Abstract
Photoreceptor cells are remarkable in their ability to adjust their sensitivity to light over a wide range of intensities. Rapid termination of the photoresponse is achieved in part by shuttling proteins in and out of the light-transducing compartment of the photoreceptor cells. One protein that undergoes light-dependent translocation is the rhodopsin regulatory protein arrestin. However, the mechanisms coupling rhodopsin to arrestin movement are poorly understood. Here we show that light-dependent shuttling of the major arrestin in Drosophila photoreceptor cells, Arrestin2 (Arr2), occurs independently of known elements of the phototransduction cascade. Disruptions of the trimeric G protein, phospholipase Cβ, the TRP channel, or the Na+/Ca2+ exchanger did not influence Arr2 localization. Rather, we found that loss of the small GTPase Rac2 severely impaired Arr2 movement and prolonged the termination of the photoresponse. Our findings demonstrate that light-induced translocation of Arr2 occurs through a noncanonical rhodopsin/Rac2 pathway, which is distinct from the classical phototransduction cascade.
Keywords: adaptation, photoreceptor cell, phototransduction rhodopsin, small GTPase
Activity-dependent shuttling of signaling proteins between the cell surface and intracellular compartments is a widespread phenomenon which contributes to the magnitude and duration of signaling in neurons and many other cell types. One of the earliest demonstrations of activity-dependent translocation of signaling proteins from one cell compartment to another was the light-induced translocation of visual arrestin from the inner to the outer segments of rod photoreceptor cells over the course of a few minutes (1). Light-dependent shuttling of signaling proteins is an evolutionarily conserved phenomenon, as photostimulation also triggers the movement of the Drosophila visual arrestins from the cell bodies to the fly counterpart to rod outer segments, the rhabdomeres (2, 3). The trimeric G proteins that function in mammalian and Drosophila phototransduction undergo light-dependent translocation as well, as does the Drosophila transient receptor potential-like (TRPL) channel (4 –6). However, in contrast to the arrestins, these latter proteins shuttle out of the outer segments and rhabdomeres in response to light. The movements of these signaling proteins have important physiological consequences, as they contribute to light adaptation and termination of the photoresponse (3, 5, 6) and thus are crucial for the ability of photoreceptor cells to adjust their sensitivity to the surrounding light conditions.
The mechanisms and signaling pathways controlling the translocation of the Drosophila arrestins, G protein, and TRPL proteins have been explored but are incompletely understood. The light-dependent movement of the major visual arrestin, referred to as Arrestin2 (Arr2), requires interaction with PIP3 (3). In addition, the NINAC myosin III has been reported to contribute to the spatial reorganization of Gq (7), TRPL (8), and Arr2 (9), although Arr2 depends on NINAC only under blue (9) but not white light (10). Because light triggers the translocations, they would be expected to require activity of the phototransduction cascade. In flies, light-activated rhodopsin engages a heterotrimeric G protein, Gq, leading to stimulation of a phospholipase C (PLC) and opening of the TRP and TRPL cation channels (11). Visual arrestin binds to rhodopsin and attenuates signaling by dislodging the heterotrimeric G protein associated with the light-activated rhodopsin. Indeed, movement of TRPL requires Gq and PLC (8, 12), although the light-dependent shuttling of Gq has been reported to occur independently of PLC, TRP, or TRPL (7).
In the current work, we found that the dynamic spatial redistribution of Arr2 from the cell bodies to the rhabdomeres required rhodopsin, but did not depend on any of the other known components of the phototransduction cascade. These include Gq, PLC, TRP, TRPL, the Na+/Ca2+ exchanger (CalX), and protein kinase C. Rather, we found that the small GTPase Rac2 interacted with rhodospsin and was essential for the translocation of Arr2 into the rhabdomeres. As is the case with photoreceptor cells expressing Arr2 derivatives that do not translocate efficiently (3), mutations in rac2 cause a defect in termination of the photoresponse. These data indicate that the light-dependent movement of Arr2 depends on a parallel phototransduction cascade that is initiated by coupling of rhodopsin to Rac2.
Results
Arr2 Shuttling Depends on Rh1 but Not on Other Phototransduction Proteins.
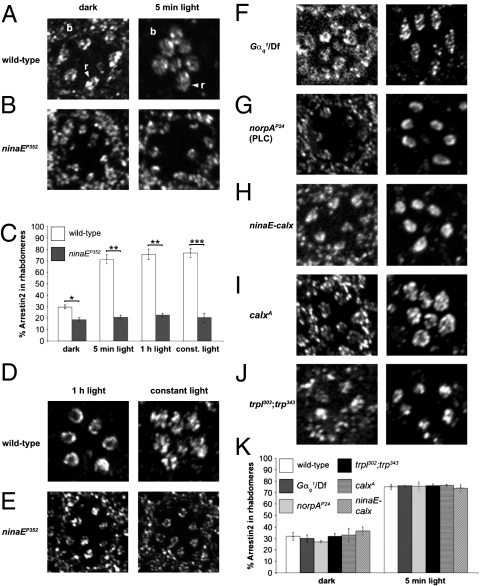
Arr2 shuttling is a light-dependent process and therefore requires a light sensor. The major Drosophila light receptor is Rhodopsin1 (Rh1), which is encoded by the ninaE gene (13, 14). To address whether Rh1 is essential for light-dependent movement of Arr2 from the cell body to the phototransducing compartment of the photoreceptor cells, the rhabdomere, we performed immunohistochemistry. The fly compound eye contains ∼800 repetitive units, the ommatidia, each of which includes seven photoreceptor cells in any plane of section (11). Rh1 is expressed in the six largest photoreceptor cells, R1–6. To examine the requirement for Rh1 for light-dependent shuttling of Arr2, we used two hypomorphic ninaE alleles (ninaEP352 and ninaEP334), which express <1% wild-type Rh1 levels (15). We did not use null ninaE alleles, because complete loss of Rh1 causes severe defects in eye morphology (16).
