Abstract
The immune response to vaccination with Bacillus Calmette-Guerin (BCG), the only tuberculosis vaccine available, has not been fully characterized. We used multiparameter flow cytometry to examine specific T cell cytokine production and phenotypic profiles in blood from 10-week old infants, routinely vaccinated with BCG at birth. Ex vivo stimulation of whole blood with BCG for 12 hours induced expression of predominantly IFN-γ, IL-2 and TNF-α in CD4+ T cells, in 7 distinct cytokine combinations. IL-4 and IL-10 expression were detected in CD4+ T cells at low frequencies, and only in cells that did not co-express Type 1 cytokines. Specific CD8+ T cells were less frequent than CD4+ T cells, and produced mainly IFN-γ and/or IL-2, and less TNF-α, IL-4 and IL-10. Importantly, many mycobacteria-specific CD4+ and CD8+ T cells did not produce IFN-γ. The predominant phenotype of BCG-specific Type 1 T cells was that of effector cells, i.e., CD45RA–CCR7–CD27+, which may reflect persistence of M. bovis BCG in infants until 10 weeks of age. Among 5 phenotypic patterns of CD4+ T cells, central memory cells were more likely to be IL-2+, and effector cells more likely to be IFN-γ+. We concluded that neonatal vaccination with BCG induces T cells with a complex pattern of cytokine expression and phenotypes. Measuring IFN-γ production alone underestimates the magnitude and complexity of the host cytokine response to BCG vaccination, and may not be an optimal readout in studies of BCG and novel tuberculosis vaccination.
Keywords: Human, Th1/Th2 Cells, T cells, Cytokines, Memory, Vaccination
Introduction
Nearly one-third of the global population is latently infected with Mycobacterium tuberculosis, and approximately 2 million people die of tuberculosis (TB) disease every year (Tuberculosis Fact Sheet, 2005, World Health Organization, Geneva, Switzerland). Bacille Calmette-Guerin (BCG), the only vaccine against tuberculosis currently available, has variable efficacy in preventing pulmonary disease (1), but 80% efficacy in preventing childhood miliary disease and meningitis (2). Our knowledge of immunity induced by BCG vaccination is incomplete, particularly after human newborn vaccination. However, infants will be targets of novel, safer and more efficacious tuberculosis vaccines in the future, and a better understanding of the immune response induced by newborn BCG vaccination is likely to facilitate development of improved vaccines.
Experimental evidence suggests that both CD4+ and CD8+ T cells are important for protection against mycobacteria (3-6). In humans, the role of CD4+ T cells has been highlighted by a increased risk of disease after infection with M. tuberculosis when CD4+ T cell numbers decline in HIV-infected persons (7). A primary function of both CD4+ and CD8+ T cells is to produce the Type 1 cytokine IFN-γ. The critical role of this cytokine has been demonstrated by characteristic severe mycobacterial disease in patients with mutations of the IFN-γ receptor (8, 9). Other Type 1 cytokines, such as TNF-α and IL-2, may also be important in protection against tuberculosis: the role of TNF-α has been underscored by high rates of reactivation of latent tuberculosis following treatment of rheumatoid arthritis patients with specific inhibitors of this cytokine (10, 11); T cell IL-2 expression has been associated with long-term memory (12, 13), which is the aim of protective immunity. BCG vaccination of human newborns does indeed induce specific CD4+ and CD8+ T cells, capable of producing IFN-γ (14-16) Previous studies have also shown that BCG vaccination of infants induces TNF-α which is detectable in plasma by ELISA (17). However, the pattern of production of all Type 1 cytokines, on a single cell basis, has not been delineated.
Our aim was to also describe expression of Type 2 cytokines such as IL-4, thought to reflect a suboptimal immune response to mycobacteria (18, 19). Although BCG vaccination of infants has been shown to induce low levels of Type 2 cytokines (15, 16, 20), the detection was in plasma, and cell-associated expression has not been reported. We also wished to assess T cell IL-10 expression, as this cytokine is likely to be an important regulator of effector T cell responses against tuberculosis (21) and is induced by newborn BCG vaccination (15, 16, 22).
The memory phenotype of T cells induced by BCG vaccination of newborns has not been described. Antigen-experienced cells may be categorized based on expression of surface markers (13, 23-26). Central memory cells express CCR7 but not CD45RA and are likely to represent a long-lived population, which expands rapidly in lymph nodes following subsequent antigen encounter (23, 27). In contrast, effector cells are both CCR7– and CD45RA– and act immediately following antigen exposure, but have limited proliferative capacity (13, 23). A third subset, terminally differentiated memory cells, are CD45RA+ and CCR7– and the most differentiated subpopulation, based on short telomere length and function (26, 28). Naïve, or non-antigen-experienced T cells, characteristically express both CD45RA and CCR7 (13, 26, 28). Combination of markers other than CCR7 and CD45RA may also differentiate subsets of antigen-experienced cells. Fritsch et al. recently proposed the phenotypic classification of CD4+ T cell populations based on expression of CD27 and CCR7 (26). Central memory T cells were defined as CD27+ and CCR7+, effectors as CD27+ and CCR7–, and terminally differentiated T cells as CD27– and CCR7– (26). Our aim was to evaluate expression of all these markers among antigen-experienced T cells induced by BCG. Although several investigators have characterized mycobacteria-specific immune responses by four color flow cytometry (29-31), these studies could only measure two cytokines or phenotypic markers at a time and thus most likely underestimated the complexity of the response.
Our hypothesis was that BCG vaccination of newborns would induce both CD4+ and CD8+ T cells capable of producing multiple cytokines, and that a central memory phenotype of specific cells would be dominant 10 weeks after vaccination. We used an intracellular cytokine assay with multiparameter flow cytometry to comprehensively characterize these variables. To achieve our goals, we established clinical structures and optimized techniques (32) to overcome hurdles common to investigation of immunity in infants.
Materials and Methods
Study participants and blood collection
Healthy 10-week old infants, routinely vaccinated intradermally with BCG (Statens Serum Institut, Copenhagen) at birth, were enrolled in the Cape Town region of South Africa. This area has a very high TB disease incidence in under 5 year-olds, exceeding 2% per year in certain areas (32). Infants born to HIV-positive mothers, infants known to be HIV positive, infants with suspected or confirmed TB disease, infants with possible exposure to TB disease and infants with any other active or chronic illnesses at the time of enrollment, were excluded. Human participation was according to the US Department of Health and Human Services and good clinical practice guidelines. This included protocol approval by the University of Cape Town Research Ethics Committee and written informed consent. Sodium heparinized blood was collected from each infant. Two different cohorts were enrolled to preserve blood volume collected for assessment with 2 different flow cytometric protocols (see below): 29 infants to assess cytokine expression of T cells, and 27 infants to assess the phenotype of IFN-γ and IL-2-expressing T cells.
