Abstract
Several reports have focused on the involvement of the endocannabinoid system in hyperexcitability, particularly in seizure and epilepsy models. Our laboratory recently characterized a novel plasticity change of the cannabinoid type 1 (CB1) receptor in hippocampi of epileptic rats following pilocarpine-induced status epilepticus (SE). This long-term redistribution included selective layer-specific changes in CB1 receptor expression within distinct hippocampal subregions. However, the temporal characteristics of this redistribution during the development of epilepsy had not been examined. Therefore, this study was initiated to evaluate the time course by which pilocarpine-induced SE produced changes in CB1 receptor expression. Immunohistochemical analysis demonstrated that within 1 week following SE, there was a pronounced loss in CB1 receptor expression throughout the hippocampus, while staining in many interneurons was preserved. By 1 month post-SE, pilocarpine-treated animals began to display epileptic seizures, and CB1 receptor expression was characteristic of the redistribution observed in long-term epileptic rats, with decreases in CB1 receptor immunoreactivity in the stratum pyramidale neuropil and dentate gyrus inner molecular layer, and increases in the strata oriens and radiatum of CA1–3. Observed changes in CB1 receptor expression were confirmed at multiple time points by western blot analysis. The data indicate that overall decreases in expression following SE preempt a long-lasting CB1 receptor redistribution, and that differential responses occur within the hippocampus to initial CB1 receptor losses. This suggests a role for dysregulation of the endocannabinoid system during epileptogenesis and indicates that the CB1 receptor redistribution temporally correlates with the emergence of epileptic seizures.
Keywords: Redistribution, Immunohistochemistry, Plasticity, Epileptogenesis, Time-course
1. Introduction
Approximately 1–3% of people will be diagnosed with epilepsy at some point during their lifetime, and a large number of epileptic patients are refractory to conventional anticonvulsant treatment (Hauser, 1990). Cannabinoid compounds have been demonstrated to have cannabinoid type 1 (CB1) receptor-dependent anticonvulsant effects in several in vitro and in vivo seizure models (Blair et al., 2006; Marsicano et al., 2003; Wallace et al., 2002, 2001) and the endogenous cannabinoid system has been shown to play a role in controlling seizure frequency and duration in the rat pilocarpine model of chronic epilepsy (Wallace et al., 2003). In this model, rats exhibit pilocarpine-induced status epilepticus (SE), followed by a latent epileptogenic period, prior to developing spontaneous recurrent seizures (SRS) that persist for the lifetime of the animals (Mello et al., 1993). During the latency period, numerous morphological changes have been shown to occur in the hippocampus, including neuronal degeneration, mossy fiber sprouting, and dentate granule cell dispersion, all of which are believed to contribute to epileptogenesis (Morimoto et al., 2004).
Alterations in the expression of hippocampal CB1 receptors have recently been examined in epilepsy, both in epileptic patients (Ludanyi et al., 2008) and in rodent models (Falenski et al., 2007). Our laboratory has demonstrated that epileptic rats exhibit a long-term redistribution of CB1 receptors within the hippocampus following pilocarpine-induced SE (Falenski et al., 2007). In this model, immunohistochemical analyses demonstrated selective increases in CB1 receptor immunoreactivity (IR) in the CA1–CA3 strata oriens and radiatum, with concomitant decreases in the dentate gyrus inner molecular layer and stratum pyramidale. This redistribution is hypothesized to serve as a compensatory mechanism to dampen excessive neuronal excitability that occurs with epilepsy (Falenski et al., 2007). Although CB1 receptor expression has been evaluated during the chronic phase; to date no studies have examined CB1 receptor expression during the epileptogenic phase in this model. Therefore, the aim of the present study was to evaluate the time course of CB1 receptor reorganization that occurs in the hippocampus following pilocarpine-induced SE.
After pilocarpine-induced SE in the rat, time points ranging from 4 days to chronic (greater than 6 months) were evaluated using immunohistochemical and western blot analyses to determine changes in CB1 receptor expression. Examining the distribution of the CB1 receptor during epileptogenesis is crucial for understanding the role of the endocannabinoid system in the pathophysiology of epilepsy.
2. Results
In order to evaluate changes in CB1 receptor expression following pilocarpine-induced SE, immunohistochemistry was performed according to previously established methods (Falenski et al., 2007; Tsou et al., 1998). Naïve controls for all time points (4 days, 1 week, 2 weeks, 1 month, 4 months, and chronic) were each paired with an age-matched treated animal on a given slide, and run through the procedure in parallel. Immunohistochemical analysis of control tissue did not result in visually identifiable qualitative changes in hippocampal CB1 receptor immunoreactivity (IR), regardless of what time point they were sacrificed, and illustrated a distribution of CB1 receptor-IR well characterized in control animals (Figs. 1-7, left panels). In particular, CB1 receptor-IR was intense in the dense neuropil of the stratum pyramidale, on interneuron somata, and in the dentate gyrus inner molecular layer, with lower levels of dendritic CB1 receptor-IR in the stratum oriens, radiatum, and lacunosum-moleculare (Figs. 1-7, left panels).
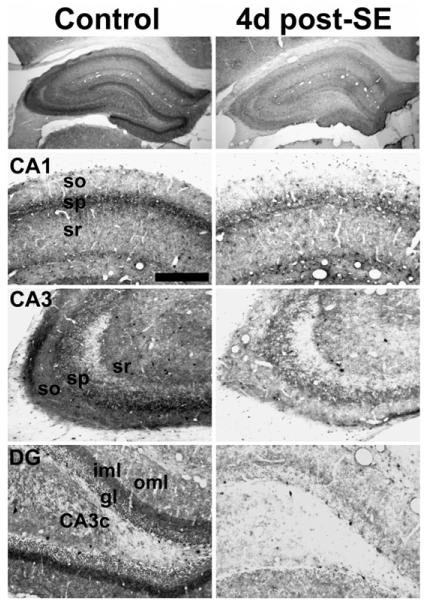
Fig. 1.
