Abstract
Background and Purpose
We investigated whether neuroglobin (Ngb), a neuronal protein that protects neurons from hypoxic-ischemic injury, is upregulated in ischemic stroke.
Methods
Ngb immunoreactivity was measured in brain tissue from control subjects and patients with ischemic stroke.
Results
Ngb was detected in several brain areas and its expression was increased in the cortical peri-infarct region following stroke.
Conclusions
Ischemic stroke increases expression of the neuroprotective protein Ngb, suggesting Ngb may represent a novel target for stroke therapy.
Keywords: neuroglobin, ischemia, neuroprotection
Neuroglobin (Ngb) is an oxygen-binding heme protein found in the brains of vertebrates, including humans 1. Ngb expression is increased in rodent brain neurons following hypoxia in vitro and focal ischemia in vivo 2, suggesting that Ngb may have a role in the brain’s response to hypoxic-ischemic injury. Additional evidence for such a role includes the observation that forced overexpression of Ngb reduces, whereas knockdown of Ngb expression exacerbates, hypoxic and ischemic neuronal damage in rodents 2–5. Although human epidemiologic studies have suggested a relationship between Ngb polymorphisms and risk for cerebrovascular disease 6, little is known about the relationship between acute stroke and Ngb expression. To investigate this question, we examined the expression of Ngb in normal human brain and in brain tissue from patients with a histopathological diagnosis of ischemic cerebral infarction.
Materials and Methods
Control brain tissue was obtained at autopsy from patients (ages 25–48 years) without neurological disease. Ischemic and adjacent, nonischemic brain tissue was obtained from six patients (ages 34–74 years) undergoing biopsy for possible ischemic stroke, in whom the diagnosis was confirmed by histopathology. Additional data regarding the age, sex, nature and duration of symptoms, and location of infarcts has been published as part of another study 7. Informed consent was obtained and the study was approved by the Institutional Research Review Board at Huashan Hospital of Fudan University, China. Western blotting with rabbit polyclonal anti-Ngb (1:500; Sigma) and mouse monoclonal anti-actin (1:2000; Sigma), single-label immunohistochemistry with rabbit polyclonal anti-Ngb (1: 500; Sigma), and dual-label fluorescence immunohistochemistry with rabbit polyclonal anti-Ngb (1:200; Sigma), mouse monoclonal anti-NeuN (1:200; Chemicon) and mouse monoclonal anti-GFAP (1:150; Sigma) primary and FITC-conjugated rat-absorbed donkey anti-mouse and rhodamine-conjugated donkey anti-goat (Jackson ImmunoResearch; 1:200) secondary antibodies were performed as described previously 2–4. In some cases, nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Controls included omitting or preabsorbing primary or omitting secondary antibody. Fluorescence signals were detected with a Nikon E800 microscope and results were recorded with a Magnifire digital camera (ChipCoolers, Warwick, RI). Slides were examined using an LSM 510 NLO Confocal Scanning System mounted on an Axiovert 200 inverted microscope (Carl Zeiss Ltd) equipped with a two-photon Chameleon laser (Coherent Inc.). Images were acquired using LSM 510 Imaging Software (Carl Zeiss Ltd).
Results
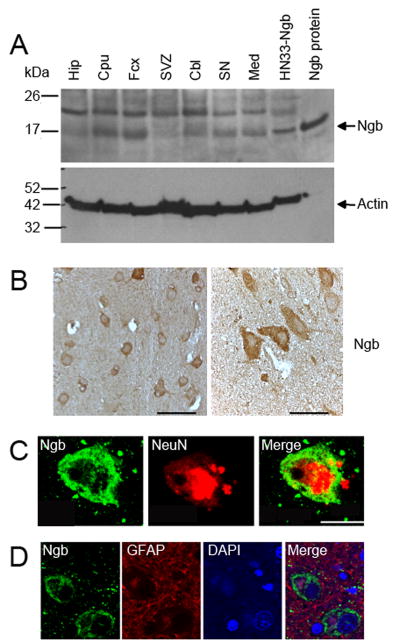
Ngb immunoreactivity, assessed by western blotting, was detectable in normal human brain, with regional variations. Of seven regions studied, Ngb levels were highest in cerebral cortex and caudatoputamen, intermediate in cerebellum, substantia nigra and medulla, and lowest in hippocampus and subventricular zone (Figure 1A). This resembles the distribution of human Ngb mRNA reported previously 8. Single-label immunohistochemistry with an anti-Ngb antibody showed that Ngb was expressed in cells with neuronal morphology (Figure 1B). This was confirmed by double-label fluorescence immunohistochemistry, which demonstrated a strong correlation between Ngb immunoreactivity and cellular expression of the neuronal marker NeuN (Figure 1C), but not the astroglial marker GFAP (Figure 1D).
