71]. DPDZ-GEF deficiency results in a stem cell loss phenotype in which selectively GSCs and SSCs are lost whereas the hub remains relatively intact. In agreement with this, DPDZ-GEF mutant germ cells can be seen to drift away from the hub and loose their identity, probably owing to a lack of stem cell regulating factors, such as ligands of the Jak/Stat and TGFβ family that are produced by the hub. A mild drifting effect produced by partial loss of DPDZ-GEF expression is markedly amplified by either Rap1 or Rap2l (encoding the fly Rap2 orthologue) heterozygozity [70••]. Importantly, the hub/stem cell interface in wild type testis displays high levels of AJ components [72]. These are markedly reduced in DPDZ-GEF mutant testis, whereas AJs between hub cells appear unaffected. Expression of constitutively active Rap1V12 is able to significantly rescue both AJ protein levels at the interface and stem cell loss. The defects are also counteracted by ectopic expression of E-cadherin, which strongly suggests a scenario in which DPDZ-GEF/Rap signaling recruits AJ components to the hub/stem cell interface [70••]. DPDZ-GEF mutant females carry supernumerary spermathecae, the receptacles that store the male sperm. Although not investigated with cellular resolution yet, genetic evidence also here suggests a signal transducing mechanism that operates via DPDZ-GEF and Rap GTPases to control AJs [73]. Interestingly, AJs at the hub/stem cell interface are sensitive to DPDZ-GEF loss, suggesting an as yet not understood asymmetry in junction formation. Knox and Brown have demonstrated an indispensable role for Rap1 in AJ remodeling between sister cells late in cytokinesis. Rap1 mutant cells fail to locally reconstitute AJs and start to abnormally disperse within the epithelium [62••]. Certainly, adhesion of stem cells to their niche and AJ remodeling in the final stages of cytokinesis are vastly different from each other, one depending on adhesion of one cell type to another, and the other representing a fundamental element of epithelial cell proliferation. It will, however, be interesting to see if future research will identify common factors that regulate AJs in both developmental processes. One obvious candidate for a Rap1 effector in both processes is Cno/AF-6, which localizes to epithelial AJs [63•,74]. Underscoring the relevance of AF-6 for AJ biology are observations that AF-6 gene disruptions in mice cause severe failures in AJ formation and/or maintenance [75,76]. AF-6 with its PDZ-domain binds to the cytoplasmic tail of Nectins, and Cno at least in vitro can associate with Echinoid, a Nectin-like protein in Drosophila AJs [77,78]. In addition, AF-6 interacts with the actin regulator profilin and associates with F-actin filaments [79,80]. In light of these interactions, the role of AF-6 and Cno in AJs will have to be further defined.
Conclusions
Rap1 function is integral to signaling pathways that regulate various aspects of different adhesion systems. Different Rap1 GEFs often operate in both integrin and cadherin-based junctional complexes suggesting still unappreciated cross-talking mechanisms. Through its ability to activate several key effector proteins such as RIAM, KRIT-1/CCM and AF-6/Cno, Rap1 can help assemble adhesion-relevant protein complexes. The functions of individual GEFs and effectors are beginning to be resolved and open up experimental opportunities for further expansion into the Rap1 signaling network. Genetic modeling of diseases, like CCM in mice and zebrafish and mutational analysis in invertebrates start to yield in vivo evidence for particular Rap1 responsive machineries. Integrated with advanced cell biological techniques and biochemical assays, they will help to further unravel the Rap1 signaling network in biologically and clinically important processes.
