Abstract
Mesenchyme is generally believed to play critical roles in “secondary induction” during organogenesis. Because of the complexity of tissue interactions in secondary inductions, however, little is known about the precise mechanisms at the cellular and molecular levels. We have demonstrated that, in mouse oviductal development, the mesenchyme determines the fate of undetermined epithelial cells to become secretory or cilial cells. We have established a model for studying secondary induction by establishing clonal epithelial and mesenchymal cell lines from perinatal p53−/− mouse oviducts. The signal sequence trap method collected candidate molecules secreted from mesenchymal cell lines. Naive epithelial cells exposed to Follistatin-like-1 (Fstl1), one of the candidates, became irreversibly committed to expressing a cilial epithelial marker and differentiated into ciliated cells. We concluded that Fstl1 is one of the mesenchymal factors determining oviductal epithelial cell fate. This is a unique demonstration that the determination of epithelial cell fate is induced by a single diffusible factor.
Keywords: cell fate determination, epithelial–mesenchymal interaction, Follistatin-like-1, oviduct
In the early gastrula of Xenopus, the anterior portion of the dorsal blastopore lip induces the dorsal axis. Hans Spemann called this phenomenon “primary induction” (1, 2). The mechanism behind primary induction now has been explained with diffusible molecules such as chordin, noggin, Follistatin, Xnr3, and BMP4 (3, 4). After primary induction is completed, “secondary (reciprocal) induction” takes place in various organ anlagen.
Since the 1950s, secondary inductions have been extensively studied. Various organ anlagen were dissected and enzymatically separated into epithelia and mesenchyme. Then epithelia alone or in combination with homologous or heterologous mesenchyme were cultured in vitro or grafted into the anterior eye chamber or under the kidney capsule. It was concluded that the mesenchyme plays critical roles in organogenesis in kidney (5), pancreas (6, 7), tooth (8, 9), mammary gland (10), lung (11), gastrointestine (12), male urogenital tract (13, 14), and female reproductive tract (15, 16) tissues. In the 1970s, a search for epithelial cell-fate-determining factors of mesenchymal origin was begun, but has been without success (17–19).
The oviduct develops from the Müllerian duct and has a unique epithelium containing two different epithelial cells, secretory cells, and cilial cells in contrast to simple columnar uterine epithelium and stratified squamous vaginal epithelium. Our previous study suggested that the oviduct contains at least two distinct epithelial cell populations rather than a single population with a transitional phenotype (20, 21). How are their fates determined?
Secondary induction is commonly transient and takes place on a small scale within a limited number of cells. To circumvent problems of small tissue scale and to develop reproducible in vitro models, we established epithelial and mesenchymal clonal cell lines and attempted to investigate the mechanism of how mesenchyme determines the fate of epithelial cells in the oviduct. We have already demonstrated that the p53−/− mouse is a useful source for establishing clonal cell lines with tissue-specific phenotypes (20, 22–25) and developmental-stage-specific phenotypes (21, 26–29).
In the present study, we have established clonal cell lines from perinatal oviducts and have developed an in vitro system in which epithelial fate determination can be studied. The system provided evidence that epithelial fate was determined by mesenchymal diffusible factors. Then “signal sequence trap,” a retrovirus-mediated expression screening method (SST–REX) (30), was applied to identify the determining factors. Finally, we isolated Follistatin-like-1 (Fstl1) as a diffusible factor from fate-determining mesenchymal cell lines.
Results
Clonal Cell Lines Established from Perinatal Oviducts.
Our recent study has revealed that undetermined epithelial cells coexist with cilial and secretory epithelial cells in perinatal oviducts (31), suggesting that epithelial fate-determining mechanisms become activated around postnatal days 3–5 (P3–P5). Accordingly, clonal epithelial cell lines E1 (Fig. S1 A–C) and B1 (Fig. S1 D–F) were established from oviducts at embryonic day 18 (E18) as a source of undetermined epithelial cells. Mesenchymal cell lines S1 (Fig. S1 G–I) and S10B (Fig. S1 J–L) were established from oviducts at postnatal day 3 (P3) as a source of fate-determining mesenchymal cells.
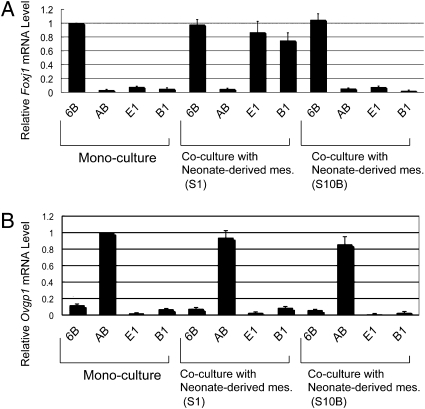
Both E1 and B1 epithelial cells were stained positive with anti-cytokeratin 18 monoclonal antibody. Cytokeratin formed fiber networks around the nucleus (Fig. S1 B and E). Both S1 and S10B mesenchymal cells were positive for anti-vimentin monoclonal antibody. Vimentin formed a fiber network in the cytoplasm (Fig. S1 I and L). As expected, E1 and B1 epithelial cells did not express Foxj1 or Ovgp1 (Fig. 1 A and B). Ovgp1 is one of the secretory proteins of the mouse oviduct (32, 33), and Foxj1 is a transcription factor specifically involved in ciliogenesis (34–36). 6B, a cilial epithelial cell line, and AB, a secretory epithelial cell line, which were established from adult oviducts in our previous study (20), expressed Foxj1 and Ovgp1, respectively (Fig. 1 A and B).
Fig. 1.
Marker expressions of epithelial cell lines cocultured with mesenchymal cell lines. Epithelial cell lines and mesenchymal cell lines were cocultured for 24 h on a culture insert and on a dish, respectively. (A) Foxj1 (ciliogenesis marker) expression of epithelial cell lines was analyzed by real-time RT–PCR. In monoculture, cilial epithelial cells (6B, positive control) had Foxj1 expression, but secretory epithelial cells (AB, negative control) and E1 and B1 epithelial cells had no Foxj1 expression. In coculture with S1 mesenchymal cells, E1 cells and B1 cells expressed Foxj1 to the same level as the positive control did. (B) Ovgp1 expression of epithelial cell lines analyzed by real-time RT–PCR. Secretory epithelial cells (AB) had Ovgp1 expression in monoculture (positive control). Error bars show the SD (n = 3).
