Abstract
The authors examined the association of ‘g’ (general intelligence) factor and 5 specific cognitive measures assessed in 1997-1999 with mortality till 2006 (mean follow-up of 8 years) in the middle-aged Whitehall II cohort study. In age- and sex-adjusted analysis, a decrease in one standard-deviation in memory (Hazard Ratio (HR)=1.19, 95% Confidence Interval (CI): 1.02, 1.39) and in AH4-I (HR=1.16, 95%CI: 1.01, 1.35) was found to be associated with higher mortality. The association with ‘g’ factor, phonemic and semantic fluency did not reach significance at p<0.05. No association was found with vocabulary. Out of education, health behaviours and health measures, it was health behaviours that explained the greater part of the association between cognition and mortality, ranging from 21% for memory to 70% for semantic fluency. All the covariates taken together explained only 26% of the association with memory and between 33-90% for the other cognitive measures. This study suggests that ‘g’ type composite measure of cognition might not be enough to understand the associations between cognition and health.
Keywords: cognitive function, ‘g’ factor, memory, reasoning, mortality, risk factor, cognitive epidemiology
1. Introduction
There are two distinct strands of research on the association between cognition and mortality. Low cognitive scores in childhood and early adulthood, usually on intelligence tests, have been shown to be associated with shortened survival (Batty et al., 2007; Hart et al., 2005; Holsinger et al., 2007; Kuh et al., 2004; Martin and Kubzansky, 2005; Whalley and Deary, 2001). On the other hand, cognition in the elderly has also been shown to be associated with mortality (Bassuk et al., 2000; Bennett et al., 2002; Dewey and Saz, 2001; Eagles et al., 1990; Fried et al., 1998; Gale et al., 1996; Gussekloo et al., 1997; Hassing et al., 2002; Hunderfund et al., 2006; Kelman et al., 1994; Korten et al., 1999; Liu et al., 1990; Neale et al., 2001; Nguyen et al., 2003; Palmer et al., 2002; Shipley et al., 2006; Small et al., 2003; Small and Backman, 1997; Smits et al., 1999; Swan et al., 1995; Tuokko et al., 2003). Besides age, a major difference between these two strands of research is the conceptualisation of cognitive function. Among children and adolescents, cognitive function is generally measured by tests of intelligence (Batty et al., 2007; Hart et al., 2005; Holsinger et al., 2007; Kuh et al., 2004; Martin and Kubzansky, 2005; Whalley and Deary, 2001), a single composite measure is used to assess global cognitive ability and referred to as ‘g’ or the general intelligence factor (Deary and Batty, 2007). Among the elderly, multiple cognitive domains, like memory (Hassing et al., 2002; Shipley et al., 2006; Small et al., 2003; Small and Backman, 1997; Smits et al., 1999), digit symbol substitution test (Fried et al., 1998; Swan et al., 1995), others measures of processing speed (Anstey et al., 2001; Hassing et al., 2002; Korten et al., 1999; Smits et al., 1999), visuospatial abilities (Hassing et al., 2002; Shipley et al., 2006; Small et al., 2003), vocabulary (Anstey et al., 2001; Rabbitt et al., 2002), verbal fluency (Small et al., 2003) or global cognitive function, like the Mini-Mental State Examination (Anstey et al., 2001; Bassuk et al., 2000; Eagles et al., 1990; Gale et al., 1996; Gussekloo et al., 1997; Kelman et al., 1994; Neale et al., 2001; Nguyen et al., 2003; Palmer et al., 2002; Small et al., 2003), have been used. However, it remains unclear whether the association between cognition and mortality is specific to a particular cognitive domain or to intelligence in general. Another issue that remains debated is whether the association between cognition and mortality in adults is linear or restricted to those at the lower end of the distribution of cognitive scores (Kuh et al., 2004).
The objective of the present study is to examine, in a middle-aged population, whether the association between cognition and mortality is best captured by specific cognitive measures or by the “g” factor. We also examine whether this association applies across the continuum of the distribution of cognitive scores. A further objective is to identify the extent to which education, health behaviours and health measures explain the association between cognition and mortality.
2. Methods
2.1. Study population
Data are drawn from the Whitehall II study, established in 1985 as a longitudinal study to examine the socioeconomic gradient in health and disease among 10,308 civil servants (6,895 men and 3,413 women) (Marmot et al., 1991). All civil servants aged 35-55 years in 20 London based departments were invited to participate by letter, and 73% agreed. Baseline examination (Phase 1) took place during 1985-1988, and involved a clinical examination and a self-administered questionnaire containing sections on demographic characteristics, health, lifestyle factors as smoking habits, work characteristics, social support and life events. Clinical examination included measures of blood pressure, anthropometry, biochemical measurements, neuroendocrine function, and subclinical markers of cardiovascular disease. Subsequent phases of data collection have alternated between postal questionnaire alone (Phases 2 (1988-1990), 4 (1995-1996), 6 (2001) and 8 (2006)) and postal questionnaire accompanied by a clinical examination (Phases 3 (1991-1994), 5 (1997-1999) and 7 (2002-2004)). Participants gave written consent to participate in the study and the University College London ethics committee approved the study.
2.2. Measures of cognitive function
Cognition was assessed at the clinical examination at Phase 5 (1997-1999) using a battery of five tests, described below.