Acknowledgements
We are grateful for Jim Duffy for help with preparation of the figure. Our work described in this review was supported by NIH grant R01-CA096882 to L.V.A., and B.B. is a recipient of an NIH postdoctoral training grant (S T32-CA009176). We apologize to those colleagues whose work we did not discuss here.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest published within the period of review have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Bos JL. Linking Rap to cell adhesion. Curr Opin Cell Biol. 2005;17:123–128. doi: 10.1016/j.ceb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Kooistra MR, Dube N, Bos JL. Rap1: a key regulator in cell-cell junction formation. J Cell Sci. 2007;120:17–22. doi: 10.1242/jcs.03306. [DOI] [PubMed] [Google Scholar]
- 3.Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–1615. doi: 10.1182/blood-2004-04-1257. [DOI] [PubMed] [Google Scholar]
- 4. Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, Puzon-McLaughlin W, Lafuente EM, Boussiotis VA, Shattil SJ, et al. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr Biol. 2006;16:1796–1806. doi: 10.1016/j.cub.2006.08.035. This study reports the reconstitution of a platelet-specific Rap1-dependent signaling pathway in CHO cells and describes how Rap1 and its effector RIAM can effectively activate talin and integrin signaling. The epistatic relationships between Rap1, RIAM and talin are elegantly demonstrated.
- 5.Critchley DR. Cytoskeletal proteins talin and vinculin in integrin-mediated adhesion. Biochem Soc Trans. 2004;32:831–836. doi: 10.1042/BST0320831. [DOI] [PubMed] [Google Scholar]
- 6.Priddle H, Hemmings L, Monkley S, Woods A, Patel B, Sutton D, Dunn GA, Zicha D, Critchley DR. Disruption of the talin gene compromises focal adhesion assembly in undifferentiated but not differentiated embryonic stem cells. J Cell Biol. 1998;142:1121–1133. doi: 10.1083/jcb.142.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Jiang G, Cai Y, Monkley SJ, Critchley DR, Sheetz MP. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat Cell Biol. 2008;10:1062–1068. doi: 10.1038/ncb1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrich BG, Marchese P, Ruggeri ZM, Spiess S, Weichert RA, Ye F, Tiedt R, Skoda RC, Monkley SJ, Critchley DR, et al. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med. 2007;204:3103–3111. doi: 10.1084/jem.20071800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Critchley DR. Genetic, biochemical and structural approaches to talin function. Biochem Soc Trans. 2005;33:1308–1312. doi: 10.1042/BST0331308. [DOI] [PubMed] [Google Scholar]
- 10.Bertagnolli ME, Locke SJ, Hensler ME, Bray PF, Beckerle MC. Talin distribution and phosphorylation in thrombin-activated platelets. J Cell Sci. 1993;106(Pt 4):1189–1199. doi: 10.1242/jcs.106.4.1189. [DOI] [PubMed] [Google Scholar]
- 11.Franke B, Akkerman JW, Bos JL. Rapid Ca2+-mediated activation of Rap1 in human platelets. Embo J. 1997;16:252–259. doi: 10.1093/emboj/16.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lafuente EM, van Puijenbroek AA, Krause M, Carman CV, Freeman GJ, Berezovskaya A, Constantine E, Springer TA, Gertler FB, Boussiotis VA. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell. 2004;7:585–595. doi: 10.1016/j.devcel.2004.07.021. Identification of RIAM as a Rap1 effector and first assessment of its potential to induce actin poymerization via Ena/VASP family proteins.
- 13. Watanabe N, Bodin L, Pandey M, Krause M, Coughlin S, Boussiotis VA, Ginsberg MH, Shattil SJ. Mechanisms and consequences of agonist-induced talin recruitment to platelet integrin alphaIIbbeta3. J Cell Biol. 2008;181:1211–1222. doi: 10.1083/jcb.200803094. A life cell study in which bimolecular fluorescence complementation with integrin subunits is achieved after activation of a thrombin pathway that signals via Rap1, RIAM and talin. The system by Han et al. is expanded herein by additional engineering of thrombin/PAR receptors into CHO cells.
