Abstract
Plant litter decomposition is a critical step in the formation of soil organic matter, the mineralization of organic nutrients, and the carbon balance in terrestrial ecosystems. Biotic decomposition in mesic ecosystems is generally negatively correlated with the concentration of lignin, a group of complex aromatic polymers present in plant cell walls that is recalcitrant to enzymatic degradation and serves as a structural barrier impeding microbial access to labile carbon compounds. Although photochemical mineralization of carbon has recently been shown to be important in semiarid ecosystems, litter chemistry controls on photodegradative losses are not understood. We evaluated the importance of litter chemistry on photodegradation of grass litter and cellulose substrates with varying levels of lignin [cellulose-lignin (CL) substrates] under field conditions. Using wavelength-specific light attenuation filters, we found that light-driven mass loss was promoted by both UV and visible radiation. The spectral dependence of photodegradation correlated with the absorption spectrum of lignin but not of cellulose. Field incubations demonstrated that increasing lignin concentration reduced biotic decomposition, as expected, but linearly increased photodegradation. In addition, lignin content in CL substrates consistently decreased in photodegradative incubations. We conclude that lignin has a dual role affecting litter decomposition, depending on the dominant driver (biotic or abiotic) controlling carbon turnover. Under photodegradative conditions, lignin is preferentially degraded because it acts as an effective light-absorbing compound over a wide range of wavelengths. This mechanistic understanding of the role of lignin in plant litter decomposition will allow for more accurate predictions of carbon dynamics in terrestrial ecosystems.
Keywords: carbon cycle, cellulose, photodegradation, semiarid grasslands, ultraviolet radiation
Lignin is second only to cellulose in abundance in plant-synthesized compounds of terrestrial origin and represents nearly 30% of the carbon sequestered in plant materials annually (1). Lignin is typically considered a recalcitrant material that is resistant to microbial decomposition; only specialized biota, predominantly fungi, are able to synthesize extracellular enzymes that break down these structures into biologically usable forms (2). As a result, lignin turnover is distinctly different from that of the other major cell-wall constituents (cellulose and hemicelluloses) (3), and lignin is known to limit microbial enzyme accessibility to these more labile cell-wall polysaccharides (4). As such, lignin plays a key role in both terrestrial (5) and oceanic carbon cycles (6, 7).
The importance of litter chemistry, particularly the concentration of nutrients and carbon compounds, as a major control on litter decomposition has been explored extensively in mesic ecosystems; lignin and lignin:nutrient ratios have been identified as key characteristics determining the rate of organic matter turnover (3, 8–11) and extracellular enzymatic activity in forests (12, 13). These correlations are much less obvious in low rainfall ecosystems, where standard indicators of litter chemistry do not predict decomposition rates and nutrient release (14–17). Recent experimental evidence has shown that, in semiarid ecosystems, abiotic photodegradation can be a dominant control on decomposition of decaying plant material (18). Solar radiation causes mass loss from plant litter in low rainfall ecosystems (18–21); photodegradation produces volatile carbon compounds (22–24) and alters the chemistry of the remaining material (21, 25, 26).
The characteristics of plant litter and the light-absorbing compounds that control this photochemical process in terrestrial ecosystems have not been identified, which limits the possibility of predicting the effects of changes in litter chemistry, climate, and plant community structure on photodegradation. Studies focused on degradation of wood and paper products have demonstrated that lignin content is an important determinant of their photosensitivity (27, 28). On the other hand, studies of photodegradation of senesced leaves have attributed light-driven CO and CO2 emissions from these materials to photooxidation of cellulose (23, 24). Litter decomposition experiments carried out in the field using various natural litter types have shown mixed results with respect to the relationships between initial litter chemistry and photodegradative losses (21, 24). Some litter decomposition studies have demonstrated a modest decrease in lignin content during exposure to UV-B radiation (290–315 nm) (20, 26, 29), although others have shown no effect of UV radiation (290–400 nm) on litter lignin (19, 30). Litter types used in these previous experiments varied in a number of characteristics other than lignin and cellulose concentrations, which makes it difficult to draw definitive conclusions about the roles of these polymers in the process of photodegradation. We directly addressed the role of cellulose and lignin as controllers of light-driven mass loss by combining a field-based action-spectroscopy approach with comparative abiotic and biotic incubations of material with controlled variation in lignin concentration.