Antigens and Antibodies
BCG was reconstituted from the vaccine vial (SSI) at 1.8 × 106 organisms/mL, as previously described (32). The positive control streptococcal enterotoxin B (SEB, Sigma) was used at 10μg/ml. The co-stimulatory antibodies anti-CD28 and anti-CD49d (both from BD Biosciences) were each used at 1μg/ml. Cytokine profiles of BCG-specific T cells were examined using the following conjugated antibodies: anti-CD3 Amcyan (SK7) and anti-IL-2 Alexa 610-PE (5344.111), both custom-conjugated at BD Biosciences; and anti-CD4 AlexaFluor 700 (RPAT4), anti-CD8 Cy5.5-PerCP (SK1), anti-IL-4 FITC (MP4-25D2), anti-IFN-γ PE (25723.11), anti-IL-10 APC (JES3-19F1), and anti-TNF-α Cy7-PE (MAb11), all obtained from BD Biosciences. A separate protocol was used for assessing T cell phenotypes, using the following conjugated antibodies: anti-CD3 PacBlu (UCHT1), anti-CD4 Cy5.5PerCP (SK3), anti-CD8 Cy5.5PerCP (SK1), anti-CD45RA Cy7PE (L48), anti-CD27 PE (MT271), anti-IFN-γ AlexaFluor 700 (B27) and anti-IL-2 FITC (5344.111), all obtained from BD Biosciences, and anti-CCR7 APC (150503), obtained from R&D Systems.
Whole Blood Intracellular Cytokine Detection Assay
To determine cell-associated cytokine production, 1mL heparinized whole blood was incubated with BCG and anti-CD28 and anti-CD49d, as described before (32). Blood incubated with SEB and co-stimulants, or with co-stimulatory antibodies alone (UNS), served as positive and negative controls, respectively. Brefeldin A (10μg/mL, Sigma) was added during the last 5 hours of incubation to capture cytokines intracellularly. After a total incubation of 12 hours, red blood cells were lysed and white cells fixed with FACS Lysing Solution (BD Biosciences), followed by cryopreservation.
Cell staining and flow cytometric analysis
To detect intracellular cytokines, cryopreserved cells were thawed, washed in PBS, permeabilized with Perm/Wash solution (BD Biosciences) and incubated at 4°C with fluorescence-conjugated antibodies for an hour. To assess T cell memory phenotypes, a two-step staining method, resulting in optimal staining, was used: cells were thawed, washed in PBS, and permeabilized with Perm/Wash solution, and then incubated with surface marker antibodies for an hour at 4°C, followed by an additional hour with antibodies specific for intracellular cytokines. Flow cytometric acquisition was completed on a LSRII flow cytometer (BD Biosciences) configured for 3 lasers and 12 detectors. All the cells in the tube were acquired. Analysis was performed using FACSDiva software (BD Biosciences). Although automated compensation with mouse IgG κ-beads was applied, compensation settings were assessed manually after acquisition, and adjusted if necessary. Multiparameter panel development (not shown) included evaluation of appropriate staining controls, of antibody and fluorochrome interactions and of spectral overlap, using control blood samples (33, 34).
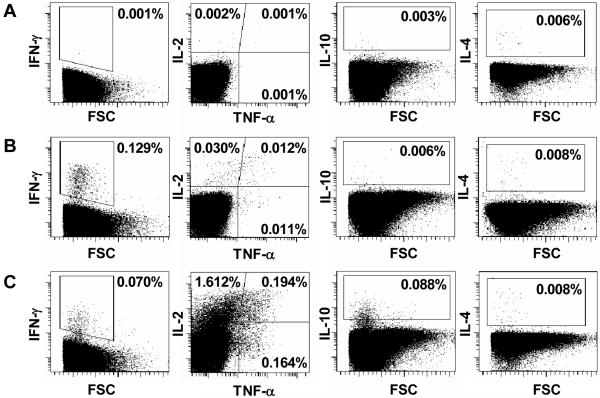
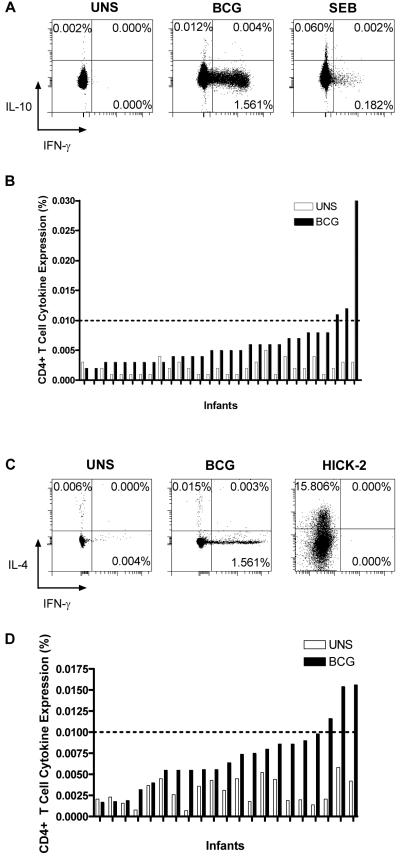
Cut-offs to determine positive cytokine expression in CD4+ and CD8+ T cells from blood incubated with BCG were set using cells from blood incubated with co-stimulatory antibodies alone (negative control) (Fig. 1A). Angled cut-off lines were necessary for some fluorochromes, because of data spread at higher fluorescence intensities, following instrument compensation (e.g., Figure 1). SEB was an excellent positive control for induction of all cytokines, except IL-4. IL-4-expressing HICK-2 cells (BD Biosciences), processed as per manufacturer’s protocol, were used as positive control for IL-4. Where distinction between positive and negative surface marker populations was not clear, isotype-matched control antibody staining was used to set cut-offs for phenotypic markers.
Figure 1.