Hippocampal CB1 receptor immunoreactivity in control (left) and 4 day post-SE (right) animals. Staining is decreased following SE throughout the hippocampus in CA1 and CA3 of Ammon's horn as well as the CA3c located between the blades of the dentate gyrus (DG). Animals sacrificed did not exhibit behavioral seizures as determined by video monitoring prior to sacrifice. so — stratum oriens, sp — stratum pyramidale, sr — stratum radiatum, oml — outer molecular layer, iml — inner molecular layer, gl — granule cell layer. Scale bar for bottom three panels — 200 μm.
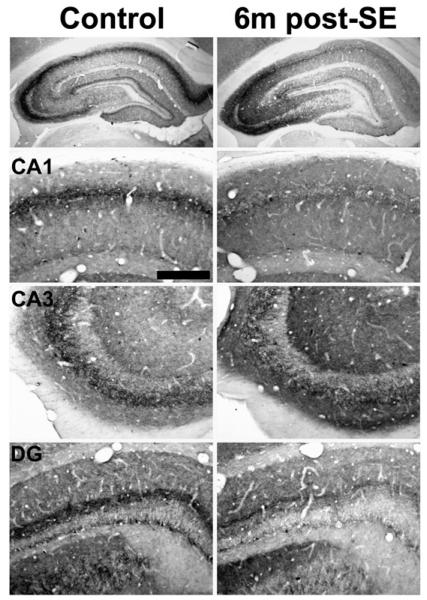
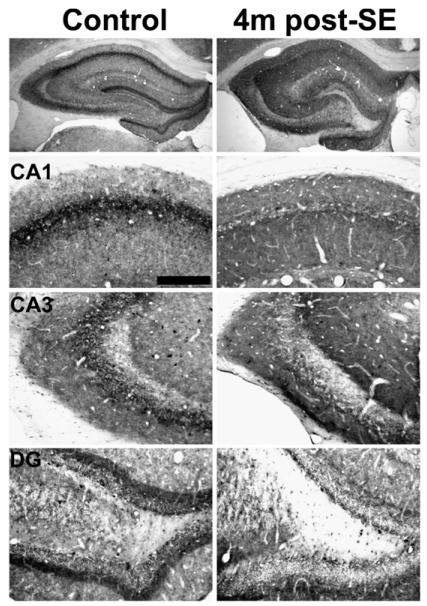
Fig. 7.
Hippocampal CB1 receptor immunoreactivity in control (left) and 6 month post-SE (right) animals. This time point represents the chronic time point post-SE, since CB1 receptor immunostaining at subsequent time points sampled out to 2 years post-SE was not different from the 6 month post-SE condition. All animals used exhibited spontaneous recurrent seizures. CB1 receptor immunostaining was decreased in epileptic animals in the stratum pyramidale of CA1 and CA3, as well as the dentate gyrus inner molecular layer. However, CB1 receptor immunoreactivity in the stratum radiatum and oriens of CA1–CA3 was increased in epileptic animals. Scale bar = 200 μm.
Four days following pilocarpine-induced SE, discernible changes in hippocampal CB1 receptor-IR were observed. Overall, there was a prominent decrease in CB1 receptor expression throughout the hippocampus (Fig. 1, right panels). The loss in CB1 receptor expression occurred in all layers of CA1–3 of Ammon's horn, particularly with a loss of punctate staining surrounding the stratum pyramidale, which has been previously reported with chronic studies (Falenski et al., 2007) (Fig. 1, CA1 and CA3). In the dentate gyrus, the largest reduction in CB1 receptor-IR occurred in the stratum oriens and radiatum of the CA3c, but was also evident in the outer and inner molecular layers (Fig. 1, DG). This group of animals did not exhibit spontaneous, recurrent seizures (SRS) 24 h prior to sacrifice as determined by continuous video monitoring (data not shown).
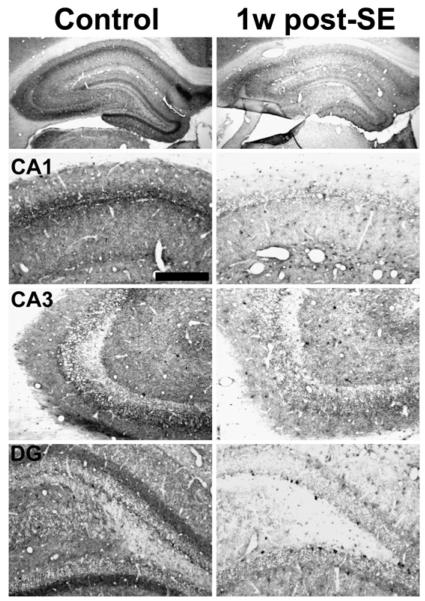
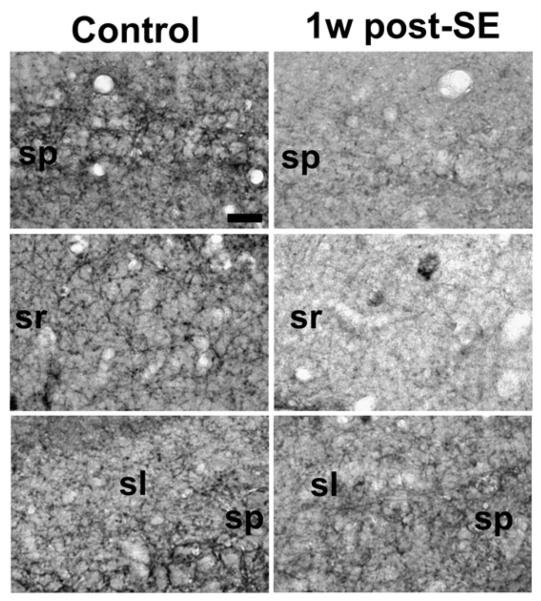
At 1 week post-SE, the diffuse decrease in CB1 receptor-IR observed throughout the hippocampus when compared to control was the most pronounced (Fig. 2, right panels); therefore, this time point was chosen for further analysis. Higher magnification images of this time point (Fig. 3) reveal that the loss in CB1 receptor immunostaining is punctuate in nature throughout the hippocampus, notably in the CA1 stratum pyramidale (Fig. 3, top panels), CA1 stratum radiatum (Fig. 3, middle panels), as well as the CA3 stratum pyramidale (Fig. 3, bottom panels). Western blot analysis also revealed a marked decrease in expression of the CB1 receptor protein at the one-week post-SE time point (Fig. 9A), and densitometric analysis confirmed a significant decrease in CB1 receptor expression (Fig. 9B). In order to assess whether the pyramidal cell loss that occurred as a result of SE could fully account for the loss in CB1 receptor-IR observed, Nissl staining on adjacent sections was also performed. Neuronal cell counts of the CA1 region of the hippocampus at the 1-week time point revealed cell losses of 19.6+/−3.4% when compared with age-matched controls (206.9+/−8.2 cells/0.5 mm for control, 