Short-term verbal memory was assessed with a 20-word free recall test. Participants were presented a list of 20 one or two syllable words at two second intervals and then had two minutes to recall in writing as many of the words in any order.
The AH4-I (Alice Heim 4-I) was used to assess reasoning (fluid intelligence). This test is composed of a series of 65 verbal and mathematical reasoning items of increasing difficulty (Heim, 1970). It tests inductive reasoning, measuring the ability to identify patterns and infer principles and rules. Participants had 10 minutes to do this section.
Vocabulary was assessed using the Mill Hill Vocabulary test (Raven, 1965). We used the test in its multiple format, consisting of a list of 33 stimulus words ordered by increasing difficulty and six response choices.
We used two measures of verbal fluency: phonemic and semantic (Borkowski JG et al., 1967). Phonemic fluency was assessed via “S” words and semantic fluency via “animal” words. Subjects were asked to recall in writing as many words beginning with “S” and as many animal names as they could. One minute was allowed for each test.
Principal component analysis of these 5 cognitive measures was used to construct a composite measure of the general intelligence (‘g’) factor (Plomin, 1999). The first factor accounted for 56% of the variance and the factor loadings were 0.32 for memory, 0.50 for AH4-I, 0.46 for Mill Hill, 0.45 for phonemic fluency and 0.49 for semantic fluency.
2.3. Mortality
A total of 10,301 respondents (99.9%) were traced for mortality through the national mortality register kept by the National Health Services Central Registry, by using the National Health Service identification number assigned to each British citizen. In our analysis, mortality follow-up began at the cognitive test assessment (Phase 5) and ended on July 31, 2006.
2.4. Covariates
Demographic variables used were age and sex.
Socioeconomic variables used were education and socioeconomic position. Education was measured using a 5-level hierarchical variable (no or lower primary school, lower secondary school, higher secondary school, university, and higher university degree). Socioeconomic position (SEP) in this white-collar cohort was assessed using the British civil service employment grade (Marmot et al, 1991). This was a three level variable that represents SEP hierarchy within the civil service: high (administrative grades), intermediate (professional or executive grades) and low (clerical or support grades) grades. People in different grades differ with respect to salary, social status and level of responsibility.
Health behaviours were drawn from Phase 5 and assessed using smoking status, alcohol consumption, frequency of fruit and vegetable consumption, and hours of physical activity. Smoking status was assessed using questions on current smoking status (current, past, never). Alcohol consumption was assessed via questions on the number of alcoholic drinks (“measures” of spirits, “glasses” of wine, and “pints” of beer) consumed in the last seven days. This was converted to number of units (8 grams) of alcohol consumed in the last week. The frequency of fruit and vegetable consumption was assessed on an 8-point scale, ranging from ‘seldom or never’ to ‘two or more times a day’. Physical activity was calculated as the sum of the hours of weekly mild, moderate, and vigorous physical activities in response to a 20-item questionnaire on the frequency and duration of participation in walking, cycling, sports, gardening, housework, and home maintenance.
Health measures were drawn from Phase 5. Coronary heart disease prevalence was based on clinically verified events and included myocardial infarction and definite angina (Ferrie et al., 2006). Stroke was assessed using a self-reported measure of physician diagnosis. Diabetes measure was based on self-reports and glucose tolerance test using the WHO criteria (World Health Organization, 1999). Blood pressure, systolic and diastolic, was measured at the Phase 5 clinical examination, twice in the sitting position after 5 minutes rest with an automated Omron 907 device. The average of two measures was taken to be the measured blood pressure. Serum cholesterol was measured within 72 h in serum stored at 4°C using enzymatic colorimetric methods (World Health Organization, 2008).
2.5. Statistical methods
We first assessed the univariate differences on all covariates between those who were alive and those who had died at the end of the follow-up period using t-tests for continuous variables and chi-square tests for categorical variables.
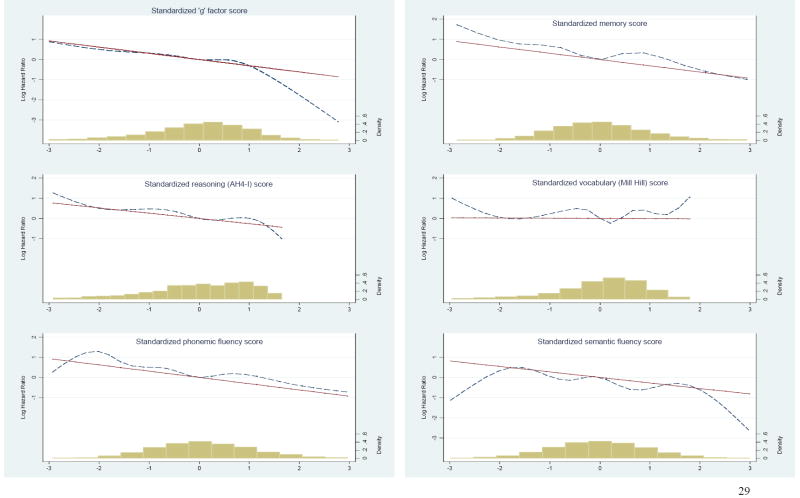
We then plotted the associations between cognition and mortality in order to test whether this association was linear. This was done for all 6 measures of cognition (‘g’ factor, memory, AH4-I, Mill Hill, phonemic, and semantic fluency) and mortality. We used the linear regression and the regression curve (estimated with restricted cubic spline (Heinzl and Kaider, 2007)) of the log Hazard Ratio (HR) for mortality plotted against the standardized cognitive z-scores (mean=0, Standard deviation (SD)=1) with the mean of each cognitive test as the reference. Knots for the cubic spline were chosen at the 5th, 27.5th, 50th, 72.5th and 95th percentile of each cognitive score (Harrell, 2005). The graphs were restricted at -3/+3 SD of the population. We added to this graph the population density histogram (similar to frequency histogram except heights of the rectangles are calculated by dividing relative frequency by class width). Test for non-linearity in the associations between cognitive measures and mortality was performed using the SAS macro rcs.mac (Heinzl and Kaider, 2007).