In dark-adapted wild-type, ninaEP352 (Fig. 1 A–C), and ninaEP334 flies (Fig. S1 A and B), Arr2 was distributed throughout the photoreceptor cells and was not concentrated in the rhabdomeres (Fig. 1 and Fig. S1 A and B). Upon exposure of wild-type flies to 5 min of white light (2500 lx), Arr2 translocated from the cell bodies and was restricted primarily to the rhabdomeres of R1–6 cells (Fig. 1 A and C and Fig. S1A). Arr2 translocation to the R7 cells was more variable (Fig. 1D Left), presumably because these cells are most responsive to UV light (11). Light-dependent translocation was also effective at lower light intensities of 100 and 1000 lx, but not at 10 lx (Fig. S1C). However, in ninaE flies, the levels of Arr2 in the rhabdomeres did not increase, even after constant exposure to light for >12 h (Fig. 1 C and E and Fig. S1E).
Fig. 1.
Requirement of rhodopsin and elements of the classical phototransduction cascade for light-dependent movement of Arr2. Localization of Arr2 in tangential sections of adult compound eyes. Flies were dark-adapted for ≥10 h and kept in the dark or exposed to white light for 5 min, 1 h, or ≥12 h (constant light). (A, B, and D–J) Confocal images of representative ommatidia stained with anti-Arr2 antibodies. The seven oval structures near the middle of the ommatidia are rhabdomeres (r), whereas the cell bodies (b) are located near the periphery of the ommatidia (indicated in A). (C and K) Quantification of Arr2 immunoreactivity in the rhabdomeres (C) from A, B, D, and E, and K from F–J. n = 11–12 ommatidia each. Error bars represent the SEM. Asterisks indicate statistically significant differences (Student's two-tailed, unpaired t test; *P < 0.01, **P < 0.005, ***P < 0.0005).
Because Rh1 was necessary for Arr2 movement into the rhabdomeres, we investigated whether other proteins that function downstream of Rh1 activation were involved in triggering light-dependent Arr2 movement. This was an open question, because light-dependent shuttling of TRPL but not Gqα requires the signaling proteins known to function subsequent to activation of Rh1 (7, 8). Therefore, we examined photoreceptor cells from flies expressing ∼1% wild-type levels of the G protein (Gαq1) that couples to photoactivated rhodopsin (17). It has been reported that Arr2 translocation is not impaired in these mutant flies (7). However, Gαq1 flies still respond to light, although they show a dramatic decrease in light sensitivity (17). Moreover, a level of 1% Gαq is sufficient to trigger TRPL translocation (8). To reduce the amount of Gαq further, we placed Gαq1 in trans with a deficiency that spanned the Gαq1 locus (Gαq1/Df). After a 5-min exposure to light, Arr2 translocated to the rhabdomeres to the same extent as in wild-type flies (Fig. 1 F and K).
Because Gαq1 flies show reduced rather than absent light-induced currents (17), it is possible that this residual activity of the phototransduction cascade might be sufficient to trigger Arr2 movement. Therefore, we examined photoreceptor cells from norpAP24 flies, which did not express the phospholipase Cβ required for phototransduction (18). The norpAP24 flies are blind and do not display a response to light (18, 19). We found that light-induced movement of Arr2 into the rhabdomeres occurred normally in norpAP24 flies (Fig. 1 G and K). Arr2 translocation was also not impaired in flies harboring a null mutation in the gene encoding eye-enriched protein kinase C (inaCP209) essential for the deactivation of the light response (20) (Fig. S1F).
Ca2+ is an intracellular messenger which regulates many cellular processes, including vesicular trafficking. Therefore, we examined Arr2 localization in flies with mutations or transgenes that increased or decreased Ca2+ levels in photoreceptor cells. To decrease intracellular Ca2+, we overexpressed the Na+/Ca2+ exchanger CalX, thereby increasing Ca2+ extrusion (21). To address the consequences of increasing intracellular Ca2+, we analyzed the spatial distribution of Arr2 in flies with a null mutation in the Na+/Ca2+ exchanger (calxA) (21). We found that increasing Ca2+ extrusion (ninaE-calx) or elevating intracellular Ca2 levels (calxA) did not impair Arr2 movement (Fig. 1 H, I, and K). To provide further evidence that activation of TRP channels and the subsequent increase in intracellular Ca2+ concentration had no influence on Arr2 localization, we examined Arr2 in flies with null mutations affecting the TRP and TRPL channels (22, 23). As in norpAP24 flies, trpl302;trpP343 photoreceptor cells are unresponsive to light (24, 25). We found that Arr2 localization was indistinguishable from wild-type in trpl302;trpP343 photoreceptor cells (Fig. 1 J and K).
Requirement for Rac2 for Arr2 Translocation.
Our results indicate that Rh1 is necessary for light-induced Arr2 translocation, but silencing of the phototransduction cascade or inducing changes in intracellular Ca2+ has no effects. Consequently, the identities of the signaling proteins that couple light activation of Rh1 to Arr2 translocation were unclear. We considered whether small GTPases of the Rho family might couple to Rh1 and function in Arr2 movement, because interactions between G-protein–coupled receptors, including rhodopsin, and Rho family members have been reported (26). In murine photoreceptor cells, Rac1 coprecipitates with rhodopsin (27). In Drosophila photoreceptor cells, Rac1 has been reported to function downstream of rhodopsin in organizing the actin cytoskeleton during morphogenesis (28).
