Abstract
Ribosomal protein S5 is critical for small ribosomal subunit (SSU) assembly and is indispensable for SSU function. Previously, we identified a point mutation in S5, (G28D) that alters both SSU formation and translational fidelity in vivo, which is unprecedented for other characterized S5 mutations. Surprisingly, additional copies of an extraribosomal assembly factor, RimJ, rescued all the phenotypes associated with S5(G28D), including fidelity defects, suggesting that the effect of RimJ on rescuing the miscoding of S5(G28D) is indirect. To understand the underlying mechanism, we focused on the biogenesis cascade and observed defects in processing of precursor 16S (p16S) rRNA in the S5(G28D) strain, which were rescued by RimJ. Analyses of p16S rRNA-containing ribosomes from other strains further supported a correspondence between the extent of 5′ end maturation of 16S rRNA and translational miscoding. Chemical probing of mutant ribosomes with additional leader sequences at the 5′ end of 16S rRNA compared to WT ribosomes revealed structural differences in the region of helix 1. Thus, the presence of additional nucleotides at the 5′ end of 16S rRNA could alter fidelity by changing the architecture of 16S rRNA in translating ribosomes and suggests that fidelity is governed by accuracy and completeness of the SSU biogenesis cascade.
Keywords: R-protein S5, ram mutations, ribosome biogenesis, 16S rRNA processing
One of the most remarkable feats of the ribosome is the ability to decode genetic information accurately in a process that involves the interaction of aminoacyl-transfer RNAs (aa-tRNAs) to the aa-tRNA binding site (A site) on the small ribosomal subunit (SSU; 30S). However, this process is not fully error proof and missense errors occur at a frequency of 10-3 to 10-4 per amino acid synthesized (1). Decoding is thought to involve a number of local and global conformational changes in the SSU upon binding of a cognate aa-tRNA to the A site. These structural changes result in a transition from the “open” to the “closed” form whereby the head of the SSU rotates toward the shoulder and the shoulder toward the platform (2). The r-proteins S4, S5, and S12 along with helices 27 and 44 of 16S rRNA are implicated in fidelity; mutations in S4 and S5 can lead to ribosome ambiguity (ram) or miscoding, whereas specific mutations in S12 lead to hyperaccuracy (3). Based on the recent crystal structures (2), the r-protein ram mutations map at the interface of S4 and S5 and disrupt a number of salt bridges that are present in the open SSUs. These changes could destabilize the open state, thereby perturbing the equilibrium to promote the closed state and allowing decreased discrimination in the decoding process (4). Some additional biochemical and structural data support this model (5); nonetheless, other data are hard to incorporate into this scheme. A few mutations in S4 can confer “restrictive” phenotypes to Salmonella typhimurium (6) and surprisingly these hyperaccurate alleles of S4 suppress the hyperaccurate phenotypes of S12 mutants. Recent work suggests that the ram mutations may not alter stability of the S4/S5 interface (7). Thus, control of translational accuracy may be governed by additional means.
In an earlier study, we identified a unique mutation in r-protein S5 [at a universally conserved position (28 in Escherichia coli) where glycine is mutated to aspartate] (8) that is distinct from other identified S5 ram mutations (9, 10). The mutation, G28D, not only decreases translational fidelity but also results in cold sensitivity and accumulation of precursor SSUs, both characteristics of defects in the SSU biogenesis cascade (8). Interestingly, a single extragenic high copy suppressor of the cold-sensitive phenotype associated with S5(G28D) was isolated and was identified to be rimJ (11), the gene that encodes S5 N-terminal acetyltransferase (12). However, acetylation of S5 is not a factor in the observed suppression as an acetyltransferase-deficient form of RimJ is sufficient to suppress the phenotypic changes associated with S5(G28D) (11). Although it might be expected that this suppressor would allow the decoupling of the biogenesis and fidelity effects associated with S5(G28D), overexpression of RimJ rescued not only biogenesis associated defects observed in this S5 mutant strain, but also rescued the miscoding phenotype near to WT levels (11). Hence, we hypothesized that there is a previously unexplored link between SSU biogenesis and translational fidelity and RimJ rescues the fidelity defects of S5(G28D) by rescuing the SSU biogenesis defects of the S5 mutant strain.
In the present study, to test our hypothesis, we further examined the link between SSU biogenesis and translational accuracy. We show that the S5(G28D) strain is defective in 16S rRNA maturation and that overexpression of RimJ relieves this maturation defect. In a number of alternative sources of ribosomes containing precursor 16S (p16S: encompassing all possible precursor forms; see below) rRNA, we observed that these strains have impaired translational accuracy as well. Furthermore, we have provided mechanistic insights into how the presence of the extra nucleotides (NTs) at the 5′ end of 16S rRNA might affect fidelity. Structures involving p16S rRNA that form at the expense of helix 1 have been predicted in assembling subunits (13, 14) but not in the translating population; herein we demonstrate that the presence of 16S rRNA leader sequences affects SSU structure around helix 1 in a functionally important region (15). Moreover, we demonstrate that base pairing between leader and mature elements is critical to changes in fidelity. Overall, our results delineate a functional link between translational fidelity and 16S rRNA maturation during SSU biogenesis.
Results
Maturation of 16S rRNA in the S5(G28D) Strain.