Results
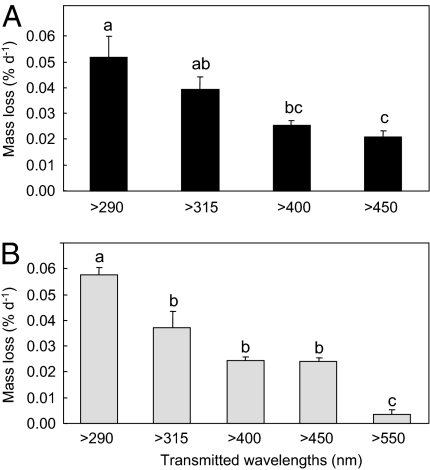
In a semiarid temperate grassland, plant litter was isolated from the soil surface and subjected to different radiation treatments using cutoff filters that attenuated specific wavelengths of natural solar radiation (Figs. S1 and S2). Grass litter samples placed under treatments that blocked the UV component of sunlight and received visible radiation only (λ > 400 nm) lost significantly less mass during the incubation period than those that received the full solar spectrum (λ > 290 nm, P < 0.001; Fig. 1A). However, samples under the filters that eliminated all UV and a significant fraction of blue light quanta (λ > 450 nm), still showed measurable mass loss. To obtain a better resolution in the activity spectrum for photodegradation, we exposed lignin-enriched (10%) cellulose (CL) substrates to sunlight filtered through a series of cutoff filters in the field. Photodegradative mass loss of these substrates was quantitatively comparable to that measured in the senescent grass litter (% d−1) and showed a very similar pattern of spectral dependence (Fig. 1). Moreover, extending the spectral range of light treatments showed that it was necessary to block all UV and blue-green quanta, with a 550-nm cutoff filter, to halt photodegradation (Fig. 1B). These results are consistent with studies demonstrating that visible light can drive CO (23) and CO2 emissions (24) from senescent plant material, and with the results of experiments showing that wood samples can readily produce free radicals, the initial step in the process of photodegradation, when exposed to low-irradiance visible light (27).
Fig. 1.
Experimental attenuation of specific regions of the solar spectrum demonstrates that both UV and visible radiation can drive photodegradation in the field. (A) Mass loss of grass litter in a semiarid grassland. Initial lignin concentration of litter was 7.3%. (B) Mass loss of cellulose-lignin (CL) substrates with 10% lignin concentration. Bars denote treatment means (n = 5 + SEM). Different letters indicate significant differences for Tukey HSD posthoc comparisons.
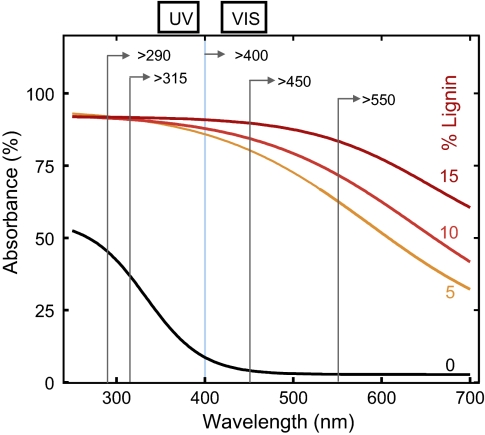
Our spectral analysis of photodegradation indicates that the wavelength dependence of mass loss observed in the field incubations with grass litter and CL substrates cannot be explained with cellulose as the principal light absorber. This is because strong light absorption by cellulose is restricted to the UV-B region of the solar spectrum and drops off to essentially zero in the visible region (Fig. 2). Additions of biologically relevant amounts of lignin (5–15%) to cellulose substrates dramatically increased light absorbance and extended the range of absorbed wavelengths well into the visible region of the solar spectrum. High light absorption over an extended range of wavelengths is common to all lignins and has been attributed to the presence of several light-absorbing groups within the molecular network of this polymer (27). Comparison of the absorption spectra of CL substrates (Fig. 2) with the activity spectrum for mass loss (Fig. 1) indicates that lignin is the most plausible light-absorbing compound to initiate the process of photodegradation in plant litter.