Figure 1. Ngb expression in normal human brain.
(A) Western blot of Ngb (~17 kDa) protein expression in normal human brain. The band at ~23 kDa is unidentified. Hip, hippocampus; Cpu, caudatoputamen; Fcx, frontal cortex; SVZ, subventricular zone; Cbl, cerebellum; SN, substantia nigra; Med, medulla; HN33-Ngb, Ngb-overexpressing HN33 (clonal mouse hippocampal neuron × neuroblastoma) cells; Ngb protein, authentic human Ngb. Actin was measured to control for variations in protein loading. in cerebral cortex at low (left) and high (right) magnification. Scale bars are 50 (left) and 20 (right) μm. (C) Double-label immunohistochemistry in cerebral cortex showing expression of Ngb (left, green) and NeuN (center, red), and merged image (right). Scale bar is 10 μm. (D) Double-label immunohistochemistry in cerebral cortex showing expression of Ngb (left, green) and GFAP (center left, red), DAPI-stained nuclei (center right, blue) and merged image (right). Scale bar is 10 μm.
Hematoxylin and eosin staining of biopsy specimens from patients with stroke showed regions of neuronal shrinkage and cell loss consistent with ischemic injury, surrounded by zones of hypercellularity reflecting infiltration of inflammatory cells, and adjacent normal tissue (Figure 2A). Ngb immunoreactivity in 6/6 patients was highest in the intermediate, peri-infarct region (Figure 2A) and essentially absent from the infarct core (Figure 2C), which is similar to the distribution of increased Ngb immunoreactivity following focal cerebral ischemia in rats 2. As observed in normal human brain, Ngb in brains of 6/6 patients with stroke was localized primarily to neurons, identified by staining for NeuN (Figure 2D), rather than GDAP-expressing astrocytes (Figure 2E). Thus, induction of neuronal Ngb expression appears to be one component of the brain’s response to ischemia in human stroke.
Figure 2. Ngb expression in post-stroke human brain.
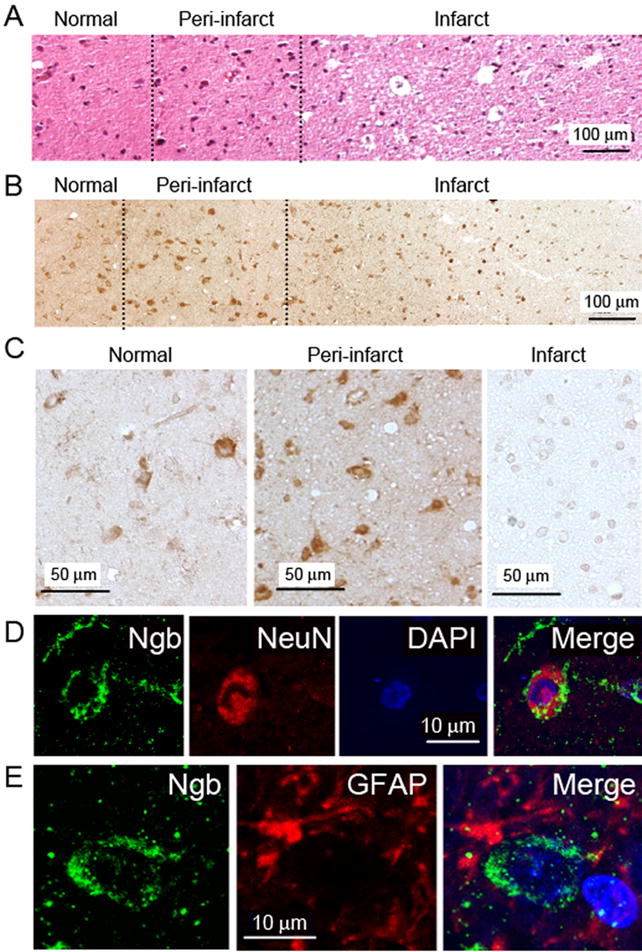
(A) Hematoxylin- and eosin-stained section from cerebral cortex of infarcted brain. (B) Ngb immunohistochemistry in cerebral cortex of infarcted brain. (C) Ngb immunohistochemistry in cerebral cortex of infarcted brain at higher magnification. (D) Double-label immunohistochemistry in cerebral cortex of infarcted brain showing expression of Ngb (left, green) and NeuN (center, red), DAPI-stained nuclei (center right, blue) and merged image (right). (D) Double-label immunohistochemistry in cerebral cortex of infarcted brain showing expression of Ngb (left, green) and GFAP (center, red), and merged image (right). Images shown are representative of similar findings in 6/6 patients studied.