Subsequently, we calculated the increase in mortality risk for one standard-deviation decrease in each measure of cognition. These associations were examined using Cox regression to model survival time subsequent to the assessment of cognition for each individual. These analyses were adjusted for age and then for age and sex. Interaction between cognitive function and survival time was tested to check for the proportional hazards assumption of the Cox model.
Then we examined the extent to which the association between cognitive measures and mortality was explained by the socioeconomic variables, health behaviours and health, sequentially and then together. For this, we first added socioeconomic variables to the model including age and sex (model 1). The reduction in model 1 was attributed to socioeconomic variables and was calculated using the following formula 100 × (HR controlling for age and sex + covariates − HR controlling for age and sex)/(HR controlling for age and sex − 1). We then repeated this analysis for health behaviours and then for measures of health. In the final model we added all covariates to model 1, allowing us to judge the extent to which these covariates explained the association between cognition and mortality. All the graphs were performed using STATA version 10 and the analyses using SAS statistical software, version 9.
3. Results
3.1. Sample description and missing data
Among the 9931 persons alive at Phase 5, 7785 participated in Phase 5, either the questionnaire or the clinical examination or both. Among them, 5572 had data on all cognitive tests and covariates. Compared to the 2213 individuals who had completed only a part of Phase 5, participants included in our study had a lower rate of mortality (3.28% versus 5.60%, p<0.0001), were younger (55.7 years versus 56.6 years, p<0.0001), composed of fewer women (28.0% versus 36.5%, p<0.0001) and had a higher education (30.2% had university degree or higher versus 20.6%, p<0.0001). Differences were more marked compared to non participants at Phase 5 for whom mortality was even higher (7.27%), population was composed of more women (42.4%) and fewer individuals (14.6%) had a university degree or higher (p<0.0001).
During a mean follow-up period of 8.4 years (SD=0.5), starting from assessment of cognition at Phase 5, 183 participants had died. Characteristics of the study participants are shown in Table 1.
Table 1.
Means or proportions of covariates measured at Phase 5 (1997-1999) by survival status*
| Covariates | Alive at end of follow-up† (N=5389) | Dead at end of follow-up (N=183) | P value |
|---|---|---|---|
| Socio-demographic measures | |||
| Age (M, SD) | 55.4 (6.0) | 59.2 (5.7) | <0.0001 |
| Women (N, %) | 1509 (28.0%) | 52 (28.4%) | 0.90 |
| Lower secondary school (N, %) | 2340 (43.4%) | 95 (51.9%) | 0.03 |
| Lower socioeconomic position (N, %) | 719 (13.3%) | 33 (18.0%) | 0.07 |
| Health behaviours | |||
| Current smokers (N, %) | 790 (14.7%) | 47 (25.7%) | <0.0001 |
| Consumption of fruits & vegetable‡ (N, %) | 4005 (74.3%) | 116 (63.4%) | <0.001 |
| Alcohol units/ week (M, SD) | 13.7 (15.0) | 16.9 (22.2) | 0.05 |
| Hours of physical activity/week (M, SD) | 22.0 (15.3) | 21.8 (16.2) | 0.88 |
| Health measures | |||
| CHD (N, %) | 308 (5.7%) | 26 (14.2%) | <0.0001 |
| Diabetes (N, %) | 865 (16.1%) | 47 (25.7%) | <0.001 |
| Stroke (N, %) | 39 (0.7%) | 5 (2.7%) | 0.01 |
| Systolic blood pressure (mmHg) (M, SD) | 122.0 (16.2) | 125.9 (18.9) | <0.0001 |
| Diastolic blood pressure (mmHg) (M, SD) | 77.4 (10.4) | 79.3 (12.2) | 0.04 |
| Cholesterol (mmol/l) (M, SD) | 5.93 (1.04) | 5.95 (1.03) | 0.75 |
Analysis restricted to people with at least one cognitive test completed and complete data on other variables (N=5572).
End of follow-up was defined as 31 of July, 2006 or date of censoring, whichever occurred first.
Denotes at least daily consumption of fruits and vegetables.