The S5(G28D) strain has phenotypes characteristic of SSU assembly defects and is spectinomycin resistant (8) and thus is distinct from other ram mutants with substitutions at positions 103 or 111 of E. coli S5 (9). We hypothesized that RimJ rescued fidelity defects of S5(G28D) by rescuing its biogenesis defects, suggesting a link between SSU biogenesis and translational fidelity. Given that changes in rRNA processing correlate with ribosome biogenesis defects, we examined 16S rRNA maturation in S5(G28D) ribosomes. In E. coli, each of the seven rDNA operons is transcribed as a precursor that contains 16S rRNA, 23S rRNA, and 5S rRNA as well as tRNAs. RNase III acts to cleave the primary transcript to generate a 17S precursor form of 16S rRNA (16) which contains an extra 115 residues at the 5′ end (leader) and 33 extra residues at the 3′ end (trailer) (16S rRNA with 115 NTs at 5′ end is denoted as long precursor 16S rRNA: lp16S rRNA) (Fig. 1 A). In subsequent reactions, RNase E cleaves to generate a leader with 66 residues upstream of the mature 16S rRNA sequence (16S rRNA with an additional 66 NTs at the 5′ end is denoted as short precursor 16S rRNA: sp16S rRNA) and a subsequent cleavage by RNase G, a homolog of RNase E, yields the mature 5′ end of 16S rRNA (17, 18) (Fig. 1 A). The identity of the RNase that generates the mature 3′ end of 16S rRNA is currently unknown as is the exact order of the processing events in vivo.
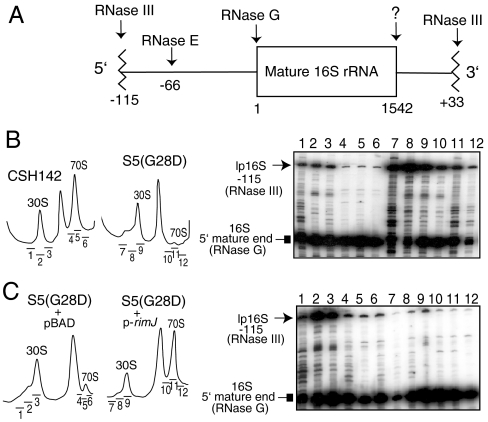
Fig. 1.
Maturation of 16S rRNA in S5(G28D) and effect of RimJ overexpression. (A) A schematic of the processing of p16S rRNA in prokaryotes (16). (B) Accumulation of p16S rRNA in S5(G28D) strain as analyzed by primer extension. Left panels show the ribosome profiles of the WT strain (CSH142) and S5(G28D) grown at nonpermissive temperature. An autoradiogram of a sequencing gel is shown on the right with lanes corresponding to the sucrose fractions from the ribosome profiles on left as indicated. Lanes 1–3 and 7–9 represent the 30S subunits of WT and S5(G28D) strains, respectively, and lanes 4–6 and 10–12 indicate the 70S ribosomes of WT and S5(G28D) strains, respectively. (C) Overexpression of RimJ rescues the 16S rRNA maturation defect of S5(G28D) strain as analyzed by primer extension. Lane representation is similar to B.
Maturation of 16S rRNA was examined in ribosomal fractions from S5(G28D) and its parental CSH142. We found that maturation of 16S rRNA is incomplete in the S5(G28D)-containing SSUs, the defect being exacerbated at nonpermissive temperature (Fig. 1 B). Interestingly, more lp16S rRNA (relative to mature 16S rRNA) is assembled in S5(G28D) 70S ribosomes than parental 70S ribosomes (Fig. 1 B, compare lanes 4–6 and 10–12) although there is a lower proportion of lp16S rRNA in the 70S ribosomes compared to the free SSUs in both strains (Fig. 1 B). In contrast to 16S rRNA processing, 23S rRNA processing is very similar in both S5(G28D) and parental strains (Fig. S1, compare lanes 5 and 6) ruling out any contribution of 23S rRNA 5′ end processing in fidelity changes. Thus, the presence of lp16S rRNA in 70S ribosomes could account for fidelity differences between these strains.
As stated above, in earlier work we demonstrated that RimJ, when overexpressed in the S5(G28D) background, mitigated the fidelity errors evident in the S5(G28D) strain (11). Therefore, a role of RimJ in rescuing 16S rRNA processing defects of the S5(G28D) strain was assessed. Overexpression of RimJ substantially decreased the levels of lp16S rRNA in both SSUs and 70S ribosomes isolated from S5(G28D) (Fig. 1 C). An increase in mature 16S rRNA in 70S ribosomes correlates with increased translational fidelity in the S5(G28D) background, yet RimJ does not associate with translating ribosomes (11).
Effect of p16S rRNA in 70S Ribosomes on Translational Fidelity in Other Backgrounds.
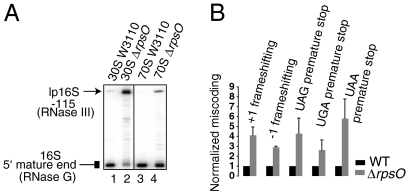
To further examine whether our observations of fidelity changes were linked with rRNA maturation or were a consequence of S5(G28D) mutation, we examined other ribosomes with defects in 16S rRNA maturation. Previous work has shown that, in a strain lacking the gene encoding r-protein S15 (rpsO), lp16S rRNA accumulates in pre-SSUs and SSUs (19), however neither translational fidelity nor 16S rRNA 5′ end maturation in 70S ribosomes was examined in this strain. Whereas 70S ribosomes isolated from a parental strain (W3110) appear to contain undetectable levels of p16S rRNA (Fig. 2 A, lane 3), both free SSUs and 70S ribosomes isolated from ΔrpsO strain contained substantial amounts of lp16S rRNA (Fig. 2 A, lanes 2 and 4). Next, we investigated miscoding in the ΔrpsO strain. [The S5(G28D) mutation did not significantly alter the fidelity of translation initiation (8), thus we did not investigate changes in initiation in other mutant strains used in the present study]. In the ΔrpsO strain, we observed a substantial increase in frameshifting and stop-codon readthrough relative to the isogenic parent (Fig. 2 B). These data suggest that decreased fidelity conferred by rpsO deletion could be due to defects in 16S rRNA maturation. Furthermore, similar to the S5(G28D) strain, the ΔrpsO strain showed no defects in processing the 5′ end of 23S rRNA (Fig. S1, compare lanes 7 and 8).