166.3+/−7.0 cells/0.5 mm for 1 week post-SE). This number was significant (p< 0.05, n=4–5/group, unpaired Student's t-test) and was consistent with previous reports from our laboratory (Falenski et al., 2007; Rice and DeLorenzo, 1998). Quantitation of the loss in CB1 receptor immunostaining was also conducted in several hippocampal layers, notably CA1. After normalization of background staining, significant decreases in CB1 receptor-IR when compared to control were observed in several regions, including CA1 stratum pyramidale (47.4 +/−21.1%), CA1 stratum oriens (45.1 +/−24.0%), and whole hippocampus (34.0 +/−3.3%). The results from both analyses reveal that the minor cell loss in the CA1 region of these animals is not large enough to fully account for the amount of decrease observed in CB1 receptor-IR. This group of animals also did not exhibit spontaneous, recurrent seizures (SRS) 24 h prior to sacrifice (data not shown).
Fig. 2.
Hippocampal CB1 receptor immunoreactivity in control (left) and 1 week post-SE (right) animals. Decreases in staining intensity are very evident at this time point following SE in CA1, CA3, and in the dentate gyrus (DG). Animals did not exhibit behavioral seizures as determined by video monitoring prior to sacrifice. Scale bar for bottom three panels — 200 μm.
Fig. 3.
Higher magnification of hippocampal CB1 receptor immunoreactivity in control (left) and 1 week post-SE (right) animals in select hippocampal regions, including the CA1 stratum pyramidale (top), CA1 stratum radiatum (middle), and CA3 stratum pyramidale (bottom). Decreases in staining intensity are evident as demonstrated by the reduction in CB1 receptor immunopositive puncta in all of these regions, with little change in the largely immunonegative stratum lucidum. Scale bar=50 μm.
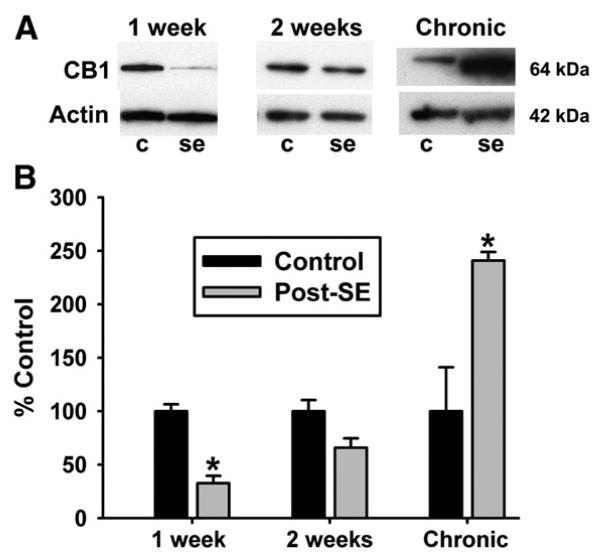
Fig. 9.
Western blot analysis of hippocampal CB1 receptor expression following pilocarpine-induced SE. Representative blots (A) and densitometric analysis of western blotting (B) are presented. The data demonstrate that expression of the 64-kDa CB1 receptor is visually reduced at 1- and 2-weeks post-SE, and decreased in comparison to control animals at 1-week post-SE (n = 4, p < 0.001). Analysis at the chronic time point revealed a significant increase in hippocampal CB1 receptor expression in post-SE animals when compared to controls (n = 5, p < 0.01).
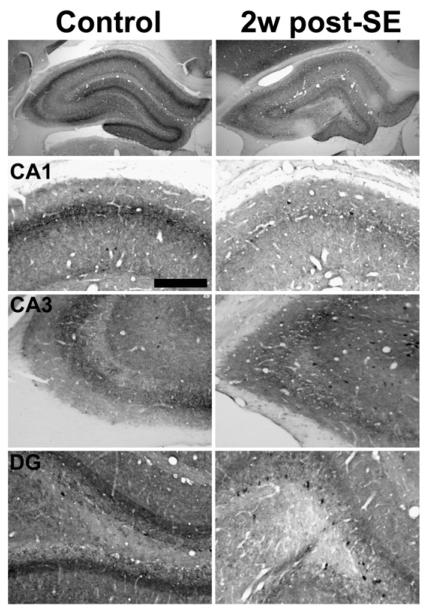
By two weeks post-SE, CB1 receptor-IR qualitatively appeared more intense relative to control than at the four-and seven-day time points (Fig. 4, right panels). CB1 receptor-IR in control and SE-animals was similar in many of the hippocampal strata throughout Ammon's horn including strata oriens and radiatum (Fig. 4, CA1 and CA3). However, the drop in CB1 receptor-IR in several layers of the dentate gyrus was still quite evident when compared to control (Fig. 4, DG). Western blot analysis illustrated a slight visual decrease in CB1 receptor protein expression at the two-week post-SE time point (Fig. 8A), but densitometric analysis revealed that this decrease in CB1 receptor expression was not statistically significant when compared to control (Fig. 9B). At this time point only one of six animals was documented to have SRS (data not shown), and the CB1 receptor immunoreactivity from this animal did not appear qualitatively different from any other animal from the two week post-SE time point.