Fig. 2.
Radiation absorbance spectra of CL substrates enriched with different quantities of lignin (0, 5, 10, and 15%). The cutoffs of the spectral filters used for the sunlight attenuation experiments (Fig. 1) are indicated for comparison.
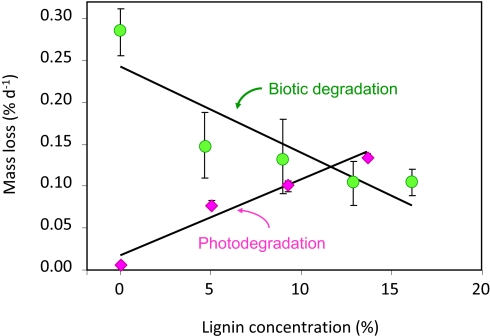
We then tested the quantitative importance of lignin on photodegradative mass loss using CL substrates enriched with increasing concentrations of lignin (Fig. 3). There was a highly significant and positive linear relationship between mass loss and lignin concentration when the samples were exposed to solar radiation (Fig. 3). In contrast, when similar samples were incubated under outdoor field conditions without solar radiation exposure, we found a negative linear correlation between lignin concentration and biotic mass loss (Fig. 3) at rates similar to those observed for grass leaf litter in this site (31). This direct comparison of abiotic and biotic litter decomposition in substrates that differed only in lignin concentration clearly demonstrates a driver-dependent role of lignin controlling the rate of litter decomposition.
Fig. 3.
Dual role of lignin in litter decomposition. Decomposition of CL substrates under abiotic conditions of full solar radiation (pink diamonds) and under biotic conditions (green circles), both under field conditions during summer and early fall. Symbols indicate mean values (n = 5 ± SEM). Solid lines are least-squares fits of the original data, which in both cases were simple linear regressions. Equations: Photodegradation = 0.0091 × (%lignin) + 0.0002. r2 = 0.92; Biotic decomposition = −0.0105 × (%lignin) + 0.0024. r2 = 0.45.
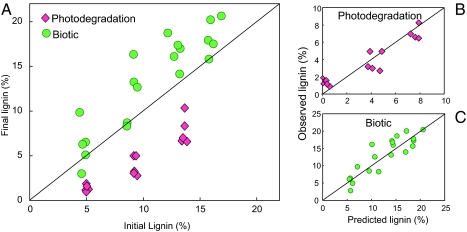
Spectral data indicate that lignin is the most likely light absorber but do not demonstrate that lignin is the polymer that is degraded to produce the observed mass loss in sunlight-exposed plant material. To address this issue, we studied the variation in lignin concentration during the experimental incubations of CL substrates. Biotic incubations resulted in increased lignin concentration (Fig. 4A). This observation is consistent with the results of previous experiments carried out in mesic ecosystems showing increased lignin concentration with biotic degradation as a consequence of preferential consumption of cellulose by detritivores (2, 3). In contrast, during photodegradation, substrates consistently lost lignin (Fig. 4A). Furthermore, when we estimated changes in lignin concentration—assuming that the only mass that was lost as a result of photodegradation was lignin (Fig. 4B) and that lignin was not lost as a result of biotic degradation (Fig. 4C)—the relationships between predicted and observed changes in lignin concentration did not depart significantly from a 1:1 relationship in either case. These results suggest that carbon losses caused by photochemical reactions were derived predominantly from lignin, whereas those resulting from microbial respiration were derived predominantly from cellulose. Studies of wood photodegradation suggest that the photon energy absorbed by cellulose in the UV range is likely to delocalize and transfer to lignin, causing degradation of the latter (27). The presence of lignin is therefore actually predicted to protect cellulose from photodegradation, a phenomenon that has been documented to some extent and attributed to the strong light absorption of lignin and its high capacity for autoxidation (27, 32). Our results support the idea that lignin was the primary loss product due to photodegradation in our standard substrates, and they are consistent with observations indicating the disappearance of lignin in litter exposed to high natural UV-B radiation (20) and increased biotic lability of plant litter after solar radiation exposure (21, 26).