- 14.Lee HS, Lim CJ, Puzon-McLaughlin W, Shattil SJ, Ginsberg MH. RIAM Activates Integrins by Linking Talin to Ras GTPase Membrane-targeting Sequences. J Biol Chem. 2009;284:5119–5127. doi: 10.1074/jbc.M807117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crittenden JR, Bergmeier W, Zhang Y, Piffath CL, Liang Y, Wagner DD, Housman DE, Graybiel AM. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med. 2004;10:982–986. doi: 10.1038/nm1098. [DOI] [PubMed] [Google Scholar]
- 16.Eto K, Murphy R, Kerrigan SW, Bertoni A, Stuhlmann H, Nakano T, Leavitt AD, Shattil SJ. Megakaryocytes derived from embryonic stem cells implicate CalDAG-GEFI in integrin signaling. Proc Natl Acad Sci U S A. 2002;99:12819–12824. doi: 10.1073/pnas.202380099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause M, Leslie JD, Stewart M, Lafuente EM, Valderrama F, Jagannathan R, Strasser GA, Rubinson DA, Liu H, Way M, et al. Lamellipodin, an Ena/VASP ligand, is implicated in the regulation of lamellipodial dynamics. Dev Cell. 2004;7:571–583. doi: 10.1016/j.devcel.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Chang C, Adler CE, Krause M, Clark SG, Gertler FB, Tessier-Lavigne M, Bargmann CI. MIG-10/lamellipodin and AGE-1/PI3K promote axon guidance and outgrowth in response to slit and netrin. Curr Biol. 2006;16:854–862. doi: 10.1016/j.cub.2006.03.083. [DOI] [PubMed] [Google Scholar]
- 19. Lyulcheva E, Taylor E, Michael M, Vehlow A, Tan S, Fletcher A, Krause M, Bennett D. Drosophila pico and its mammalian ortholog lamellipodin activate serum response factor and promote cell proliferation. Dev Cell. 2008;15:680–690. doi: 10.1016/j.devcel.2008.09.020. Genetic analysis of Pico, a RIAM/Lpd homologue in Drosophila. The authors establish a genetic link between Pico and the SRF response, both probably being coupled by shifts in F-actin levels in various cells. This relationship has wider implementations for organismal growth control.
- 20.Bivona TG, Wiener HH, Ahearn IM, Silletti J, Chiu VK, Philips MR. Rap1 up-regulation and activation on plasma membrane regulates T cell adhesion. J Cell Biol. 2004;164:461–470. doi: 10.1083/jcb.200311093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katagiri K, Maeda A, Shimonaka M, Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat Immunol. 2003;4:741–748. doi: 10.1038/ni950. [DOI] [PubMed] [Google Scholar]
- 22.Katagiri K, Ohnishi N, Kabashima K, Iyoda T, Takeda N, Shinkai Y, Inaba K, Kinashi T. Crucial functions of the Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat Immunol. 2004;5:1045–1051. doi: 10.1038/ni1111. [DOI] [PubMed] [Google Scholar]
- 23.Ohba Y, Ikuta K, Ogura A, Matsuda J, Mochizuki N, Nagashima K, Kurokawa K, Mayer BJ, Maki K, Miyazaki J, et al. Requirement for C3G-dependent Rap1 activation for cell adhesion and embryogenesis. Embo J. 2001;20:3333–3341. doi: 10.1093/emboj/20.13.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakkab D, Lewitzky M, Posern G, Schaeper U, Sachs M, Birchmeier W, Feller SM. Signaling of hepatocyte growth factor/scatter factor (HGF) to the small GTPase Rap1 via the large docking protein Gab1 and the adapter protein CRKL. J Biol Chem. 2000;275:10772–10778. doi: 10.1074/jbc.275.15.10772. [DOI] [PubMed] [Google Scholar]
- 25.Huelsmann S, Hepper C, Marchese D, Knoll C, Reuter R. The PDZ-GEF dizzy regulates cell shape of migrating macrophages via Rap1 and integrins in the Drosophila embryo. Development. 2006;133:2915–2924. doi: 10.1242/dev.02449. [DOI] [PubMed] [Google Scholar]
- 26.Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem. 2005;280:11675–11682. doi: 10.1074/jbc.M412595200. [DOI] [PubMed] [Google Scholar]
- 27.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 29.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMPactivated exchange factor for Rap GTPase. Blood. 2005;105:1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- 30.Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Doskeland SO, Blank JL, Bos JL. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–906. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- 31.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579:4966–4972. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- 33.Ponsioen B, Gloerich M, Ritsma L, Rehmann H, Bos JL, Jalink K. Direct spatial control of Epac1 by cAMP. Mol Cell Biol. 2009 doi: 10.1128/MCB.01630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rehmann H, Arias-Palomo E, Hadders MA, Schwede F, Llorca O, Bos JL. Structure of Epac2 in complex with a cyclic AMP analogue and RAP1B. Nature. 2008;455:124–127. doi: 10.1038/nature07187. [DOI] [PubMed] [Google Scholar]