M: Mean, SD: Standard deviation, CHD Coronary heart disease
3.2. Association between cognition and mortality
Figure 1 presents the log HR as a function of standardized cognitive scores. For all six measures, across the greater part of the cognitive distribution (between -2 and +2 SD from the mean), the observed regression curve follows a linear trend. At the extremes of the cognitive distribution, the observed regression curve diverges from the linear trend but the density histogram shows that this observation is based on very few individuals. As the test for nonlinearity showed no evidence of non-linearity in the associations between cognition and mortality (p=0.72 for ‘g’ factor, p=0.25 for memory, p=0.78 for AH4-I, p=0.14 for vocabulary, p=0.61 for phonemic and p=0.35 for semantic fluency), we used z-scores to model this association (Table 2). One standard-deviation decrease in memory corresponded to 2 (out of 20) words, 11 points (out of 65) for the AH4-I, 4 words (out of 33) for the Mill Hill, and 4 words for the phonemic (out of 35) and semantic (out of 36) fluency tests. There were no gender differences in the association between measures of cognitive function and mortality (p for all tests >0.20), leading us to combine men and women in the analysis. In age and sex adjusted analysis, one SD decrease in memory was associated with a higher risk of mortality (HR, 1.19; 95% confidence interval (CI), 1.02-1.39). This was also true for the AH4-I (HR, 1.16; 95% CI, 1.01-1.35). The association with ‘g’ factor (HR, 1.16; 95% CI, 0.99-1.34), phonemic (HR, 1.15; 95% CI, 0.98-1.34) and semantic fluency (HR, 1.10; 95% CI, 0.95-1.28) did not reach significance at p<0.05. There was no association between the Mill Hill vocabulary test and mortality (HR 1.01; 95% CI, 0.87-1.17).
Figure 1.
Log Hazard Ratio as a function of standardized cognitive scores
Table 2.
The association between standardized cognitive scores and subsequent mortality in the Whitehall II study
| Adjustments | General intelligence ‘g’ factor | Memory | AH4-I | Mill Hill | Phonemic fluency | Semantic fluency |
|---|---|---|---|---|---|---|
| (N=5572) | (N=5572) | (N=5572) | (N=5572) | (N=5572) | (N=5572) | |
| HR* (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Age | 1.14 (0.99, 1.31) | 1.19 (1.02, 1.39) | 1.14 (0.99, 1.30) | 1.00 (0.87, 1.15) | 1.15 (0.98, 1.34) | 1.10 (0.95, 1.28) |
| Age and sex | 1.16 (0.99, 1.34) | 1.19 (1.02, 1.39) | 1.16 (1.01, 1.35) | 1.01 (0.87, 1.17) | 1.15 (0.98, 1.34) | 1.10 (0.95, 1.28) |
HR hazard ratio associated with a 1 SD decrease in cognitive score, CI confidence interval
Table 3 presents the results aimed at identifying the extent to which covariates explained the association between cognitive function and mortality. The lack of association between Mill Hill and mortality (p=0.90) led us not to pursue further analysis for this measure. Health behaviours explained a considerable portion of the association between cognition and mortality: 63% for the ‘g’ factor, 21% for memory, 50% for the AH4-I, 33% for phonemic and 70% for semantic fluency. All the covariates taken together explained 63% of association between mortality and the ‘g’ factor, 26% with memory, 56% with AH4-I, 33% with phonemic fluency and 90% with semantic fluency.
Table 3.
The role of covariates in explaining the association between cognitive z-scores and mortality
| General intelligence ‘g’ factor | % change* | Memory | % change* | AH4-I | % change* | Phonemic fluency | % change* | Semantic fluency | % change* | |
|---|---|---|---|---|---|---|---|---|---|---|
| (N=5572) | (N=5572) | (N=5572) | (N=5572) | (N=5572) | ||||||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||||
| Model 1: age and sex | 1.16 (0.99, 1.34) | 1.19 (1.02, 1.39) | 1.16 (1.01, 1.35) | 1.15 (0.98, 1.34) | 1.10 (0.95, 1.28) | |||||
| Model 1+ education + SEP | 1.15 (0.96, 1.37) | -6% | 1.18 (1.01, 1.38) | -5% | 1.16 (0.98, 1.38) | 0% | 1.13 (0.96, 1.33) | -13% | 1.08 (0.92, 1.27) | -20% |
| Model 1 + health behaviours† | 1.06 (0.91, 1.24) | -63% | 1.15 (0.98, 1.34) | -21% | 1.08 (0.93, 1.25) | -50% | 1.10 (0.95, 1.29) | -33% | 1.03 (0.88, 1.20) | -70% |
| Model 1 + health‡ | 1.11 (0.96, 1.29) | -31% | 1.18 (1.01, 1.38) | -5% | 1.12 (0.97, 1.30) | -25% | 1.11 (0.96, 1.30) | -27% | 1.07 (0.92, 1.24) | -30% |
| Fully adjusted model | 1.06 (0.89, 1.27) | -63% | 1.14 (0.98, 1.34) | -26% | 1.07 (0.90, 1.27) | -56% | 1.10 (0.93, 1.29) | -33% | 1.01 (0.86, 1.19) | -90% |
Percentage change= 100× (HR controlling for age and sex + covariate group − HR controlling for age and sex)/(HR controlling for age and sex − 1)
Smoking status, alcohol consumption, fruit and vegetable consumption, hours of physical activity
Diabetes, stroke, coronary heart disease, systolic and diastolic blood pressure, and cholesterol HR hazard ratio, CI confidence interval, SEP socioeconomic position
4. Discussion
This study presents three key findings. In a large prospective cohort study of middle-aged British civil servants, memory and reasoning (fluid intelligence) were linearly associated with mortality followed up over eight years in analysis adjusted for age and sex. Out of education, health behaviours and health, it was health behaviours that explained the greater portion of the association between cognition and mortality. Finally, only 26% of the association between memory and mortality was explained by multiple covariates, compared to between 33-90% for the other measures of cognition.