Flow cytometric detection of CD4+ T cell cytokine expression in whole blood incubated with BCG for 12 hours, from a single 10-week old BCG-vaccinated infant. The cut-off gates for cytokine expression were determined using unstimulated T cells from whole blood incubated with co-stimulants only (A). Cytokine expression in CD4+ T cells from whole blood incubated with BCG (B), and with SEB (C) is shown. IL-2/TNF-α subset gating was based on patterns of cytokine expression in SEB-stimulated whole blood. Dotplots are gated on CD3+CD4+ T cells, and are representative of 29 infants.
Plasma Cytokine Detection
Plasma was collected from the stimulated whole blood after 7 hours, and cryopreserved. Later, thawed plasma was used to measure levels of IL-2, IL-4, IL-10 and IFN-γ with multiplex beads following the manufacturer’s instructions (Bio-Rad Laboratories), and read on a luminometer (Luminex). The range of detection for all cytokines was 1.95–32,000pg/mL. The optimal plasma dilution for our assay, determined in pilot experiments, was 1:4. Background cytokine levels measured in plasma harvested from unstimulated blood were subtracted from BCG-stimulated blood.
Statistical considerations
Negative control (background) values for Type 1 cytokine expression were not subtracted from BCG-induced responses, as the median backgrounds for all Type 1 CD4+ T cell subsets was 0.001% (range, 0.000%-0.01%) and for CD8+ T cell subsets 0.000% (range, 0.000%-0.01%). We used an empiric cut-off value of 0.01% as positive: given that a median of 508,509 CD4+ T cells and 188,498 CD8+ T cells were collected, this cut-off was predicted to be >90% different from background, at an alpha of 0.05 (35). IL-4 and IL-10 expression were reported with backgrounds (see below). Nonparametric tests were used to compare differences in cytokine expression and phenotypic profiles between CD4+ and CD8+ T cells. Associations between cellular expression of cytokines and plasma levels of these were assessed by the nonparametric Spearman test. Data were considered statistically significant when p<0.05. Statistical analysis was performed using GraphPad Prism 4 (GraphPad Software).
Results
BCG-specific CD4+ and CD8+ T cell Type 1 cytokine production
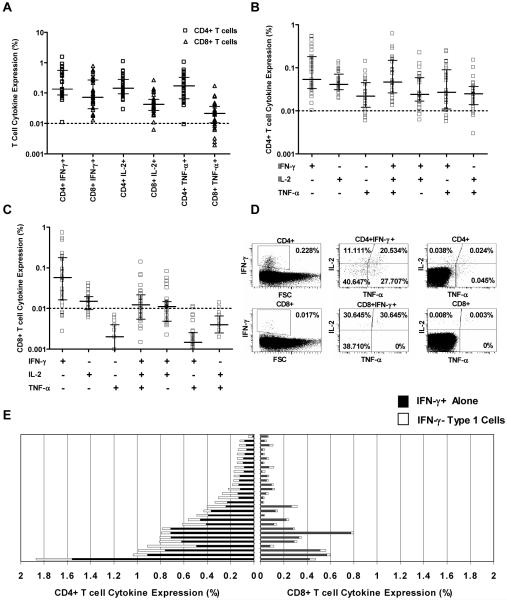
Intracellular expression of 3 Type 1 cytokines thought to be critical for protective immunity against mycobacteria, IFN-γ, IL-2 and TNF-α (36, 37), was evaluated by incubating blood from 29 BCG-vaccinated infants with BCG for 12 hours (Figure 1). The frequencies of CD4+ T cells expressing either IFN-γ or IL-2 or TNF-α were similar (Figure 2A). Lower frequencies of CD8+ T cells expressed IFN-γ or IL-2, compared with CD4+ T cells (p<0.05), while TNF-α was expression was very low in CD8+ T cells (Figure 2A). There was a strong positive correlation between the frequencies of CD4+ and CD8+ T cells expressing IFN-γ, and between the frequencies of these cells expressing IL-2 (R=0.770 R=0.879, respectively, both p<0.0001, Spearman test).
Figure 2.
Cytokine profiles of BCG-specific T cells in 10-week old infants, vaccinated at birth. (A) Frequency of CD4+ and of CD8+ T cells expressing individual Type 1 cytokines following incubation of blood with BCG for 12 hours, in 29 infants. Responses above 0.01% were considered positive. The horizontal line indicates the median and the whiskers the interquartile range. (B) Frequency of BCG-specific CD4+ T cells expressing different combinations of Type 1 cytokines. (C) Frequency of BCG-specific CD8+ T cells expressing different combinations of Type 1 cytokines. (D) Representative staining of intracellular Type 1 cytokines in BCG-specific CD4+ T cells and CD8+ T cells, from a single 10-week old infant. (E) Comparison of frequency of CD4+ and of CD8+ T cells expressing IFN-γ (dark bars) with frequency of T cells expressing IL-2 and/or TNF-α without IFN-γ (light bars), in 29 BCG-vaccinated infants.
Analysis of simultaneous expression of IFN-γ, IL-2 and TNF-α on a single cell level revealed 7 distinct Type 1 cytokine-expressing CD4+ T cell populations (Figure 2B). Most cytokine-producing CD4+ T cells expressed 2 of the 3 cytokines. Among CD8+ T cells, the dominant population expressed IFN-γ only; 3 other populations were discernable (Figure 2C). Importantly, a substantial proportion of CD4+ T cells expressing IL-2 and/or TNF-α did not co-express IFN-γ (Figure 2 D). Similarly, among CD8+ T cells, a proportion of IL-2 expressing T cells also did not co-express IFN-γ (Figure 2 D).
All 3 Type 1 cytokines could also be detected in plasma (data not shown). There was a significant correlation between plasma levels of IFN-γ, IL-2 and TNF-α and frequencies of CD4+ T cells producing these cytokines (Table I). Plasma IFN-γ and IL-2 also correlated with frequencies of CD8+ T cells producing IFN-γ and IL-2, respectively (Table I).
Table I.
Association between type 1 cytokine levels in plasma, and frequencies of CD4+ or CD8+ T cells expressing these cytokines, after incubation of whole blood with BCG. A Spearman test was used to assess correlation in 29 infants
| CD4+ T cells expressing cytokine |
CD8+ T cells expressing cytokinea |
|||
|---|---|---|---|---|
| r | p | r | p | |
| Plasma IFN-γ | 0.6793 | <0.0001 | 0.6305 | 0.0004 |
| Plasma IL-2 | 0.6441 | 0.0003 | 0.6013 | 0.0009 |
| Plasma TNF-α | 0.4034 | 0.01 | 0.3187 | 0.1052 |
Taken together, we concluded that BCG vaccination of newborns induces multiple Type 1 T cell subsets defined by expression of distinct cytokine combinations.
