Abstract
Abundant plant biomass has the potential to become a sustainable source of fuels and chemicals. Realizing this potential requires the economical conversion of recalcitrant lignocellulose into useful intermediates, such as sugars. We report a high-yielding chemical process for the hydrolysis of biomass into monosaccharides. Adding water gradually to a chloride ionic liquid-containing catalytic acid leads to a nearly 90% yield of glucose from cellulose and 70–80% yield of sugars from untreated corn stover. Ion-exclusion chromatography allows recovery of the ionic liquid and delivers sugar feedstocks that support the vigorous growth of ethanologenic microbes. This simple chemical process, which requires neither an edible plant nor a cellulase, could enable crude biomass to be the sole source of carbon for a scalable biorefinery.
Keywords: biofuel, carbohydrate, ethanol fermentation, ionic liquid, lignocellulose
As the primary component of lignocellulosic biomass, cellulose is the most abundant organic compound on earth and has the potential to be a renewable source for energy and chemicals. The estimated global annual production of biomass is 1 × 1011 tons, sequestering 2 × 1021 J (1, 2). For comparison, annual petroleum production amounts to 2 × 1020 J, whereas the technically recoverable endowment of conventional crude oil is 2 × 1022 J (1). Hence, in only one decade, Earth’s plants can renew in the form of cellulose, hemicellulose, and lignin all of the energy stored as conventional crude oil. The challenge for scientists is to access these polymers and convert them into fuels and building blocks for civilization.
Sugars are natural intermediates in the biological and chemical conversion of lignocellulosic biomass (3 –11), but access to sugars is hindered by the recalcitrance of plant cell walls (4, 12). The majority of glucose in lignocellulose is locked into highly crystalline cellulose polymers. Hemicellulose—a branched polymer of glucose, xylose, and other sugars—and lignin—a complex aromatic polymer—encase the cellulose, fortifying and protecting the plant. Deriving sugars from this heterogeneous feedstock requires both physical and chemical disruption. Enzymatic methods of saccharification are the most common, and use physical and chemical pretreatment processes (13) followed by hydrolysis with cellulases to produce sugars. The proper combination of pretreatment and enzymes for a given feedstock enables high yields of sugars from both hemicellulose and cellulose components (14, 15). Nonetheless, the costs of both pretreatment and enzymes [one-third of the cost of ethanol production from cellulose (16)] and low rates of hydrolysis are potential drawbacks to enzymatic hydrolysis.
Chemistry provides an alternative means to hydrolyze biomass. As early as 1819, Braconnot demonstrated that linen dissolved in concentrated H2SO4, diluted with water, and heated was transformed into a fermentable sugar (17, 18). The concentrated acid plays a dual role in biomass hydrolysis. By disrupting its network of intra- and interchain hydrogen bonds, strong acids decrystallize cellulose and make it accessible to reagents (19); and by catalyzing the hydrolysis of glycosidic bonds, strong acids cleave cellulose and hemicellulose into sugars (Fig. 1) (4). Bergius took advantage of these attributes of HCl in the development of a commercial process that operated in Germany from 1935 to 1948 (20, 21). In the United States, several related processes using H2SO4 have been developed, typically with 80–90% conversion of cellulose and hemicellulose into sugars (22 –27). Nevertheless, the hazards of handling concentrated acids and the complexities of recycling them have limited the adoption of this technology.
Fig. 1.
Hydrolysis reactions of cellulose and xylan. Chemical hydrolysis of cellulose and hemicellulose into monomeric sugars proceeds through oligomers and is accompanied by side reactions that form furans and other degradation products.
Less hazardous and more tractable cellulose solvents would facilitate lignocellulose hydrolysis. Room-temperature ionic liquids, that is, salts with melting points near or below ambient temperature, show promise as cellulose solvents for nonwoven fiber production (28) and chemical derivatization (29 –31). Like concentrated acids, ionic liquids containing chloride, acetate, and other moderately basic anions disrupt the hydrogen bond network of cellulose and enable its dissolution (30 –34). Although ionic liquids have been used to pretreat biomass for enzymatic hydrolysis (35 –37), chemical deconstruction to produce glucose in ionic liquids has produced only moderate yields (38 –43) that contrast with the nearly quantitative yields of glucose attainable from cellulose in concentrated acids and other cellulose solvents (44).
Noting that highly efficient hydrolyses employ much higher water concentrations than those used in ionic-liquid-phase hydrolysis, we reasoned that water could play an important role in delivering high glucose yields. Our investigation of the reactivity of glucose and cellulose in mixtures of an ionic liquid and water confirmed this hypothesis and enabled a high-yielding process for the hydrolysis of cellulose and lignocellulosic biomass. Moreover, this process generates easily recovered sugars that are superb feedstocks for microbial growth and biocatalytic ethanol production.
Results
Cellulose Reactivity in Ionic Liquids.
We began by investigating the intrinsic reactivity of cellulose and sugars under acidic conditions in ionic liquids. First, we reacted cellulose with H2SO4 and HCl in 1-ethyl-3-methylimidazolium chloride ([EMIM]Cl), an ionic liquid that is an effective solvent for cellulose (39). We observed the production of 5-hydroxymethylfurfural (HMF), but only moderate yields of glucose (Table 1). HMF inhibits microbial growth and fermentation (45).
Table 1.