Fig. 4.
Hippocampal CB1 receptor immunoreactivity in control (left) and 2 week post-SE (right) animals. CB1 receptor staining is still decreased in all regions, but at 2 weeks post-SE staining in the CA1 and CA3 strata oriens and radiatum is closer to control levels when compared to the previous two time points. However, staining in the dentate gyrus was still decreased when compared to control. Scale bar=200 μm.
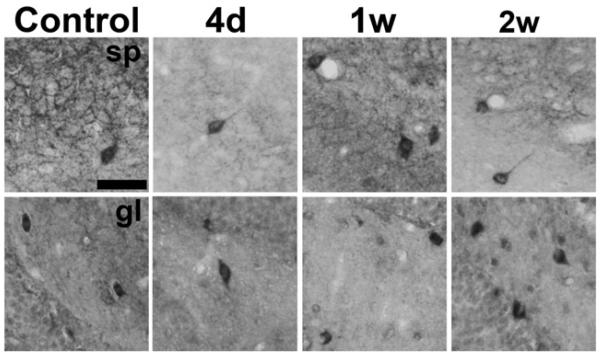
Fig. 8.
Hippocampal CB1 receptor immunoreactivity in representative interneurons sampled from the border of the stratum pyramidale (sp, top panels) and the dentate gyrus granule cell layer (gl, bottom panels) at several time points following pilocarpine-induced SE. Although decreased CB1 receptor immunostaining occurred throughout the hippocampus, interneuron immunoreactivity in many interneurons was preserved at 4 days (4d), 1 week (1w), and 2 weeks (2w) after SE. Scale bar = 50 μm.
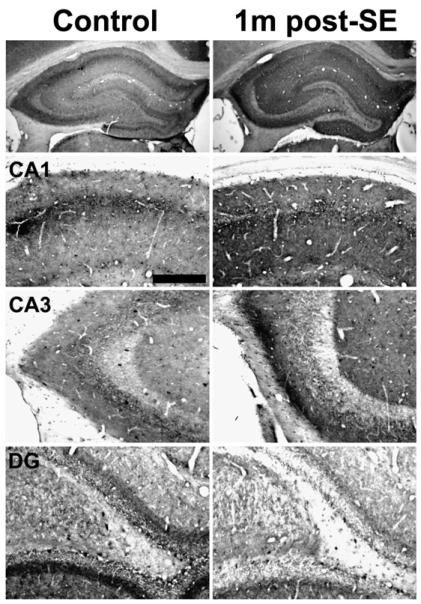
At 1-month post-SE, all pilocarpine-treated animals that exhibited SE displayed behavioral epileptic seizures. Immunohistochemical localization of the CB1 receptor in these animals illustrated a pattern of hippocampal CB1 receptor expression that was similar to the pattern described previously for long-term epileptic animals (Falenski et al., 2007) (Fig. 5, right panels). In particular, there was a noted increase in CB1 receptor-IR in the strata oriens and radiatum of CA1–CA3 of Ammon's horn (Fig. 5, CA1, CA3) when compared to control. However, the characteristic staining of the stratum pyramidale and inner molecular layer of the dentate gyrus was decreased in epileptic animals (Fig. 5, CA1, DG). Similar results were observed following pilocarpine-induced SE at 4 months (Fig. 6) and the chronic time point of greater than 6 months (Fig. 7), where all animals were documented to exhibit behavioral seizures prior to sacrifice (data not shown). Western blots of the chronic time point illustrate an increase in expression of the CB1 receptor protein (Fig. 9A), with densitometry revealing a significant increase when compared to control (Fig. 9B), supporting previous findings from the laboratory (Wallace et al., 2003).
Fig. 5.
Hippocampal CB1 receptor immunoreactivity in control (left) and 1 month post-SE (right) animals. At 1 month post-SE, the epileptic distribution is apparent — CB1 receptor immunostaining is decreased in the stratum pyramidale of CA1 and CA3 and the inner molecular layer of the dentate gyrus (DG). However, the surrounding synaptic regions of the stratum oriens and radiatum are increased in 1 month post-SE animals in both the CA1 and CA3 regions. By this time post-SE, all animals monitored displayed spontaneous, recurrent seizures as confirmed by video monitoring. Scale bar = 200 μm.
Fig. 6.
Hippocampal CB1 receptor immunoreactivity in control (left) and 4 month post-SE (right) animals. All animals studied exhibited spontaneous recurrent seizures. CB1 receptor immunostaining was decreased in epileptic animals in the stratum pyramidale of CA1 and CA3, as well as the dentate gyrus inner molecular layer. However, CB1 receptor immunoreactivity in the stratum radiatum and oriens of CA1–CA3 was increased in epileptic animals. Scale bar = 200 μm.
In the hippocampus of untreated animals, CB1 receptors have been localized at very high levels on populations of cholecystokinin-containing interneurons (Tsou et al., 1999). Interestingly, CB1 receptor-IR was still expressed in many interneuronal somata throughout the time-course following pilocarpine-induced SE. Specifically, intense CB1-immunopositive somata were located adjacent to the stratum pyramidale (Fig. 8,top panels) and at the base of the dentate gyrus granule cell layer (Fig. 8, bottom panels), even while expression in surrounding layers was reduced, such as in the 4 days and 1 week post-SE time points. Because CB1 receptor immunoreactivity was so prominent in these interneurons, counts of CB1 receptor immunopositive somata were performed along the dentate granule cell layer to determine whether status epilepticus increased the number of CB1 receptor expressing interneurons at these time points. The number of CB1 receptor positive interneurons in this region averaged 13.3+/−1.6 for control and 14.0+/−1.0 at 4 days post-SE. At one-week post-SE, the number of interneurons was determined to be 12.5+/−1.0 for control and 15.0+/−0.7 for pilocarpine-treated (unpaired Student's t-test, n=4/group, p=0.080). These results suggest that SE does not seem to alter the expression of CB1 receptors in many interneurons.