BCG-specific IL-10 and Type 2 cytokine production
The frequency of T cells expressing IL-10 or IL-4 following incubation of whole blood with BCG was low (Figure 3). Few donors had responses above 0.01%, our cut-off for a positive Type 1 cytokine response, but IL-10 and IL-4 production were consistently above the background expression levels found in blood not incubated with BCG (Figure 3B and 3D). CD4+ T cell expression of both cytokines was slightly higher than that of CD8+ T cells (CD4+ T cell expression shown in Figure 3; CD8+ T cells: a median 0.004%, range 0.001%-0.024%, expressed IL-10 and 0.005%, 0.000%-0.011%, IL-4). IL-10 and IL-4 were never co-expressed by cells making Type 1 cytokines (Figure 3A and 3C). Intracellular IL-4 could be readily detected in HICK-2 cytokine-expressing cells, which served as positive control (Figure 3C). IL-4 and IL-10 were detected at low levels in plasma of whole blood incubated with BCG (Figure 4).
Figure 3.
Expression of IL-10 (A) and of IL-4 (C) in CD4+ T cells from whole blood from a single vaccinated infant, incubated with co-stimulants (UNS), BCG or SEB. Frequency of CD4+ T cells expressing IL-10 (B) and of IL-4 (D) following incubation of blood with co-stimulants only (UNS) (light bars) and BCG (dark bars) for 12 hours, in 29 infants.
Figure 4.
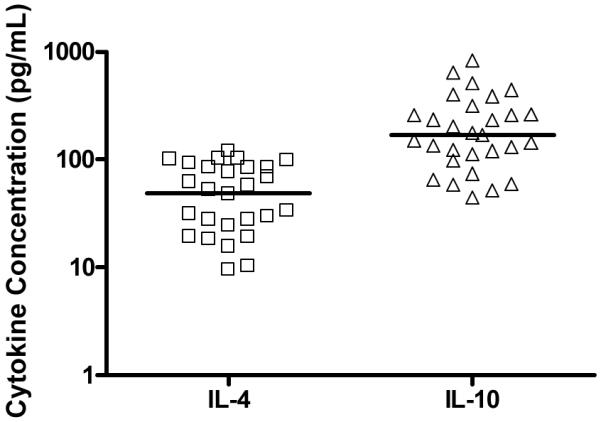
Levels of IL-4 and IL-10, in plasma from whole blood incubated with BCG for 7 hours. The horizontal line represents the median. Background cytokine levels were subtracted.
We concluded that BCG vaccination of newborns induces low levels of IL-4 and IL-10 expression.
Phenotypic profiles of specific Type 1 T cell subsets
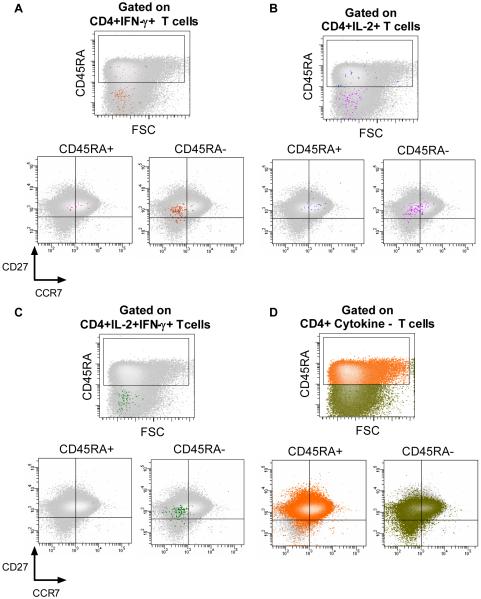
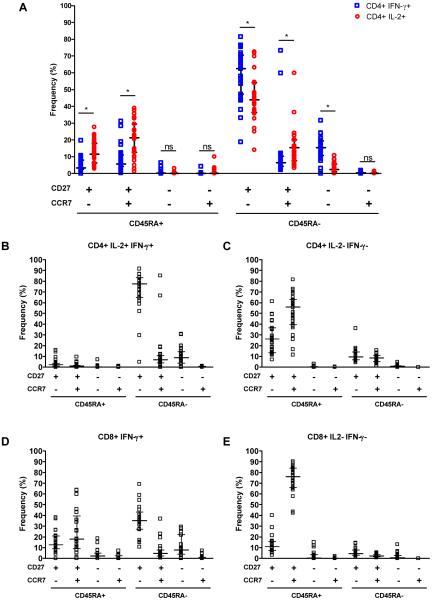
Studies in other infectious disease models have shown that distinct populations of antigen-experienced T cells may be associated with long-lived protection (38). We therefore examined the phenotypic profiles of BCG-induced T cells. Specific CD4+ T cells were defined as either IFN-γ or IL-2-expressing (Figure 5A-D); frequencies of cells expressing other cytokines were too low to reliably delineate the phenotype. Five major and distinct antigen-experienced CD4+ T cells subsets could be discerned, based on expression of CD45RA, CCR7, and CD27 (Figure 6A-C). By far the most common phenotype of both IFN-γ and IL-2-expressing CD4+ T cells was CD45RA–CCR7–CD27+ (Figure 6A), a phenotype that has been reported to be characteristic of effector T cells (13). The second most common phenotype among IFN-γ-expressing CD4+ T cells was CD45RA–CCR7–CD27–, also characteristic of effector T cells (Figure 6A). Among IL-2-expressing CD4+ T cells, the latter population was significantly less frequent (Figure 6A), while central memory phenotypes were more common: IL-2+ cells were more likely to be CD45RA–CCR7+CD27+, CD45RA+CCR7–CD27+, or CD45RA+CCR7+CD27+, compared with IFN-γ-expressing CD4+ T cells (Figure 6A). CD4+ T cells that expressed both IFN-γ and IL-2 were predominantly effector memory cells (CD45RA–CCR7–CD27+ or CD45RA–CCR7+CD27+; Figure 6B).
Figure 5.
Phenotype of BCG-specific CD4+ T cells in 10-week old infants, vaccinated at birth. Antigen-specific CD4+ T cells were identified by expression of intracellular IFN-γ (A), IL-2 (B), or both cytokines (C), and the expression of CD45RA, CCR7 and CD27 determined in each case. The plots illustrate the distribution of cytokine-expressing cells (in color; foreground) in relation to the entire CD4+ T cell population (grey; background, also shown in D).