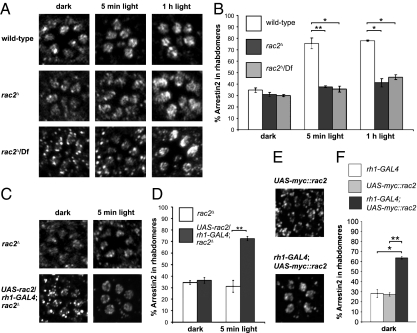
In Drosophila, there are three genes encoding Rac GTPases: Drosophila rac1, rac2, and mig-2-like (Mtl) (29). We found that loss of Rac2 had a profound effect on the light-dependent redistribution of Arr2. In null rac2 mutant photoreceptor cells (rac2Δ) (30) from dark-adapted flies, Arr2 was dispersed throughout the photoreceptor cells, similar to wild-type (Fig. 2 A and B). However, of significance here, after a 5-min exposure to light, Arr2 remained distributed throughout the cell bodies and rhabdomeres (Fig. 2 A and B). The deficit in light-dependent Arr2 translocation was not due to a background mutation, because we obtained the same results when rac2Δ was placed in trans with a deficiency chromosome that uncovered the rac2 locus (rac2Δ/Df) (Fig. 2 A and B). We repeated the immunostainings after exposing the flies to light for 1 h. Even after this prolonged light stimulation, Arr2 was not concentrated in the rhabdomeres (Fig. 2 A and B). We rescued the impairment in Arr2 translocation upon expression of a wild-type rac2 transgene in the rac2Δ mutant photoreceptor cells, under control of the GAL4/UAS system (31) (UAS-rac2 and rh1-GAL4) (Fig. 2 C and D). When we increased Rac2 expression by introducing UAS-rac2 and rh1-GAL4 in a wild-type (rac2+) background, there was a significant increase in Arr2 levels in the rhabdomeres of dark-adapted flies (Fig. 2 E and F).
Fig. 2.
Defects in light-induced translocation of Arr2 in rac2Δ mutant flies. (A and C) Flies were maintained in the dark for ≥10 h and either kept in the dark or exposed to light for 5 min or 1 h. Shown are the spatial distributions of Arr2 in tangential sections of adult compound eyes using anti-Arr2 antibodies and confocal microscopy. (A) Ommatidia from wild-type (ry506), rac2Δ, and rac2Δ/Df flies. (B) Quantification of Arr2 immunoreactivity in the rhabdomeres from A. n = 11–12 ommatidia. Error bars represent the SEM. (C) Ommatidia from rac2Δ flies with and without a rac2 cDNA transgene expressed in R1–6 photoreceptor cells under control of the rh1 promoter (UAS-rac2/rh1-GAL4;rac2Δ). (D) Quantification of Arr2 staining in the rhabdomeres from C. n = 12 ommatidia each. Error bars represent the SEM. (E) Ommatidia from dark-adapted flies overexpressing Myc-tagged Rac2 under the control of rh1-GAL4. Flies containing only the UAS-myc::rac2 transgene served as the negative control. Flies were maintained in the dark for ≥10 h. (F) Quantification of the Arr2 immunoreactivity from E. n = 11–12 ommatidia. Error bars represent the SEM and asterisks indicate significant differences using the Student's t test (*P < 0.01, **P < 0.001).
In contrast to the impairment in Arr2 shuttling in rac2Δ flies, mutations or transgenes affecting the activities of other Rho families had no impact on Arr2 movement. Mutations affecting members of the Rho family of small GTPases can disrupt eye morphology (28, 32, 33). Therefore, we examined Arr2 localization in flies expressing constitutively active and dominant-negative Rho family members in the eye (GMR-GAL4) using a modified GAL4/UAS system that allows temporal as well as spatial control of gene expression (31, 34). In this system, a temperature-sensitive protein (GAL80ts) inhibits GAL4 transcriptional activity at a permissive temperature (18 °C). Thus, GAL4 is activated by shifting the flies to the nonpermissive temperature (29 °C). We found that constitutively active Rho or Rac1, or dominant-negative Rho, Cdc42, or Rac1, had no effect on light-induced Arr2 translocation (Fig. S2A). This lack of effect did not appear to be due to ineffective induction of these transgenes, because mutant flies raised at the nonpermissive temperature for GAL80ts (29 °C; Fig. S3A) exhibited rough-eye morphology (Fig. S3B). We also generated genetically mosaic flies in which eyes were composed exclusively of cells deficient for mtl. However, loss of mtl did not impair Arr2 translocation (Fig. S2B).
Rho-family GTPases are known to regulate the actin cytoskeleton (35). Therefore, Drosophila rac2 might be involved in establishing photoreceptor cell morphology, as has been reported for Drosophila rac1 (28) and affect Arr2 localization nonspecifically. Therefore, we analyzed the eyes of rac2Δ flies by transmission electron microscopy (EM) and found that the morphology of rac2Δ photoreceptor cells closely resembled wild-type (Fig. S4A). Additionally, we stained the actin cytoskeleton with fluorophore-conjugated phalloidin and found that there were no apparent differences between rac2Δ and wild-type flies (Fig. S4B). To provide further evidence that the impairment in dynamic shuttling of Arr2 in rac2Δ was not due to a morphological defect, we used a heat shock-GAL4 to express UAS-rac2 exclusively during the pupal period when photoreceptor cell morphogenesis takes place (Fig. S4C). We found that Arr2 did not undergo light-dependent translocation in these flies (Fig. S4D). The localizations of other rhabdomere-enriched proteins examined, such as Rh1 and TRP, were indistinguishable in wild-type and rac2Δ flies (Fig. S5A). Furthermore, the concentrations of Rh1 or Arr2 were not decreased in rac2Δ flies (Fig. S5 C and D).