Hydrolysis of cellulose in [EMIM]Cl
| Water content, wt% | ||||||||||
| Cellulose, wt% | HCl, wt% | 0′ | 5′ | 10′ | 20′ | 30′ | 60′ | Time, h | Glucose yield, % | HMF yield, % |
| 5 | 20* | 5 | 5 | 5 | 5 | 5 | 5 | 1 | 40 | 19 |
| 5 | 20 | 5 | 5 | 5 | 5 | 5 | 5 | 1 | 45 | 17 |
| 5 | 20 | 5 | 33 | 33 | 33 | 33 | 33 | 1 | 14 | ND |
| 2 | 29 | ND | ||||||||
| 5 | 20 | 5 | 5 | 20 | 20 | 20 | 20 | 1 | 31 | ND |
| 2 | 64 | 19 | ||||||||
| 3 | 51 | 25 | ||||||||
| 4 | 36 | 30 | ||||||||
| 5 | 20 | 5 | 5 | 20 | 20 | 33 | 33 | 1 | 40 | ND |
| 2 | 84 | 7 | ||||||||
| 3 | 81 | 10 | ||||||||
| 4 | 77 | 8 | ||||||||
| 5 | 20 | 5 | 5 | 20 | 25 | 33 | 33 | 1 | 44 | ND |
| 2 | 86 | 7 | ||||||||
| 3 | 83 | 10 | ||||||||
| 4 | 77 | 13 | ||||||||
| 5 | 20 | 5 | 5 | 20 | 25 | 33 | 43 | 1 | 38 | ND |
| 2 | 85 | 5 | ||||||||
| 3 | 87 | 6 | ||||||||
| 4 | 89 | 7 | ||||||||
| 10 | 10 | 5 | 5 | 20 | 25 | 33 | 43 | 3 | 71 | ND |
Cellulose was reacted in [EMIM]Cl at 105 °C after its dissolution at 105 °C for 12 h. HCl loading is relative to cellulose mass. Water content is relative to the total mass of the reaction mixture and is shown at time points (minutes) following the initiation of hydrolysis. Yields are molar yields based on HPLC analysis, and are relative to the glucose monomers contained in the cellulose. ND, not determined.
*H2SO4.
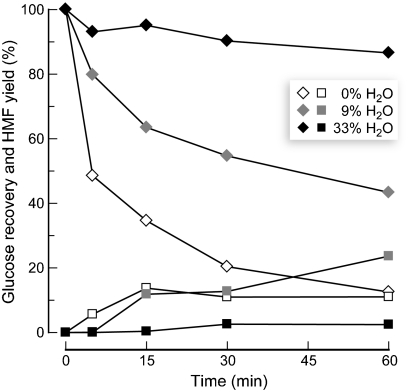
The production of HMF at the expense of glucose suggested to us nascent glucose was being dehydrated to form HMF. To test this hypothesis, we reacted glucose in [EMIM]Cl containing varying amounts of water (Fig. 2, Table S1 in the SI Appendix). In the absence of both acid and water, glucose was recovered intact. On the other hand, adding H2SO4 led to the rapid decay of glucose into HMF and other products in ionic liquid containing little or no water. Most significantly, we observed that increasing the water content to 33 wt% in this same acidic solution enabled nearly 90% of the glucose to remain intact after 1 h.
Fig. 2.
Acid-catalyzed degradation of glucose in [EMIM]Cl. In acidic [EMIM]Cl, glucose (diamonds) disappears rapidly at 100 °C, forming HMF (squares) and other degradation products. Increased water content slows glucose loss. Reaction conditions: Glucose, 10 wt%; H2SO4, 4 wt% relative to glucose; H2O, 0–33 wt% relative to the total mass of the reaction mixture. Product levels are reproducible to within ± 4%.
Why does glucose degrade rapidly under nonaqueous conditions in [EMIM]Cl but not in the presence of a high concentration of water? Le Chatelier’s principle (46) tells us that water disfavors dehydrative reactions, such as glucose oligomerization and conversion into HMF. Additionally, the highly nucleophilic chloride anions of [EMIM]Cl coordinate strongly to carbohydrates (47, 48), accelerating acid-catalyzed dehydration reactions (49). High concentrations of water solvate chloride and thus distract it from interacting with carbohydrates.
Effect of Water on Celluose Reactivity in Ionic Liquids.
We suspected that increasing the water concentration in an [EMIM]Cl reaction mixture would enhance glucose yields from cellulose. We were, however, aware that water precipitates cellulose from ionic liquids (32). Indeed, we observed that a 5 wt% solution of cellulose in [EMIM]Cl formed an intractable gel when the solution was diluted to 10 wt% water, making homogeneous hydrolysis of cellulose in ionic liquid/water solutions impossible. Instead, we attempted to balance cellulose solubility and glucose stability by adding water gradually during hydrolysis, expecting that cellulose solubility would increase as the reaction progresses. In these experiments, we chose to use HCl as the acid so as to match the anion with that of the ionic liquid. [EMIM]Cl containing 5 wt% cellulose was first treated with HCl and a small amount of water at 105 °C to allow hydrolysis of the cellulose into shorter, more soluble segments (Table 1). After a delay, additional water was added to stabilize the glucose product.