Figure 6.
Frequency of different subsets of BCG-specific CD4+ T cells, based on expression of CD45RA, CCR7 and CD27 among IFN-γ+ and IL-2+ (A), and IFN-γ+IL-2+ (B), expressing CD4+ T cells. (C) Frequency of these cells among cytokine-negative, i.e., mostly mycobacteria-non-specific, CD4+ T cells. The horizontal line represents the median and the whiskers the interquartile range. *, p<0.05; ns, p≥0.05.
Phenotypes of BCG-specific CD8+ T cells could reliably be detected only for IFN-γ-producing cells, as the frequencies of IL-2-producing CD8+ T cells were too low. CD8+IFN-γ+ T cells also displayed a pre-dominant CD45RA–CCR7–CD27+ effector phenotype (Figure 6D). Unlike CD4+IFN-γ+ T cells, a central memory population (CD45RA+CCR7+CD27+) was the second most common.
We concluded that the majority of specific T cells induced by BCG vaccination of newborns has an effector phenotype, and that IL-2 expression is more likely to be associated with a central memory phenotype.
Discussion
We showed that BCG vaccination of human newborns induces a diverse set of T cells, delineated by distinct cytokine production and phenotypic profiles. BCG-specific T cells produced mainly Type 1 cytokines, as has been demonstrated before (14-16, 20, 22). However, we showed that not only IFN-γ is produced. A considerable number of IFN-γ-negative CD4+ T cells were present, expressing the other Type 1 cytokines IL-2 and TNF-α. Similarly, many CD8+ T cells produced IL-2 in the absence of IFN-γ. The most commonly used measure of mycobacteria-induced immunity today is IFN-γ production, be this to diagnose latent infection via IFN-γ release assays (39, 40) or to describe human immune responses to novel tuberculosis vaccines (41). Recent experimental data suggest that measuring IFN-γ may not correlate with vaccination-induced protection against tuberculosis (42-44). This strongly supports measurement of all 3 cytokines on a cellular level to delineate a mycobacteria-specific Type 1 response. Measuring a single component of the immune response may underestimate the magnitude and complexity of BCG-induced immunity.
A BCG-induced CD8+ T cell response was readily detectable, as Type 1 cytokine-producing cells. Murine studies suggest that CD8+ T cells play an important role in control of M. tuberculosis infection and contribute substantially to total IFN-γ production (4). Although a BCG-induced CD8+ T cell response has been described before (14, 45), we now show that the response is characterized by both IFN-γ and IL-2 producing subsets. An interesting observation was that CD8+ T cells produced very little TNF-α. Smith, et al., detected similar frequencies of CD8+ T cells expressing TNF-α and IFN-γ following incubation of PBMC with BCG for 6 days (45). The contrasting low frequency of TNF-α expression observed in our study could be due to differences in assays, as we measured cytokine expression 12 hours after incubation of whole blood with BCG. In longer term assays such as those performed by Smith, et al., TNF-α production may be derived from newly differentiated effector T cells, whereas our short term assay measures cytokine producing potential directly ex vivo. Our results also contrast with data from HIV-infected adults, whose HIV-specific CD8+ T cells readily express TNF-α in short-term intracellular cytokine assays (46, 47), implying that BCG-specific CD8+ T cells in newborns express little TNF-α.
The memory phenotype of BCG-induced T cells has not been reported. We identified five phenotypically distinct subsets within BCG-specific Type 1 T cells, based on expression of CD45RA, CCR7 and CD27. The predominant phenotype of both IFN-γ and IL-2-expressing CD4+ and CD8+ T cells was CD45RA–CCR7–CD27+. CCR7− effector cells are characteristic of persistent activation of T cells seen in chronic viral infections where antigen is not cleared (48-50). Similarly, increased numbers of effector T cells are observed in children with active TB (51). It is possible that M. bovis BCG had persisted up to 10 weeks of age, the time at which blood was collected from infants, resulting in this predominant phenotype.
Distinct differences in phenotypes were observed among CD4+ IFN-γ and IL-2-expressing cells. IL-2-expressing cells were significantly more likely to have a central memory phenotype, e.g., CD45RA–CCR7+CD27+, compared with IFN-γ-expressing cells. IL-2-expressing T cells induced by BCG vaccination therefore follows patterns similar to those previously described for purified human central memory populations, which are likely to express IL-2 (13). However, our results, from 10 weeks after vaccination with BCG, contrast with results obtained 8 weeks after vaccination with tetanus toxoid; specific IL-2-expresing central memory cells were present but in a relatively small population, whereas this population was predominant after tetanus vaccination (52). Tetanus toxoid is not a persistent antigen; we therefore hypothesize that viable M. bovis BCG persisted following vaccination, driving differentiation predominantly into IFN-γ-expressing effector cells, and preventing differentiation into IL-2-expressing central memory cells. An alternate hypothesis is that continuous exposure to environmental mycobacteria may contribute to chronic immune activation, and therefore a predominance of effector T cells. The infants in this study were from a region with high rates of environmental mycobacteria, and unpublished data suggest that exposure may occur even within the first 10 weeks of life. It has been proposed that exposure to environmental mycobacterial antigens in tropical regions may undermine protective immunity induced by BCG (53). A third hypothesis is that the time point at which we measured the host response was too early for full differentiation into the central memory phenotype. A central memory/IL-2 phenotype has been associated with improved prognosis of chronic human viral infections such as HIV (49), and in experimental settings of chronic intracellular bacterial infection with long-lived protection (38). It remains to be determined whether a central memory phenotype is also associated with successful vaccination against tuberculosis.
We found that a significant amount of specific CD4+ and CD8+ T cells had a phenotype traditionally regarded as naïve, i.e., CD45RA+ and CCR7+. In a recent study of children with tuberculosis, Caccamo et al. also described this population, identified as specific by MHC Class I pentamers of antigen-85A (54). We propose that this CD45RA+CCR7+ population reflects early differentiation into antigen-specific cells, prior to loosing CD45RA expression.