Fig. 4.
Lignin concentration decreases with photodegradation and increases during biotic decomposition. (A) Initial and final lignin concentrations for photodegradation and biotic incubation experiments. CL substrates exposed to solar radiation consistently demonstrated a reduction in lignin concentration, whereas biotically incubated CL substrates overall showed increased lignin concentrations. Each data point corresponds to an individual sample exposed to full solar radiation (pink diamonds) or biotic conditions (green circles). (B) Predicted and observed changes in lignin for photodegradation: predicted changes were calculated assuming that all mass loss was due to the photodegradation of lignin. Regression for data points shown: Observed % = 0.99 × (Predicted %), r2 = 0.88, which does not deviate significantly from the 1:1 line. (C) Predicted and observed changes in lignin for biotic decomposition: predicted changes were calculated assuming that all mass loss was due to cellulose respiration. Regression for data points shown: Observed % = 1.06 × (Predicted %), r2 = 0.77, which does not deviate significantly from the 1:1 line.
Discussion
Lignin has traditionally been considered as a recalcitrant compound that retards biotic breakdown of organic matter (2). Although our results support this inhibitory role for biotic decomposition, they demonstrate a previously undescribed function of lignin as a facilitator of carbon turnover in terrestrial ecosystems. The net effect of lignin appears to be “driver-dependent,” that is, determined by whether photochemical effects or strictly biotic factors are controlling decomposition.
In ecosystems where exposure of plant litter and standing dead material to solar radiation is high, such as arid zones (18), clear-cut forests (33), fire-prone ecosystems, or agroecosystems, the photodegradation of lignin could directly result in carbon loss. In addition, and perhaps more importantly, the demonstration that the most recalcitrant cell-wall polymer is precisely the target of photo-destruction suggests the potential for significant positive effects of photodegradation on subsequent biological turnover (20, 21, 26). In decaying plant tissues, lignin serves as a structural barrier impeding access of enzymes to the bulk of labile carbon compounds (4, 34). Therefore, photodegradation not only would release carbon from lignin, but also would increase the accessibility to biotic degradation of many other compounds locked in lignin linkages within the cell walls, potentially changing the carbon-to-nitrogen ratios perceived by decomposers (35). Thus, although lignin represents a relatively small proportion of the total senescent plant material, its degradation by sunlight may have an inordinately large effect, increasing the potential for biotic decomposition of the carbohydrates present in litter.
Other plant attributes such as phenology, plant form, leaf architecture, and lignin distribution may also modify photodegradation potentials (24, 36). Furthermore, given the complex free-radical nature of the process of photodegradation, the participation of the various constituents of plant litter is likely to depend on a number of environmental factors such as humidity and the concentration of reactive gases such as oxygen. Additional work is necessary to identify the full suite of characteristics that control the susceptibility of plant litter to photodegradation.
Projected future shifts to warmer and dryer conditions in terrestrial ecosystems (37) suggest that photodegradation of plant litter could become a major pathway for carbon loss from arid and semiarid ecosystems (38). Plant traits that include allocation to lignin could have positive effects on carbon sequestration in mesic ecosystems (39), but could have lesser effects on carbon sequestration in deserts (40, 41) because of the offsetting effects of lignin on photodegradation. The identification of lignin as the key light absorber initiating photodegradation of plant litter has implications for our understanding of which aspects of litter chemistry control the rate of photodegradation and how this chemistry is altered during exposure to sunlight. In addition, our results (Fig. 4) predict a decline in lignin concentration after light exposure, which could have implications for models of litter decomposition and soil organic matter. Lignin content of litter inputs is a key variable in many terrestrial ecosystem carbon models (42, 43), and recent empirical models of long-term decomposition demonstrate that lignin content is the best determinant of the recalcitrant litter pools (44). If changes in initial lignin concentration of senescent plant material due to photodegradation are not considered, errors in the estimation of these recalcitrant litter pools could occur. As such, a better understanding of the basic mechanisms controlling photodegradation is a valuable step forward in the generation of more accurate predictions of carbon dynamics in terrestrial ecosystems.