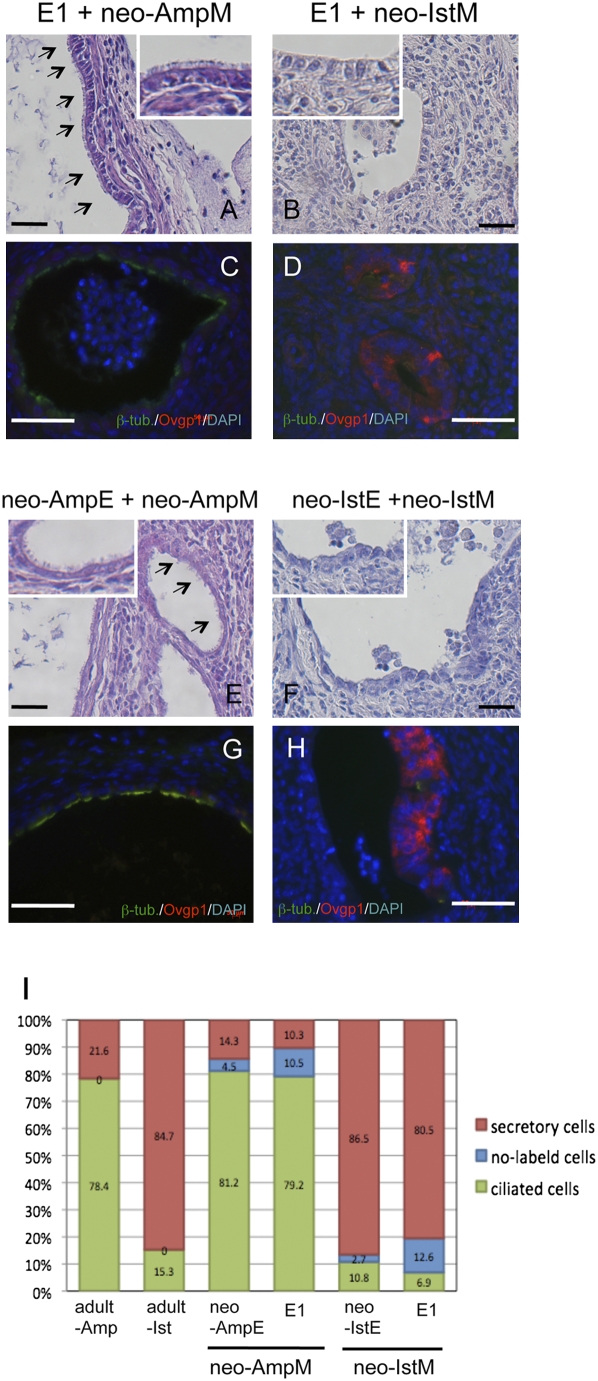
If both E1 and B1 epithelial cells are undetermined epithelial cells, their fates must be determined by factors provided by the oviduct mesenchyme around P3–P5. To examine this question, we recently established an experimental method in which undetermined oviductal epithelial cells can differentiate into ciliated cells and secretory cells when recombined with mesenchyme and grown under the kidney capsule (31). This method was adopted to evaluate whether E1 and B1 epithelial cells are undetermined. E1 epithelial cells were recombined with ampullar mesenchymal tissues of P3 oviducts (neo-AmpM) and grown under the kidney capsule for 4 weeks. Miniature oviducts developed from the recombinants, and the epithelium contained abundant ciliated cells (Fig. 2A). Immunohistochemical analyses demonstrated the presence of β-tubulin IV (an essential component of cilia)-positive epithelial cells and Ovgp1-positive epithelial cells at 79.2 ± 7.7% and at 10.3 ± 2.6%, respectively (n = 8) (Fig. 2 C and I). The ratio of the two types of epithelial cells was similar to those of the recombinants of neo-AmpE and neo-AmpM and the adult ampullar region (Fig. 2 E, G, and I).
Fig. 2.
Histology and immunohistochemistry of recombinant tissues. Neo-AmpM (mesenchyme of ampulla) and neo-IstM (mesenchyme of isthmus) of P3 oviducts were recombined with E1 epithelial cells and grown under the kidney capsule for 4 weeks. As control, neo-AmpE and neo-IstE (epithelium of P3 oviducts) were recombined with neo-AmpM and neo-IstM, respectively. (Scale bars: 100 μm.) (A) E1 epithelial cells combined with neo-AmpM developed epithelia with numerous cilia (arrows). (B) E1 epithelial cells combined with neo-IstM developed epithelia of simple columnar cells without cilia. (C) Double immunostaining for β-tubulin IV (green) and Ovgp1 (red). β-Tubulin IV-positive cells occupied the epithelium. (D) Double immunostaining for β-tubulin IV and Ovgp1 of reconstructed oviducts with E1 epithelial cells and neo-IstM. Ovgp1-positive cells occupied the epithelium. (E) Neo-AmpE was recombined with neo-AmpM. Numerous cilia were observed at the surface of the epithelium (arrows). (F) Neo-IstE was recombined with neo-IstM. The epithelium was occupied mainly by simple columnar cells without cilia. Insets in A, B, E, and F are partial enlargements (×4). (G) Double immunostaining for β-tubulin IV and Ovgp1 of reconstructed oviducts with neo-AmpE and neo-AmpM. β-Tubulin IV-positive cells occupied the epithelium. (H) Double immunostaining for β-tubulin IV and Ovgp1 of reconstructed oviducts with neo-IstE and neo-IstM. Ovgp1-positive cells occupied the epithelium. (I) Percentage of β-tubulin IV-positive cells and Ovgp1-positive cells in epithelia of adult oviducts and reconstructed oviducts. To determine the ratio of ciliated epithelial cells (β-tubulin IV-positive) and secretory epithelial cells (Ovgp1-positive), the numbers of β-tubulin IV-positive epithelial cells, Ovgp1-positive epithelial cells, and double-negative epithelial cells were counted on the screen in three frames for each specimen (>400 total epithelial cells). Results were based on an analysis of 8–24 tissue recombinants per group. In ampulla of adult oviducts, epithelia had β-tubulin IV-positive cells (78.4 ± 6.2%) and Ovgp1-positive cells (21.6 ± 3.1%) (n = 3). In the isthmus of adult oviducts, epithelia had β-tubulin IV-positive cells (15.3 ± 1.4%) and Ovgp1-positive cells (84.7 ± 5.8%) (n = 3). In recombinants of neo-AmpE and neo-AmpM, epithelia had β-tubulin IV-positive cells (81.2 ± 7.3%) and Ovgp1-positive cells (14.3 ± 2.6%). Double-negative cells were 4.5 ± 1.3% (n = 8). In recombinants of E1 cells and neo-AmpM, epithelia had β-tubulin IV-positive cells (79.2 ± 7.7%) and Ovgp1-positive cells (10.3 ± 2.6%). Double-negative cells were 10.5 ± 1.6% (n = 12). In recombinants of neo-IstE and neo-IstM, epithelia had β-tubulin IV-positive cells (10.8 ± 1.2%) and Ovgp1-positive cells (86.5 ± 8.6%). Double-negative cells were 2.7 ± 0.3% (n = 8). In recombinants of E1 epithelial cells and neo-IstM, epithelia had β-tubulin IV-positive cells (6.9 ± 1.1%) and Ovgp1-positive cells (80.5 ± 5.5%). Double-negative cells were 12.6 ± 2.7% (n = 12).