- 35.Sakurai A, Fukuhara S, Yamagishi A, Sako K, Kamioka Y, Masuda M, Nakaoka Y, Mochizuki N. MAGI-1 is required for Rap1 activation upon cell-cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol Biol Cell. 2006;17:966–976. doi: 10.1091/mbc.E05-07-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei P, Satoh T, Edamatsu H, Aiba A, Setsu T, Terashima T, Kitazawa S, Nakao K, Yoshikawa Y, Tamada M, et al. Defective vascular morphogenesis and midgestation embryonic death in mice lacking RA-GEF-1. Biochem Biophys Res Commun. 2007;363:106–112. doi: 10.1016/j.bbrc.2007.08.149. [DOI] [PubMed] [Google Scholar]
- 37.Serebriiskii I, Estojak J, Sonoda G, Testa JR, Golemis EA. Association of Krev-1/rap1a with Krit1, a novel ankyrin repeat-containing protein encoded by a gene mapping to 7q21–22. Oncogene. 1997;15:1043–1049. doi: 10.1038/sj.onc.1201268. [DOI] [PubMed] [Google Scholar]
- 38.Labauge P, Denier C, Bergametti F, Tournier-Lasserve E. Genetics of cavernous angiomas. Lancet Neurol. 2007;6:237–244. doi: 10.1016/S1474-4422(07)70053-4. [DOI] [PubMed] [Google Scholar]
- 39.Craig HD, Gunel M, Cepeda O, Johnson EW, Ptacek L, Steinberg GK, Ogilvy CS, Berg MJ, Crawford SC, Scott RM, et al. Multilocus linkage identifies two new loci for a mendelian form of stroke, cerebral cavernous malformation, at 7p15-13 and 3q25.2–27. Hum Mol Genet. 1998;7:1851–1858. doi: 10.1093/hmg/7.12.1851. [DOI] [PubMed] [Google Scholar]
- 40.Dubovsky J, Zabramski JM, Kurth J, Spetzler RF, Rich SS, Orr HT, Weber JL. A gene responsible for cavernous malformations of the brain maps to chromosome 7q. Hum Mol Genet. 1995;4:453–458. doi: 10.1093/hmg/4.3.453. [DOI] [PubMed] [Google Scholar]
- 41.Liquori CL, Berg MJ, Squitieri F, Leedom TP, Ptacek L, Johnson EW, Marchuk DA. Deletions in CCM2 are a common cause of cerebral cavernous malformations. Am J Hum Genet. 2007;80:69–75. doi: 10.1086/510439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clatterbuck RE, Eberhart CG, Crain BJ, Rigamonti D. Ultrastructural and immunocytochemical evidence that an incompetent blood-brain barrier is related to the pathophysiology of cavernous malformations. J Neurol Neurosurg Psychiatry. 2001;71:188–192. doi: 10.1136/jnnp.71.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong JH, Awad IA, Kim JH. Ultrastructural pathological features of cerebrovascular malformations: a preliminary report. Neurosurgery. 2000;46:1454–1459. doi: 10.1097/00006123-200006000-00027. [DOI] [PubMed] [Google Scholar]
- 44.Eerola I, Plate KH, Spiegel R, Boon LM, Mulliken JB, Vikkula M. KRIT1 is mutated in hyperkeratotic cutaneous capillary-venous malformation associated with cerebral capillary malformation. Hum Mol Genet. 2000;9:1351–1355. doi: 10.1093/hmg/9.9.1351. [DOI] [PubMed] [Google Scholar]
- 45.Plummer NW, Gallione CJ, Srinivasan S, Zawistowski JS, Louis DN, Marchuk DA. Loss of p53 sensitizes mice with a mutation in Ccm1 (KRIT1) to development of cerebral vascular malformations. Am J Pathol. 2004;165:1509–1518. doi: 10.1016/S0002-9440(10)63409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guzeloglu-Kayisli O, Amankulor NM, Voorhees J, Luleci G, Lifton RP, Gunel M. KRIT1/cerebral cavernous malformation 1 protein localizes to vascular endothelium, astrocytes, and pyramidal cells of the adult human cerebral cortex. Neurosurgery. 2004;54:943–949. doi: 10.1227/01.neu.0000114512.59624.a5. discussion 949. [DOI] [PubMed] [Google Scholar]
- 47. Glading A, Han J, Stockton RA, Ginsberg MH. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J Cell Biol. 2007;179:247–254. doi: 10.1083/jcb.200705175. Using cultured endothelial cells the authors demonstrate a function for KRIT-1 as a Rap1 effector in VE-cadherin based junctions. They show that KRIT-1's adaptor role relies on Rap1 activation levels and that KRIT-1 governs endothelial barrier function.