Materials and Methods
Field Experiments.
One of the study sites was located in a perennial grassland in the province of Córdoba, Argentina (31° 04 S, 64° 31 W) in a matrix of open woodland vegetation. The vegetation is dominated by perennial tussock grasses, including Festuca hieronymi and Stipa trichotoma. Senescent, standing-dead plant litter was collected from the field site in spring (October in Argentina). Material was carefully selected as only recently senesced material (yellow) from the previous year's growth. Samples were placed in a drying oven at 65 °C for 7 days to minimize microbial growth without altering the structure or chemical composition of the litter. Senescent material (0.750 g) was placed in an uncovered plastic polystyrene box measuring 8 cm wide × 13 cm long × 2.5 cm high (Fig. S1). The plastic box with the litter was then placed within an oblong chicken wire cage (30 cm long × 15 cm wide × 10 cm high) in which the bottom consisted of a 2-mm fiberglass mesh fabric, the ends were blocked with a porous polyurethane sponge, and the top was covered with a plastic filter. The cage was suspended 30 cm above the soil surface on metal stakes to prevent soil contact and provide adequate possibility for free air movement. Plastic filters that attenuated specific wavelengths of natural solar radiation were placed on top of the chicken wire cage and used to establish the following treatments (see Fig. S2 for transmission spectra of plastic filters): (i) control (transparent filter, 95% transmittance of all solar radiation); (ii) attenuation of UV-B (280–315 nm, Mylar filter); (iii) attenuation of all UV radiation (280–400 nm, Costech UV filter); and (iv) attenuation of UV and blue wavelengths (280–450 nm, Costech UV filter and Rosco Lime no. 96). Litter was protected from all rainfall events and was not in contact with the soil surface during the period of the incubation. Litter was incubated for 120 days in the field, and there were eight replicates of each attenuation treatment. Litter from each box was collected, extraneous debris was removed, and litter was oven-dried at 65 °C for 48 h and weighed to determine mass loss. Initial litter quality measurements for carbon chemistry, made by using a standard acid detergent method (45), were the following: cellulose and sugars, 49.1 ± 0.4%; hemicellulose, 37.3 ± 0.2%; and lignin,7.3 ± 0.2%.
Two photodegradation experiments and one biotic incubation experiment were conducted using cellulose-lignin (CL) substrates at the second study site, the experimental station at the College of Agronomy in Buenos Aires (34° 35 S, 58° 29 W). Cellulose filter papers (Whatman no.42) were cut in quarters (≈250 mg and 31 cm2 for each filter) for impregnation with a lignin solution. The solution consisted of purified sugarcane lignin powder (Sigma Aldrich) dissolved in dioxane at a concentration of 3% lignin. The solution was applied in 400 μL doses to achieve substrates with different concentrations of lignin (≈5, 10, 15, 20%). In addition, dioxane was added in the same dose to the 0% lignin (pure cellulose) substrates. CL substrates were left overnight to ensure complete evaporation of dioxane from the filter paper. These model substrates were similar to a thin senescent plant leaf, although some of the lignin–cellulose relationships may be lacking from these substrates when compared to actual plant litter. For the biotic incubation, before the lignin impregnation, a solution of ammonium nitrate was applied to the cellulose filter paper to achieve a concentration of 1.5% dry weight of nitrogen. CL substrates of 0, 5, 10, and 15% lignin