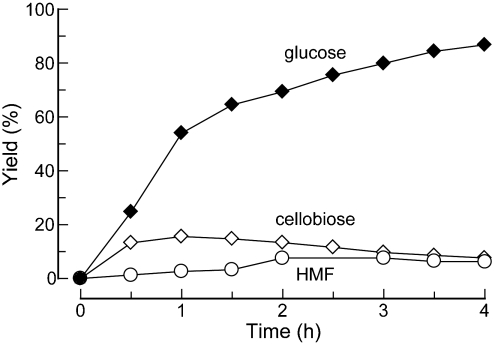
The timing of water addition was critical for high glucose yields. When the reaction mixture was diluted to 33% water after 5 min, cellulose precipitated, resulting in low yields. Delaying dilution until after 10 min prevented cellulose precipitation, and gradually increasing the water content to 43% within 60 min allowed glucose yields of nearly 90% in 2–4 h. These yields are nearly twice as high as the previous best in ionic liquids and approach those achieved through enzymatic hydrolysis. We also examined the effect of the time for dissolution of cellulose in the ionic liquid prior to hydrolysis, finding that 6 h was optimal (Table S2 in the SI Appendix). Longer times probably led to increased byproduct formation (50, 51), which was indicated by discoloration of the reaction mixture, whereas shorter times were insufficient for complete solvation of the cellulose. With this optimized procedure, more concentrated cellulose solutions (10 wt%) could be hydrolyzed in high yields. Tiny cellulose fibers visible in these reaction mixtures suggest that glucose yields were slightly lower due to incomplete cellulose breakdown prior to water addition. It is likely that cellulose is converted into a mixture of glucose and soluble oligomers within the first 30–60 min of reaction, and that these oligomers hydrolyze subsequently into glucose. Monitoring the production of glucose and cellobiose (which is a glucose dimer) during cellulose hydrolysis revealed that cellobiose concentrations peaked at 1 h and decayed as glucose concentrations increased (Fig. 3). Experiments with other ionic liquids indicate that chloride-based ionic liquids alone are able to balance cellulose solubility and hydrolytic activity through strong interactions with cellulose coupled with weak basicity (Table 2).
Fig. 3.
Production of glucose, HMF, and cellobiose production during cellulose hydrolysis under optimized reaction conditions. Product levels are reproducible to within ± 4%.
Table 2.
Hydrolysis of cellulose in ionic liquids
| Ionic liquid | Cellulose concentration, wt% | Glucose yield, % |
| [EMIM]OAc | 2 | 0 |
| [EMIM]OAc | 5 | 0 |
| [EMIM]NO3 | 2 | 0 |
| 1,3-dimethylimidazolium dimethylphosphate | 2 | 0 |
| 1,3-dimethylimidazolium dimethylphosphate | 5 | 0 |
| [EMIM]Br | 2 | 4 |
| [BMIM]BF4 | 2 | 0 |
| [EMIM]OTf | 2 | 5 |
| [BMIM]Cl | 5 | 66 |
| 1-butyl-4-methylpyridinium chloride | 5 | 73 |
| 1-ethylpyridinium chloride | 5 | 69 |
| 1-ethyl-2,3-dimethylimidazolium chloride | 5 | 46 |
Cellulose was reacted in ionic liquid for 3 h at 105 °C after mixing at 105 °C for 6 h. HCl loading was 20 wt% relative to cellulose weight. The water content of the reaction began at 5 wt% and was increased as follows: 20% (10 min), 25% (20 min), 33% (30 min), 43% (60 min). Yields are molar yields based on HPLC analysis and are relative to the glucose monomers contained in the cellulose.
Hydrolysis of Crude Biomass.
Complex and heterogeneous, lignocellulosic biomass presents a more significant challenge for hydrolysis than does cellulose. In addition to intractable crystalline cellulose, lignocellulosic biomass such as corn stover includes protective hemicellulose and lignin, heterogeneous components that are major obstacles to many biomass hydrolysis processes (4, 12). Nonetheless, chloride ionic liquids are excellent solvents for lignocellulosic biomass, suggesting that they would easily disrupt these polymeric barriers. Moreover, preliminary experiments demonstrated that xylan, a hemicellulose, was readily hydrolyzed under our conditions, producing xylose in 77% yield.
We attempted to extend our process for cellulose hydrolysis to crude lignocellulosic biomass. Untreated corn stover that had been mixed with [EMIM]Cl was hydrolyzed with 10 wt% HCl at 105 °C with the same water-dilution process used for pure cellulose. This process produced a 71% yield of xylose and 42% yield of glucose based on the xylan and cellulose content of the stover (Table 3). Dilution of the reaction mixture to 70% water caused precipitation of unhydrolyzed polysaccharides and lignin. These residues were then dissolved in [EMIM]Cl and subjected to an identical second-stage hydrolysis, which released additional xylose and glucose, leaving behind lignin-containing solids. Combined, these two steps resulted in a 70% yield of glucose and a 79% yield of xylose using only simple chemical reagents. Given the well-documented versatility of ionic liquids as biomass solvents, we anticipate that this process will be amenable to other biomass sources, such as wood and grasses. The brown residue remaining after two-stage corn stover hydrolysis likely includes lignin, insoluble polysaccharides, any insoluble hydrolysis byproducts, protein, and ash. We have not yet characterized the lignin byproduct, but it is likely to resemble lignin produced by other acid hydrolysis processes, e.g., Klason lignin (43, 52).
Table 3.