The association between cognition and mortality appears robust and most measures of cognition have been shown to be associated with mortality (Anstey et al., 2001; Hassing et al., 2002; Korten et al., 1999; Pavlik et al., 2003; Portin et al., 2001; Shipley et al., 2006; Small et al., 2003; Smits et al., 1999). However, comparisons between different cognitive measures in the elderly reveal, in one study, visuospatial reasoning, verbal fluency and short-term but not working memory to be associated with mortality (Small et al., 2003). In another study, verbal reasoning, processing speed and memory were shown to be associated with mortality but not verbal knowledge (Anstey et al., 2001). A study on middle-aged adults, did not find an association with ‘verbal knowledge’ (a composite score on tests of similarities and working memory from the Weschsler Adult Intelligence Scale) but found an association with short-term memory and visuo-spatial reasoning (Portin et al., 2001). These results are comparable to ours in terms of the populations studied and the results showing associations with short-term memory and reasoning (fluid intelligence) but not vocabulary or other tests of crystallized intelligence which are thought to be more robust to the effects of age.
The precise structure and definition of cognitive function continues to be debated (Deary and Batty, 2007). One view is that a single general factor best represents the diverse cognitive abilities of an individual. This view is reflected in the studies on the association between cognition and mortality in childhood/ early adulthood (Batty et al., 2007; Hart et al., 2005; Holsinger et al., 2007; Kuh et al., 2004; Martin and Kubzansky, 2005; Whalley and Deary, 2001). Here, cognitive abilities are seen to be represented by the ‘g’ factor, typically derived from principal component analysis using a battery of cognitive tests (Plomin, 1999; Spearman, 1904). Horn and Cattell proposed a modification by identifying two aspects to ‘g’: fluid and crystallized (Horn and Cattell, 1967). Fluid intelligence is seen to represent basic information processing, declines with age and in our tests is measured by the AH4-I. Crystallized intelligence assesses knowledge, learnt over time and does not much decline with age and is measured here by the Mill Hill. Finally, research on older adults almost always assesses several cognitive domains. In recent times, the emergence of cognitive epidemiology (Deary and Batty, 2007) which explores the association between cognition and human health has reopened the debate on the precise structure of human cognition. We were able to examine the associations with mortality for the ‘g’ factor, fluid intelligence and several individual measures of cognition. Our results do not support the single factor theory of cognition, at least in the associations with mortality, as the results show reasoning (fluid intelligence) and memory, in particular, to be important.
Four principal mechanisms have been proposed to explain the association between cognition at early ages and subsequent mortality.(Whalley and Deary, 2001) These are: cognition as a predictor of entry into safer environments via its association with education, as related to healthy behaviours, as an indictor of system integrity and as a record of bodily insults. We examined the importance of these mechanisms in our middle-aged cohort by exploring the contribution of education and socioeconomic position, health behaviours, and health (to assess system integrity and bodily insults). Our results show that health behaviours explained a considerable part of the association between cognition and mortality among middle-aged individuals. Many previous studies have either not examined the role of health behaviours (Anstey et al., 2001; Frisoni et al., 1999; Hart et al., 2005; Hassing et al., 2002; Kelman et al., 1994; Liu et al., 1990; Nguyen et al., 2003; Portin et al., 2001; Rabbitt et al., 2002; Small et al., 2003; Smits et al., 1999; Whalley and Deary, 2001) or not separated their effects from those of socioeconomic variables (Shipley et al., 2006). Measures of health, as indirect measures of system integrity, explain less of the association with memory compared to the other measures of cognition. It should be noted that the measures of health used are not complete. Furthermore, the calculations of the percent attenuation should not be used to make causal inferences.
The explanatory variables previously examined to explore the association between cognition and mortality have included socioeconomic variables (Anstey et al., 2001; Hart et al., 2005; Hassing et al., 2002; Korten et al., 1999; Kuh et al., 2004; Pavlik et al., 2003; Portin et al., 2001; Shipley et al., 2006; Small et al., 2003; Small and Backman, 1997; Smits et al., 1999; Whalley and Deary, 2001), health behaviours (Holsinger et al., 2007; Korten et al., 1999; Kuh et al., 2004; Pavlik et al., 2003; Shipley et al., 2006) and measures of health (Anstey et al., 2001; Hassing et al., 2002; Holsinger et al., 2007; Korten et al., 1999; Kuh et al., 2004; Pavlik et al., 2003; Portin et al., 2001; Shipley et al., 2006; Small et al., 2003; Smits et al., 1999). The different results on the role played by explanatory variables are probably due to differences in measures of cognition and the covariates examined. Our results, using multiple measures of cognition, show that the association between memory and mortality had the least amount of attenuation when adjustments were made for the explanatory factors. In effect, 74% of this association remained unexplained. Memory deficits are critical to the diagnosis of mild cognitive impairment (Brayne, 2007; Gauthier et al., 2006) which is itself linked to progression to dementia (Tschanz et al., 2006; Tyas et al., 2007). More than a half people with mild cognitive impairment progress to dementia within 5 years (Gauthier et al, 2006). Dementia is known to be a predictor of mortality (Tschanz et al., 2004). In a recent study, survival time in people with dementia has been estimated to be 4.5 years (Xie et al, 2008). Thus, our results highlight the importance of poor memory as a predictor for mortality in a reasonable healthy and high functioning middle-aged cohort.