were exposed to full solar radiation for evaluation of direct photodegradation effects (lignin dosage experiment, n = 5 for each lignin treatment). In addition, CL substrates of 10% lignin were subjected to light attenuation treatments (spectral attenuation experiment, n = 5 for each spectral attenuation treatment). The CL substrates for the lignin dosage and spectral attenuation experiments were suspended at 7 cm above the ground surface with small wire alligator clips and placed under a clear plastic film (Stretch, >90% UV-visible transmittance) to avoid wetting during rain events (n = 5 replicates for each lignin concentration). In the case of the spectral attenuation experiment, a cutoff plastic filter was placed above and below the CL substrate to attenuate the desired wavelengths (Fig. S2). In addition to the treatments mentioned for the grassland incubation experiment described above, a fifth treatment that attenuated UV and visible radiation up to 550 nm (Rosco no. 21 Orange) was added, with 5 attenuation treatments in total (n = 5 for each light treatment). The biotic decomposition experiment consisted of 5 levels of lignin concentrations (0, 5, 10, 15, and 20%), with two pieces of cellulose substrates (total initial mass ∼500 mg) of identical lignin concentration placed in a 2-mm fiberglass mesh litterbag of 12 × 12 cm and placed on the soil surface (n = 5 for all treatments). All litterbags were then covered with two layers of a 3- × 3-m 75% shade cloth. The substrates were incubated for 75 days for the spectral attenuation of 10% CL substrates from December to March, for 30 days for the varying CL substrates in January and for 120 days for biotic decomposition from January to April.
Spectral Analysis of Lignin.
The absorption spectra of pure cellulose (Whatman no.42) and CL substrates were obtained using a microspectrometer (model USB4000-UV-VIS), with optics and grating optimized for measurements in the UV range and a pulsed Xe-arc lamp (model PX-2), both from Ocean Optics. To evaluate changes in lignin concentration during the incubation, CL substrates used in the lignin dosage and biotic decomposition experiments were analyzed for visible and infrared (VIS-IR) reflectance with a FieldSpec Pro spectrophotometer (Analytical Spectral Devices). The reflectance measurements were then converted to absorbance (log 1/R). Unexposed CL substrates in the range of 0–20% lignin, identical to those used in the experiments, were scanned to generate calibration regressions using the integral of absorbance in a 550- to 650-nm waveband. This calibration yielded a highly predictive polynomial regression equation: %lignin = 0.00003(abs)2 − 0.0002(abs) − 0.0036; r2 = 0.98, P < 0.0001, which was used to calculate the final lignin concentrations in the experimental substrates.
Supplementary Material
Acknowledgments
We thank IFEVA and the College of Agronomy for use of the experimental station; C. Mazza for help with determination of absorption spectrum of CL substrates;, M. Silva, G. Irisarri, M. Durante, L. Gherardi, P. Culaciati, A. Grasso, and M. Cargnel for technical and field assistance; three anonymous reviewers for constructive comments on a previous version of this manuscript; and L. Vivanco and C. Mazza for discussions. Financial support came from the Fundación Antorchas, the Agencia Nacional de Promoción Científica y Tecnológica (PICT 21247/4, 21798/4, 31970/5, 01296/6), and the University of Buenos Aires of Argentina (G034, G812).
Footnotes
*This Direct Submission article had a prearranged editor.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909396107/DCSupplemental.