Western blot analysis was performed on hippocampal membrane fractions from several time points of interest to confirm the immunohistochemical analysis. Fig. 9A illustrates a representative blot from several time points, demonstrating reduction in CB1 receptor protein 1 week following SE, and an increase in expression of the 64-kDa CB1 receptor at the chronic time point. Densitometric analysis (one-way ANOVA) was performed, and confirmed that a significant loss in CB1 receptor expression occurs at the 1 week post-SE time point (67.6 +/− 8.1%, n = 4–5 per group, p < 0.001) (Fig. 9B), while no significant change in CB1 receptor expression was observed 2 weeks following SE with this analysis (34.4 +/− 11.3%, n = 5 per group, p = 0.05) (Fig. 9B). However, expression of CB1 receptors significantly increased in chronically epileptic animals (140.8+/− 8.1%, n = 5 per group, p < 0.01) (Fig. 9B).
3. Discussion
Results from this study demonstrate for the first time that the CB1 receptor undergoes temporal plasticity changes during epileptogenesis in the pilocarpine model of acquired epilepsy. Furthermore, our findings confirm an essentially permanent redistribution of the hippocampal CB1 receptor in long-term epileptic animals. Within 4 days following pilocarpine-induced SE, there was a marked decrease in CB1 receptor expression throughout all strata of the hippocampus. The greatest loss in receptor expression relative to control occurred at approximately 1-week post-SE, and by 1 month post-SE increases in expression of this receptor in these layers became apparent. In other regions, including the CA1–3 stratum pyramidale and dentate gyrus inner molecular layer, CB1 receptor expression never returned to control levels. At 1–4-months post-SE, as well as our chronic time point of greater than 6 months, CB1 receptor expression was increased in several regions within the hippocampus, specifically in the strata radiatum and oriens, and decreased in the CA1–3 stratum pyramidale and dentate gyrus inner molecular layer, characteristic of the CB1 receptor redistribution previously described in epileptic animals (Falenski et al., 2007).
The results from this study suggest that the characteristic redistribution of CB1 receptor expression temporally correlates with the emergence of SRS, which has been reported to be anywhere between 4 and 44 days with a mean latency of 14.8 days (Leite et al., 1990; Raza et al., 2004). By one month post-SE, however, animals in our study exhibited both behavioral epileptic seizures as well as the characteristic CB1 receptor redistribution previously observed in the chronic phase (Falenski et al., 2007). The documented anticonvulsant effects of CB1 receptor activation in this model (Wallace et al., 2003) suggest that the presence of this redistributed receptor population is a compensatory effect for increased excitability that occurs with epilepsy. However, it would be of interest in future studies to determine whether this long-term plasticity change is due to an underlying mechanism that occurs as a result of epileptogenesis or merely maintained by the presence of SRS.
An interesting finding of this study refers to the differential regulation of the receptor within the hippocampus that occurs between 1 and 2 weeks post-SE, after the initial reduction in hippocampal CB1 receptor staining. CB1 receptors located on the CA1–3 stratum pyramidale appear to stay depressed, while CB1 receptors located on terminals in the strata oriens and radiatum appear to recover and ultimately overshoot control levels. Although the mechanisms underlying this differential regulation have not been fully elucidated, a possible explanation could be a loss of CB1 receptor-dependent regulation of synaptic transmission at the stratum pyramidale, which has been demonstrated to contain primarily GABAergic terminals (Hajos et al., 2000; Tsou et al., 1999), and an increase in CB1 receptor-dependent regulation of synaptic transmission throughout the strata oriens and radiatum, which is in accordance with axon terminals of glutamatergic neurons (Boulland et al., 2007) recently found to contain CB1 receptors (Katona et al., 2006; Kawamura et al., 2006). In our model, this could ultimately result in an increase in GABAergic neuro-transmission, and a decrease in glutamatergic transmission, substantiating the anticonvulsant effect of CB1 receptor activation. Furthermore, studies using the Cre/loxP system to generate conditional knockout mouse lines found that CB1 receptors located specifically on glutamatergic neurons are responsible for mediating the suppressive effects of cannabinoids in kainic acid induced seizures (Marsicano et al., 2003; Monory et al., 2006) as well as several parameters of the mouse tetrad including hypolocomotion and hypothermia (Monory et al., 2007), indicating that a separation of CB1 receptor modulation of these neurotransmitter systems can occur.
It is hypothesized that glutamate itself may play a role in the regulation of CB1 mRNA, as a study in the caudateputamen indicated that administration of MK801, the NMDA receptor antagonist, significantly altered CB1 mRNA expression in quantitative in situ hybridization (Mailleux and Vanderhaeghen, 1994). NMDA receptor activation has been found to be necessary for both the induction of epilepsy in the pilocarpine model (Rice and DeLorenzo, 1998) as well as the resultant CB1 receptor redistribution (Falenski, unpublished observations). Given that the enhancement in glutamatergic systems is a hallmark of epilepsy (Morimoto et al., 2004), it is conceivable that increased NMDA receptor activation could cause alterations in CB1 receptor mRNA production, as endocannabinoid production has recently been shown to be NMDA receptor-dependent (Ohno-Shosaku et al., 2007).