- 48.Hayashi S, Takeuchi K, Suzuki S, Tsunoda T, Tanaka C, Majima Y. Effect of thrombin on permeability of human epithelial cell monolayers. Pharmacology. 2006;76:46–52. doi: 10.1159/000089718. [DOI] [PubMed] [Google Scholar]
- 49.Stockton RA, Schaefer E, Schwartz MA. p21-activated kinase regulates endothelial permeability through modulation of contractility. J Biol Chem. 2004;279:46621–46630. doi: 10.1074/jbc.M408877200. [DOI] [PubMed] [Google Scholar]
- 50.Voss K, Stahl S, Schleider E, Ullrich S, Nickel J, Mueller TD, Felbor U. CCM3 interacts with CCM2 indicating common pathogenesis for cerebral cavernous malformations. Neurogenetics. 2007;8:249–256. doi: 10.1007/s10048-007-0098-9. [DOI] [PubMed] [Google Scholar]
- 51.Zawistowski JS, Stalheim L, Uhlik MT, Abell AN, Ancrile BB, Johnson GL, Marchuk DA. CCM1 and CCM2 protein interactions in cell signaling: implications for cerebral cavernous malformations pathogenesis. Hum Mol Genet. 2005;14:2521–2531. doi: 10.1093/hmg/ddi256. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Rigamonti D, Dietz HC, Clatterbuck RE. Interaction between krit1 and malcavernin: implications for the pathogenesis of cerebral cavernous malformations. Neurosurgery. 2007;60:353–359. doi: 10.1227/01.NEU.0000249268.11074.83. discussion 359. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J, Clatterbuck RE, Rigamonti D, Chang DD, Dietz HC. Interaction between krit1 and icap1alpha infers perturbation of integrin beta1-mediated angiogenesis in the pathogenesis of cerebral cavernous malformation. Hum Mol Genet. 2001;10:2953–2960. doi: 10.1093/hmg/10.25.2953. [DOI] [PubMed] [Google Scholar]
- 54.Chang DD, Wong C, Smith H, Liu J. ICAP-1, a novel beta1 integrin cytoplasmic domain-associated protein, binds to a conserved and functionally important NPXY sequence motif of beta1 integrin. J Cell Biol. 1997;138:1149–1157. doi: 10.1083/jcb.138.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zawistowski JS, Serebriiskii IG, Lee MF, Golemis EA, Marchuk DA. KRIT1 association with the integrin-binding protein ICAP-1: a new direction in the elucidation of cerebral cavernous malformations (CCM1) pathogenesis. Hum Mol Genet. 2002;11:389–396. doi: 10.1093/hmg/11.4.389. [DOI] [PubMed] [Google Scholar]
- 56. Whitehead KJ, Chan AC, Navankasattusas S, Koh W, London NR, Ling J, Mayo AH, Drakos SG, Marchuk DA, Davis GE, et al. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nat Med. 2009;15:177–184. doi: 10.1038/nm.1911. This work provides evidence for CCM2, a direct interaction partner of CCM1, in regulation of Rho signaling in endothelial cells.