In contrast, miniature oviducts developed from recombinants of E1 epithelial cells and the isthmus mesenchymal tissues of P3 oviducts (neo-IstM) contained epithelia occupied mainly by simple columnar cells (Fig. 2B). Immunohistochemical analyses demonstrated Ovgp1-positive epithelial cells and β-tubulin IV-positive epithelial cells at 80.5 ± 5.5% and at 6.9 ± 1.1%, respectively (n = 12) (Fig. 2 D and I). The ratio of the two types of epithelial cells was similar to those of the recombinants of neo-IstE and neo-IstM and the adult isthmus region (Fig. 2 F, H, and I). The oviductal epithelium is surrounded by muscular layers. Immunohistochemistry confirmed α-actin-positive muscle layers (Fig. S2A) surrounding the epithelia in reconstructed miniature oviducts. To examine further whether E1 epithelial cells are undetermined, E1 epithelial cells were recombined with mesenchymal tissues of P3 vagina (neo-VaM) and grown under the kidney capsule. E1 epithelial cells formed stratified squamous epithelia (Fig. S2B) as shown in the tissue recombinant experiment; uterine epithelia at P3 (undetermined) become stratified squamous epithelia when recombined with mesenchymal tissues of P3 vagina and grown under kidney capsule (16). Thus, the results demonstrated that (1) the tissue–tissue recombinant method was successfully adapted to the tissue–cells recombinant experiment, (2) E1 cells were undetermined as perinatal epithelial cells, and (3) the fate of E1 epithelial cells was determined by mesenchyme.
Fate Determination of Epithelial Cells by Mesenchymal Cell Lines in Vitro.
The in vivo tissue recombinant experiments demonstrated that the fate of oviductal epithelial cells is determined by mesenchymal factors during a neonatal period. To define the factors, we attempted to demonstrate epithelial fate determination in vitro. Epithelial cells were seeded on a Millicell cell culture insert (Millipore), and mesenchymal cells were layered on a plastic surface. Physical contact was not allowed for epithelial and mesenchymal cells in this coculture system. When undetermined epithelial cells (E1 and B1) were cocultured with mesenchymal cells (S1 or S10B) established from a neonatal oviduct, Foxj1 (ciliogenesis marker), expression became clearly detectable in E1 and B1 epithelial cells cocultured with S1 mesenchymal cells. In contrast, coculture with S10B mesenchymal cells did not induce Foxj1 expression in either E1 or B1 epithelial cells (Fig. 1A). None of mesenchymal cell lines induced Ovgp1 expression in either E1 and B1 epithelial cells (Fig. 1B). A recent study demonstrated that neural stem cells transdifferentiated into mammary epithelial cells when they were transplanted into adult mammary mesenchyme (37). Therefore, the questions of whether the epithelial fate determination is reversible or irreversible and whether once-determined cilial cells can transdifferentiate into secretory cells were addressed. If epithelial fate determination elicited by mesenchymal cells has proceeded, then the epithelial cells must express Foxj1 (ciliogenesis marker) without further determining factors. Therefore, E1 epithelial cells exposed to S1 mesenchymal cells in coculture were singly cultured as a subline d-E1 (determined E1) for 5 days to deprive them of the determining factors. As shown in Fig. S3A, d-E1 cells continuously expressed Foxj1 at almost the same level as seen in the parental line. In previous studies (20, 21), we established an adult cilial epithelial cell line (6B) and a mesenchymal cell line (S3B) from an adult oviduct. S3B mesenchymal cells are capable of enhancing Foxj1 (ciliogenesis marker) expression on adult 6B epithelial cells in the coculture system described above. S3B mesenchymal cells significantly and specifically increased Foxj1 expression in d-E1 epithelial cells in coculture (Fig. S3B). Furthermore, coculture with S3B mesenchymal cells had no detectable induction of Foxj1 expression in E1 epithelial cells (Fig. S3B).
Epithelial Fate Determination Is Irreversible.