The present study demonstrated that at 4- and 7-days post-SE, there is a widespread loss in CB1 receptor expression throughout the hippocampus. Initial cell loss (Covolan and Mello, 2000) and delayed neuronal cell death (Weise et al., 2005) are found to occur in this model, and may account in part for decreases in CB1 receptor immunoreactivity. Our results have agreed with previous studies conducted in the laboratory, where a small degree of pyramidal cell loss was observed in the CA1 region following pilocarpine-induced SE (Rice and DeLorenzo, 1998). However, our findings demonstrate overall increases in CB1 receptor expression in long-term epileptic rats (Wallace et al., 2003), substantiated by increases in [3H]WIN55,212-2 binding and agonist-stimulated [35S]GTPgammaS autoradiography (Falenski et al., 2007). Thus, it is important to consider other mechanisms by which CB1 receptor-IR is initially decreased following pilocarpine-induced SE. Future investigations of CB1 receptor mRNA expression following pilocarpine-induced SE are warranted to determine if this dropout in CB1 receptor expression is due to alterations in transcriptional regulation. Furthermore, it would be of interest to determine whether the decreases in CB1 receptor expression observed following SE translate to more functional changes in [3H]WIN55,212-2 binding and WIN55,212-stimulated [35S]GTPgammaS autoradiography, as observed in the chronic state.
Interestingly, CB1 receptor-IR on many interneurons, particularly those at the border of the dentate gyrus granule cell layer, was preserved at these early time points, and in some instances appear more numerous or intense than in the corresponding controls. Colocalization studies have demonstrated that these CB1 receptor-positive interneurons represent a subtype of inhibitory cells that are also immunoreactive for cholecystokinin, while interneurons containing either parvalbumin (Katona et al., 1999; Marsicano and Lutz, 1999; Tsou et al., 1999) or somatostatin (McDonald and Mascagni, 2001) are largely CB1 receptor-immunonegative. In the pilocarpine model of epilepsy, several studies have demonstrated a selective loss of parvalbumin- and somatostatin-immunoreactive interneurons in the CA1 stratum oriens and dentate gyrus (Andre et al., 2001; Dinocourt et al., 2003; Kobayashi and Buckmaster, 2003). The consistent levels of CB1 receptor immunoreactivity present on many interneurons throughout this time-course, as well as the consistent number of CB1 receptor immunopositive interneurons observed in the dentate gyrus, suggest that the interneurons within this subtype GABAergic interneuron population are spared. Further studies are undoubtedly required to more fully characterize this population of cells following pilocarpine-induced SE; nevertheless, these results illustrate that the loss in CB1 receptor IR observed is not all-encompassing.
Overall, these results indicate that decreased hippocampal CB1 receptor expression, and thus diminished endocannabinoid tone, may contribute to the pathophysiological mechanisms underlying epileptogenesis, and are consistent with findings from other studies implicating the endocannabinoid system in regulation of excitability in both normal and pathological conditions. Exogenous administration of the endocannabinoid anandamide (Wallace et al., 2002), as well as manipulation of the endocannabinoid levels using inhibitors of the anandamide-degrading enzyme fatty acid amide hydrolase (Coomber et al., 2008; Naderi et al., 2008), are effective in producing anticonvulsant effects in several acute seizure models, although one study has implicated anandamide as being proconvulsant (Clement et al., 2003). Furthermore, kainic acid administration has been shown to produce elevated anandamide levels in mice (Marsicano et al., 2003). Evidence for the role of the endocannabinoid 2-Arachidonylglycerol exist as well, as levels are elevated after both high frequency stimulation in vitro (Stella et al., 1997) and following 30 min of pilocarpine-induced SE (Wallace et al., 2003). Disruption of the endocannabinoid system by the CB1 receptor antagonist SR141716A prior to febrile seizures has recently been shown to prevent later CB1 receptor upregulation and the potentiation of depolarization-induced suppression of inhibition (DSI) (Chen et al., 2003, 2007), indicating that endocannabinoid system activation during an excitotoxic insult can initiate long-lasting plasticity changes. Administration of SR141716A has also been found to lower seizure threshold (Wallace et al., 2002) and increase seizure frequency in the pilocarpine model (Wallace et al., 2003) demonstrating the importance of an intact endocannabinoid system in maintaining baseline excitability. Understanding the nature of this plasticity and the role of the endocannabinoid system in epileptogenesis may ultimately lead to the development of novel therapeutic interventions for the treatment of acquired epilepsy.
4. Experimental procedures
4.1. Animals and reagents
All procedures were approved by and in accordance with the Virginia Commonwealth University Animal Care and Use Committee (IACUC) guidelines and the NIH Guide for Care and Use of Laboratory Animals, and all attempts were made to minimize animal suffering and reduce the number of animals utilized for these studies. Adult male Sprague-Dawley rats (approximately 250 grams) (Harlan, Indianapolis) were housed in single cages in a temperature-controlled environment (20–22 °C) on a 12-hour light/dark cycle and were provided with food and water ad libitum. All drugs were dissolved in distilled water or isotonic (0.9%) saline. Reagent-grade chemicals were purchased from Sigma (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA) unless otherwise noted.
4.2. Induction of SE and acquired epilepsy
Pilocarpine-induced SE and acquired epilepsy was produced according to previously established methods (Mello et al., 1993) as routinely conducted in our laboratory (Wallace et al., 2003). Briefly, 30 min after administration of methylscopola-mine nitrate (1 mg/kg i.p.), pilocarpine nitrate (375 mg/kg i.p.) was administered to induce SE. Onset of SE was determined by the presence of continuous class 4–5 level seizures assessed using the Racine scale (Racine, 1972). After 60 min of SE, these animals were ‘rescued’ by three consecutive injections of diazepam (5 mg/kg, i.p.) (VCU Health Systems Pharmacy, Richmond, VA), given 2 h apart. For these studies, 4–5 rats were sacrificed with an equal number of age-matched controls at the following time points after pilocarpine-induced SE: 4 days, 1 week, 2 weeks, 1 month, 4 months, and chronic (6 months). Animals were video monitored for behavioral seizure activity for 24 h prior to sacrifice and the presence of SRS was noted.