The base of the rhabdomeres undergoes massive morphological rearrangements upon illumination, which affects Gαq translocation (4). To determine whether Rac2 plays a role in regulating those morphological changes, we performed transmission EM on wild-type and rac2Δ photoreceptor cells, following dark adaptation and 1-h exposure to light. Although there were some light-dependent morphological changes at the base of the rhabdomeres, there were no clear differences between wild-type and rac2Δ flies (Fig. S5B).
Association of Rac2 with Rh1 in Photoreceptor Cells.
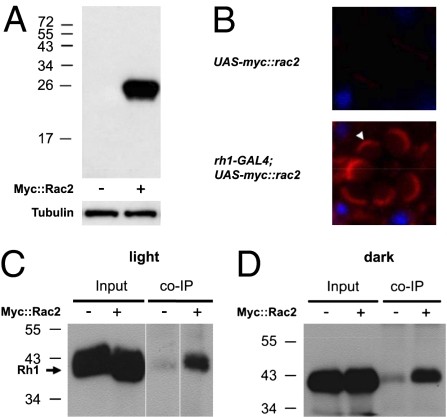
In vertebrate photoreceptors, Rac1 has been reported to coprecipitate with rhodopsin (27). Thus, we reasoned that in Drosophila photoreceptor cells, Rac2 might form a complex with Rh1. Because there is very high (92%) sequence identity between Rac1 and Rac2 (33), to specifically detect Rac2, we generated a transgene (UAS-myc::rac2) encoding Myc-tagged Rac2, which we expressed under the control of the rh1 (ninaE) promoter (Fig. 3A) and performed immunostaining with anti-Myc antibodies. The immunoreactivity was strongest at the base of the rhabdomeres (Fig. 3B).
Fig. 3.
Spatial distribution and interaction of Myc::Rac2 with Rh1. (A) Western blot containing head extracts from flies either overexpressing Myc-tagged Rac2 under the control of rh1-GAL4 (+) or containing the UAS-myc::rac2 transgene only (−). The blot was probed with anti-Myc antibodies and reprobed with anti-Tubulin antibodies. The positions of protein size markers are indicated. (B) Confocal images of single ommatidia stained with anti-Myc. Frozen eye sections were obtained from flies carrying the UAS-myc::rac2 transgene only (upper; UAS-myc::rac2) and flies overexpressing myc::rac2 (lower; rh1-GAL4;UAS-myc::rac2). Immunostaining was performed with anti-Myc (red). The nuclei were stained with DAPI (blue). Strong Myc immunoreactivity was detected at the base of the rhabdomeres (arrowhead). (C and D) Rh1 coimmunoprecipitated with Myc::Rac2 in vivo. Head extracts were prepared from flies expressing UAS-myc::rac2 under the control of rh1-GAL4 (+) or flies carrying the UAS-myc::rac2 transgene only (−), which had been either exposed to light (C) or dark-adapted (D) before the experiment. Immunoprecipitations (IPs) were performed with anti-Myc and the western blot was probed with anti-Rh1 antibodies. (Left) 0.5% inputs are shown.
To address whether Rac2 was present in a complex with Rh1, we performed coimmunoprecipitations using head extracts prepared from flies reared in light and dark. We used anti-Myc to precipitate Myc::Rac2 and probed the western blots with anti-Rh1. We found that Rh1 coimmunoprecipitated with Myc::Rac2 and did so equally well in light and dark (Fig. 3 C and D). These results suggest that Rac2 associates with a subset of the Rh1 pool at the base of the rhabdomeres and that the interaction was not light-dependent. Consistent with this conclusion, Rh1 has also been reported to be present at the base of the rhabdomeres and is even concentrated at this location in newly eclosed flies (16).
Rh1/Rac2 Cascade Required for Normal Termination of the Photoresponse.
In Drosophila, light-dependent translocation of Arr2 is required for rapid termination of the photoresponse (3). In dark-adapted flies, the termination of the photoresponse is slower than after preexposure to light (3). Mutations that interfere with the rate of Arr2 translocation from the cell bodies to the rhabdomeres cause corresponding defects in the termination rate of the light response (3, 9).