Fig. 2.
Correlation of 16S rRNA processing and translational fidelity in a strain lacking r-protein S15. (A) Primer extension analysis of the 5′ end of 16S rRNA isolated from sucrose gradient purified 30S subunits (lanes 1 and 2) and 70S ribosomes (lanes 3 and 4) from either the parental W3110 (lanes 1 and 3) or ΔrpsO strain (lanes 2 and 4). (B) Effect of deletion of rpsO on frameshifting errors and stop-codon readthrough in vivo. The ratio of units of β-galactosidase activity (obtained with the mutant lacZ reporters) to the β-galactosidase units (obtained with WT lacZ) in ΔlacZ ΔrpsO strain is normalized to that in an isogenic lacZ deficient MC252 strain. Error bars represent SD.
In vivo auxiliary extraribosomal factors aid in ribosome assembly by acting as chaperones, in rRNA and r-protein modification, or in an other yet to be characterized manner (for review see ref. 20). Deletion or mutation of some of these E. coli factors leads to the accumulation of p16S rRNA as previously demonstrated for SSU assembly factors, RimM and KsgA (21, 22). We observed elevated levels of stop-codon readthrough in strains lacking either RimM or KsgA (SI Text and Fig. S2), further corroborating the link between translational fidelity and presence of p16S rRNA in translating ribosomes.
Miscoding in a Strain Lacking RNase G.
Although the data correlating decreased translational accuracy with the presence of p16S rRNA in 70S ribosomes are strong, in a number of the examined cases, integral components of the ribosomes were mutant. To directly assess the role of 16S rRNA maturation in altering translational accuracy, a strain bearing a deletion of the gene encoding RNase G was investigated. RNase G is not an integral part of the functional ribosome, but is responsible for processing of the precise 5′ end of 16S rRNA (see Fig. 1 A). Previous work demonstrated that a strain lacking rng (gene encoding RNase G also known as cafA) is defective in processing the 5′ end of 16S rRNA, but the 3′ end of 16S rRNA was fully mature (17, 18). When deletion of rng is combined with a temperature-sensitive allele of RNase E, the double mutant strain accumulates lp16S rRNA in the 70S ribosomes (17). However, the status of incorporation of the p16S rRNA in 70S ribosomes generated in the Δrng strain alone was not reported. Three forms of p16S rRNA were observed in specific ribosomal fractions from the RNase G deletion strain (JW3216-1); these correspond to sp16S rRNA and lp16S rRNA (see Figs. 1 A and 3 A). In addition, more mature-like forms of 16S rRNA that are processed to within 3–4 NTs of the mature end are observed, but fully mature 16S rRNA is completely lacking. Interestingly, we observed that only the sp16S rRNA and the mature-like forms of p16S rRNA are found in the 70S ribosomes (Fig. 3 A). Additionally, no defects in 23S rRNA (5′ end) processing in this strain were observed (Fig. S1, compare lanes 1 and 2). In the 70S ribosomes with only the 5′ end of 16S rRNA extended (17) (Fig. 3 A), translational fidelity analysis of the Δrng strain revealed that nonsense readthrough and frameshifting levels were elevated compared to the parental strain (Fig. 3 B).
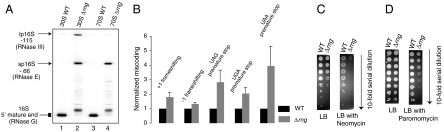
Fig. 3.
Effects of deletion of RNase G on 16S rRNA maturation, translational fidelity, and aminoglycoside susceptibility. (A) Primer extension analysis of the 5′ end of 16S rRNA isolated from sucrose gradient purified 30S subunits (lanes 1 and 2) and 70S ribosomes (lanes 3 and 4) from either the WT BW25113 (lanes 1 and 3) or Δrng strain (lanes 2 and 4). The mature 5′ end and the precursor forms (lp16S and sp16S rRNA) are indicated. (B) Effect of deletion of rng on frameshifting errors and stop-codon readthrough in vivo monitored by β-galactosidase assay in Δrng and parental BW25113 strains as described in Fig. 2 B. (C) Dilution plating (10-fold serial dilutions) of MG1655 (parent of Δrng) and Δrng strains on LB plates as indicated in presence of neomycin (1 ug/mL). (D) Same as C in presence of paromomycin (1 ug/mL).
To corroborate the miscoding phenotype of the RNase G deletion strain, we tested its sensitivity with aminoglycoside antibiotics. Aminoglycoside antibiotics such as neomycin and paromomycin interact with 16S rRNA near the SSU decoding site and result in codon misreading (23). Both neomycin and paromomycin exacerbated the growth of the Δrng strain relative to its parental strain (Fig. 3 C and D), suggesting that the fidelity changes attributed to 16S rRNA processing and aminoglycosides are additive. This additive effect of aminoglycosides on growth of the Δrng strain is similar to that for the S5(G28D) strain (Fig. S3). All of these observations indicate that maturation of the 5′ end of 16S rRNA and translational fidelity are functionally linked.
Alternative 16S rRNA Conformations in Ribosomes with Leader Sequences.
Several models to explain the functional interaction between 16S rRNA 5′ end maturation and translational accuracy are possible. One possibility is that the leader would be positioned at the subunit interface and perturb the interaction and critical communication between the two subunits. We observed no differences in the magnesium concentration dependent dissociation of 70S ribosomes from WT, S5(G28D) or Δrng mutant strains (Fig. S4). These data are consistent with earlier analysis of ribosomes from a rng/rne double mutant strain (17). Hence, it seems that 16S rRNA leader sequences do not dramatically alter interaction of the subunits.