4.3. Tissue preparation
For immunohistochemical studies, animals were briefly anesthetized by halothane then injected with a ketamine/ xylazine cocktail (75 mg/kg, i.p.) (VCU Health Systems Pharmacy). Animals were transcardially flushed with saline, then perfused with 4% PF in a 100 mM sodium phosphate buffer. Brains were then postfixed overnight in 4% PF, followed by cryoprotection in 30% sucrose. Twenty micron coronal sections were cut on a Leitz cryostat (Leica Microsystems, Wetzlar, Germany) maintained at −20 °C and mounted onto gelatin-subbed slides. Both a control and an SE-treated brain from the same time point were placed on a slide to reduce variability in staining. Slides were stored at −80 °C. For western blot analysis, animals were briefly anesthetized with halothane anesthesia prior to sacrifice, and hippocampal tissue was harvested on ice. Hippocampi were homogenized in buffer containing 50 mM Tris–HCl pH 7.4, 7 mM EGTA, 5 mM EDTA, and 320 mM sucrose, and hippocampal neuronal membranes were isolated by centrifugation as previously described (Wallace et al., 2003). Protein concentration was determined by the Bradford assay (BioRad, Hercules, CA).
4.4. Immunohistochemisty
Immunohistochemistry was performed according to previously published techniques (Tsou et al., 1998)(Falenski et al., 2007) with minor modifications. Briefly, slides, each containing a brain of a pilocarpine-treated animal with its age-matched control run in parallel, were brought to room temperature and blocked in SuperBlock blocking buffer, (SBB, Pierce, Rockford, IL, 1 h RT). An N-terminus CB1 receptor antibody to amino acid residues 1–77 (courtesy of Dr. Ken Mackie; 1:1000 dilution, 72 h, 4 °C) was then applied. The sections were then incubated in biotinylated anti-Rabbit IgG (Vector Laboratories, Burlingame, CA, 1:200, 30 min RT) and avidin-biotin complex (Vector, 1:100, 30 min RT) and visualized using 3′3′ diaminobenzidine (DAB) peroxidase (Vector). Adjacent sections were Nissl stained. Controls included absence of primary antibody, as well as coabsorption with an immunizing peptide (1 μg/mL).
4.5. Western blotting
Western blotting of hippocampal membrane fractions was carried out as previously described (Morris et al., 2000; Wallace et al., 2003). Briefly, hippocampal membrane fractions (15 μg protein/lane) were separated by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Immobilon, Millipore Corp., Bedford, MA). Membranes were blocked for 1 h in PBS containing 0.05% Tween-20 and 3% BioRad blocking reagent and incubated in CB1 receptor primary antibody (Cayman Chemical Company, Ann Arbor, MI) diluted 1:500 in blocking solution overnight at 4 °C. The membranes were then washed 5 times with PBS followed by incubation for 1 h with goat anti-rabbit IgG antibody conjugated to horseradish peroxidase (1:1000, Santa Cruz Biotechnology Inc., Santa Cruz, CA). The blots were washed 5 times with PBS and bound secondary antibody was detected by enhanced chemiluminescence (SuperSignal, Pierce, Rockford, IL) and exposure to Kodak X-Omat Blue XB-1 X-ray film (Eastman Kodak, Rochester, NY).
4.6. Data analysis
After mounting, digitized images of immunohistochemically stained slides were visualized using Analysis software (Soft Imaging System, Lakewood, CO) and Adobe Photoshop. All camera settings including exposure time, gain, and offset were held constant throughout capturing, and figures contain equally contrasted-enhanced images of control and treated animals always taken from the same slide. For densitometric analysis of CB1 receptor immunostains, selected hippocampal layers from CA1 from four to five animals per group were evaluated. Following acquisition of high-resolution digitized grayscale images, mean pixel intensity per area (0–255) above normalized background (consisting of white matter areas including corpus collosum) was measured for each circled layer and averaged across both hemispheres. Data were normalized to % of age-matched controls.
Pyramidal cell counts of the CA1 region of Nissl-stained slides per 0.5 mm area were also averaged across hemispheres and compared between control and 1 week post-SE animals, similar to techniques previously described by our laboratory (Rice and DeLorenzo, 1998). Cell counts were expressed as #cells/0.5 mm as well as normalized to % of age-matched controls. The location of the hippocampus used for staining and histology was nearly identical between animals as determined by comparison to the Paxinos and Watson atlas (Paxinos, 1986); however, stereological and volumetric techniques were not employed and thus more subtle changes in cell number may not be detected.
Western blot film images were digitized using a gel scanner and analyzed by computer-assisted densitometry. Membranes were stripped and re-blotted with anti-beta-actin antibody (Sigma-Aldrich, St. Louis, MO) at a dilution of 1:5000 to assess correct protein loading. Densitometric analysis of the western blot was obtained using Image J software. Bands were normalized with the beta-actin loading control, and for analysis each treated group was normalized to % of the corresponding age-matched control. Epileptic densities across time points were compared using a one-way ANOVA with a post-hoc Dunnett's test.
Acknowledgments
This work was supported by U01-NS058213-01, R01-NS052529-01, and R01-NS051505-01 to RJD.
The authors would like to gratefully thank Dr. Ken Mackie (DA11322 awarded to Ken Mackie) for providing the antisera.