Strengths & limitations
The primary advantage of conducting this study in a middle-aged population is that age-related physical health deterioration, which is associated with cognitive decline (Ivan et al., 2004; Solfrizzi et al., 2004) and mortality, is less likely to be a confounder in our analysis. A further strength of the study is the use of the graphic method and an explicit test for nonlinearity in order to examine whether the association between cognition and mortality is linear across the cognitive distribution or restricted to low cognitive scores as suggested in a previous paper (Kuh et al., 2004). Our results lead us to conclude that this association is evident all along the continuum of the cognitive scores.
The main limitation of the present study is that although the sample covered a wide socioeconomic range, data are from white-collar civil servants and cannot be assumed to represent the general population. Second, as in many observational studies, mortality was higher among non-participants. Consequently, results are based on healthier participants which could imply that the association between cognition and mortality was underestimated if non participants also had lower cognitive scores (Tyas et al., 2006). Finally, due to the age of the participants the number of deaths was small and we could not explore associations with cause-specific mortality.
In conclusion, our results show a linear association of reasoning (fluid intelligence) and memory with subsequent mortality in a middle-aged population. A considerable part of the associations between different measures of cognition (‘g’ factor, memory, reasoning (fluid intelligence), vocabulary, and verbal fluency) and mortality was explained by health behaviours suggesting that it might be an important pathway through which cognition is associated with health outcomes. This study also suggests that composite measures of cognition, like the ‘g’ factor, might mask the true relationship between cognition and health.
Acknowledgments
AS-M is supported by a “Chaire d’excellence” award from the French Ministry of Research and a “European Young Investigator Award” from the European Science Foundation. MGM is supported by an MRC research professorship. MJS is supported by a grant from the British Heart Foundation. The Whitehall II study has been supported by grants from the British Medical Research Council (MRC); the British Heart Foundation; the British Health and Safety Executive; the British Department of Health; the National Heart, Lung, and Blood Institute (grant HL36310); the National Institute on Aging (grant AG13196); the Agency for Health Care Policy and Research (grant HS06516); and the John D. and Catherine T. MacArthur Foundation Research Networks on Successful Midlife Development and Socioeconomic Status and Health.
We thank all of the participating civil service departments and their welfare, personnel, and establishment officers; the British Occupational Health and Safety Agency; the British Council of Civil Service Unions; all participating civil servants in the Whitehall II study; and all members of the Whitehall II study team.
Footnotes
Disclosure statement All authors confirm that there are no conflicts of interest with regard to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anstey KJ, Luszcz MA, Giles LC, Andrews GR. Demographic, health, cognitive, and sensory variables as predictors of mortality in very old adults. Psychol Aging. 2001;16:3–11. doi: 10.1037/0882-7974.16.1.3. [DOI] [PubMed] [Google Scholar]
- Bassuk SS, Wypij D, Berkman LF. Cognitive impairment and mortality in the community-dwelling elderly. Am J Epidemiol. 2000;151:676–688. doi: 10.1093/oxfordjournals.aje.a010262. [DOI] [PubMed] [Google Scholar]
- Batty GD, Deary IJ, Gottfredson LS. Premorbid (early life) IQ and later mortality risk: systematic review. Ann Epidemiol. 2007;17:278–288. doi: 10.1016/j.annepidem.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologica. 1967;5:135–140. [Google Scholar]
- Brayne C. The elephant in the room - healthy brains in later life, epidemiology and public health. Nat Rev Neurosci. 2007;8:233–239. doi: 10.1038/nrn2091. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Batty GD. Cognitive epidemiology. J Epidemiol Community Health. 2007;61:378–384. doi: 10.1136/jech.2005.039206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey ME, Saz P. Dementia, cognitive impairment and mortality in persons aged 65 and over living in the community: a systematic review of the literature. Int J Geriatr Psychiatry. 2001;16:751–761. doi: 10.1002/gps.397. [DOI] [PubMed] [Google Scholar]
- Eagles JM, Beattie JA, Restall DB, Rawlinson F, Hagen S, Ashcroft GW. Relation between cognitive impairment and early death in the elderly. BMJ. 1990;300:239–240. doi: 10.1136/bmj.300.6719.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrie JE, Langenberg C, Shipley MJ, Marmot MG. Birth weight, components of height and coronary heart disease: evidence from the Whitehall II study. Int J Epidemiol. 2006;35:1532–1542. doi: 10.1093/ije/dyl184. [DOI] [PubMed] [Google Scholar]
- Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF, Robbins JA, Gardin JM. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Fratiglioni L, Fastbom J, Viitanen M, Winblad B. Mortality in nondemented subjects with cognitive impairment: the influence of health-related factors. Am J Epidemiol. 1999;150:1031–1044. doi: 10.1093/oxfordjournals.aje.a009927. [DOI] [PubMed] [Google Scholar]
- Gale CR, Martyn CN, Cooper C. Cognitive impairment and mortality in a cohort of elderly people. BMJ. 1996;312:608–611. doi: 10.1136/bmj.312.7031.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, Belleville S, Brodaty H, Bennett D, Chertkow H, Cummings JL, de LM, Feldman H, Ganguli M, Hampel H, Scheltens P, Tierney MC, Whitehouse P, Winblad B. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- Gussekloo J, Westendorp RG, Remarque EJ, Lagaay AM, Heeren TJ, Knook DL. Impact of mild cognitive impairment on survival in very elderly people: cohort study. BMJ. 1997;315:1053–1054. doi: 10.1136/bmj.315.7115.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell FE. Restricted cubic splines. Regression modeling strategies,, Anonymous) 2005:20–26. [Google Scholar]
- Hart CL, Taylor MD, Smith GD, Whalley LJ, Starr JM, Hole DJ, Wilson V, Deary IJ. Childhood IQ and all-cause mortality before and after age 65: prospective observational study linking the Scottish Mental Survey 1932 and the Midspan studies. Br J Health Psychol. 2005;10:153–165. doi: 10.1348/135910704X14591. [DOI] [PubMed] [Google Scholar]
- Hassing LB, Johansson B, Berg S, Nilsson SE, Pedersen NL, Hofer SM, McClearn G. Terminal decline and markers of cerebro- and cardiovascular disease: findings from a longitudinal study of the oldest old. J Gerontol B Psychol Sci Soc Sci. 2002;57:268–276. doi: 10.1093/geronb/57.3.p268. [DOI] [PubMed] [Google Scholar]
- Heim AW. AH 4 group test of general Intelligence. NFER-Nelson Publishing Company Ltd.; Windsor, UK: 1970. [Google Scholar]
- Heinzl H, Kaider A. Manual for the SAS-Macro RCS. 2007 http://www.meduniwien.ac.at/msi/biometrie/programme/Rcs.htm.