We could detect only very low intracellular expression of the Type 2 cytokine IL-4. Expression of Type 2 cytokines has been associated with a suboptimal immune response to mycobacteria (55). For example, Ordway, et al., showed that long-term control of latent M. tuberculosis infection in humans appeared to be associated with optimal Type 1 cytokine production and absence of detectable Type 2 cytokine production (56). They showed that high percentages of IL-4 expressing CD8+ and γδ T cells soon after M.tb infection were associated with ultimate development of TB disease. The IL-4-expressing cells were detected after incubation of PBMC for 6 days, which contrasted with our 12-hour assay; longer-term assays may be required to detect these cells in the setting of BCG vaccination of the newborn. Intracellular expression of IL-10 could also be detected in our cohort, was low, and, like IL-4 expression, was never co-expressed with any Type 1 cytokine. We propose that the IL-10-expressing T cells were induced regulatory T cells, which are expected to be present at low frequencies. Regulatory T cells control conventional effector immune responses, are induced by infections (57), and likely also by vaccination with BCG.
Our results clearly demonstrate the advantages of complex multiparameter flow cytometric analysis for deciphering a vaccination-induced immune response. However, this technology is not readily available. It is therefore important to note, from our findings, that when detection of IFN-γ or IL-2 is the aim and flow cytometry is not available, plasma levels may serve as surrogates of T cell cytokine production. We showed that plasma levels of Type 1 cytokines correlated strongly with intracellular expression despite the fact that non-T cells also have the ability to make these cytokines (58, 59). When only 4-color flow cytometry is available, inclusion of all 3 Type 1 cytokines in one color channel for detecting the total Type 1 response may be the most useful. This proposal is supported by observations in HIV infection, where presence of “polyfunctional” CD8+ T cell populations, i.e., HIV-specific CD8+ T cells that co-express multiple cytokines, was associated with better clinical outcome (46). The observation in a mouse model of Leishmania major infection that “polyfunctional” T cell induction is also associated with the best outcome (60) suggests that measurement of these cells may also be important following BCG vaccination, as the mechanisms of immune protection are very similar for Leishmania and mycobacteria. Regardless, measurement of all 3 Type 1 cytokines in one color would still not delineate the complexity of cytokine expression profiles. This may be important, as other studies HIV infection showed that IL-2 expression, rather than IFN-γ or dual expression, correlated with the best clinical outcome (49). We therefore propose that delineation of multiple cytokine-expressing subsets, individually, will be important to investigate protective immunity against tuberculosis, either following natural infection, or following vaccination.
Acknowledgments
The authors acknowledge the infants who took part in this study, their families, and the support of an excellent team of researchers at our field site. We would like to thank Dr N. Goonetilleke and Professor S. Gordon from the University of Oxford for initial assistance with the Luminex assay.
Funding
TJS is a Wellcome Trust Senior Clinical Research Fellow. AH, GDH and WAH are supported by the Aeras Global Tuberculosis Vaccine Foundation, and by the European Commission, within the 6th Framework Programme. GDH is supported by the NIH, D43-TW007115, as is GK, RO1-AI66046, RO1-AI065653, NO1-AI70022 and RO1-HL55936, and WAH, RO1-AI065653 and NO1-AI70022. WAH is supported by the Bill & Melinda Gates Foundation through Grand Challenges in Global Health Grants #37772 and #37885, the Dana Foundation, and the European and Developing Countries Trials Partnership.
Footnotes
Disclosures
None.
References
- 1.Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, Fineberg HV. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995;96:29–35. [PubMed] [Google Scholar]
- 2.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 3.Flory CM, Hubbard RD, Collins FM. Effects of in vivo T lymphocyte subset depletion on mycobacterial infections in mice. J. Leukoc. Biol. 1992;51:225–229. doi: 10.1002/jlb.51.3.225. [DOI] [PubMed] [Google Scholar]
- 4.Lazarevic V, Nolt D, Flynn JL. Long-term control of Mycobacterium tuberculosis infection is mediated by dynamic immune responses. J. Immunol. 2005;2:1107–1117. doi: 10.4049/jimmunol.175.2.1107. [DOI] [PubMed] [Google Scholar]
- 5.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, Flynn JL. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol. 1999;162:5407–5416. [PubMed] [Google Scholar]
- 7.Elliott AM, Hodsdon S, Kyosiimire J, Quigley MA, Nakiyingi JS, Namujju PB, Watera C, French N, Gilks CF, Dockrell HM, Whitworth JA. Cytokine responses and progression to active tuberculosis in HIV-1-infected Ugandans: a prospective study. Trans. R. Soc. Trop. Med. Hyg. 2004;98:660–670. doi: 10.1016/j.trstmh.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Ottenhoff TH, De Boer T, van Dissel JT, Verreck FA. Human deficiencies in type-1 cytokine receptors reveal the essential role of type-1 cytokines in immunity to intracellular bacteria. Adv. Exp. Med. Biol. 2003;531:279–294. doi: 10.1007/978-1-4615-0059-9_24. [DOI] [PubMed] [Google Scholar]
- 9.van de Vosse E, Hoeve MA, Ottenhoff TH. Human genetics of intracellular infectious diseases: molecular and cellular immunity against mycobacteria and salmonellae. Lancet Infect. Dis. 2004;12:739–749. doi: 10.1016/S1473-3099(04)01203-4. [DOI] [PubMed] [Google Scholar]
- 10.Saliu OY, Sofer C, Stein DS, Schwander SK, Wallis RS. Tumor-necrosis-factor blockers: differential effects on mycobacterial immunity. J. Infect. Dis. 2006;194:486–492. doi: 10.1086/505430. [DOI] [PubMed] [Google Scholar]