Abbreviations
- CB1
Cannabinoid type 1
- DG
dentate gyrus
- IR
immunoreactivity
- SE
status epilepticus
- SRS
spontaneous recurrent seizures
REFERENCES
- Andre V, et al. Alterations of hippocampal GAbaergic system contribute to development of spontaneous recurrent seizures in the rat lithium-pilocarpine model of temporal lobe epilepsy. Hippocampus. 2001;11:452–468. doi: 10.1002/hipo.1060. [DOI] [PubMed] [Google Scholar]
- Blair RE, et al. Activation of the cannabinoid type-1 receptor mediates the anticonvulsant properties of cannabinoids in the hippocampal neuronal culture models of acquired epilepsy and status epilepticus. J. Pharmacol. Exp. Ther. 2006;317:1072–1078. doi: 10.1124/jpet.105.100354. [DOI] [PubMed] [Google Scholar]
- Boulland JL, et al. Changes in vesicular transporters for gamma-aminobutyric acid and glutamate reveal vulnerability and reorganization of hippocampal neurons following pilocarpine-induced seizures. J. Comp. Neurol. 2007;503:466–485. doi: 10.1002/cne.21384. [DOI] [PubMed] [Google Scholar]
- Chen K, et al. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron. 2003;39:599–611. doi: 10.1016/s0896-6273(03)00499-9. [DOI] [PubMed] [Google Scholar]
- Chen K, et al. Prevention of plasticity of endocannabinoid signaling inhibits persistent limbic hyperexcitability caused by developmental seizures. J. Neurosci. 2007;27:46–58. doi: 10.1523/JNEUROSCI.3966-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement AB, et al. Increased seizure susceptibility and proconvulsant activity of anandamide in mice lacking fatty acid amide hydrolase. J. Neurosci. 2003;23:3916–3923. doi: 10.1523/JNEUROSCI.23-09-03916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomber B, et al. Inhibition of endocannabinoid metabolism attenuates enhanced hippocampal neuronal activity induced by kainic acid. Synapse. 2008;62:746–755. doi: 10.1002/syn.20547. [DOI] [PubMed] [Google Scholar]
- Covolan L, Mello LE. Temporal profile of neuronal injury following pilocarpine or kainic acid-induced status epilepticus. Epilepsy Res. 2000;39:133–152. doi: 10.1016/s0920-1211(99)00119-9. [DOI] [PubMed] [Google Scholar]
- Dinocourt C, et al. Loss of interneurons innervating pyramidal cell dendrites and axon initial segments in the CA1 region of the hippocampus following pilocarpine-induced seizures. J. Comp. Neurol. 2003;459:407–425. doi: 10.1002/cne.10622. [DOI] [PubMed] [Google Scholar]
- Falenski KW, et al. Status epilepticus causes a long-lasting redistribution of hippocampal cannabinoid type 1 receptor expression and function in the rat pilocarpine model of acquired epilepsy. Neuroscience. 2007;146:1232–1244. doi: 10.1016/j.neuroscience.2007.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, et al. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur. J. Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hauser H. Epilepsy: Frequency, Causes and Consequences. Demos; New York: 1990. [Google Scholar]
- Katona I, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J. Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, et al. Molecular composition of the endocannabinoid system at glutamatergic synapses. J. Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, et al. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J. Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J. Neurosci. 2003;23:2440–2452. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite JP, et al. Spontaneous recurrent seizures in rats: an experimental model of partial epilepsy. Neurosci. Biobehav. Rev. 1990;14:511–517. doi: 10.1016/s0149-7634(05)80076-4. [DOI] [PubMed] [Google Scholar]
- Ludanyi A, et al. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J. Neurosci. 2008;28:2976–2990. doi: 10.1523/JNEUROSCI.4465-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Glutamatergic regulation of cannabinoid receptor gene expression in the caudate-putamen. Eur. J. Pharmacol. 1994;266:193–196. doi: 10.1016/0922-4106(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur. J. Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Marsicano G, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: high concentrations in a subpopulation of cholecystokinin-containing interneurons. Neuroscience. 2001;107:641–652. doi: 10.1016/s0306-4522(01)00380-3. [DOI] [PubMed] [Google Scholar]
- Mello LE, et al. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia. 1993;34:985–995. doi: 10.1111/j.1528-1157.1993.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Monory K, et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monory K, et al. Genetic dissection of behavioural and autonomic effects of Delta(9)-tetrahydrocannabinol in mice. PLoS Biol. 2007;5:e269. doi: 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto K, et al. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog. Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Morris TA, et al. Chronic DeltaFosB expression and increased AP-1 transcription factor binding are associated with the long term plasticity changes in epilepsy. Brain Res. Mol. Brain Res. 2000;79:138–149. doi: 10.1016/s0169-328x(00)00112-1. [DOI] [PubMed] [Google Scholar]
- Naderi N, et al. Evaluation of interactions between cannabinoid compounds and diazepam in electroshock-induced seizure model in mice. J. Neural. Transm. 2008;115(11):1501–1511. doi: 10.1007/s00702-008-0076-x. Nov. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, et al. Endocannabinoid signalling triggered by NMDA receptor-mediated calcium entry into rat hippocampal neurons. J. Physiol. 2007;584:407–418. doi: 10.1113/jphysiol.2007.137505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos W. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1986. [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Raza M, et al. Evidence that injury-induced changes in hippocampal neuronal calcium dynamics during epileptogenesis cause acquired epilepsy. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17522–17527. doi: 10.1073/pnas.0408155101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice AC, DeLorenzo RJ. NMDA receptor activation during status epilepticus is required for the development of epilepsy. Brain Res. 1998;782:240–247. doi: 10.1016/s0006-8993(97)01285-7. [DOI] [PubMed] [Google Scholar]
- Stella N, et al. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Tsou K, et al. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Tsou K, et al. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience. 1999;93:969–975. doi: 10.1016/s0306-4522(99)00086-x. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, et al. Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur. J. Pharmacol. 2001;428:51–57. doi: 10.1016/s0014-2999(01)01243-2. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, et al. Evidence for a physiological role of endocannabinoids in the modulation of seizure threshold and severity. Eur. J. Pharmacol. 2002;452:295–301. doi: 10.1016/s0014-2999(02)02331-2. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, et al. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J. Pharmacol. Exp. Ther. 2003;307:129–137. doi: 10.1124/jpet.103.051920. [DOI] [PubMed] [Google Scholar]
- Weise J, et al. Expression time course and spatial distribution of activated caspase-3 after experimental status epilepticus: contribution of delayed neuronal cell death to seizure-induced neuronal injury. Neurobiol. Dis. 2005;18:582–590. doi: 10.1016/j.nbd.2004.10.025. [DOI] [PubMed] [Google Scholar]