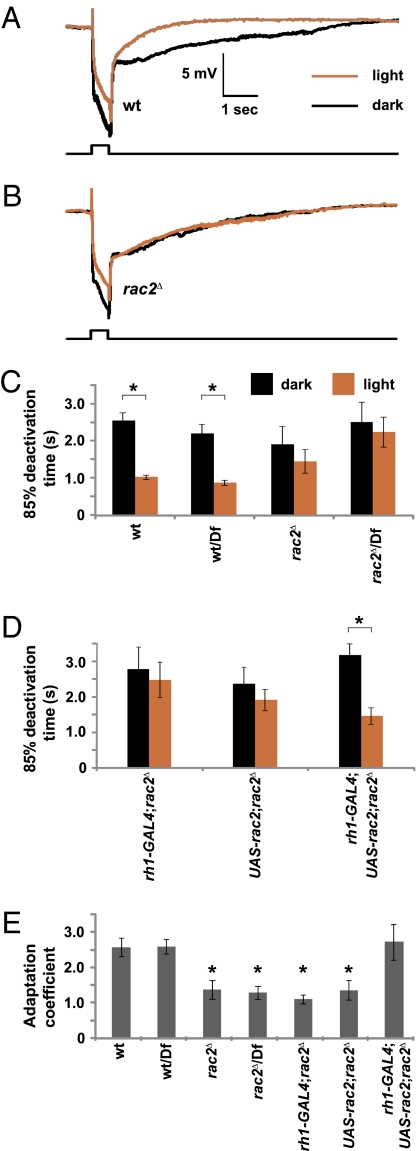
To test whether loss of rac2 reduced the ability of the flies to adapt the termination rate to increased exposure to light, we used electroretinogram (ERG) recordings. This assay measures the summed light-evoked responses in the whole retina. We compared the ERG responses of flies that were either dark-adapted or preexposed to light for 2 min (Fig. 4 A and B). The times required for an 85% recovery after termination of the light stimulus (t 85) were similar in dark-adapted wild-type, rac2Δ, and rac2Δ/Df flies (Fig. 4C; t 85: wild-type, 2.55 ± 0.20 s; rac2Δ, 1.91 ± 0.48 s; rac2Δ/Df, 2.51 ± 0.54 s). After exposure to light, the termination rate in wild-type flies was much faster (Fig. 4 A, C, and E; t85: wild-type, 1.02 ± 0.05 s; P = 0.0001). However, after the same light stimulation, the termination rate in rac2Δ and rac2Δ/Df flies was not significantly faster than in dark-adapted flies (Fig. 4 B, C, and E; t85: rac2Δ, 1.45 ± 0.32 s, P = 0.17; rac2Δ/Df, 2.24 ± 0.40 s, P = 0.57). We rescued the defect in light adaptation by expressing a wild-type rac2 transgene in R1–6 photoreceptor cells using the GAL4/UAS system (UAS-rac2 and rh1-GAL4) (Fig. 4 D and E; dark t85: rh1-GAL4;rac2Δ, 2.76 ± 0.62 s; UAS-rac2;rac2Δ, 2.35 ± 0.45 s; rh1-GAL4, UAS-rac2;rac2Δ, 3.15 ± 0.31 s; light t85: rh1-GAL4;rac2Δ, 2.45 ± 0.50 s; UAS-rac2;rac2Δ, 1.88 ± 0.30 s; rh1-GAL4, UAS-rac2;rac2Δ, 1.44 ± 0.23 s). Our results indicated that loss of Rac2 impaired the ability of the flies to undergo rapid response termination after a preexposure to light (Fig. 4E).
Fig. 4.
ERGs showing a requirement of Rac2 for long-term adaptation. Dark-adapted flies (black) of the indicated genotypes were exposed to a 0.5-s pulse of orange light. A second ERG recording was performed on the same fly after exposure to bright white light for 2 min and a recovery period of 30 s. (A and B) Representative ERGs. Light pulses are indicated by the event marker below the ERGs. (C and D) Quantification of the time required for an 85% recovery after termination of the light response in each genotype. (E) Adaptation coefficients. The adaptation coefficients compare the termination time in flies that are preexposed to light versus the time in dark-adapted flies. A value of 1.0 resulted if light preexposure did not accelerate the termination time. To obtain the adaptation coefficient, the 85% deactivation time in the dark-adapted flies was divided by the 85% deactivation time in flies that were preexposed to light. Error bars represent SEM. The asterisks in C and D indicate significant differences using the paired Student's t test (P < 0.05). The asterisks in E represent significant differences from wild-type, wild-type/Df, or rh1-GAL4;UAS-rec2;rac2 Δ using the unpaired Student's t test (P < 0.05). n = 10–12.
Discussion
Rhodopsin is the archetypal G-protein-coupled receptor, which defines a family of highly related visual pigments. Before the current work, all light-activated pathways known to be physiologically required in photoreceptor cells functioned through heterotrimeric G proteins. Alternative candidate effectors for rhodopsin were small GTPases, because mammalian rhodopsin interacts with a Rho family member and activates this small GTPase in a light-dependent fashion (27). However, no role has been ascribed for transducing light activity through a rhodopsin/small GTPase pathway.
We found that the light-induced movement of Arr2 from the cell bodies to the rhabdomeres was strictly dependent on Rac2. Moreover, termination of the photoresponse was severely impaired in rac2Δ flies. Thus, our findings indicate a signaling mechanism underlying one form of light adaptation which entails transduction of the light signal through a small GTPase, rather than engaging Rh1 with the Gq/PLC/TRP cascade.
In addition to its role as a light receptor, rhodopsin plays a structural role during photoreceptor cell morphogenesis (16). This light-independent function of rhodopsin is mediated by Rac1 (28). In contrast, the requirement for Rac2 for Arr2 movement described in this report did not appear to be due to a morphological defect because the ultrastructures of wild-type and rac2Δ null mutant photoreceptor cells were indistinguishable. Thus, although the roles of Rac1 and Rac2 are often considered interchangeable, Rac2 is specifically required for light-dependent translocation of Arr2 translocation in adult photoreceptor cells, whereas Rac1 has a structural role during development. Other nonredundant functions of Rac2 have been described in the Drosophila cellular immune response (36).