- 57.Mably JD, Mohideen MA, Burns CG, Chen JN, Fishman MC. heart of glass regulates the concentric growth of the heart in zebrafish. Curr Biol. 2003;13:2138–2147. doi: 10.1016/j.cub.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 58. Kleaveland B, Zheng X, Liu JJ, Blum Y, Tung JJ, Zou Z, Chen M, Guo L, Lu MM, Zhou D, et al. Regulation of cardiovascular development and integrity by the heart of glass-cerebral cavernous malformation protein pathway. Nat Med. 2009;15:169–176. doi: 10.1038/nm.1918. Genetics in mouse and zebrafish are combined to elegantly link the function of the heart of glass (Het1) transmembrane protein with the CCM complex. It also provides a first model of how CCM proteins are biochemically tethered to Het1.
- 59.Lecuit T. Adhesion remodeling underlying tissue morphogenesis. Trends Cell Biol. 2005;15:34–42. doi: 10.1016/j.tcb.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Quintin S, Gally C, Labouesse M. Epithelial morphogenesis in embryos: asymmetries, motors and brakes. Trends Genet. 2008;24:221–230. doi: 10.1016/j.tig.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 61.Asha H, de Ruiter ND, Wang MG, Hariharan IK. The Rap1 GTPase functions as a regulator of morphogenesis in vivo. Embo J. 1999;18:605–615. doi: 10.1093/emboj/18.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Knox AL, Brown NH. Rap1 GTPase regulation of adherens junction positioning and cell adhesion. Science. 2002;295:1285–1288. doi: 10.1126/science.1067549. Investigating genetically mosaic Drosophila wing imaginal discs the authors demonstrate for the first time a vital function for Rap1 in reconstituting AJs during the late stages of cytokinesis. A failure in this process causes cells in mitotic clones to loose cohesion and to aberrantly disperse into wild type tissue.
- 63. Boettner B, Harjes P, Ishimaru S, Heke M, Fan HQ, Qin Y, Van Aelst L, Gaul U. The AF-6 homolog canoe acts as a Rap1 effector during dorsal closure of the Drosophila embryo. Genetics. 2003;165:159–169. doi: 10.1093/genetics/165.1.159. In vivo demonstration of a Rap1 effector function for Canoe (Cno) in ectodermal cells involved in cell migration and closure of the Drosophila embryo.
- 64. Boettner B, Van Aelst L. The Rap GTPase activator Drosophila PDZ-GEF oregulates cell shape in epithelial migration and morphogenesis. Mol Cell Biol. 2007;27:7966–7980. doi: 10.1128/MCB.01275-07. Identification of PDZ-GEF as a relevant Rap1GEF in a morphogenetic process using genetic approaches in Drosophila. By linking PDZ-GEF function to Cno, this study also provides an example for specific Rap1 effector selection and MyosinII regulation by a GEF in an in vivo process.
- 65.Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 66. Dube N, Kooistra MR, Pannekoek WJ, Vliem MJ, Oorschot V, Klumperman J, Rehmann H, Bos JL. The RapGEF PDZ-GEF2 is required for maturation of cell-cell junctions. Cell Signal. 2008;20:1608–1615. doi: 10.1016/j.cellsig.2008.05.006. Description of a role for mammalian PDZ-GEF2 in adherens junctional maturation. The study, by employing RNAi and fluorescence microscopy concludes that not initial formation of AJs but rather their differentiation into a tightly sealed state is specifically affected by PDZ-GEF2.
- 67. Frische EW, Pellis-van Berkel W, van Haaften G, Cuppen E, Plasterk RH, Tijsterman M, Bos JL, Zwartkruis FJ. RAP-1 and the RAL-1/exocyst pathway coordinate hypodermal cell organization in Caenorhabditis elegans. Embo J. 2007;26:5083–5092. doi: 10.1038/sj.emboj.7601922. In this RNAi screen the authors uncover a link between Rap1 function and the exocyst complex in the C. elegans hypodermis. Synthetic loss of Rap1 and individual exocyst dosages compromises AJ levels and ventral closure of the hypodermis.