Hydrolysis of untreated corn stover in [EMIM]Cl
| Water content, wt% | |||||||||||
| Stover, wt% | Stage | HCl, wt% | 0′ | 5′ | 10′ | 20′ | 30′ | 60′ | Time, h | Glucose yield, % | Xylose yield, % |
| 5 | 1 | 20 | 5 | 5 | 20 | 25 | 33 | 43 | 2.5 | 42 | 71 |
| 2 | 20 | 5 | 5 | 20 | 25 | 33 | 43 | 3.0 | 28 | 8 | |
| Overall | 70 | 79 | |||||||||
| 10 | 1 | 10 | 5 | 5 | 20 | 25 | 33 | 43 | 3.5 | 19 | 74 |
| 2 | 10 | 5 | 5 | 20 | 25 | 33 | 43 | 3.0 | 47 | 1 | |
| Overall | 66 | 75 | |||||||||
| 10 | 1 | 10 | 5 | 5 | 20 | 25 | 33 | 43 | 1.0 | 17 | 60 |
| 1 | 1.5 | 21 | 73 | ||||||||
| 1 | 2.0 | 25 | 80 | ||||||||
| 1 | 2.5 | 27 | 82 | ||||||||
| 2 | 10 | 5 | 5 | 20 | 25 | 33 | 43 | 1.5 | 37 | 5 | |
| 2 | 2.0 | 42 | 5 | ||||||||
| 2 | 2.5 | 44 | 5 | ||||||||
| 2 | 3.0 | 48 | 5 | ||||||||
Untreated corn stover was reacted in [EMIM]Cl at 105 °C after its dissolution at 105 °C for 6 h. HCl loading is relative to stover weight. Water content is relative to the total mass of the reaction mixture and is shown at time points (minutes) following the initiation of hydrolysis. Yields are molar yields based on HPLC analysis and are relative to the glucose and xylose monomers contained in the stover.
Sugar and Solvent Recovery.
A practical biomass hydrolysis process requires efficient means for sugar and solvent recovery. We found that ion-exclusion chromatography enables separation of the sugars and ionic liquid from the corn stover hydrolysis reaction mixture. In this technique, a mixture containing electrolyte and nonelectrolyte solutes is separated by passing it through a charged resin (53). Charged species, such as the ionic liquid, are excluded from the resin, while nonelectrolytes, such as sugars, are retained. Nonpolar species such as HMF and furfural are adsorbed more strongly than sugars, and elute later. Passing the corn stover hydrolyzate through a column of [EMIM]-exchanged Dowex® 50 resin allowed laboratory-scale separation of the ionic-liquid solvent from the sugars, with > 95% recovery of the ionic liquid, 94% recovery of glucose, and 88% recovery of xylose. Additionally, this chromatographic step removed inhibitory compounds such as HMF and furfural. We suspect that ionic-liquid and sugar recovery are limited by the difficulties intrinsic in small-scale separations and would be improved upon scale-up.
Microbial Fermentation of Biomass Hydrolyzate.
To support bioconversion, biomass hydrolyzate sugars must be free of contaminants that inhibit microbial growth and fermentation. We found that sugars derived from corn stover through our process are excellent feedstocks for an ethanologenic bacterium and yeast. Whereas wild-type Escherichia coli ferments a range of sugars into a mixture of ethanol and organic acids, the engineered KO11 strain produces ethanol selectively (54). Serving as the sole carbon source, the hydrolyzate sugars from corn stover enabled aerobic growth of E. coli KO11 at a rate comparable to that of a control glucose/xylose mixture (Fig. 4 A). Moreover, under oxygen-deficient conditions, E. coli KO11 produced a (79 ± 4)% yield of ethanol from stover hydrolyzate sugars and a (76 ± 3)% yield from pure glucose and xylose, demonstrating that sugars from our hydrolysis process can be converted readily into ethanol.
Fig. 4.
Aerobic growth of ethanologenic microbes on corn stover hydrolyzate and pure sugars as their sole carbon source. (A) Escherichia coli strain KO11: hydrolyzate (●), 5 replicates; sugars (○), 20 replicates. (B) Pichia stipitis: hydrolyzate (●), 2 replicates; sugars (○), 6 replicates. Data are mean values (± SE) at 0.5 h intervals. Under anaerobic conditions, the cultures produced ethanol with the following yields based on sugar conversion (three replicates each). Escherichia coli strain KO11: hydrolyzate, (79 ± 4)%; sugars, (76 ± 3)%. Pichia stipitis: hydrolyzate, (70 ± 2)%; sugars, (72 ± 1)%.
Engineered bacteria show promise for biofuel production, but yeast fermentation predominates today (55, 56). Pichia stipitis, which has an innate ability to ferment xylose, is an especially viable candidate for bioconversion of lignocellulose-derived sugars (57 –59). Corn stover hydrolyzate sugars are an excellent carbon source for the growth of this yeast (Fig. 4 B), and P. stipitis efficiently converts them into ethanol. Fermenting xylose and glucose, the yeasts produced a (70 ± 2)% yield of ethanol from hydrolyzate and a (72 ± 1)% yield from pure sugars.
Discussion
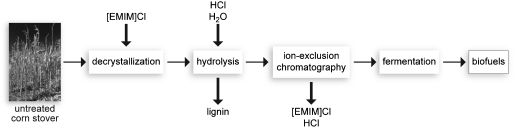
We have demonstrated an efficient system for polysaccharide hydrolysis as well as means to separate and ferment the resulting sugars. By balancing cellulose solubility and reactivity with water, we produce sugars from lignocellulosic biomass in yields that are severalfold greater than those achieved previously in ionic liquids and approach those of enzymatic hydrolysis. Furthermore, the hydrolyzate products are readily converted into ethanol by microorganisms. Together, these steps comprise an integrated process for chemical hydrolysis of biomass for biofuel production (Fig. 5). First, lignocellulosic biomass such as corn stover is decrystallized through mixing with [EMIM]Cl. With their defense against chemical assault breached by the ionic liquid, the hemicellulose and cellulose are hydrolyzed by treatment with HCl and water. The residual lignin and cellulose solids are subjected to a second hydrolysis, while the liquid hydrolyzate is separated through ion-exclusion chromatography. Ionic liquid recovered in the ion-exclusion step is stripped of water and recycled, while hydrolyzate sugars are fermented into fuels and other products.
Fig. 5.