It appears that two phototransduction pathways contribute to light-dependent movements of signaling proteins in Drosophila photoreceptor cells. The classical pathway is required for shuttling of TRPL because it is dependent on most elements of the phototransduction cascade including the PLC (8, 12). However, the movement of Arr2 depends on a second noncanonical, Rac2-dependent pathway. Light-dependent shuttling of the Gαq may also function through this second pathway because it occurs normally in mutants missing PLC, protein kinase C, or TRP (7).
The current work raises questions concerning the nature of the proteins that function in concert with Rh1/Rac2 signaling in photoreceptor cells. Finally, because the mammalian Rac1, which is the GTPase most related to Drosophila Rac2, is activated in a light-dependent manner (27), we propose that rhodopsin/Rac signaling may be an evolutionarily conserved mechanism controlling light-induced arrestin translocation and light adaptation in mammalian rods and cones.
Methods
Fly Stocks and Genetics.
The following fly strains were used: w1118, ry506, Canton S, w;;ninaEP334, w;;ninaEP352, w;Gαq1, Df(2R)vg135, w,norpAP24, w;trpl343;trp302, w;inaCP209, calxA, Rac2Δ ry506, Df(3L)pbl-X1, P[hsFLP]22 y1,w*; P[rh1-GAL4]2/CyO;TM2/TM6B, y1,w*; P[UAS-Rac1V12]1, y1,w*; P[UAS-Rac1N17]1, w*; P[UAS-Rho1V14]2.1, w*; P[UAS-Rho1N19]2.1, w[*]; P[w(+mC)=UAS-Cdc42.F89]3, y1,w*; P[neoFRT]82B Mtl/TM3,Sb1, and w*;nocSco/CyO; P[tubP-GAL80ts]7. Except if noted otherwise, the experiments were performed with flies (<3 days posteclosion) reared at 25 °C under a 12-h light/12-h dark cycle. In those cases in which the strain underwent rapid retinal degeneration (ninaEP334, ninaEP352), we used flies 1 day posteclosion. We generated the P[UAS-rac2] and P[UAS-myc::rac2] transgenic flies by subcloning the rac2 cDNA between the EcoRI and XhoI restriction sites of pUAST (31) and pUASTmyc, respectively. The mosaic mtlΔ eyes were generated as described (37). To express Myc-tagged Rac2 during development under the control of hsp-GAL4, we exposed the flies for 30 min to 37 °C, starting at the third-instar stage until the early pupae. Newly eclosed flies were kept at 18 °C for 3 days before the experiment.
Immunohistochemistry and Quantitative Analysis.
To perform the immunohistochemistry, we used flies that were dark-adapted for >10 h and subsequently exposed to bright white light (∼2500 lx) or maintained in the dark. Specimens were prepared as described (3). Antibodies were diluted in phosphate-buffered saline (PBS) plus 5% normal goat serum (PBSN). Sections were incubated with primary antibodies overnight at 4 °C with Alexa Fluor 568-labeled secondary (1:500) antibodies (Invitrogen) for 1 h at room temperature. Anti-Arr2 antibodies (1:500) were a gift from Dr. S. Subramanium (Bethesda, MD), mouse monoclonal anti-Rh1 (4C5) was from the Developmental Studies Hybridoma Bank (University of Iowa), and the rabbit anti-TRP antibodies were described previously (38). Sections were mounted in Vectashield media (Vector Labs) and confocal images were acquired on an LSM 510 META laser scanning microscope (Zeiss). For red-eyed flies, we used Alexa Fluor 647-conjugated secondary antibodies (1:250). Autofluorescence was eliminated using the emission fingerprinting function of the LSM 510 META microscope. Pictures were acquired as a λ stack and subsequently the linear unmixing function was applied using the extracellular space between the rhabdomeres as reference for the background. Quantification of the relative amount of Arr2 in the rhabdomeres was performed as described (3). Each data set was based on 11–16 ommatidia from ≥3 different flies. Statistical analyses were performed using Microsoft Excel and Student's t test (two-tailed, two-sample, unequal variance).
To visualize the subcellular localization of Myc-tagged-Rac2, we prepared frozen sections of the compound eye. Heads were hemisected and fixed in ice-cold 4% paraformaldehyde with 0.15 M sodium phosphate (pH 7.4) as buffer for ≤2 h. The specimens were then incubated overnight in 30% sucrose for cryoprotection, and embedded in Tissue-Tek OCT compound. Six- to eight-micrometer sections were incubated with rabbit anti-Myc antibodies (Santa Cruz Biotechnology) diluted in PBSN with 0.2% Triton X-100 (Sigma) overnight at 4 °C, and subsequently with Alexa Fluor 568-conjugated secondary (1:500) antibodies (Invitrogen) for 1 h at room temperature. Sections were mounted with Vectashield media with DAPI, and confocal images were acquired on an LSM 510 META microscope using a 100× objective. Images were processed with Adobe Photoshop 7.0 software for assembling the figures. For visualization of the actin cytoskeleton, fly heads were treated as described above. Instead of cutting the specimens, they were incubated with Alexa Fluor 568–conjugated phalloidin (Invitrogen) overnight. We then detached the retina from the overlying cuticle and mounted the specimens with Vectashield media containing DAPI.
Transmission Electron Microscopy.
Transmission EM was performed as described previously (39), except that 0.1 M sodium phosphate (pH 7.4) was used as the buffer. Tangential sections (85 nm) were obtained at a consistent depth, counterstained with uranyl acetate and lead citrate, and examined by transmission EM using a Zeiss electron microscope.
Western Blot Analyses.