A second possibility is that, as proposed in pre-30S particles (13, 14), the presence of the leader could promote formation of competing, alternative helical structures (Fig. 4 A). Such structures, if present in the 70S ribosomes, could preclude the appropriate folding of the mature elements of 16S rRNA and thereby affect translational fidelity. In ribosomes containing at least 10 additional NTs at the 5′ end, such as lp16S rRNA [+115 NTs, as found in the S5(G28D) and ΔrpsO strains] or sp16S rRNA (+66 NTs, as found in the Δrng strain), NTs 7–16 in the mature 16S rRNA are predicted to base pair with the first 10 NTs of the leader sequence [Fig. 4 A(I)] and form an alternative helix that would compete with mature helix 1 formation (13). Additionally, in ribosomes containing lp16S rRNA [as found in the S5(G28D) and ΔrpsO strains], a second proposed alternative structure could form utilizing NTs starting around -108 in the leader and those from 21 to 29 of mature 16S rRNA (24) and thus would also preclude helix 1 formation [Fig. 4 A(II)]. Lastly, RNA folding prediction (25) indicates that the lowest free energy structure of a 16S rRNA fragment containing NTs from -115 to 50 (numbered with respect to the mature 5′ end) involves base pairing between the leader and helix 1 elements. To test the possibility of the additional leader elements destabilizing helix 1 in S5(G28D), ΔrpsO and Δrng strains, we performed structural probing of SSUs and 70S ribosomes. Nucleotides A7 and A8 in mature 16S rRNA are free and accessible to DMS when mature helix 1 is formed, whereas formation of the proposed alternative helix [Fig. 4 A(I)] engages these NTs in base pairing so that they are no longer fully accessible to DMS modification. Our data show that A7 and A8 are less prone to DMS modification in the S5(G28D), ΔrpsO, and Δrng strains compared to their corresponding parental strains, suggesting that alternative structures are favored over helix 1 in the mutant strains (Fig. 4 B). Indeed, the intensity of modification is proportional to the amount and length (lp vs. sp) of the p16S rRNA leader in SSUs and 70S ribosomes (Fig. 4 B and C). It is difficult to directly access increased occupancy of the alternative structures due to lack of control structures within parental strains as they lack the leader. Overall, our data are consistent with the presence of p16S rRNA inducing errors in translational fidelity by altering helix 1 formation.
Fig. 4.
Structural considerations for the leader sequence of 16S rRNA and translational fidelity. (A) Secondary structure of E. coli 16S rRNA (40) with the region containing the mature 5′ end and the central pseudoknot in box. Two sets (I and II) of possible base-pairing configurations are shown in the box to form alternative helices #1 and #2 (see text). Mature 16S rRNA is shown in blue and leader sequence is shown in brown. Helices 1 and 2 as observed in mature 16S rRNA [derived from the crystal structure of 70S ribosome of E. coli (41)] are shown in equilibrium with leader specific helices (prehelices) which precludes the formation of helix 1 and may hinder formation of helix 2 (see ref. 14). Nucleotides in parenthesis in panel II are absent in lp16S rRNA (see text), but present in the primary transcript. Solid lines denote Watson–Crick bonding, whereas solid circles denote canonical non-Watson–Crick interactions (40) and empty circles denote additional interactions based on the X-ray crystal structure of E. coli 70S ribosome 41. (B) Primer extension analysis of RNA extracted from SSUs and 70S ribosomes modified with DMS and isolated from S5(G28D), ΔrpsO, and Δrng strains (and their parental strains). A and G correspond to the dideoxy sequencing lanes. Two control lanes are unmodified W3110 SSUs and 70S ribosomes. Lanes 1–12 are modified by DMS (see Methods). Lanes 1, 2, 5, 6, 9, and 10 are SSUs and lanes 3, 4, 7, 8, 11, and 12 are 70S ribosomes from CSH142 (lanes 1 and 3), S5(G28D) (lanes 2 and 4), W3110 (lanes 5 and 7), ΔrpsO (lanes 8 and 10), BW25113 (lanes 9 and 11), or Δrng (lanes 10 and 12). Altered reactivity in S5(G28D), ΔrpsO, and Δrng mutant particles relative to parental particles is marked with a solid box-headed arrow. As shown, 70S ribosomes from the parental strains lack p16S rRNAs and thus do not extend beyond the mature 5′ end. (C) Structural changes in positions A7 and A8 of 16S as indicated by the solid box-headed arrow in B are quantified using ImageJ (National Institutes of Health). Protections in S5(G28D) (brown bars), ΔrpsO (green bars), and Δrng (purple bars) are denoted as percentages of their parental strains for both the SSU and 70S ribosomes.
Effects of Removal of Alternative Helix #1.