- Holsinger T, Helms M, Plassman B. Intelligence in early adulthood and life span up to 65 years later in male elderly twins. Age Ageing. 2007;36:286–291. doi: 10.1093/ageing/afm016. [DOI] [PubMed] [Google Scholar]
- Horn JL, Cattell RB. Age differences in fluid and crystallized intelligence. Acta Psychol. 1967;26:107–129. doi: 10.1016/0001-6918(67)90011-x. [DOI] [PubMed] [Google Scholar]
- Hunderfund AL, Roberts RO, Slusser TC, Leibson CL, Geda YE, Ivnik RJ, Tangalos EG, Petersen RC. Mortality in amnestic mild cognitive impairment: a prospective community study. Neurology. 2006;67:1764–1768. doi: 10.1212/01.wnl.0000244430.39969.5f. [DOI] [PubMed] [Google Scholar]
- Ivan CS, Seshadri S, Beiser A, Au R, Kase CS, Kelly-Hayes M, Wolf PA. Dementia after stroke: the Framingham Study. Stroke. 2004;35:1264–1268. doi: 10.1161/01.STR.0000127810.92616.78. [DOI] [PubMed] [Google Scholar]
- Kelman HR, Thomas C, Kennedy GJ, Cheng J. Cognitive impairment and mortality in older community residents. Am J Public Health. 1994;84:1255–1260. doi: 10.2105/ajph.84.8.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korten AE, Jorm AF, Jiao Z, Letenneur L, Jacomb PA, Henderson AS, Christensen H, Rodgers B. Health, cognitive, and psychosocial factors as predictors of mortality in an elderly community sample. J Epidemiol Community Health. 1999;53:83–88. doi: 10.1136/jech.53.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh D, Richards M, Hardy R, Butterworth S, Wadsworth ME. Childhood cognitive ability and deaths up until middle age: a post-war birth cohort study. Int J Epidemiol. 2004;33:408–413. doi: 10.1093/ije/dyh043. [DOI] [PubMed] [Google Scholar]
- Liu IY, LaCroix AZ, White LR, Kittner SJ, Wolf PA. Cognitive impairment and mortality: a study of possible confounders. Am J Epidemiol. 1990;132:136–143. doi: 10.1093/oxfordjournals.aje.a115625. [DOI] [PubMed] [Google Scholar]
- Marmot MG, Smith GD, Stansfeld S, Patel C, North F, Head J, White I, Brunner E, Feeney A. Health inequalities among British civil servants: the Whitehall II study. Lancet. 1991;337:1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- Martin LT, Kubzansky LD. Childhood cognitive performance and risk of mortality: a prospective cohort study of gifted individuals. Am J Epidemiol. 2005;162:887–890. doi: 10.1093/aje/kwi300. [DOI] [PubMed] [Google Scholar]
- Neale R, Brayne C, Johnson AL. Cognition and survival: an exploration in a large multicentre study of the population aged 65 years and over. Int J Epidemiol. 2001;30:1383–1388. doi: 10.1093/ije/30.6.1383. [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Black SA, Ray LA, Espino DV, Markides KS. Cognitive impairment and mortality in older mexican americans. J Am Geriatr Soc. 2003;51:178–183. doi: 10.1046/j.1532-5415.2003.51055.x. [DOI] [PubMed] [Google Scholar]
- Palmer K, Wang HX, Backman L, Winblad B, Fratiglioni L. Differential evolution of cognitive impairment in nondemented older persons: results from the Kungsholmen Project. Am J Psychiatry. 2002;159:436–442. doi: 10.1176/appi.ajp.159.3.436. [DOI] [PubMed] [Google Scholar]
- Pavlik VN, de Moraes SA, Szklo M, Knopman DS, Mosley TH, Jr, Hyman DJ. Relation between cognitive function and mortality in middle-aged adults: the atherosclerosis risk in communities study. Am J Epidemiol. 2003;157:327–334. doi: 10.1093/aje/kwf209. [DOI] [PubMed] [Google Scholar]
- Plomin R. Genetics and general cognitive ability. Nature. 1999;402:C25–C29. doi: 10.1038/35011520. [DOI] [PubMed] [Google Scholar]
- Portin R, Muuriaisniemi ML, Joukamaa M, Saarijarvi S, Helenius H, Salokangas RK. Cognitive impairment and the 10-year survival probability of a normal 62-year-old population. Scand J Psychol. 2001;42:359–366. doi: 10.1111/1467-9450.00247. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Watson P, Donlan C, Mc IL, Horan M, Pendleton N, Clague J. Effects of death within 11 years on cognitive performance in old age. Psychol Aging. 2002;17:468–481. doi: 10.1037//0882-7974.17.3.468. [DOI] [PubMed] [Google Scholar]