Fly heads were homogenized in SDS sample buffer. The proteins were fractionated by SDS/PAGE and transferred overnight to nitrocellulose membranes (Amersham). Western blots were probed with anti-Myc antibodies followed by peroxidase-conjugated secondary antibodies (Sigma). Signals were detected using ECL reagents (Amersham). The blots were reprobed with anti-Tubulin antibodies (Sigma).
Coimmunoprecipitations.
The Rac2 and Rh1 coimmunoprecipitations were performed as described with minor modifications (40). Twelve-milligram fly heads were homogenized in 1 mL buffer A [20 mM Tris-HCl (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 10% sucrose, 1% glycerol, 1 mM EDTA, and Complete protease inhibitors containing 1% CHAPS]. The extracts were centrifuged at 16,000 × g for 30 min at 4 °C. Two hundred-microliter aliquots of the supernatant were diluted with 800 μL buffer A without CHAPS. These extracts were precleared by incubating with 30 μL of preblocked protein A beads for 3 h at 4 °C, the addition of 4 μg of anti-c-Myc antibodies (Santa Cruz Biotechnology), and incubating overnight at 4 °C. Twenty microliters of preblocked protein A beads was added to the immune complexes and incubated for 2 h at 4 °C. After three washes with buffer A containing 0.2% CHAPS, the immune complexes were eluted with 2× SDS sample buffer and the western blots were probed with anti-Rh1 monoclonal antibodies (Developmental Studies Hybridoma Bank) and peroxidase-conjugated anti-mouse IgG secondary antibodies (Sigma). We detected the signals using ECL reagents (GE Healthcare Life Sciences).
Electroretinogram Recordings.
ERG recordings were performed essentially as described previously (9). Briefly, flies were fixed under a dim red photographic safety light, and two glass microelectrodes filled with Ringer's solution were inserted into small drops of electrode cream placed on the surfaces of the eye and thorax. A Newport Oriel Apex illuminator was used to stimulate the eyes with orange light. The ERGs were amplified with a Warner Electrometer IE-210 and recorded with a Powerlab 4/30 A/D converter and the LabChart v6.0 program (AD Instruments).
To establish the paradigm, we compared the termination times in dark- and light-adapted wild-type flies over a range of conditions. We found that the termination time following a second pulse of light approached the minimum when wild-type flies were treated for 2 min with bright light followed by a 30-s recovery period. Therefore, we used these conditions to compare the relative termination times displayed by wild-type flies and other fly strains. We used 85% recovery to the baseline because this resulted in the most reproducible results.
We performed ERG recordings on 10–12 flies of each genotype. We compared the termination times between the dark- and light-adapted flies using the Student's paired t test because each fly was used twice for ERG recordings (after dark adaptation and after the light treatment). We used unpaired t tests when comparing the adaptation coefficients across two genotypes, and ANOVA when comparing more than two genotypes.
Supplementary Material
Acknowledgments
We thank the Bloomington Stock Center for fly stocks, Dr. S. Subramanium for the anti-Arr2 antibodies, H. Shim for help with generating transgenic flies, M. Sepanski for preparing sections of compound eyes, and Drs. Y. Kwon, K. Venkatachalam, and D. Wasserman for helpful discussions. D.K. was supported in part by a predoctoral fellowship from the American Heart Association. This work was supported by a grant to C.M. from the National Eye Institute (EY010852).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.-W.Y. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906386107/DCSupplemental.
References
- 1.Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Jr, Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006;16:560–568. doi: 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Kiselev A, et al. A molecular pathway for light-dependent photoreceptor apoptosis in Drosophila . Neuron. 2000;28:139–152. doi: 10.1016/s0896-6273(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Xu H, Kang LW, Amzel LM, Montell C. Light adaptation through phosphoinositide-regulated translocation of Drosophila visual arrestin. Neuron. 2003;39:121–132. doi: 10.1016/s0896-6273(03)00390-8. [DOI] [PubMed] [Google Scholar]
- 4.Kosloff M, et al. Regulation of light-dependent Gqα translocation and morphological changes in fly photoreceptors. EMBO J. 2003;22:459–468. doi: 10.1093/emboj/cdg054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sokolov M, et al. Massive light-driven translocation of transducin between the two major compartments of rod cells: A novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- 6.Bähner M, et al. Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron. 2002;34:83–93. doi: 10.1016/s0896-6273(02)00630-x. [DOI] [PubMed] [Google Scholar]
- 7.Cronin MA, Diao F, Tsunoda S. Light-dependent subcellular translocation of Gqα in Drosophila photoreceptors is facilitated by the photoreceptor-specific myosin III NINAC. J Cell Sci. 2004;117:4797–4806. doi: 10.1242/jcs.01371. [DOI] [PubMed] [Google Scholar]