- 11.Stenger S. Immunological control of tuberculosis: role of tumour necrosis factor and more. Ann. Rheum. Dis. 2005;64:24–28. doi: 10.1136/ard.2005.042531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouneaud C, Garcia Z, Kourilsky P, Pannetier C. Lineage relationships, homeostasis, and recall capacities of central- and effector-memory CD8 T cells in vivo. J. Exp. Med. 2005;201:579–590. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nat. Immunol. 1999;14:659–660. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 14.Murray RA, Mansoor N, Harbacheuski R, Soler J, Davids V, Soares A, Hawkridge A, Hussey GD, Maecker H, Kaplan G, Hanekom WA. Bacillus Calmette Guerin Vaccination of Human Newborns Induces a Specific, Functional CD8+ T cell Response. J. Immunol. 2006;177:5647–5651. doi: 10.4049/jimmunol.177.8.5647. [DOI] [PubMed] [Google Scholar]
- 15.Ota MO, Vekemans J, Schlegel-Haueter SE, Fielding K, Sanneh M, Kidd M, Newport MJ, Aaby P, Whittle H, Lambert PH, McAdam KP, Siegrist CA, Marchant A. Influence of Mycobacterium bovis bacillus Calmette-Guerin on antibody and cytokine responses to human neonatal vaccination. J. Immunol. 2002;168:1919–1925. doi: 10.4049/jimmunol.168.2.919. [DOI] [PubMed] [Google Scholar]
- 16.Marchant A, Goetghebuer T, Ota MO, Wolfe I, Ceesay SJ, De Groote D, Corrah T, Bennett S, Wheeler J, Huygen K, Aaby P, McAdam KP, Newport MJ. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J. Immunol. 1999;163:2249–2255. [PubMed] [Google Scholar]
- 17.Davids V, Hanekom WA, Mansoor N, Gamieldien H, Gelderbloem SJ, Hawkridge A, Hussey GD, Hughes EJ, Soler J, Murray RA, Ress SR, Kaplan G. The effect of bacille Calmette-Guerin vaccine strain and route of administration on induced immune responses in vaccinated infants. J. Infect. Dis. 2006;193:531–536. doi: 10.1086/499825. [DOI] [PubMed] [Google Scholar]
- 18.Ordway DJ, Martins MS, Costa LM, Freire MS, Arroz MJ, Dockrell HM, Ventura FA. Increased IL-4 production in response to virulent Mycobacterium tuberculosis in tuberculosis patients with advanced disease. Acta. Med. Port. 2005;18:27–36. [PubMed] [Google Scholar]
- 19.Lienhardt C, Azzurri A, Amedei A, Fielding K, Sillah J, Sow OY, Bah B, Benagiano M, Diallo A, Manetti R, Manneh K, Gustafson P, Bennett S, D’Elios MM, McAdam K, Del Prete G. Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. Eur. J. Immunol. 2002;32:1605–1613. doi: 10.1002/1521-4141(200206)32:6<1605::AID-IMMU1605>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Hussey GD, Watkins ML, Goddard EA, Gottschalk S, Hughes EJ, Iloni K, Kibel MA, Ress SR. Neonatal mycobacterial specific cytotoxic T-lymphocyte and cytokine profiles in response to distinct BCG vaccination strategies. Immunology. 2002;105:314–324. doi: 10.1046/j.1365-2567.2002.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boussiotis VA, Tsai EY, Yunis EJ, Thim S, Delgado JC, Dascher CC, Berezovskaya A, Rousset D, Reynes JM, Goldfeld AE. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J. Clin. Invest. 2000;105:1317–1375. doi: 10.1172/JCI9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vekemans J, Amedei A, Ota MO, D’Elios MM, Goetghebuer T, Ismaili J, Newport MJ, Del Prete G, Goldman M, McAdam KP, Marchant A. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur. J. Immunol. 2001;31:1531–1535. doi: 10.1002/1521-4141(200105)31:5<1531::AID-IMMU1531>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Ann. Rev. Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 24.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 25.Song K, Rabin RL, Hill BJ, De Rosa SC, Perfetto SP, Zhang HH, Foley JF, Reiner JS, Liu J, Mattapallil JJ, Douek DC, Roederer M, Farber JM. Characterization of subsets of CD4+ memory T cells reveals early branched pathways of T cell differentiation in humans. Proc. Natl. Acad. Sci. USA. 2005;102:7916–7921. doi: 10.1073/pnas.0409720102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fritsch RD, Shen X, Sims GP, Hathcock KS, Hodes RJ, Lipsky PE. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J. Immunol. 2005;175:6489–6497. doi: 10.4049/jimmunol.175.10.6489. [DOI] [PubMed] [Google Scholar]
- 27.Huster KM, Koffler M, Stemberger C, Schiemann M, Wagner H, Busch DH. Unidirectional development of CD8+ central memory T cells into protective Listeria-specific effector memory T cells. Eur. J. Immunol. 2006;36:1453–1464. doi: 10.1002/eji.200635874. [DOI] [PubMed] [Google Scholar]
- 28.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 29.Sester M, Sester U, Clauer P, Heine G, Mack U, Moll T, Sybrecht GW, Lalvani A, Kohler H. Tuberculin skin testing underestimates a high prevalence of latent tuberculosis infection in hemodialysis patients. Kidney Int. 2004;65:1826–1834. doi: 10.1111/j.1523-1755.2004.00586.x. [DOI] [PubMed] [Google Scholar]
- 30.Hughes AJ, Hutchinson P, Gooding T, Freezer NJ, Holdsworth SR, Johnson PD. Diagnosis of Mycobacterium tuberculosis infection using ESAT-6 and intracellular cytokine cytometry. Clin. Exp. Immunol. 2005;142:132–139. doi: 10.1111/j.1365-2249.2005.02884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tesfa L, Koch FW, Pankow W, Volk HD, Kern F. Confirmation of Mycobacterium tuberculosis infection by flow cytometry after ex vivo incubation of peripheral blood T cells with an ESAT-6-derived peptide pool. Cytometry B Clin. Cytom. 2004;60:47–53. doi: 10.1002/cyto.b.20007. [DOI] [PubMed] [Google Scholar]
- 32.Hanekom WA, Hughes J, Mavinkurve M, Mendillo M, Watkins M, Gamieldien H, Gelderbloem SJ, Sidibana M, Mansoor N, Davids V, Murray RA, Hawkridge A, Haslett PA, Ress S, Hussey GD, Kaplan G. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J. Immunol. Methods. 2004;291:185–195. doi: 10.1016/j.jim.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Maecker HT, Frey T, Nomura LE, Trotter J. Selecting Fluorochrome Conjugates for Maximum Sensitivity. Cytometry. 2004;62 A:169–173. doi: 10.1002/cyto.a.20092. [DOI] [PubMed] [Google Scholar]
- 34.Perfetto SP, Chattopadhyay PK, Roederer M. Seventeen-colour flow cytometry: unravelling the immune system. Nat. Rev. Immunol. 2004;4:648–655. doi: 10.1038/nri1416. [DOI] [PubMed] [Google Scholar]
- 35.Motulsky H. Intuitive Biostatistics. Oxford University Press; New York, NY: 1995. Choosing an Appropriate Sample Size; pp. 199–200. [Google Scholar]