- 68.Pellis-van Berkel W, Verheijen MH, Cuppen E, Asahina M, de Rooij J, Jansen G, Plasterk RH, Bos JL, Zwartkruis FJ. Requirement of the Caenorhabditis elegans RapGEF pxf-1 and rap-1 for epithelial integrity. Mol Biol Cell. 2005;16:106–116. doi: 10.1091/mbc.E04-06-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee JH, Cho KS, Lee J, Kim D, Lee SB, Yoo J, Cha GH, Chung J. Drosophila PDZ-GEF, a guanine nucleotide exchange factor for Rap1 GTPase, reveals a novel upstream regulatory mechanism in the mitogen-activated protein kinase signaling pathway. Mol Cell Biol. 2002;22:7658–7666. doi: 10.1128/MCB.22.21.7658-7666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang H, Singh SR, Zheng Z, Oh SW, Chen X, Edwards K, Hou SX. Rap-GEF signaling controls stem cell anchoring to their niche through regulating DE-cadherin-mediated cell adhesion in the Drosophila testis. Dev Cell. 2006;10:117–126. doi: 10.1016/j.devcel.2005.11.004. The study provides the first example for a RapGEF mutation causing a stem cell defect. It shows that the loss of male stem cells in PDZ-GEF mutant testis is due to insufficient adhesion between stem and somatic cells at the stem cell/ hub interface.
- 71.Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- 72.Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- 73.Singh SR, Oh SW, Liu W, Chen X, Zheng Z, Hou SX. Rap-GEF/Rap signaling restricts the formation of supernumerary spermathecae in Drosophila melanogaster. Dev Growth Differ. 2006;48:169–175. doi: 10.1111/j.1440-169X.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 74.Takahashi K, Matsuo T, Katsube T, Ueda R, Yamamoto D. Direct binding between two PDZ domain proteins Canoe and ZO-1 and their roles in regulation of the jun N-terminal kinase pathway in Drosophila morphogenesis. Mech Dev. 1998;78:97–111. doi: 10.1016/s0925-4773(98)00151-8. [DOI] [PubMed] [Google Scholar]
- 75.Ikeda W, Nakanishi H, Miyoshi J, Mandai K, Ishizaki H, Tanaka M, Togawa A, Takahashi K, Nishioka H, Yoshida H, et al. Afadin: A key molecule essential for structural organization of cell-cell junctions of polarized epithelia during embryogenesis. J Cell Biol. 1999;146:1117–1132. doi: 10.1083/jcb.146.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhadanov AB, Provance DW, Jr, Speer CA, Coffin JD, Goss D, Blixt JA, Reichert CM, Mercer JA. Absence of the tight junctional protein AF-6 disrupts epithelial cell-cell junctions and cell polarity during mouse development. Curr Biol. 1999;9:880–888. doi: 10.1016/s0960-9822(99)80392-3. [DOI] [PubMed] [Google Scholar]
- 77.Takahashi K, Nakanishi H, Miyahara M, Mandai K, Satoh K, Satoh A, Nishioka H, Aoki J, Nomoto A, Mizoguchi A, et al. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with Afadin, a PDZ domain-containing protein. J Cell Biol. 1999;145:539–549. doi: 10.1083/jcb.145.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei SY, Escudero LM, Yu F, Chang LH, Chen LY, Ho YH, Lin CM, Chou CS, Chia W, Modolell J, et al. Echinoid is a component of adherens junctions that cooperates with DE-Cadherin to mediate cell adhesion. Dev Cell. 2005;8:493–504. doi: 10.1016/j.devcel.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 79.Boettner B, Govek EE, Cross J, Van Aelst L. The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. Proc Natl Acad Sci U S A. 2000;97:9064–9069. doi: 10.1073/pnas.97.16.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, et al. Afadin: A novel actin filamentbinding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139:517–528. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]