Integrated process for biofuel production using ionic-liquid biomass hydrolysis. Ionic-liquids solvents enable efficient biomass decrystallization and hydrolysis. Ion-exclusion chromatography separates the ionic liquids for recycling and the hydrolyzate sugars for fermentation. (Photograph copyright 2001, Department of Energy/National Renewable Energy Laboratory; credit, Jim Yost.)
In comparison to extant enzymatic and chemical processes to biomass hydrolysis, ours has several attractive features. Like concentrated acid processes, it uses inexpensive chemical catalysts rather than enzymes and avoids an independent pretreatment step. Working in concert, [EMIM]Cl and HCl produce high sugar yields in hours at just 105 °C, whereas enzymatic hydrolysis can take days (16) and many pretreatment methods require temperatures of 160–200 °C (13). Also, lignocellulose solubilization by the ionic liquid allows processing at high concentrations, which can be a problem in enzymatic hydrolysis. On the other hand, our process improves on typical acid hydrolysis methods by avoiding the use of hazardous concentrated acid. Using catalytic amounts of dilute acid removes the complexity and danger of recycling large volumes of concentrated acid. The ionic liquid used in its place is likely to be far easier to handle. Despite this difference, our process is similar to commercial processes using concentrated acid hydrolysis (20, 27) and consequently can exploit proven engineering and equipment for facile scale-up, particularly for separations and recycling (vide infra).
Still, important challenges remain for implementation of an ionic-liquid biomass hydrolysis process. Highly viscous biomass-ionic-liquid mixtures might require special handling, and larger scale fermentation of hydrolyzate sugars might reveal the presence of inhibitors not detected in our demonstration experiments. Moreover, the sugar concentration resulting from stover hydrolysis (about 1%) is too low for practical fermentation and is decreased even further during the chromatographic separation, leading to water-evaporation costs. Methods allowing a higher starting biomass loading and strategies to concentrate rather than dilute the sugars during separation from the ionic liquid would overcome this problem.
Separations and ionic-liquid recycling could pose additional challenges to commercialization. Our separation method—ion-exclusion chromatography—is complex, especially with acid, ionic liquid, multiple sugars, and other soluble species in the hydrolyzate. Nonetheless, this technique is already used in continuous carbohydrate processing on a large scale, notably for the separation of glucose and fructose in corn wet mills (60). The separation of electrolytes such as ionic liquids from sugars is likely to be easier than this industrial application, and is currently being scaled up by BlueFire Ethanol, Inc. with sulfuric acid in two demonstration plants. Lessons from these facilities can aid in the design of efficient processes based on ionic-liquid hydrolysis.
Ionic liquids are expensive and will likely remain so in comparison to other solvents, even as expanded use reduces their prices to near those of their raw materials [∼$2.50/kg for dialkylimidazolium chlorides (61)]. Consequently, virtually complete solvent recovery will be required to make biomass processing with ionic liquids economical. For instance, one target for the cost of ionic liquid lost in biomass processing might be $0.13/L (= $0.50/gal) ethanol, a near-term target for the cost of hydrolytic enzymes. An optimized process using 1 kg of ionic liquid for each kilogram of biomass processed would then need 98% recovery of the ionic liquid in each cycle (see SI Text). This level of solvent recovery might be feasible in an optimized process, considering that reported sulfuric acid losses in a pilot-scale concentrated acid process are only about 38 g/kg biomass, despite the much lower incentive to recover sulfuric acid (62). We note, however, that the accumulation of impurities in an ionic-liquid recycle loop could be problematic. Future studies will need to address this issue.
Our chemical hydrolysis method offers flexibility for an integrated biomass conversion process. Because the ionic-liquid solvent makes biomass polysaccharides readily accessible for chemical reactions, this process is likely to be compatible with a broad range of biomass feedstocks. Downstream, the sugars produced by lignocellulose hydrolysis are flexible feedstocks for production of a nearly infinite range of fuels and chemicals. E. coli, which readily use the hydrolyzate sugars, have been engineered to produce not only fuel ethanol but also 1-butanol, 2-butanol, branched alcohols, fatty acids, isoprenoids, and even hydrogen (63 –65). Furthermore, the aqueous stream of sugars can also be converted by catalytic processes into fuels (66) or chemical intermediates. In contrast with enzymatic hydrolysis reactions that often require coupling to fermentation (simultaneous saccharification and fermentation) to prevent product inhibition (15, 16), this chemical process can be paired with any downstream conversion. Finally, the lignin recovered from ionic-liquid biomass hydrolysis could be a valuable coproduct. After washing to remove ionic liquid, this residue could be burned to generate process heat and electricity. The lignin residue from similar acid hydrolysis processes has a heating value similar to that of wood, making it a useful feed for a solid fuel boiler (62). Alternatively, the lignin could be an excellent feedstock for high-value products (43, 67). As a result, our process, which uses simple chemical reagents to overcome biomass recalcitrance and liberate valuable sugars, has the potential to underlie a biorefinery.
Materials and Methods
A description of general materials and methods, the hydrolysis of cellulose and xylan, the recovery of sugars and [EMIM]Cl, the reaction of glucose, experiments with nonchloride ionic liquids, microbial growth and fermentation, the economics of ionic liquid usage, and Tables S1 and S2 are provided in SI Text.
Representative Procedure for Hydrolysis of Corn Stover.
Corn stover (26.7 mg, 54 μmol glucose units, 44 μmol xylose units) and [EMIM]Cl (502 mg) were mixed at 105 °C for 6 h. To this mixture was added aqueous HCl (1.66 M, 29 μL, equivalent to 5 mg conc. HCl), and the reaction mixture was stirred vigorously at 105 °C. After 10 min, deionized water (100 μL) was added with stirring, followed by additional aliquots at 20 min (50 μL), 30 min (75 μL), and 60 min (125 μL). After a total reaction time of 2.5 h, the solution was diluted with water (750 μL). Insoluble materials were removed by centrifugation, rinsed twice with water (200 μL), and dried. The liquid products (2.046 g) were analyzed by HPLC (2.0 mg/g glucose, 42% yield; 2.3 mg/g xylose, 71% yield).