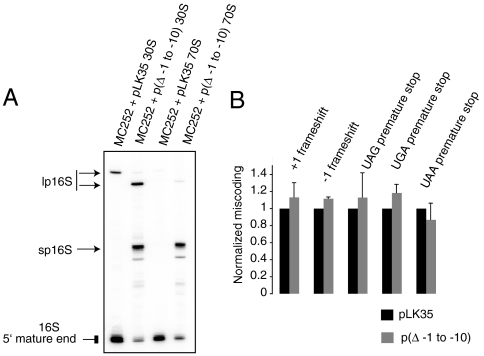
Given our observation that p16S rRNA decreases occupancy of helix 1 and thus may decrease translational fidelity, we investigated the effect of p16S rRNA lacking the ability to form alternative helix #1. Using a plasmid-borne copy of the rrnB operon (pLK35), we generated 16S rRNA lacking the 10 NTs upstream of the 5′ end (Δ - 1 to -10). Analysis of SSUs from a strain with this 10 NT deletion revealed accumulation of lp16S rRNAs and sp16S rRNAs with only sp16S rRNA being incorporated in 70S ribosomes (Fig. 5 A). The 70S ribosomes with this form of sp16S rRNA can form neither of the alternative helices; the base-pairing elements for alternative helix #1 have been deleted and, as these 70S ribosomes lack lp16S rRNA, alternative helix #2 cannot form. Investigation of translational fidelity in presence of (Δ - 1 to -10) 16S rRNA revealed no defects in translational fidelity when compared with WT 16S rRNA (Fig. 5 B). These results indicate that the presence of p16S rRNA alone is not sufficient to induce errors in fidelity and that base pairing plays a significant role.
Fig. 5.
Effects of mutations in alternative helix #1 on processing and fidelity. (A) Primer extension analysis of RNA extracted from 30S subunits and 70S ribosomes from MC252 strain with a WT rrnB operon (pLK35) or with a rrnB operon producing 16S rRNA with deletion of 10 NTs upstream of the mature 5′ end [p(Δ - 1 to - 10)]. The lp16S rRNA in the MC252 strain transformed with p(Δ - 1 to - 10) is shorter by 10 NTs than lp16S rRNA in the MC252 strain transformed with pLK35 and thus runs as a distinct product. (B) β-Galacosidase assay to monitor translational fidelity in MC252 containing p(Δ - 1 to - 10) as described in Fig. 2 B.
Discussion
In the presence of S5(G28D), maturation of 16S rRNA during ribosome biogenesis is affected. A p16S rRNA species, lp16S rRNA, which is cleaved by RNase III but not further end matured, accumulates in free SSUs and 70S ribosomes. Overexpression of RimJ increases the level of mature 16S rRNA and restores translational fidelity and thus 16S rRNA maturation is correlated with changes in fidelity. Another mutant strain lacking r-protein S15 also accumulates lp16S rRNA in 70S ribosomes (Fig. 2 A) and exhibits defects in translational fidelity (Fig. 2 B). Furthermore, deletion of rng (RNase G), which blocks processing of only the 5′ end of 16S rRNA (17), (18) (Fig. 3 A) also increases miscoding. In addition to the Δrng strain, we show that deletion of extraribosomal assembly factors, RimM and KsgA, which result in the accumulation of p16S rRNA (21, 22) also leads to decreased translational fidelity (Fig. S2). Early work revealed that deletion of the rimM (yfjA) gene led to a decrease in growth and translation efficiency (21). Our data suggest that the accumulation of p16S rRNA affects fidelity in the ΔrimM strain and thus could account for the earlier findings. Similarly, previous studies have suggested that strains lacking the functional dimethyltransferase activity of KsgA (nature of mutations were uncharacterized) were associated with high levels of stop-codon readthrough, frameshifting (26), and non-AUG (start) codon intitiation errors (27). Consistent with earlier reports, we observe fidelity defects now using a strain with a clean deletion of ksgA (Fig. S2). Interestingly, none of these SSU biogenesis factors bind 70S ribosomes but do interact with free SSUs and/or pre-SSUs (22, 28). Hence, these factors cannot modulate fidelity of the 70S ribosomes directly. Chemical probing of SSUs and 70S ribosomes from parental, S5(G28D), ΔrpsO, and Δrng strains revealed that helix 1 is not well formed in the p16S rRNA-containing particles. These data support the formation of alternative helical structures, which are formed by base pairing between leader and mature 16S rRNA sequences (13, 14) (see Fig. 4); formation of these structures would compete with mature helix 1 formation and affect translational fidelity. Furthermore, we show that deletion of 10 bps upstream of the 5′ end of 16S rRNA which result in accumulation of sp16S rRNA but not lp16S rRNA in 70S ribosomes, does not affect translational fidelity as expected by their inability to destabilize helix 1 (Fig. 5). Overall, our data suggest that formation of helix 1 as a result of appropriate 16S rRNA maturation during SSU biogenesis is critical for synthesis of accurate ribosomes.
Although mutations in S5 have previously been linked to changes in translational fidelity, we propose a unique role of S5 in this process. Although mutation of G28 to D in S5 confers a ram phenotype, position 28 of S5 does not map to the S4/S5 interface (Fig. S5). Recently, it has been shown in a yeast two-hybrid study that S5(G28D) does not destabilize S4–S5 interaction (7). Interestingly, investigation of 16S rRNA 5′ end processing in a S4 ram mutant and a S12 restrictive mutant revealed no significant processing changes in these strains, although in the S4 ram mutant a slight accumulation of p16S rRNA is evident compared to the parental strain (Fig. S6). Hence, ambiguity can be achieved via distinct mechanisms and our data indicate that fidelity is dependent on 16S rRNA processing during SSU biogenesis.