Discussion
The major finding of this study is that ischemic stroke leads to an increase in the expression of Ngb in the peri-infarct cerebral cortex, compared to the adjacent normal brain and ischemic core. Ngb was discovered recently based on its homology to hemoglobin and myoglobin 8. Ngb is localized primarily to neurons and binds O2, CO and NO, but its physiological function is uncertain 9. However, the observation that Ngb expression is induced by hypoxia and ischemia 2 is consistent with a role in neurocellular responses to these insults. The significance of increased Ngb expression in clinical stroke is unclear, but in rodents, overexpression of Ngb is associated with reduction of infarct size and improved functional outcome 3, 4, 10. Accordingly, Ngb might serve a similar neuroprotective role in humans. Although direct evidence for this is lacking, one study has shown an association between Ngb polymorphisms and susceptibility to stroke in the Southern Chinese Han population 6. Ngb can be detected in human cerebrospinal fluid 11, which might facilitate future clinical studies on the relationship between Ngb levels and stroke severity or outcome. Given the neuroprotective effect of Ngb in animal models of stroke 3, 4, 10 and other neurological diseases 12, drugs that stimulate Ngb expression could be therapeutically useful in these disorders.
Acknowledgments
Supported by USPHS grant NS62040 (D.A.G.).
Footnotes
Conflicts of Interest/Disclosure
None
References
- 1.Trent JT, 3rd, Watts RA, Hargrove MS. Human neuroglobin: A hexacoordinate hemoglobin that reversibly binds oxygen. J Biol Chem. 2001;276:30106–30110. doi: 10.1074/jbc.C100300200. [DOI] [PubMed] [Google Scholar]
- 2.Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is upregulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci U S A. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci USA. 2003;100:3497–3500. doi: 10.1073/pnas.0637726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan AA, Wang Y, Sun Y, Mao XO, Xie L, Miles E, Graboski J, Chen S, Ellerby LM, Jin K, Greenberg DA. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc Natl Acad Sci U S A. 2006;103:17944–17948. doi: 10.1073/pnas.0607497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan AA, Mao XO, Banwait S, DerMardirossian CM, Bokoch GM, Jin K, Greenberg DA. Regulation of hypoxic neuronal death signaling by neuroglobin. FASEB J. 2008;22:1737–1747. doi: 10.1096/fj.07-100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin Y, Fang L, Xue XH, Murong SX, Wang N, Wu ZY. Association between Ngb polymorphisms and ischemic stroke in the Southern Chinese Han population. BMC Med Genet. 2008;9:110. doi: 10.1186/1471-2350-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg DA, Jin K, Khan AA. Neuroglobin: An endogenous neuroprotectant. Curr Opin Pharmacol. 2008;8:20–24. doi: 10.1016/j.coph.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Liu J, Zhu H, Tejima E, Tsuji K, Murata Y, Atochin DN, Huang PL, Zhang C, Lo EH. Effects of neuroglobin overexpression on acute brain injury and long-term outcomes after focal cerebral ischemia. Stroke. 2008;39:1869–1874. doi: 10.1161/STROKEAHA.107.506022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casado B, Pannell LK, Whalen G, Clauw DJ, Baraniuk JN. Human neuroglobin protein in cerebrospinal fluid. Proteome Sci. 2005;3:2. doi: 10.1186/1477-5956-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan AA, Mao XO, Banwait S, Jin K, Greenberg DA. Neuroglobin attenuates beta-amyloid neurotoxicity in vitro and transgenic Alzheimer phenotype in vivo. Proc Natl Acad Sci U S A. 2007;104:19114–19119. doi: 10.1073/pnas.0706167104. [DOI] [PMC free article] [PubMed] [Google Scholar]