- 36.Flynn JL. Immunology of tuberculosis and implications in vaccine development. Tuberculosis. 2004;84:93–101. doi: 10.1016/j.tube.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Kaufmann SH. Recent findings in immunology give tuberculosis vaccines a new boost. Trends Immunol. 2005;26:660–667. doi: 10.1016/j.it.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Zaph C, Uzonna J, Beverley SM, Scott P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat. Med. 2004;10:1104–1110. doi: 10.1038/nm1108. [DOI] [PubMed] [Google Scholar]
- 39.Mahomed H, Hughes EJ, Hawkridge T, Minnies D, Simon E, Little F, Hanekom WA, Geiter L, Hussey GD. Comparison of mantoux skin test with three generations of a whole blood IFN-gamma assay for tuberculosis infection. Int. J. Tuberc. Lung Dis. 2006;10:310–316. [PubMed] [Google Scholar]
- 40.Janssens JP, Roux-Lombard P, Perneger T, Metzger M, Vivien R, Rochat T. Quantitative scoring of a {gamma}-interferon assay for differentiating active from latent tuberculosis. Eur. Respir. J. 2007 doi: 10.1183/09031936.00028507. [DOI] [PubMed] [Google Scholar]
- 41.McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K, Fletcher HA, Hill AV. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 2004;10:1240–1244. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 42.Hovav AH, Mullerad J, Davidovitch L, Fishman Y, Bigi F, Cataldi A, Bercovier H. The Mycobacterium tuberculosis recombinant 27-kilodalton lipoprotein induces a strong Th1-type immune response deleterious to protection. Infect. Immun. 2003;71:3146–3154. doi: 10.1128/IAI.71.6.3146-3154.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majlessi L, Simsova M, Jarvis Z, Brodin P, Rojas MJ, Bauche C, Nouze C, Ladant D, Cole ST, Sebo P, Leclerc C. An Increase in Antimycobacterial Th1-Cell Responses by Prime-Boost Protocols of Immunization Does Not Enhance Protection against Tuberculosis. Infect. Immun. 2006;74:2128–2137. doi: 10.1128/IAI.74.4.2128-2137.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hope JC, Thom ML, Villarreal-Ramos B, Vordermeier HM, Hewinson RG, Howard CJ. Vaccination of neonatal calves with Mycobacterium bovis BCG induces protection against intranasal challenge with virulent M. bovis. Clin. Exp. Immunol. 2005;139:48–56. doi: 10.1111/j.1365-2249.2005.02668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith SM, Malin AS, Lukey PT, Atkinson SE, Content J, Huygen K, Dockrell HM. Characterization of human Mycobacterium bovis bacille Calmette-Guerin-reactive CD8+ T cells. Infect. Immun. 1999;67:5223–5230. doi: 10.1128/iai.67.10.5223-5230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Castro Cunha RM, Rodrigues DS, Nascimento Burattini M, Salomao R. Interferon-gamma and tumour necrosis factor-alpha production by CD4+ T and CD8+ T lymphocytes in AIDS patients with tuberculosis. Clin. Exp. Immunol. 2005;140:491–497. doi: 10.1111/j.1365-2249.2005.02796.x. K. E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baars PA, Sierro S, Arens R, Tesselaar K, Hooibrink B, Klenerman P, van Lier RA. Properties of murine (CD8+)CD27− T cells. Eur. J. Immunol. 2005;35:3131–3141. doi: 10.1002/eji.200425770. [DOI] [PubMed] [Google Scholar]
- 49.Harari A, Petitpierre S, Vallelian F, Pantaleo G. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood. 2004;103:966–972. doi: 10.1182/blood-2003-04-1203. [DOI] [PubMed] [Google Scholar]
- 50.Harari A, Vallelian F, Pantaleo G. Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Eur. J. Immunol. 2004;34:3525–3533. doi: 10.1002/eji.200425324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacobsen M, Detjen AK, Mueller H, Gutschmidt A, Leitner S, Wahn U, Magdorf K, Kaufmann SHE. Clonal Expansion of CD8+ Effector T Cells in Childhood Tuberculosis. J. Immunol. 2007;179:1331. doi: 10.4049/jimmunol.179.2.1331. [DOI] [PubMed] [Google Scholar]
- 52.Harari A, Vallelian F, Meylan PR, Pantaleo G. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J. Immunol. 2005;174:1037–1045. doi: 10.4049/jimmunol.174.2.1037. [DOI] [PubMed] [Google Scholar]
- 53.Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 2005;3:656–662. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- 54.Caccamo N, Meraviglia S, La Mendola C, Guggino G, Dieli F, Salerno A. Phenotypical and functional analysis of memory and effector human CD8 T cells specific for mycobacterial antigens. J. Immunol. 2006;177:1780–1785. doi: 10.4049/jimmunol.177.3.1780. [DOI] [PubMed] [Google Scholar]
- 55.Hernandez-Pando R, Orozcoe H, Sampieri A, Pavon L, Velasquillo C, Larriva-Sahd J, Alcocer JM, Madrid MV. Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology. 1996;89:26–33. [PMC free article] [PubMed] [Google Scholar]
- 56.Ordway DJ, Costa L, Martins M, Silveira H, Amaral L, Arroz MJ, Ventura FA, Dockrell HM. Increased Interleukin-4 production by CD8 and gammadelta T cells in health-care workers is associated with the subsequent development of active tuberculosis. J. Infect. Dis. 2004;190:756–766. doi: 10.1086/422532. [DOI] [PubMed] [Google Scholar]
- 57.Grazia Roncarolo M, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 58.Esin S BG, Pardini M, Favilli F, Bottai D, Maisetta G, Florio W, Vanacore R, Wigzell H, Campa M. Functional characterization of human natural killer cells responding to Mycobacterium bovis bacille Calmette-Guerin. Immunology. 2004;112:143–152. doi: 10.1111/j.1365-2567.2004.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feinberg J, Fieschi C, Doffinger R, Feinberg M, Leclerc T, Boisson-Dupuis S, Picard C, Bustamante J, Chapgier A, Filipe-Santos O, Ku CL, de Beaucoudrey L, Reichenbach J, Antoni G, Balde R, Alcais A, Casanova JL. Bacillus Calmette Guerin triggers the IL-12/IFN-gamma axis by an IRAK-4- and NEMO-dependent, non-cognate interaction between monocytes, NK, and T lymphocytes. Eur. J. Immunol. 2004;34:3276–3284. doi: 10.1002/eji.200425221. [DOI] [PubMed] [Google Scholar]
- 60.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional T(H)1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007 doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]