References
- 1.Boerjan W, Ralph J, Baucher M. Lignin biosynthesis. Annu Rev Plant Biol. 2003;54:519–546. doi: 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- 2.Swift MJ, Heal OW, Anderson JM. Decomposition in Terrestrial Ecosystems. Berkeley, CA: University of California Press; 1979. [Google Scholar]
- 3.Berg B, Johansson M-B, Meentemeyer V. Litter decomposition in a transect of Norway spruce forests: Substrate quality and climate control. Can J Res. 2000;30:1136–1147. [Google Scholar]
- 4.Pauly M, Keegstra K. Cell-wall carbohydrates and their modification as a resource for biofuels. Plant J. 2008;54:559–568. doi: 10.1111/j.1365-313X.2008.03463.x. [DOI] [PubMed] [Google Scholar]
- 5.Potter CS, Klooster SA. Global model estimates of carbon and nitrogen storage in litter and soil pools: Response to changes in vegetation quality and biomass allocation. Tellus B Chem Phys Meteorol. 1997;49:1–17. [Google Scholar]
- 6.Opsahl S, Benner R. Distribution and cycling of terrigenous dissolved organic matter in the ocean. Nature. 1997;386:480–482. [Google Scholar]
- 7.Opsahl S, Zepp R. Photochemically-induced alteration of stable carbon isotope ratios (delta C-13) in terrigenous dissolved organic carbon. Geophys Res Lett. 2001;28:2417–2420. [Google Scholar]
- 8.Melillo JM, et al. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology. 1982;63:621–626. [Google Scholar]
- 9.Meentemeyer V. Macroclimate and lignin control of litter decomposition rates. Ecology. 1978;59:465–472. [Google Scholar]
- 10.Hättenschwiler S, Gasser P. Soil animals alter plant litter diversity effects on decomposition. Proc Natl Acad Sci USA. 2005;102:1519–1524. doi: 10.1073/pnas.0404977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornwell WK, et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett. 2008;11:1065–1071. doi: 10.1111/j.1461-0248.2008.01219.x. [DOI] [PubMed] [Google Scholar]
- 12.Carreiro M, et al. Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology. 2000;81:2359–2365. [Google Scholar]
- 13.Allison SD. Cheaters, diffusion and nutrients constrain decomposition by microbial enzymes in spatially structured environments. Ecol Lett. 2005;8:626–635. [Google Scholar]
- 14.Moorhead DL, Callaghan TV. Effects of increasing UV-B radiation on decomposition and soil organic matter dynamics. A synthesis and modelling study. Biol Fertil Soils. 1994;18:19–26. [Google Scholar]
- 15.Parton W, et al. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science. 2007;315:361–364. doi: 10.1126/science.1134853. [DOI] [PubMed] [Google Scholar]
- 16.Vanderbilt K, et al. Aboveground decomposition in arid environments: Results of a long-term study in central New Mexico. J Arid Environ. 2008;72:696–709. [Google Scholar]
- 17.Austin AT, Araujo PI, Leva PE. Interaction of position, litter type and pulsed water events on decomposition of grasses from the semiarid Patagonian steppe. Ecology. 2009;90:2642–2647. doi: 10.1890/08-1804.1. [DOI] [PubMed] [Google Scholar]
- 18.Austin AT, Vivanco L. Plant litter decomposition in a semiarid ecosystem controlled by photodegradation. Nature. 2006;442:555–558. doi: 10.1038/nature05038. [DOI] [PubMed] [Google Scholar]
- 19.Brandt LA, King JY, Milchunas DG. Effects of ultraviolet radiation on litter decomposition depend on precipitation and litter chemistry in a shortgrass steppe ecosystem. Glob Change Biol. 2007;13:2193–2205. [Google Scholar]
- 20.Day TA, Zhang ET, Ruhland CT. Exposure to solar UV-B radiation accelarates mass and lignin loss of Larrea tridentata litter in the Sonoran Desert. Plant Ecol. 2007;193:185–194. [Google Scholar]
- 21.Gallo ME, et al. Photoacceleration of plant litter decomposition in an arid environment. Soil Biol Biochem. 2009;41:1433–1441. [Google Scholar]
- 22.Anesio AM, Tranvik LJ, Granéli W. Production of inorganic carbon from aquatic macrophytes by solar radiation. Ecology. 1999;80:1852–1859. [Google Scholar]
- 23.Schade GW, Hormann RM, Crutzen PJ. CO emissions from degrading plant matter. Tellus B Chem Phys Meteorol. 1999;51:899–908. [Google Scholar]