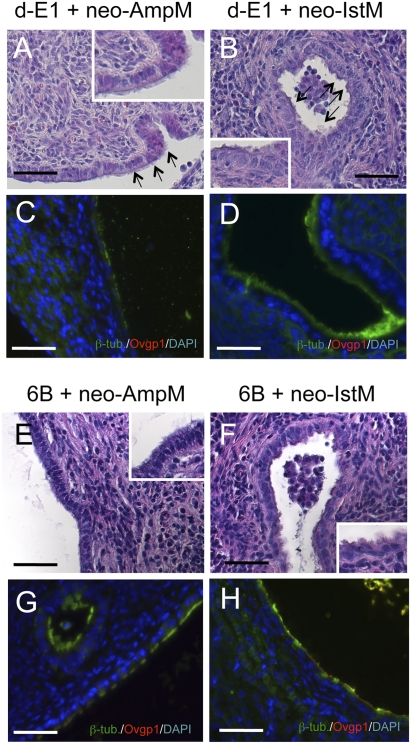
We addressed whether epithelial fate determination is reversible or irreversible and whether, once determined, cilial cells can transdifferentiate into secretory cells. To examine this question, recombinants of d-E1 epithelial cells and neo-AmpM were prepared and grown under the kidney capsule. Developed miniature oviducts had surface covered mainly with (>90%) ciliated cells (Fig. 3A). Likewise, miniature oviducts developed from recombinants of d-E1 epithelial cells, and neo-IstM had epithelia occupied by mainly (>90%) ciliated cells (Fig. 3B). In both types of recombinants, nonciliated cells were negative for both anti-Ovgp1 and β-tubulin IV antibodies (Fig. 3 C and D). Thus, the determination process is irreversible, and an additional exposure to determining mesenchyme will not induce transdifferentiation into secretory cells. To confirm this conclusion, epithelial cells of a cilial type line (6B) derived from adult oviducts were recombined with neo-AmpM and neo-IstM, and the recombinants were grown under the kidney capsule. Miniature oviducts developed from both recombinants had surface covered with mainly (>90%) ciliated cells (Fig. 3 E and F). Nonciliated cells were negative for both anti-Ovgp1 and β-tubulin IV antibodies (Fig. 3 G and H).
Fig. 3.
Fate determination is irreversible. Recombined tissues were implanted under the kidney capsule for 4 weeks. d-E1 epithelial cells were combined with neo-AmpM (P3 mesenchyme of ampulla) and neo-IstM (P3 mesenchyme of isthmus). As control, 6B cells (an adult epithelial line) were combined with neo-AmpM and neo-IstM. (Scale bars: 100 μm.) (A) d-E1 epithelial cells combined with neo-AmpM developed epithelia with numerous cilia at the surface (arrows). (B) E1 epithelial cells combined with neo-IstM also developed epithelia with numerous cilia at the surface (arrows). (C) Double immunostaining for β-tubulin IV (green) and Ovgp1 (red) of reconstructed oviducts with d-E1 epithelial cells and neo-AmpM. β-Tubulin IV-positive cells occupied the epithelium, and no Ovgp1-positive cells were observed. (D) Double immunostaining for β-tubulin IV and Ovgp1 of reconstructed oviducts with d-E1 epithelial cells and neo-IstM. β-Tubulin IV-positive cells occupied the epithelium and no Ovgp1-positive cells were observed. (E) 6B epithelial cells combined with neo-AmpM developed epithelia with numerous cilia at the surface. (F) 6B cells (an adult epithelial line) combined with neo-IstM developed epithelia with numerous cilia at the surface as observed when combined with neo-AmpM. Insets in A, B, E, and F are partial enlargements (×4). (G) Double immunostaining for β-tubulin IV and Ovgp1 of reconstructed oviducts with 6B epithelial cells and neo-AmpM. β-Tubulin IV-positive cells occupied the epithelium. (H) Double immunostaining for β-tubulin IV and Ovgp1 of reconstructed oviducts with 6B epithelial cells and neo-IstM. β-Tubulin IV-positive cells occupied the epithelium as observed when combined with neo-AmpM.
Identification of Mesenchyme-Derived Determining Factors.
The preceding experiments have demonstrated that undetermined epithelial cells were determined to cilial cells by S1 mesenchymal cells in coculture in which epithelial and mesenchymal cells were physically separated. The result strongly suggested that the determining factors are diffusible. To identify the diffusible factors, the SST method was employed using pooled poly(A) RNA from S1 mesenchymal cells (determined) and S10B mesenchymal cells (nondetermined). We screened and sequenced 123 clones from SST–REX of S1 mesenchymal cells. In addition, 96 clones were obtained from SST–REX of S10B mesenchymal cells. After clones with transmembrane domain were excluded, they were subtracted and selected as clones expressed only in S1 mesenchymal cells. Finally, seven genes were selected as candidates of mesenchymal diffusible factors derived from S1 mesenchymal cells (Table S1).
Further Screening of Mesenchymal Factors Identified by SST Method.
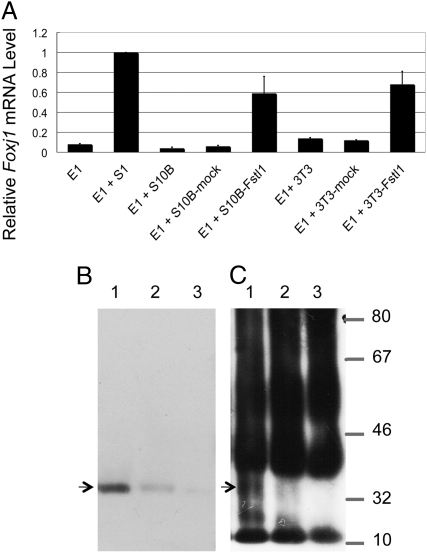
To evaluate seven candidate factors, NIH/3T3 cells and S10B mesenchymal cells were transfected with the recombinant plasmid pcDNA3.1 using a commercial system, and we established cell lines stably expressing individual candidate factors. All transfected cell lines were cultured with E1 epithelial cells in the coculture system described above. Only mesenchymal cell lines transfected with Fstl1, S10B-Fstl1, and 3T3-Fstl1 induced Foxj1 (ciliogenesis marker) expression in E1 epithelial cells (Fig. 4A). The inductive activity of Fstl1-overexpressing lines was weaker than the activity of the parental S1 mesenchymal line (as shown in Fig. 1A). To eliminate the possible long-lasting effect of Fstl1 and to confirm fate determination, E1 epithelial cells were monocultured for 5 days after coculture with Fstl1-overexpressing mesenchymal cells for 24 h. E1 epithelial cells continuously expressed Foxj1 expression without the mesenchymal cells (Fig. S4A). Longer exposure of E1 epithelial cells to Fstl1-overexpressing mesenchymal cells in coculture did not increase Foxj1 expression; rather, it decreased its expression (Fig. S4B).The results indicated that Fstl1 was secreted by transfected mesenchymal cells and determined E1 epithelial cells to cilial cells. If this is the case, culture media conditioned by Fstl1-transfected cell lines must contain Fstl1. The estimated molecular size of Fstl1 was 34 kDa. Immunoprecipitaion and Western blot analysis detected Fstl1 protein at 34 kDa in cellular extracts of 3T3-Fstl1 and S1 mesenchynmal cells (Fig. 4B, lanes 1 and 2) and in the conditioned media from 3T3-Fstl1 and S1 mesenchymal cells (Fig. 4C, lanes 1 and 2), whereas no bands were detected in the extract of S10B mesenchymal cells or in the conditioned medium from S10B mesenchymal cells (Fig. 4 B and C, lane 3).