Interestingly, in both the S5(G28D) and ΔrpsO strains, lp16S rRNA is the most prominent intermediate, thus in these strains conversion of lp16S to more mature products is inhibited. Whereas, in the Δrng strain, SSUs with very short extensions of 16S rRNA, sp16S, and lp16S rRNAs are observed. Yet, SSUs containing more mature forms of p16S rRNA are preferentially found in 70S ribosomes in the Δrng strain to the exclusion of lp16S rRNA (Fig. 3 A). Although all of these strains show an increase in miscoding (8) (Figs. 2 and 3), the effect on translational fidelity is more pronounced in the S5(G28D) and ΔrpsO strains than in the Δrng strain, suggesting that the longer leader sequence may be more deleterious. Our probing data of helix 1 are consistent with S5(G28D) and ΔrpsO having more severe effects than Δrng (Fig. 4 B and C). Additionally, both S5(G28D) and ΔrpsO are cold sensitive and have reduced growth rates even at the permissive temperature (8, 19), whereas Δrng has only a slight impact on growth (29) (Fig. 3 C and D Left) suggesting that strains with a longer leader are more susceptible to kinetic traps. Based on these data, we propose a model (Fig. S7) where incorporation of immature SSUs into the translating population alters translational fidelity and cellular fitness. The longer leader (with an additional 115 NTs; Fig. S7A) allows for the possibility of one more alternative helix than the shorter leader (with 66 additional NTs at the 5′ end; Fig. S7B) (24). Hence, three potential species could form with lp16S rRNA (Fig. S7A). On the other hand, two potential species could form with sp16S rRNA (Fig. S7B). Hence, the population of particles containing mature helix 1 could be dependent on the length and proportion of leader-containing particles. Our probing data support this hypothesis as the reactivity of residues in helix 1 is altered more in the S5(G28D) and ΔrpsO ribosomes compared to the Δrng or parental strains (Fig. 4 B and C). Previous work has suggested a functional relationship between helix 1 and the 900 tetraloop capping helix 27, which is implicated in translational fidelity (30). This present study indicates that helix 1 formation is important for SSU function and the data presented herein reveal a unique means by which helix 1 formation can be perturbed in the functional ribosomal population by inclusion of p16S rRNA-containing SSUs.
The 16S rRNA leader sequence is highly conserved among all seven operons in E. coli. Leader sequences have been shown to facilitate correct 16S rRNA folding in a chaperone-like way by forming transient RNA–RNA interactions (31). Mutations in highly conserved sequence elements of the leader (nut-like) have been found to result in cold sensitivity and impaired structure and function of 30S subunits (32). Furthermore, it has been proposed that box C sequences, part of the nut-like elements and harboring the RNase III cleavage site, might mediate the formation of the universally conserved central pseudoknot of the 30S subunit (24). Mutational studies in the pseudoknot region revealed that the mutant ribosomes were impaired for in vivo translation (15, 33), establishing the significance of the central pseudoknot to ribosome function. Formation of the universally conserved pseudoknot is believed to be preceded by another pseudoknot structure that involves base pairing of a U3 box A-like sequence between positions -104 and -122 of the 5′ leader of 16S rRNA and NTs 14–31 and 916–918 of 16S rRNA (24). Thus, the leader is likely conserved due to its critical role in the biogenesis cascade but the presence of the leader is ultimately inconsistent with mature ribosome function.
Methods
Strains and Plasmids.
Strains BW25113 [parental KEIO strain (34)], and KEIO strains, JW3216-1 [Δrng, Coli Genetic Stock Center (CGSC) no. 10436], JW5413-6 (ΔrimM, CGSC no. 11375), and JW0050-3 (ΔksgA, CGSC no. 8292) have been obtained from CGSC, Yale University (Keio strains have been created at National BioResource Project of the National Institute of Genetics). An isogenic strain carrying the rpso knockout was constructed by P1 transduction (35) from the ΔrpsO NB497 (19) to MC136 [Δ(lac-pro)thi-aroE ∷ Tn10(Tetracycline)] background [kind gift from Dr. Michael O’Connor (Kansas City, MO)]. This strain was called ΔlacZΔrpsO strain. Deletion of rpsO and lacZ in this strain was verified by PCR amplification and a β-galactosidase assay using ortho-Nitrophenyl-β-galactoside as described below. A WT lacZ deficient strain (MC252) is also a kind gift from Michael O’Connor. Strains Xac, B210 (Xac rpsL141) and B212 (Xac rpsD12) have been described previously (36) and are kind gifts from Phil Farabaugh (Baltimore, MD). The lacZ plasmids used for fidelity assays are either pSG series (tetracycline resistant) or pACYC (chloramphenicol resistant) series of plasmids and carry frameshifting mutations or premature stops at the N terminus of WT lacZ gene and are kind gifts from Michael O’Connor.
Ribosome Profiles, RNA Extraction, and Primer Extension.
Cells were grown at 37 °C (permissive temperature) to ∼0.7 OD or grown at 37 °C to ∼0.2 OD prior to being shifted to 20 °C, which is the nonpermissive temperature for S5(G28D) cells or room temperature for ΔrpsO cells and incubated for another 4 h. RimJ expression in S5(G28D) strain was done as described earlier (11). Ribosome profiles were essentially performed as described (37), with sedimentation adjusted to allow separation of SSUs and pre-SSUs at the expense of observing polysomes. RNA extraction and primer extension were performed as described (38).
β-Galactosidase Assay.
Cells to be assayed for β-galactosidase activity, as described previously (27), were grown in LB medium containing 12.5 μg/mL tetracycline for pSG plasmids or 30 μg/mL chloramphenicol for pACYC plasmids. β-Galactosidase activity from the plasmid encoding WT lacZ in the WT strains and the same in the mutant strains, were used for normalization. Units of normalized β-galactosidase activity for the ΔlacZ ΔrpsO strain and the KEIO strains (Δrng, ΔrimM, and ΔksgA) with various reporters were reported as ratios to those observed in the WT MC252 strain and BW25113, respectively. Fidelity assay for the p(Δ - 1 to - 10) mutant was performed in MC252 background grown in presence of ampicillin (100 ug/mL) and normalization was done with a plK35 plasmid in MC252.
Structure Probing of Mutant Ribosomes.