- 24.Brandt LA, Bohnet C, King JY. Photochemically induced carbon dioxide production as a mechanism for carbon loss from plant litter in arid ecosystems. J Geophys Res. 2009;114:G02004. [Google Scholar]
- 25.Kieber DJ, McDaniel J, Mopper K. Photochemical source of biological substrates in sea water: Implications for carbon cycling. Nature. 1989;341:637–639. [Google Scholar]
- 26.Henry HAL, Brizgys K, Field CB. Litter decomposition in a California annual grassland: Interactions between photodegradation and litter layer thickness. Ecosystems (NY, Print) 2008;11:545–554. [Google Scholar]
- 27.Hon DN-S. In: Wood and cellulosic chemistry. Hon DN-S, Shiraishi N, editors. Marcel Decker, New York: 2001. pp. 525–555. [Google Scholar]
- 28.George B, et al. Photodegradation and photostabilisation of wood: The state of the art. Polym Degrad Stabil. 2005;88:268–274. [Google Scholar]
- 29.Rozema J, et al. Stratospheric ozone reduction and ecosystem processes: Enhanced UV-B radiation affects chemical quality and decomposition of leaves of the dune grassland species Calamagrostis espigeios. Plant Ecol. 1997;128:284–294. [Google Scholar]
- 30.Gehrke C, et al. The impact of enhanced UV-B radiation on litter quality and decomposition processes in Vaccinum leaves from the subarctic. Oikos. 1995;72:213–222. [Google Scholar]
- 31.Vivanco L, Austin AT. Intrinsic effects of species on leaf litter and root decomposition: A comparison of temperate grass species from North and South America. Oecologia. 2006;150:97–107. doi: 10.1007/s00442-006-0495-z. [DOI] [PubMed] [Google Scholar]
- 32.Hon N-S. Formation of free-radicals in photoirradiated cellulose. 6. Effect of lignin. J Polym Sci A Polym Chem. 1975;13:2641–2652. [Google Scholar]
- 33.Whitford WG, et al. Exceptions to the AET model: Deserts and clear-cut forests. Ecology. 1981;62:275–277. [Google Scholar]
- 34.Gressel J. Transgenics are imperative for biofuel crops. Plant Sci. 2008;174:246–263. [Google Scholar]
- 35.Manzoni S, et al. The global stoichiometry of litter nitrogen mineralization. Science. 2008;321:684–686. doi: 10.1126/science.1159792. [DOI] [PubMed] [Google Scholar]
- 36.Gallo ME, Sinsabaugh RL, Cabaniss SE. The role of ultraviolet radiation in litter decomposition in arid ecosystems. Appl Soil Ecol. 2006;34:82–91. [Google Scholar]
- 37.Gangulya AR, et al. Higher trends but larger uncertainty and geographic variability in 21st century temperature and heat waves. Proc Natl Acad Sci USA. 2009;106:15555–15559. doi: 10.1073/pnas.0904495106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andrady A, et al. Environmental effects of ozone depletion and its interactions with climate change: Progress report, 2009. Photochem Photobiol Sci. 2010 doi: 10.1039/b923342n. 10.1039/b923342n. [DOI] [PubMed] [Google Scholar]
- 39.De Deyn GB, Cornelissen JHC, Bardgett RD. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol Lett. 2008;11:516–531. doi: 10.1111/j.1461-0248.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- 40.Stursova M, Sinsabaugh RL. Stabilization of oxidative enzymes in desert soil may limit organic matter accumulation. Soil Biol Biochem. 2008;40:550–553. [Google Scholar]
- 41.Schlesinger WH, Belnap J, Marion GM. On carbon sequestration in desert ecosystems. Glob Change Biol. 2009;15:1488–1490. [Google Scholar]
- 42.Schimel DS, et al. Climatic, edaphic, and biotic controls over storage and turnover of carbon in soils. Global Biogeochem Cycles. 1994;8:279–293. [Google Scholar]
- 43.Vitousek PM, et al. Litter decomposition on the Mauna Loa matrix: Patterns, mechanisms, and models. Ecology. 1994;75:418–429. [Google Scholar]
- 44.Adair EC, et al. Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Glob Change Biol. 2008;14:2636–2660. [Google Scholar]
- 45.Van Soest PJ. Use of detergents in analysis of fibrous feeds II: A rapid method for the determination of fiber and lignin. J Assoc Offic Agr Chem. 1963;46:829–835. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.