The brown solids from the first hydrolysis were then heated with [EMIM]Cl (306 mg) at 105 °C for 4.5 h. To this mixture was added aqueous HCl (1.66 M, 14.5 μL, equivalent to 2.5 mg conc. HCl), and the reaction mixure was vigorously stirred at 105 °C. After 10 min, deionized water (50 μL) was added with stirring, followed by an additional 25 μL water at 20 min, 67.5 μL water at 30 min, and 70 μL water at 60 min. After 3 h total reaction time, the solution was diluted with water (300 μL) and centrifuged to sediment insoluble materials. The liquid products (770 mg) were analyzed by HPLC (3.56 mg/g glucose, 28% yield; 0.7 mg/g xylose, 8% yield). For the two-step process, the overall yield of glucose was 70% and the overall yield of xylose was 79%.
In other cases, aliquots of the reaction mixture were removed periodically for HPLC analysis.
Supplementary Material
Acknowledgments.
We are grateful to J.E. Holladay for helpful conversations on biomass chemistry in ionic liquids; B.R. Caes for contributive discussions; J.J. Blank and A.V. Cefali for experimental assistance; W.D. Marner, G.N. Phillips, and T.J. Rutkoski for assistance with bacterial growth studies; and T.W. Jeffries and T.M. Long for assistance with yeast fermentation. This work was supported by the Department of Energy through the Great Lakes Bioenergy Research Center. J.B.B. was supported by an National Science Foundation Graduate Research Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912073107/DCSupplemental.
References
- 1.US National Petroleum Council. Facing the Hard Truths about Energy. Washington, DC: US National Petroleum Council; 2007. pp. 110, 156–158. [Google Scholar]
- 2.Smeets EMW, Faaij APC, Lewandowski IM, Turkenburg WC. A bottom-up assessment and review of global bio-energy potentials to 2050. Prog Energ Combust. 2007;33:56–106. [Google Scholar]
- 3.Hahn-Hägerdal B, Galbe M, Gorwa-Grauslund MF, Lidén G, Zacchi G. Bio-ethanol—The fuel of tomorrow from the residues of today. Trends Biotechnol. 2006;24:549–556. doi: 10.1016/j.tibtech.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Dumitriu S, editor. Polysaccharides: Structural Diversity and Functional Versatility. 2nd Ed. New York: Marcel Dekker; 2005. [Google Scholar]
- 5.Peters D. Raw materials. Adv Biochem Eng Biotechnol. 2007;105:1–30. doi: 10.1007/10_031. [DOI] [PubMed] [Google Scholar]
- 6.Lange J-P. Lignocellulose conversion: An introduction to chemistry, process and economics. Biofuel Bioprod Bior. 2007;1:39–48. [Google Scholar]
- 7.Stephanopoulos G. Challenges in engineering microbes for biofuels production. Science. 2007;315:801–804. doi: 10.1126/science.1139612. [DOI] [PubMed] [Google Scholar]
- 8.Chheda JN, Huber GW, Dumesic JA. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew Chem Int Edit. 2007;46:7164–7183. doi: 10.1002/anie.200604274. [DOI] [PubMed] [Google Scholar]
- 9.Van Haveren J, Scott EL, Sanders J. Bulk chemicals from biomass. Biofuel Bioprod Bior. 2008;2:41–57. [Google Scholar]
- 10.Christensen CH, Rass-Hansen J, Marsden CC, Taarning E, Egeblad K. The renewable chemicals industry. ChemSusChem. 2008;1:283–289. doi: 10.1002/cssc.200700168. [DOI] [PubMed] [Google Scholar]
- 11.Binder JB, Raines RT. Simple chemical transformation of lignocellulosic biomass into furans for fuels and chemicals. J Am Chem Soc. 2009;131:1979–1985. doi: 10.1021/ja808537j. [DOI] [PubMed] [Google Scholar]
- 12.Himmel ME, et al. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science. 2007;315:804–807. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- 13.Mosier N, et al. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresource Technol. 2005;96:673–686. doi: 10.1016/j.biortech.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Wyman CE, et al. Comparative sugar recovery data from laboratory scale application of leading pretreatment technologies to corn stover. Bioresource Technol. 2005;96:2026–2032. doi: 10.1016/j.biortech.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Lau MW, Dale BE. Cellulosic ethanol production from AFEX-treated corn stover using Saccharomyces cerevisiae 424A(LNH-ST) Proc Natl Acad Sci USA. 2009;106:1368–1373. doi: 10.1073/pnas.0812364106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aden A, et al. Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing Co-Current Dilute Acid Prehydrolysis and Enzymatic Hydrolysis for Corn Stover. Golden, CO: National Renewable Energy Laboratory, Department of Energy; 2002. [Google Scholar]