Fig. 4.
Coculture of undetermined epithelial cells and Fstl1-overexpressing mesenchymal cells. (A) E1 epithelial cells were cocultured with Fstl1 overexpressing cells for 24 h and subjected to real-time RT–PCR analysis of Foxj1 (ciliogenesis marker) expression. Error bars show the standard deviation (n = 3). (B) Western blot analysis of Fstl1 in lysates of 3T3-Fstl1 cells (lane 1), S1 cells (lane 2), and S10B cells (lane 3). Fstl1 protein was detected at 34 kDa in lysates of 3T3-Fstl1 cells and S1 mesenchymal cells (lanes 1 and 2), but not in the sample of S10B mesenchymal cells (lane 3). (C) Immunoprecipitation analysis detected Fstl1 protein at 34 kDa in media conditioned by 3T3-Fstl1 and S1 cells (lanes 1 and 2), but not in medium conditioned by S10B cells (lane 3).
Localization of Fstl1 in Oviducts.
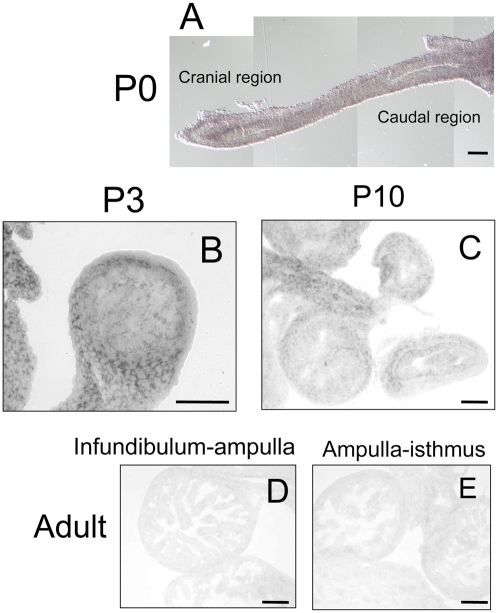
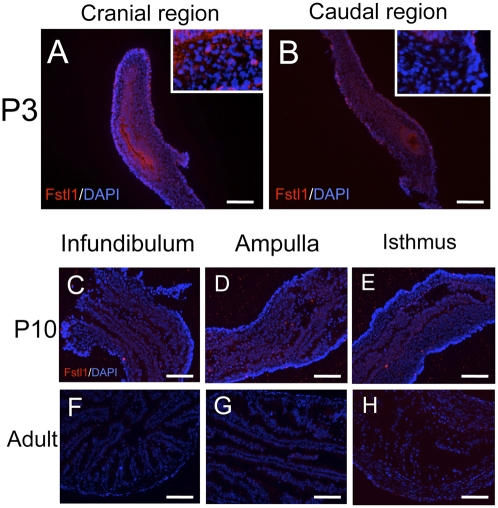
Our recent study (31) and the present study showed that the fate of oviductal epithelial cells is determined around P3–P5. Therefore, Fstl1 must be expressed during this period. In situ hybridization analyses demonstrated that Fstl1 mRNA was strongly and specifically expressed in the mesenchymal compartment of neonatal oviducts (Fig. 5 A and B). The signal was especially strong in the cranial region of the P0 oviduct (Fig. 5A). In P10 oviducts, the signal of Fstl1 became weaker when compared with the signal in P3 oviducts (Fig. 5 B and C), and Fstl1 expression was undetectable in adult oviducts (Fig. 5 D and E). Fstl1 expression was also analyzed with anti-Fstl1 polyclonal antibody. At P3, a stronger immunoreaction for Fstl1 in ampulla than in isthmus was observed, and the reaction was detected mainly in mesenchymal cells (Fig. 6 A and B) as was observed for expression of Fstl1 mRNA. The immunoreaction was also observed in the basement membrane in the ampullar region (Fig. 6A). No immunoreactions were detected in either P10 or adult oviducts (Fig. 6 C– H). These results indicate that Fstl1 is expressed and secreted from the mesenchymal cells and acts on the neighboring epithelial cells during the critical period.
Fig. 5.
Localization of FSTL1 mRNA in oviducts. In situ hybridization was performed to localize the expression of Fstl1 in oviducts. (Scale bars: 100 μm.) (A) Signals of Fstl were detected in the cranial part of P0 oviducts. (B) Signals of Fstl were strongly and specifically detected in the mesenchymal compartment of P3 oviducts. (C) Signals of Fstl became weaker in P4 oviducts. (D and E) Signals of Fstl were undetected in any region of adult oviducts.
Fig. 6.
Localization of FSTL1 in oviducts. (A and B) Stronger immunoreaction for Fstl1 (red) was observed in the cranial region than in the caudal region, and the reaction was detected mainly in mesenchymal cells of P3 oviducts. The sections were counterstained with DAPI (blue). Insets in A and B are partial enlargements (5×). (C–E) Immunoreaction was not detected in the infundibulum, ampulla, and isthmus of P10 oviducts. (F–H) Immunoreaction was not detected in the infundibulum, ampulla, and isthmus of adult oviducts.