- 8.Meyer NE, Joel-Almagor T, Frechter S, Minke B, Huber A. Subcellular translocation of the eGFP-tagged TRPL channel in Drosophila photoreceptors requires activation of the phototransduction cascade. J Cell Sci. 2006;119:2592–2603. doi: 10.1242/jcs.02986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SJ, Montell C. Light-dependent translocation of visual arrestin regulated by the NINAC myosin III. Neuron. 2004;43:95–103. doi: 10.1016/j.neuron.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Satoh AK, Ready DF. Arrestin1 mediates light-dependent rhodopsin endocytosis and cell survival. Curr Biol. 2005;15:1722–1733. doi: 10.1016/j.cub.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 11.Wang T, Montell C. Phototransduction and retinal degeneration in Drosophila . Pflugers Arch. 2007;454:821–847. doi: 10.1007/s00424-007-0251-1. [DOI] [PubMed] [Google Scholar]
- 12.Cronin MA, Lieu MH, Tsunoda S. Two stages of light-dependent TRPL-channel translocation in Drosophila photoreceptors. J Cell Sci. 2006;119:2935–2944. doi: 10.1242/jcs.03049. [DOI] [PubMed] [Google Scholar]
- 13.Zuker CS, Cowman AF, Rubin GM. Isolation and structure of a rhodopsin gene from D. melanogaster . Cell. 1985;40:851–858. doi: 10.1016/0092-8674(85)90344-7. [DOI] [PubMed] [Google Scholar]
- 14.O'Tousa JE, et al. The Drosophila ninaE gene encodes an opsin. Cell. 1985;40:839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- 15.Johnson EC, Pak WL. Electrophysiological study of Drosophila rhodopsin mutants. J Gen Physiol. 1986;88:651–673. doi: 10.1085/jgp.88.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar JP, Ready DF. Rhodopsin plays an essential structural role in Drosophila photoreceptor development. Development. 1995;121:4359–4370. doi: 10.1242/dev.121.12.4359. [DOI] [PubMed] [Google Scholar]
- 17.Scott K, Becker A, Sun Y, Hardy R, Zuker C. Gq α protein function in vivo: Genetic dissection of its role in photoreceptor cell physiology. Neuron. 1995;15:919–927. doi: 10.1016/0896-6273(95)90182-5. [DOI] [PubMed] [Google Scholar]
- 18.Bloomquist BT, et al. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 19.Ostroy SE, Wilson M, Pak WL. Drosophila rhodopsin: Photochemistry, extraction and differences in the norp AP12 phototransduction mutant. Biochem Biophys Res Commun. 1974;59:960–966. doi: 10.1016/s0006-291x(74)80073-2. [DOI] [PubMed] [Google Scholar]
- 20.Smith DP, et al. Photoreceptor deactivation and retinal degeneration mediated by a photoreceptor-specific protein kinase C. Science. 1991;254:1478–1484. doi: 10.1126/science.1962207. [DOI] [PubMed] [Google Scholar]
- 21.Wang T, et al. Light activation, adaptation, and cell survival functions of the Na+/Ca2+ exchanger CalX. Neuron. 2005;45:367–378. doi: 10.1016/j.neuron.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 22.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 23.Phillips AM, Bull A, Kelly LE. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- 24.Reuss H, Mojet MH, Chyb S, Hardie RC. In vivo analysis of the Drosophila light-sensitive channels, TRP and TRPL. Neuron. 1997;19:1249–1259. doi: 10.1016/s0896-6273(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 25.Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell R, et al. Rhodopsin-family receptors associate with small G proteins to activate phospholipase D. Nature. 1998;392:411–414. doi: 10.1038/32937. [DOI] [PubMed] [Google Scholar]
- 27.Balasubramanian N, Slepak VZ. Light-mediated activation of Rac-1 in photoreceptor outer segments. Curr Biol. 2003;13:1306–1310. doi: 10.1016/s0960-9822(03)00511-6. [DOI] [PubMed] [Google Scholar]
- 28.Chang HY, Ready DF. Rescue of photoreceptor degeneration in rhodopsin-null Drosophila mutants by activated Rac1. Science. 2000;290:1978–1980. doi: 10.1126/science.290.5498.1978. [DOI] [PubMed] [Google Scholar]
- 29.Hakeda-Suzuki S, et al. Rac function and regulation during Drosophila development. Nature. 2002;416:438–442. doi: 10.1038/416438a. [DOI] [PubMed] [Google Scholar]
- 30.Ng J, et al. Rac GTPases control axon growth, guidance and branching. Nature. 2002;416:442–447. doi: 10.1038/416442a. [DOI] [PubMed] [Google Scholar]
- 31.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 32.Go MJ. Activation of Rac1 or Cdc42 during early morphogenesis of eye discs induces ectopic antennae in Drosophila . Dev Growth Differ. 2005;47:225–231. doi: 10.1111/j.1440-169X.2005.00798.x. [DOI] [PubMed] [Google Scholar]
- 33.Hariharan IK, et al. Characterization of rho GTPase family homologues in Drosophila melanogaster: Overexpressing Rho1 in retinal cells causes a late developmental defect. EMBO J. 1995;14:292–302. doi: 10.1002/j.1460-2075.1995.tb07003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila . Sci STKE. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- 35.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Williams MJ, Ando I, Hultmark D. Drosophila melanogaster Rac2 is necessary for a proper cellular immune response. Genes Cells. 2005;10:813–823. doi: 10.1111/j.1365-2443.2005.00883.x. [DOI] [PubMed] [Google Scholar]
- 37.Stowers RS, Schwarz TL. A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics. 1999;152:1631–1639. doi: 10.1093/genetics/152.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chevesich J, Kreuz AJ, Montell C. Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex. Neuron. 1997;18:95–105. doi: 10.1016/s0896-6273(01)80049-0. [DOI] [PubMed] [Google Scholar]
- 39.Porter JA, Hicks JL, Williams DS, Montell C. Differential localizations of and requirements for the two Drosophila ninaC kinase/myosins in photoreceptor cells. J Cell Biol. 1992;116:683–693. doi: 10.1083/jcb.116.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu H, et al. A lysosomal tetraspanin associated with retinal degeneration identified via a genome-wide screen. EMBO J. 2004;23:811–822. doi: 10.1038/sj.emboj.7600112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.