- 17.Braconnot H. Memoir on the conversion of wood particles in rubber, in sugar, and in a special natural acid, by means of sulfuric acid; conversion of the same woody substance in ulmin by potash (Translated from French) Ann Chim Phys. 1819;12:172–195. [Google Scholar]
- 18.Stern AL. X. Contributions to the chemistry of cellulose. I. Celluose-sulphuric acid, and the products of its hydrolysis. J Chem Soc, Trans. 1895;67:74–90. [Google Scholar]
- 19.Xiang Q, Lee YY, Pettersson PO, Torget RW. Heterogeneous aspects of acid hydrolysis of α-cellulose. Appl Biochem Biotechnol. 2003;105:505–514. doi: 10.1385/abab:107:1-3:505. [DOI] [PubMed] [Google Scholar]
- 20.Bergius F. Conversion of wood to carbohydrates. Ind Eng Chem. 1937;29:247–253. [Google Scholar]
- 21.Schoenemann K. The perfecting of wood hydrolysis in the Rheinau process. Chim Ind (Paris) 1958;80:140–150. [Google Scholar]
- 22.Dunning JW, Lathrop EC. The saccharification of agricultural residues: A continuous process. Ind Eng Chem. 1945;37:24–29. [Google Scholar]
- 23.Tsao GT, Ladisch MR, Voloch M, Bienkowski PR. Production of ethanol and chemicals from cellulosic materials. Process Biochem. 1982;17:34–38. [Google Scholar]
- 24.Wright JD, D’Agincourt CG. Evaluation of Sulfuric Acid Hydrolysis Processes for Alcohol Fuel Production. Golden, CO: Solar Energy Research Institute, Department of Energy; 1984. [Google Scholar]
- 25.Wright JD, Power AJ. Energy from Biomass and Wastes. Vol. 10. Chicago, IL: Institute of Gas Technology; 1987. Comparative technical evaluation of acid hydrolysis processes for conversion of cellulose to alcohol; pp. 949–971. [Google Scholar]
- 26.Farina GE, Barrier JW, Forsythe ML. Fuel alcohol production from agricultural lignocellulosic feedstocks. Energ Source Part A. 1988;10:231–237. [Google Scholar]
- 27.Farone WA, Cuzens JE. US Patent 5,726,046. 1998
- 28.Hermanutz F, Meister F, Uerdingen E. New developments in the manufacture of cellulose fibers with ionic liquids. Chem Fibers Int. 2006;56:342–344. [Google Scholar]
- 29.Graenacher C. US Patent 1,943,176. 1934
- 30.Zhu S, et al. Dissolution of cellulose with ionic liquids and its application: A mini-review. Green Chem. 2006;8:325–327. [Google Scholar]
- 31.El Seoud OA, Koschella A, Fidale LC, Dorn S, Heinse T. Applications of ionic liquids in carbohydrate chemistry: A window of opportunities. Biomacromolecules. 2007;8:2629–2647. doi: 10.1021/bm070062i. [DOI] [PubMed] [Google Scholar]
- 32.Swatloski RP, Spear SK, Holbrey JD, Rogers RD. Dissolution of cellose with ionic liquids. J Am Chem Soc. 2002;124:4974–4975. doi: 10.1021/ja025790m. [DOI] [PubMed] [Google Scholar]
- 33.Mikkola J-P, et al. Ultrasound enhancement of cellulose processing in ionic liquids: From dissolution towards functionalization. Green Chem. 2007;9:1229–1237. [Google Scholar]
- 34.Pinkert A, Marsh KN, Pang S, Staiger MP. Ionic liquids and their interaction with cellulose. Chem Rev. 2009;109:6712–6728. doi: 10.1021/cr9001947. [DOI] [PubMed] [Google Scholar]
- 35.Dadi AP, Varanasi S, Schall CA. Enhancement of cellulose saccharification kinetics using an ionic liquid pretreatment step. Biotechnol Bioeng. 2006;95:904–910. doi: 10.1002/bit.21047. [DOI] [PubMed] [Google Scholar]
- 36.Kilpelainen I, et al. Dissolution of wood in ionic liquids. J Agric Food Chem. 2007;55:9142–9148. doi: 10.1021/jf071692e. [DOI] [PubMed] [Google Scholar]
- 37.Singh S, Simmons BA, Vogel KP. Visualization of biomass solubilization and cellulose regeneration during ionic liquid pretreatment of switchgrass. Biotechnol Bioeng. 2009;104:68–75. doi: 10.1002/bit.22386. [DOI] [PubMed] [Google Scholar]
- 38.Fanselow M, Holbrey JD, Seddon KR. Eur Patent Appl 1,860,201. 2007
- 39.Li C, Zhao ZK. Efficient acid-catalyzed hydrolysis of cellulose in ionic liquid. Adv Synth Catal. 2007;349:1847–1850. [Google Scholar]
- 40.Li C, Wang Q, Zhao ZK. Acid in ionic liquid: An efficient system for hydrolysis of lignocellulose. Green Chem. 2008;10:177–182. [Google Scholar]
- 41.Rinaldi R, Palkovits R, Schüth F. Depolymerization of cellulose using solid catalysts in ionic liquids. Angew Chem Int Edit. 2008;47:8047–8050. doi: 10.1002/anie.200802879. [DOI] [PubMed] [Google Scholar]
- 42.Vanoye L, Fanselow M, Holbrey JD, Atkins MP, Seddon KR. Kinetic model for the hydrolysis of lignocellulosic biomass in the ionic liquid, 1-ethyl-3-methyl-imidazolium chloride. Green Chem. 2009;11:390–396. [Google Scholar]
- 43.Sievers C, et al. Ionic-liquid-phase hydrolysis of pine wood. Ind Eng Chem Res. 2009;48:1277–1286. [Google Scholar]
- 44.Cao NJ, Xu Q, Chen LF. Acid hydrolysis of cellulose in zinc chloride solution. Appl Biochem Biotechnol. 1995;51–52:21–28. [Google Scholar]