Discussion
The present study has demonstrated that (1) the oviductal epithelium has two distinct cell populations: secretory and ciliated types of cells; (2) during the critical neonatal period, mesenchymal factors irreversibly determine undetermined epithelial cells to either of the two lineages; and (3) Fstl1 is one of the determining factors secreted by mesenchymal cells during the critical period that determines undetermined epithelial cells to cilial epithelial lineage. Recent technical advances have begun to unveil the developmental phenomenon of secondary (reciprocal) inductions, although we are still at the early stage of understanding the mechanisms in organogenesis. The major difficulties in analyzing the mechanisms of organogenesis are from the nature of the developmental events that occur three-dimensionally during a brief period in tissues of small scale. To circumvent these difficulties, we have established oviductal epithelial clonal cell lines from p53−/− mice (38) in the present and previous studies (20). These epithelial cell lines allowed us to develop model systems in which we can analyze the oviductal development in vitro and in vivo. Both secretory and ciliated cells coexist in the epithelium of the cranial oviductal region, whereas the majority are secretory cells in the caudal oviductal region. The present study dose not support an earlier idea that cilial and secretory epithelial cells are transitory throughout the estrous cycle (39–41). To explain lineage determination and the cellular distribution pattern, there must be at least two signals released from the neonatal mesenchyme. As shown in the present study, one of them is Fstl1, which induces undifferentiated epithelial cells into the cilial lineage. Presumably, there is another factor that induces the secretory lineage. Both types of epithelial cells appear at an early stage in the cranial oviductal region, suggesting that two different signals are released from the mesenchyme during P3–P5. Approximately 10% of the epithelial cells were negative for both secretory and cilial epithelial cell markers in miniature oviducts developed from recombinants of mesenchymal tissues and epithelial cells of lines. Such negative cells were also detected at a lower incidence in control miniature oviducts. Experimental procedures may perturb the determining process. For example, enzymatic treatments will severely destroy surface structures of epithelial plasma membrane, including possible receptors for determining factors.
E1 and B1 epithelial cells established from oviducts on E18 were selected at random, and they were shown to be naive and able to respond to mesenchymal signals. In cloning mesenchymal cell lines from P3 oviducts, we expected to establish multiple cell lines secreting different determining signals. Two mesenchymal cell lines were selected at random. The results showed that S1 mesenchymal cells were Fstl1-secreting and that S10B mesenchymal cells did not have determining activity. This suggests that other signal-secreting cell lines may be established from the mesenchyme of the caudal region of an oviduct on P3. Oviductal mesenchyme must have many functionally distinct groups of cells. During the critical period, the mesenchyme contains determining signal-secreting cells such as S1 cells and nondetermining cells such as S10B cells. In adult mesenchyme, such determining cells disappear (Fstl1-secreting cells were undetected). Instead, the mesenchyme has enhancer cells that enhance the expression of Ovgp1 and Foxj1 in secretory epithelial cells and in cilial epithelial cells, respectively (20). Dual origin of mesenchymal tissues is reported in mouse mammary gland embryogenesis (42). At present, there is no information available on the transition of cell populations in the oviductal mesenchyme.
Fstl1 was originally isolated from mouse osteogenic MC3T3E1 cells (43). Human follistatin-related protein (FRP) (44), chicken Flik (45), and Xenopus FRP (46) were identified as the homologs of the Follistatin-related gene. Also known as TSC-36, Fstl1 is an extracellular glycoprotein belonging to the BM-40/SPARC/osteonectin family of proteins containing both extracellular calcium-binding and Follistatin-like domains (47). Expression of Fstl1 has been reported in the developing kidney and lung (48) and the adult heart (49). In the present study, the expression of Foxj1 (ciliogenesis marker) in E1 epithelial cells was not induced by BMP antagonists (Follistatin, Noggin, and Chordin) (Fig. S5), suggesting that BMP signaling may not mediate cell fate determination of Fstl1 in oviduct. Fstl1-overexpressing cell lines showed a weaker potency than S1 mesenchymal cells in epithelial fate-determining activity or Foxj1 inductive activity (Fig. 4A). A much higher content of Fstl1 in the conditioned medium and cellular extract of 3T3-Fstl1 cells than in S1 mesenchymal cells was confirmed (Fig. 4C). Longer exposure to Fstl1-secreting cells in coculture was thought to increase in Foxj1 (ciliogenesis marker) expression or in the number of fate-determined cells. However, this was not observed in Foxj1 expression (Fig. S4B). These observations suggest that the molecular mechanism in Fstl1 action is complex and that multiple factors might be involved in epithelial cell fate determination in the oviduct.
Experimental Procedures
Animals.
CD-1 mice (Charles River Japan) and p53−/− mice (hybrid between C57BL/6 and CBA) (38) were maintained as previously described (20). Mice care and handling conformed to the National Institutes of Health guidelines for animal research. The experimental protocols were approved by the Institutional Animal Care and Use Committee.
Recombinant Tissue Preparation and Grafting Under Kidney Capsule.
The method for oviductal tissue recombination was developed in our recent study (31). See SI Experimental Procedures for details.
Coculture of Epithelial and Mesenchymal Cells.
The method for coculture of epithelial and mesenchymal cells was developed in our recent study (21). See SI Experimental Procedures for details.
Real-time RT–PCR.
See SI Experimental Procedures for details.
Construction of cDNA Library for SST–REX.
To identify cDNAs encoding secretory proteins from mesenchymal cell lines, a SST library of mesenchymal cell lines was constructed and screened according to the method described in ref. 30. See SI Experimental Procedures for more details.
Overexpression of Mesenchymal Factors in NIH/3T3 Cells.
See SI Experimental Procedures for details.
Immunohistochemistry, Immunoblotting, and Immunoprecipitation.
Cell and tissue extracts were prepared as previously described (24). See SI Experimental Procedures for more details.
Anti-Fstl1 Antibody Production.
See SI Experimental Procedures for details.
In situ Hybridization.
See SI Experimental Procedures for details.
Supplementary Material
Acknowledgments
We thank Dr. Shinichi Aizawa (REKEN Center for Developmental Biology) for p53-deficient mice and Dr. Toshio Kitamura (Tokyo University) for pMXs retroviral constructs and Plat-E cells. Dr. Teruyo Sakakura (Mie University) kindly read the manuscript and made critical comments. This work was supported by the “Academic Frontier” project for private universities to Y.T. (2003–2007) and by KAKENHI (Grant 19570062) to Y.T. and T.U.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909501107/DCSupplemental.