The folding of rRNA within the mutant ribosomes was examined using DMS modification of the N1 position of adenosine and N3 of cytosine residues. DMS modification was carried out as described previously (39). Primer extensions were done using a primer with sequence 5′-AGCTAATCCCATCTGGG-3′, which anneals near the 5′ end of 16S rRNA.
Site-Directed Mutagenesis.
Deletion of 10 NTs at the 5′ end of 16S rDNA was carried out on pLK35 plasmid that carries a rrnB operon encoding 16S rRNA. QuikChange (Stratagene) site-directed mutagenesis protocol was followed, except that an extension time of 2 min /kb was used. Primers were 5′–CGA AGT TTA ATT CTT TGA GCG AAA TTG AAG AGT TTG ATC ATG GC–3′ and its reverse complementary primer.
Supplementary Material
Acknowledgments.
We thank Dr. Michael O’Connor (University of Missouri-Kansas City) for his generous gifts of the pSG and pACYC series of plasmids that were used for the translational fidelity analyses. We thank the E. coli Genetic Stock Center at Yale and National BioResource Project of the National Institute of Genetics, Japan, for E. coli Keio strains. We thank Dr. Philip Farabaugh for gifting strains Xac, B210, and B212. We also thank Dr. Yi-Tao Yu and Dr. Elaine Sia for their critical comments; Dr. Rachel Green, Dr. Martin Pavelka, and Culver lab members for their helpful discussions. This work was supported by National Institutes of Health Grant GM062432 (to G.M.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912305107/DCSupplemental.
References
- 1.Parker J. Errors and alternatives in reading the universal genetic code. Microbiol Rev. 1989;53:273–298. doi: 10.1128/mr.53.3.273-298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogle JM, Murphy FV, Tarry MJ, Ramakrishnan V. Selection of tRNA by the ribosome requires a transition from an open to a closed form. Cell. 2002;111:721–732. doi: 10.1016/s0092-8674(02)01086-3. [DOI] [PubMed] [Google Scholar]
- 3.Kurland CG, Hughes D, Ehrenberg M. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. Neidhardt FC, et al., editors. Washington, DC: American Society for Microbiology; 1996. pp. 979–1004. [Google Scholar]
- 4.Ogle JM, Carter AP, Ramakrishnan V. Insights into the decoding mechanism from recent ribosome structures. Trends Biochem Sci. 2003;28:259–266. doi: 10.1016/S0968-0004(03)00066-5. [DOI] [PubMed] [Google Scholar]
- 5.Noller HF. Biochemical characterization of the ribosomal decoding site. Biochimie. 2006;88:935–941. doi: 10.1016/j.biochi.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Bjorkman J, Samuelsson P, Andersson DI, Hughes D. Novel ribosomal mutations affecting translational accuracy, antibiotic resistance and virulence of Salmonella typhimurium . Mol Microbiol. 1999;31:53–58. doi: 10.1046/j.1365-2958.1999.01142.x. [DOI] [PubMed] [Google Scholar]
- 7.Vallabhaneni H, Farabaugh PJ. Accuracy modulating mutations of the ribosomal protein S4-S5 interface do not necessarily destabilize the rps4-rps5 protein–protein interaction. RNA. 2009;15:1100–1109. doi: 10.1261/rna.1530509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirthi N, Roy-Chaudhuri B, Kelley T, Culver GM. A novel single amino acid change in small subunit ribosomal protein S5 has profound effects on translational fidelity. RNA. 2006;12:2080–2091. doi: 10.1261/rna.302006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito T, Wittmann HG. Amino acid replacements in proteins S5 and S12 of two Escherichia coli revertants from streptomycin dependence to independence. Mol Gen Genet. 1973;127:19–32. doi: 10.1007/BF00267779. [DOI] [PubMed] [Google Scholar]
- 10.Piepersberg W, Bock A, Wittmann HG. Effect of different mutations in ribosomal protein S5 of Escherichia coli on translational fidelity. Mol Gen Genet. 1975;140:91–100. doi: 10.1007/BF00329777. [DOI] [PubMed] [Google Scholar]
- 11.Roy-Chaudhuri B, Kirthi N, Kelley T, Culver GM. Suppression of a cold-sensitive mutation in ribosomal protein S5 reveals a role for RimJ in ribosome biogenesis. Mol Microbiol. 2008;68:1547–1559. doi: 10.1111/j.1365-2958.2008.06252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshikawa A, Isono S, Sheback A, Isono K. Cloning and nucleotide sequencing of the genes rimI and rimJ which encode enzymes acetylating ribosomal proteins S18 and S5 of Escherichia coli K12. Mol Gen Genet. 1987;209:481–488. doi: 10.1007/BF00331153. [DOI] [PubMed] [Google Scholar]
- 13.Young RA, Steitz JA. Complementary sequences 1700 nucleotides apart form a ribonuclease III cleavage site in Escherichia coli ribosomal precursor RNA. Proc Natl Acad Sci USA. 1978;75:3593–3597. doi: 10.1073/pnas.75.8.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dammel CS, Noller HF. A cold-sensitive mutation in 16S rRNA provides evidence for helical switching in ribosome assembly. Genes Dev. 1993;7:660–670. doi: 10.1101/gad.7.4.660. [DOI] [PubMed] [Google Scholar]
- 15.Poot RA, van den Worm SH, Pleij CW, van Duin J. Base complementarity in helix 2 of the central pseudoknot in 16S rRNA is essential for ribosome functioning. Nucleic Acids Res. 1998;26:549–553. doi: 10.1093/nar/26.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srivastava AK, Schlessinger D. Mechanism and regulation of bacterial ribosomal RNA processing. Annu Rev Microbiol. 1990;44:105–129. doi: 10.1146/annurev.mi.44.100190.000541. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Pandit S, Deutscher MP. RNase G (CafA protein) and RNase E are both required for the 5′ maturation of 16S ribosomal RNA. EMBO J. 1999;18:2878–2885. doi: 10.1093/emboj/18.10.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wachi M, et al. Escherichia coli cafA gene encodes a novel RNase, designated as RNase G involved in processing of the 5′ end of 16S rRNA. Biochem Biophys Res Commun. 1999;259:483–488. doi: 10.1006/bbrc.1999.0806. [DOI] [PubMed] [Google Scholar]