- Raven JC. Guide to using the Mill Hill vocabulary test with progressive matrices. HK Lewis; London, UK: 1965. [Google Scholar]
- Shipley BA, Der G, Taylor MD, Deary IJ. Cognition and all-cause mortality across the entire adult age range: health and lifestyle survey. Psychosom Med. 2006;68:17–24. doi: 10.1097/01.psy.0000195867.66643.0f. [DOI] [PubMed] [Google Scholar]
- Small BJ, Backman L. Cognitive correlates of mortality: evidence from a population-based sample of very old adults. Psychol Aging. 1997;12:309–313. doi: 10.1037//0882-7974.12.2.309. [DOI] [PubMed] [Google Scholar]
- Small BJ, Fratiglioni L, von SE, Backman L. Terminal decline and cognitive performance in very old age: does cause of death matter? Psychol Aging. 2003;18:193–202. doi: 10.1037/0882-7974.18.2.193. [DOI] [PubMed] [Google Scholar]
- Smits CH, Deeg DJ, Kriegsman DM, Schmand B. Cognitive functioning and health as determinants of mortality in an older population. Am J Epidemiol. 1999;150:978–986. doi: 10.1093/oxfordjournals.aje.a010107. [DOI] [PubMed] [Google Scholar]
- Solfrizzi V, Panza F, Colacicco AM, D’Introno A, Capurso C, Torres F, Grigoletto F, Maggi S, Del PA, Reiman EM, Caselli RJ, Scafato E, Farchi G, Capurso A. Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology. 2004;63:1882–1891. doi: 10.1212/01.wnl.0000144281.38555.e3. [DOI] [PubMed] [Google Scholar]
- Spearman C. “General Intelligence,” Objectively Determined and Measured. The American Journal of Psychology. 1904;15:201–292. [Google Scholar]
- Swan GE, Carmelli D, LaRue A. Performance on the digit symbol substitution test and 5-year mortality in the Western Collaborative Group Study. Am J Epidemiol. 1995;141:32–40. doi: 10.1093/oxfordjournals.aje.a117342. [DOI] [PubMed] [Google Scholar]
- Tschanz JT, Corcoran C, Skoog I, Khachaturian AS, Herrick J, Hayden KM, Welsh-Bohmer KA, Calvert T, Norton MC, Zandi P, Breitner JC. Dementia: the leading predictor of death in a defined elderly population: the Cache County Study. Neurology. 2004;62:1156–1162. doi: 10.1212/01.wnl.0000118210.12660.c2. [DOI] [PubMed] [Google Scholar]
- Tschanz JT, Welsh-Bohmer KA, Lyketsos CG, Corcoran C, Green RC, Hayden K, Norton MC, Zandi PP, Toone L, West NA, Breitner JC. Conversion to dementia from mild cognitive disorder: the Cache County Study. Neurology. 2006;67:229–234. doi: 10.1212/01.wnl.0000224748.48011.84. [DOI] [PubMed] [Google Scholar]
- Tuokko H, Frerichs R, Graham J, Rockwood K, Kristjansson B, Fisk J, Bergman H, Kozma A, McDowell I. Five-year follow-up of cognitive impairment with no dementia. Arch Neurol. 2003;60:577–582. doi: 10.1001/archneur.60.4.577. [DOI] [PubMed] [Google Scholar]
- Tyas SL, Salazar JC, Snowdon DA, Desrosiers MF, Riley KP, Mendiondo MS, Kryscio RJ. Transitions to mild cognitive impairments, dementia, and death: findings from the nun study. Am J Epidemiol. 2007;165:1231–1238. doi: 10.1093/aje/kwm085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyas SL, Tate RB, Wooldrage K, Manfreda J, Strain LA. Estimating the incidence of dementia: the impact of adjusting for subject attrition using health care utilization data. Ann Epidemiol. 2006;16:477–484. doi: 10.1016/j.annepidem.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Whalley LJ, Deary IJ. Longitudinal cohort study of childhood IQ and survival up to age 76. BMJ. 2001;322:1–5. doi: 10.1136/bmj.322.7290.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Report of a WHO Consultation. Geneva: World Health Organization; 1999. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. [Google Scholar]
- World Health Organization. Blood Safety and Clinical Technology. Guidelines on Standard Operating Procedures for CLINICAL CHEMISTRY. [27 of March, 2008]; http://www.searo.who.int/en/Section10/Section17/Section53/Section481_1756.htm.
- Xie J, Brayne C, Matthews FE. Survival times in people with dementia: analysis from population based cohort study with 14 year follow-up. BMJ. 2008;336:258–262. doi: 10.1136/bmj.39433.616678.25. [DOI] [PMC free article] [PubMed] [Google Scholar]