References
- 1.Spemann H. Embryonic development and induction. New Haven, CT: Yale University Press; 1938. [Google Scholar]
- 2.De Robertis EM. Spemann's organizer and self-regulation in amphibian embryos. Nat Rev Mol Cell Biol. 2006;7:296–302. doi: 10.1038/nrm1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz i Altaba A. Induction and axial patterning of the neural plate: Planar and vertical signals. J Neurobiol. 1993;24:1276–1304. doi: 10.1002/neu.480241004. [DOI] [PubMed] [Google Scholar]
- 4.Kuroda H, Wessely O, De Robertis EM. Neural induction in Xenopus: Requirement for ectodermal and endomesodermal signals via Chordin, Noggin, Catenin, and Cerberus. PLoS Biol. 2004;2 doi: 10.1371/journal.pbio.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grobstein C. Trans-filter induction of tubules in mouse metanephrogenic mesenchyme. Exp Cell Res. 1956;10:424–440. doi: 10.1016/0014-4827(56)90016-7. [DOI] [PubMed] [Google Scholar]
- 6.Golosow N, Grobstein C. Epitheliomesenchymal interaction in pancreatic morphogenesis. Dev Biol. 1962;4:242–255. doi: 10.1016/0012-1606(62)90042-8. [DOI] [PubMed] [Google Scholar]
- 7.Wessels NK, Cohen JH. Early pancreas organogenesis: Morphogenesis, tissue interactions, and mass effects. Dev Biol. 1967;15:237–270. doi: 10.1016/0012-1606(67)90042-5. [DOI] [PubMed] [Google Scholar]
- 8.Kollar EJ, Baird GR. The influence of the dental papilla on the development of tooth shape in embryonic mouse tooth germs. J Embryol Exp Morphol. 1969;21:131–148. [PubMed] [Google Scholar]
- 9.Kollar EJ, Fisher C. Tooth induction in chick epithelium: Expression of quiescent genes for enamel synthesis. Science. 1980;207:993–995. doi: 10.1126/science.7352302. [DOI] [PubMed] [Google Scholar]
- 10.Sakakura T, Nishizuka Y, Dawe CJ. Mesenchyme-dependent morphogenesis and epithelium-specific cytodifferentiation in mouse mammary gland. Science. 1976;194:1439–1441. doi: 10.1126/science.827022. [DOI] [PubMed] [Google Scholar]
- 11.Wessells NK. Mammalian lung development: Interactions in formation and morphogenesis of tracheal buds. J Exp Zool. 1970;175:455–466. doi: 10.1002/jez.1401750405. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi K, Yasugi S, Mizuno T. Pepsinogen gene transcription induced in heterologous epithelial-mesenchymal recombinations of chicken endoderms and glandular stomach mesenchyme. Development. 1988;103:725–731. doi: 10.1242/dev.103.4.725. [DOI] [PubMed] [Google Scholar]
- 13.Donjacour AA, Cunha GR. Stromal regulation of epithelial function. Cancer Treat Res. 1991;53:335–364. doi: 10.1007/978-1-4615-3940-7_16. [DOI] [PubMed] [Google Scholar]
- 14.Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation. 2002;70:473–485. doi: 10.1046/j.1432-0436.2002.700902.x. [DOI] [PubMed] [Google Scholar]
- 15.Cunha GR. Stromal induction and specification of morphogenesis and cytodifferentiation of the epithelia of the Mullerian ducts and urogenital sinus during development of the uterus and vagina in mice. J Exp Zool. 1976;196:361–370. doi: 10.1002/jez.1401960310. [DOI] [PubMed] [Google Scholar]
- 16.Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (Müllerian) epithelial differentiation. Dev Biol. 2001;240:194–211. doi: 10.1006/dbio.2001.0458. [DOI] [PubMed] [Google Scholar]
- 17.Filosa S, Pictet RL, Rutter WJ. Positive control of cyclic AMP on mesenchymal factor controlled DNA synthesis in embryonic pancreas. Nature. 1975;257:702–705. doi: 10.1038/257702a0. [DOI] [PubMed] [Google Scholar]
- 18.Thesleff I, Lehtonen E, Wartiovaara J, Saxén L. Interference of tooth differentiation with interposed filters. Dev Biol. 1977;58:197–203. doi: 10.1016/0012-1606(77)90085-9. [DOI] [PubMed] [Google Scholar]
- 19.Takiguchi-Hayashi K, Yasugi S. Transfilter analysis of the inductive influence of proventricular mesenchyme on stomach epithelial differentiation of chick embryo. Dev Genes Evol. 1990;198:460–466. doi: 10.1007/BF00399056. [DOI] [PubMed] [Google Scholar]
- 20.Umezu T, Hanazono M, Aizawa S, Tomooka Y. Characterization of newly established clonal oviductal cell lines and differential hormonal regulation of gene expression. In Vitro Cell Dev Biol Anim. 2003;39:146–156. doi: 10.1007/s11626-003-0009-9. [DOI] [PubMed] [Google Scholar]
- 21.Umezu T, Tomooka Y. An evidence of stromal cell populations functionally linked with epithelial cell populations in the mouse oviduct. Zoolog Sci. 2004;21:319–326. doi: 10.2108/zsj.21.319. [DOI] [PubMed] [Google Scholar]
- 22.Hanazono M, et al. Establishment of uterine cell lines from p53-deficient mice. In Vitro Cell Dev Biol-Animal. 1997;33:668–671. doi: 10.1007/s11626-997-0121-3. [DOI] [PubMed] [Google Scholar]
- 23.Minakawa M, Sugimoto T, Aizawa S, Tomooka Y. Cerebellar cell lines established from a p53-deficient adult mouse. Brain Res. 1998;813:172–176. doi: 10.1016/s0006-8993(98)00979-2. [DOI] [PubMed] [Google Scholar]
- 24.Hanazono M, Nozawa R, Itakura R, Aizawa S, Tomooka Y. Establishment of an androgen-responsive prostatic cell line “PEA5” from a p53-deficient mouse. Prostate. 2001;46:214–225. doi: 10.1002/1097-0045(20010215)46:3<214::aid-pros1026>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Tanahashi K, et al. Establishment and characterization of clonal cell lines from the vagina of p53-deficient young mice. In Vitro Cell Dev Biol Anim. 2002;38:547–556. doi: 10.1290/1543-706x(2002)38<547:eacocc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Horiuchi M, Tomooka Y. An attempt to generate neurons from an astrocyte progenitor cell line FBD-104. Neurosci Res. 2005;53:104–115. doi: 10.1016/j.neures.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Horiuchi H, Itoh M, Pleasure DE, Tomooka Y. Multipotency of FBD-103a, a neural progenitor cell line from the p53-deficient mouse. Brain Res. 2005;1066:24–36. doi: 10.1016/j.brainres.2005.09.061. [DOI] [PubMed] [Google Scholar]