- 19.Bubunenko M, et al. 30S ribosomal subunits can be assembled in vivo without primary binding ribosomal protein S15. RNA. 2006;12:1229–1239. doi: 10.1261/rna.2262106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson DN, Nierhaus KH. The weird and wonderful world of bacterial ribosome regulation. Crit Rev Biochem Mol Biol. 2007;42:187–219. doi: 10.1080/10409230701360843. [DOI] [PubMed] [Google Scholar]
- 21.Bylund GO, Wipemo LC, Lundberg LA, Wikstrom PM. RimM and RbfA are essential for efficient processing of 16S rRNA in Escherichia coli . J Bacteriol. 1998;180:73–82. doi: 10.1128/jb.180.1.73-82.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connolly K, Rife JP, Culver G. Mechanistic insight into the ribosome biogenesis functions of the ancient protein KsgA. Mol Microbiol. 2008;70:1062–1075. doi: 10.1111/j.1365-2958.2008.06485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schroeder R, Waldsich C, Wank H. Modulation of RNA function by aminoglycoside antibiotics. EMBO J. 2000;19:1–9. doi: 10.1093/emboj/19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennis PP, Russell AG, Moniz De Sa M. Formation of the 5′ end pseudoknot in small subunit ribosomal RNA: Involvement of U3-like sequences. RNA. 1997;3:337–343. [PMC free article] [PubMed] [Google Scholar]
- 25.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Buul CP, Visser W, van Knippenberg PH. Increased translational fidelity caused by the antibiotic kasugamycin and ribosomal ambiguity in mutants harbouring the ksgA gene. FEBS Lett. 1984;177:119–124. doi: 10.1016/0014-5793(84)80994-1. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor M, Thomas CL, Zimmermann RA, Dahlberg AE. Decoding fidelity at the ribosomal A and P sites: Influence of mutations in three different regions of the decoding domain in 16S rRNA. Nucleic Acids Res. 1997;25:1185–1193. doi: 10.1093/nar/25.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bylund GO, Persson BC, Lundberg LA, Wikstrom PM. A novel ribosome-associated protein is important for efficient translation in Escherichia coli . J Bacteriol. 1997;179:4567–4574. doi: 10.1128/jb.179.14.4567-4574.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wachi M, Umitsuki G, Nagai K. Functional relationship between Escherichia coli RNase E and the CafA protein. Mol Gen Genet. 1997;253:515–519. doi: 10.1007/s004380050352. [DOI] [PubMed] [Google Scholar]
- 30.Belanger F, Theberge-Julien G, Cunningham PR, Brakier-Gingras L. A functional relationship between helix 1 and the 900 tetraloop of 16S ribosomal RNA within the bacterial ribosome. RNA. 2005;11:906–913. doi: 10.1261/rna.2160405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theissen G, Thelen L, Wagner R. Some base substitutions in the leader of an Escherichia coli ribosomal RNA operon affect the structure and function of ribosomes. Evidence for a transient scaffold function of the rRNA leader. J Mol Biol. 1993;233:203–218. doi: 10.1006/jmbi.1993.1500. [DOI] [PubMed] [Google Scholar]
- 32.Schaferkordt J, Wagner R. Effects of base change mutations within an Escherichia coli ribosomal RNA leader region on rRNA maturation and ribosome formation. Nucleic Acids Res. 2001;29:3394–3403. doi: 10.1093/nar/29.16.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brink MF, Verbeet MP, de Boer HA. Formation of the central pseudoknot in 16S rRNA is essential for initiation of translation. EMBO J. 1993;12:3987–3996. doi: 10.1002/j.1460-2075.1993.tb06076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baba T, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol Syst Biol. 2006;2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller JH. Experiments in Molecular Genetics. Plainview, New York: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 36.Kramer EB, Farabaugh PJ. The frequency of translational misreading errors in E. coli is largely determined by tRNA competition. RNA. 2007;13:87–96. doi: 10.1261/rna.294907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maki JA, Schnobrich DJ, Culver GM. The DnaK chaperone system facilitates 30S ribosomal subunit assembly. Mol Cell. 2002;10:129–138. doi: 10.1016/s1097-2765(02)00562-2. [DOI] [PubMed] [Google Scholar]
- 38.Culver GM, Noller HF. In vitro reconstitution of 30S ribosomal subunits using complete set of recombinant proteins. Method Enzymol. 2000;318:446–460. doi: 10.1016/s0076-6879(00)18069-3. [DOI] [PubMed] [Google Scholar]
- 39.Merryman C, Noller HF. Footprinting and modification interference analysis of binding sites on RNA. In: Smith CWJ, editor. RNA: Protein Interactions: A Practical Approach. New York: Oxford University Press; 1998. pp. 237–253. [Google Scholar]
- 40.Cannone JJ, et al. The comparative RNA web (CRW) site: An online database of comparative sequence and structure information for ribosomal, intron, and other RNAs. BMC Bioinformatics. 2002;3:2. doi: 10.1186/1471-2105-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuwirth BS, et al. Structures of the bacterial ribosome at 3.5 A resolution. Science. 2005;310:827–834. doi: 10.1126/science.1117230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.