- 45.Almeida JRM, Betilsson M, Gorwa-Grauslund MF, Gorsich S, Liden G. Metabolic effects of furaldehydes and impacts on biotechnological processes. Appl Microbiol Biotechnol. 2009;82:625–638. doi: 10.1007/s00253-009-1875-1. [DOI] [PubMed] [Google Scholar]
- 46.Le Chatelier HL. On a general expression of the laws of chemical equilibrium (Translated from French) CR Hebd Seances Acad Sci. 1884;99:786–789. [Google Scholar]
- 47.Remsing RC, Swatloski RP, Rogers RD, Moyna G. Mechanism of cellulose dissolution in the ionic liquid 1-n-butyl-3-methylimidazolium chloride: A 13C and 35/37Cl NMR relaxation study on model systems. Chem Commun. 2006:1271–1273. doi: 10.1039/b600586c. [DOI] [PubMed] [Google Scholar]
- 48.Remsing RC, et al. Solvation of carbohydrates in N ′-dialkylimidazolium ionic liquids: A multinuclear NMR spectroscopy study. J Phys Chem B. 2008;112:11071–11078. doi: 10.1021/jp8042895. [DOI] [PubMed] [Google Scholar]
- 49.Mascal M, Nikitin EB. Direct, high-yield conversion of cellulose into biofuel. Angew Chem Int Edit. 2008;47:7924–7926. doi: 10.1002/anie.200801594. [DOI] [PubMed] [Google Scholar]
- 50.Ebner G, Schiehser S, Potthast A, Rosenau T. Side reaction of cellulose with common 1-alkyl-3-methylimidazolium-based ionic liquids. Tetrahedron Lett. 2008;49:7322–7324. [Google Scholar]
- 51.Vitz J, Erdmenger T, Hänsch C, Schubert US. Extended dissolution studies of cellulose in imidazolium based ionic liquids. Green Chem. 2009;11:417–424. [Google Scholar]
- 52.Hatfield RD, Jung HJG, Ralph J, Buxton DR, Weimer PJ. A comparison of the insoluble residues produced by the Klason lignin and acid detergent lignin procedures. J Sci Food Agr. 1994;65:51–58. [Google Scholar]
- 53.Asher DR. Sugar purification by ion exclusion. Ind Eng Chem. 1956;48:1465–1466. [Google Scholar]
- 54.Dien BS, Cotta MA, Jeffries TW. Bacteria engineered for fuel ethanol production: Current status. Appl Microbiol Biotechnol. 2003;63:258–266. doi: 10.1007/s00253-003-1444-y. [DOI] [PubMed] [Google Scholar]
- 55.Ho NWY, Chen Z, Brainard AP, Sedlak M. Successful design and development of genetically engineered Saccharomyces yeasts for effective cofermentation of glucose and xylose from cellulosic biomass to fuel ethanol. Adv Biochem Eng Biotechnol. 1999;65:163–192. doi: 10.1007/3-540-49194-5_7. [DOI] [PubMed] [Google Scholar]
- 56.Van Maris AJA, et al. Development of efficient xylose fermentation in Saccharomyces cerevisiae: Xylose isomerase as a key component. Adv Biochem Eng Biotechnol. 2007;108:179–204. doi: 10.1007/10_2007_057. [DOI] [PubMed] [Google Scholar]
- 57.Jeffries TW. Emerging technology for fermenting D-xylose. Trends Biotechnol. 1985;3:208–212. [Google Scholar]
- 58.Agbogbo FK, Haagensen FD, Milam D, Wenger KS. Fermentation of acid-pretreated corn stover to ethanol without detoxification using Pichia stipitis . Appl Biochem Biotech. 2008;145:53–58. doi: 10.1007/s12010-007-8056-4. [DOI] [PubMed] [Google Scholar]
- 59.Agbogbo FK, Coward-Kelly G. Cellulosic ethanol production using the naturally occurring xylose-fermenting yeast, Pichia stipitis . Biotech Lett. 2008;30:1515–1524. doi: 10.1007/s10529-008-9728-z. [DOI] [PubMed] [Google Scholar]
- 60.Colonna WJ, Clarke MA, Godshall MA, Cleary M, White JS. In: Kirk-Othmer Encyclopedia of Chemical Technology. Kroschwitz JI, editor. New York: Wiley-Interscience; 2004. p. 485. [Google Scholar]
- 61.Reddy RG. Ionic liquids: How well do we know them? J Phase Equilib Diff. 2006;27:210–211. [Google Scholar]
- 62.Cuzens JC, Miller JR. Acid hydrolysis of bagasse for ethanol production. Renew Energ. 1997;10:285–290. [Google Scholar]
- 63.Maeda T, Sanchez-Torres V, Wood TK. Enhanced hydrogen production from glucose by metabolically engineered Escherichia coli . Appl Microbiol Biotechnol. 2007;77:879–890. doi: 10.1007/s00253-007-1217-0. [DOI] [PubMed] [Google Scholar]
- 64.Atsumi S, Hanai T, Liao JC. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature. 2008;451:86–89. doi: 10.1038/nature06450. [DOI] [PubMed] [Google Scholar]
- 65.Fortman JL, et al. Biofuel alternatives to ethanol: Pumping the microbial well. Trends Biotechnol. 2008;26:375–381. doi: 10.1016/j.tibtech.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 66.Kunkes EL, et al. Catalytic conversion of biomass to monofunctional hydrocarbons and targeted liquid-fuel classes. Science. 2008;322:417–421. doi: 10.1126/science.1159210. [DOI] [PubMed] [Google Scholar]
- 67.Lora JH, Glasser WG. Recent industrial applications of lignin: A sustainable alternative to nonrenewable materials. J Polym Environ. 2002;10:39–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.