- 28.Komine A, Suenaga M, Nakao K, Tsuji T, Tomooka Y. Tooth regeneration from newly established cell lines from a molar tooth germ epithelium. Biochem Biophys Res Commun. 2007;355:758–763. doi: 10.1016/j.bbrc.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 29.Kanatsu-Shinohara M, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Kojima T, Kitamura T. A signal sequence trap based on a constitutively active cytokine receptor. Nat Biotechnol. 1999;17:487–490. doi: 10.1038/8666. [DOI] [PubMed] [Google Scholar]
- 31.Yamanouchi H, Umezu T, Tomooka Y. Reconstruction of oviduct and demonstration of the epithelial fate determination. Biol Reprod. 2010 doi: 10.1095/biolreprod.109.078329. (Epub ahead of print Nov. 11) [DOI] [PubMed] [Google Scholar]
- 32.Sendai Y, et al. Molecular cloning and characterization of a mouse oviduct-specific glycoprotein. Biol Reprod. 1995;53:285–294. doi: 10.1095/biolreprod53.2.285. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi K, et al. Mouse oviduct-specific glycoprotein gene: Genomic organization and structure of the 5′-flanking regulatory region. Biol Reprod. 2000;62:217–226. doi: 10.1095/biolreprod62.2.217. [DOI] [PubMed] [Google Scholar]
- 34.Hackett BP, et al. Primary structure of hepatocyte nuclear factor/forkhead homologue 4 and characterization of gene expression in the developing respiratory and reproductive epithelium. Proc Natl Acad Sci USA. 1995;92:4249–4253. doi: 10.1073/pnas.92.10.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim L, Zhou H, Costa RH. The winged helix transcription factor HFH-4 is expressed during choroid plexus epithelial development in the mouse embryo. Proc Natl Acad Sci USA. 1997;94:3094–3099. doi: 10.1073/pnas.94.7.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelletier GJ, Brody SL, Liapis H, White RA, Hackett BP. A human forkhead/winged-helix transcription factor expressed in developing pulmonary and renal epithelium. Am J Physiol. 1998;274:L351–L359. doi: 10.1152/ajplung.1998.274.3.L351. [DOI] [PubMed] [Google Scholar]
- 37.Booth BW, et al. The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc Natl Acad Sci USA. 2008;105:14891–14896. doi: 10.1073/pnas.0803214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsukada T, et al. Enhanced proliferative potential in culture of cells from p53-deficient mice. Oncogene. 1993;8:3313–3322. [PubMed] [Google Scholar]
- 39.Abe H. Regional variations in the ultrastructural features of secretory cells in the rat oviductal epithelium. Anat Rec. 1994;240:77–85. doi: 10.1002/ar.1092400108. [DOI] [PubMed] [Google Scholar]
- 40.Shirley B, Reeder RL. Cyclic changes in the ampulla of the rat oviduct. J Exp Zool. 1996;276:164–173. doi: 10.1002/(SICI)1097-010X(19961001)276:2<164::AID-JEZ10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 41.Abe H, Onodera M, Sugawara S, Satoh T, Hoshi H. Ultrastructural features of goat oviductal secretory cells at follicular and luteal phases of the oestrous cycle. J Anat. 1999;195:515–521. doi: 10.1046/j.1469-7580.1999.19540515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakakura T, Sakagami Y, Nishizuka Y. Dual origin of mesenchymal tissues participating in mouse mammary gland embryogenesis. Dev Biol. 1982;91:202–207. doi: 10.1016/0012-1606(82)90024-0. [DOI] [PubMed] [Google Scholar]
- 43.Shibanuma M, Mashimo J, Mita A, Kuroki T, Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur J Biochem. 1993;217:13–19. doi: 10.1111/j.1432-1033.1993.tb18212.x. [DOI] [PubMed] [Google Scholar]
- 44.Zwijsen A, et al. Characterization of a rat C6 glioma-secreted follistatin-related protein (FRP). Cloning and sequence of the human homologue. Eur J Biochem. 1994;225:937–946. doi: 10.1111/j.1432-1033.1994.0937b.x. [DOI] [PubMed] [Google Scholar]
- 45.Patel K, Connolly DJ, Amthor H, Nose K, Cooke J. Cloning and early dorsal axial expression of Flik, a chick follistatin-related gene: Evidence for involvement in dorsalization/neural induction. Dev Biol. 1996;178:327–342. doi: 10.1006/dbio.1996.0222. [DOI] [PubMed] [Google Scholar]
- 46.Okabayashi K, et al. cDNA cloning and distribution of the Xenopus follistatin-related protein. Biochem Biophys Res Commun. 1999;254:42–48. doi: 10.1006/bbrc.1998.9892. [DOI] [PubMed] [Google Scholar]
- 47.Hambrock HO, et al. Structural characterization of TSC-36/Flik: Analysis of two charge isoforms. J Biol Chem. 2004;279:11727–11735. doi: 10.1074/jbc.M309318200. [DOI] [PubMed] [Google Scholar]
- 48.Adams D, Larman B, Oxburgh L. Developmental expression of mouse follistatin-like 1 (Fstl1): Dynamic regulation during organogenesis of the kidney and lung. Gene Expr Patterns. 2007;7:491–500. doi: 10.1016/j.modgep.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oshima Y, et al. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008;117:3099–3108. doi: 10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.