Abstract
The herbicide atrazine is one of the most commonly applied pesticides in the world. As a result, atrazine is the most commonly detected pesticide contaminant of ground, surface, and drinking water. Atrazine is also a potent endocrine disruptor that is active at low, ecologically relevant concentrations. Previous studies showed that atrazine adversely affects amphibian larval development. The present study demonstrates the reproductive consequences of atrazine exposure in adult amphibians. Atrazine-exposed males were both demasculinized (chemically castrated) and completely feminized as adults. Ten percent of the exposed genetic males developed into functional females that copulated with unexposed males and produced viable eggs. Atrazine-exposed males suffered from depressed testosterone, decreased breeding gland size, demasculinized/feminized laryngeal development, suppressed mating behavior, reduced spermatogenesis, and decreased fertility. These data are consistent with effects of atrazine observed in other vertebrate classes. The present findings exemplify the role that atrazine and other endocrine-disrupting pesticides likely play in global amphibian declines.
Keywords: amphibian decline, endocrine disruption, pesticide, sex reversal
Atrazine is one of the most widely used pesticides in the world. Approximately 80 million pounds are applied annually in the United States alone, and atrazine is the most common pesticide contaminant of ground and surface water (1). Atrazine can be transported more than 1,000 km from the point of application via rainfall and, as a result, contaminates otherwise pristine habitats, even in remote areas where it is not used (2, 3). In fact, more than a half million pounds of atrazine are precipitated in rainfall each year in the United States (2).
In addition to its persistence, mobility, and widespread contamination of water, atrazine is also a concern because several studies have shown that atrazine is a potent endocrine disruptor active in the ppb (parts per billion) range in fish (4, 5), amphibians (6–12), reptiles, and human cell lines (5, 13–15), and at higher doses (ppm) in reptiles (16–18), birds (19), and laboratory rodents (20–28). Atrazine seems to be most potent in amphibians, where it is active at levels as low as 0.1 ppb (6–10). Although a few studies suggest that atrazine has no effect on amphibians under certain laboratory conditions (29, 30), in other studies, atrazine reduces testicular volume; reduces germ cell and Sertoli cell numbers (11); induces hermaphroditism (6, 8, 10); reduces testosterone (10); and induces testicular oogenesis (7–9, 31). Furthermore, atrazine contamination is associated with demasculinization and feminization of amphibians in agricultural areas where atrazine is used (32) and directly correlated with atrazine contamination in the wild (7, 9, 33, 34).
Despite the wealth of data from larvae and newly metamorphosed amphibians, the ultimate impacts of atrazine’s developmental effects on reproductive function and fitness at sexual maturity, which relate more closely to population level effects and amphibian declines, have been unexplored. In the present study, we examined the long-term effects of atrazine exposure on reproductive development and function in an all-male population of African clawed frogs (Xenopus laevis), generated by crossing ZZ females (sex-reversed genetic males) to ZZ males (SI Materials and Methods). The advantage of using this population is that 100% of the animals tested were genetic males. As a result, all hermaphrodites and females observed are ensured to be genetic males that have been altered by endocrine disruption. We examined sex ratios, testosterone levels, sexual dimorphism, reproductive behaviors, and fertility in males exposed to 2.5 ppb atrazine throughout the larval period and for up to 3 years after metamorphosis.
Results
Feminization.
All of the control animals reared to sexual maturity (n = 40) were males, on the basis of external morphology, whereas only 90% of the atrazine-treated animals (36 of 40) appeared male at sexual maturity (on the basis of the presence of keratinized nuptial pads on the forearms and the absence of cloacal labia). The other 10% of atrazine-exposed animals (n = 4) lacked visible nuptial pads on the forearms and had protruding cloacal labia, typical of females (Fig. 1). Upon dissection of two of the apparent females and laparotomy in another two, we confirmed that animals with cloacal labia were indeed females from the present study, on the basis of the presence of ovaries (Fig. 1F). To date, two atrazine-induced females have been maintained, mated with control males (Fig. 1G), and produced viable eggs (Fig. 1H). The resulting larvae were all male when raised to metamorphosis and sampled (n = 100), confirming that atrazine-induced females were, in fact, chromosomal males. Furthermore, atrazine-induced females lacked the DM-W further confirming that these atrazine-induced females were indeed chromosomal males (Fig. 2). These ZZ females expressed gonadal aromatase, as did true ZW females (n = 4, from our stock colony), but ZZ males (n = 8, control or treated) did not (Fig. 2).
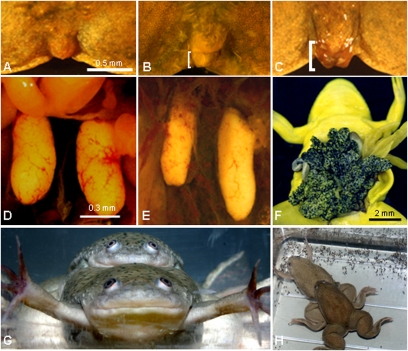
Fig. 1.
Atrazine feminized exposed males. Cloaca (A–C) and gonads (D–F) for control male (A and D), atrazine-exposed male (B and E), and atrazine-exposed female (C and F) ZZ animals (genetic males). (G) Atrazine-induced female (genetic male, ZZ) copulating with an unexposed male sibling. (H) Same pair as in G, producing eggs. Eggs (H) were viable and produced larvae that survived to metamorphosis and adulthood. Yellow coloration (F) is the result of fixation in Bouin’s solution. Brackets (B and C) indicate protruding cloacal labia. (Scale bar in A applies to A–C; in D applies to D and E.)
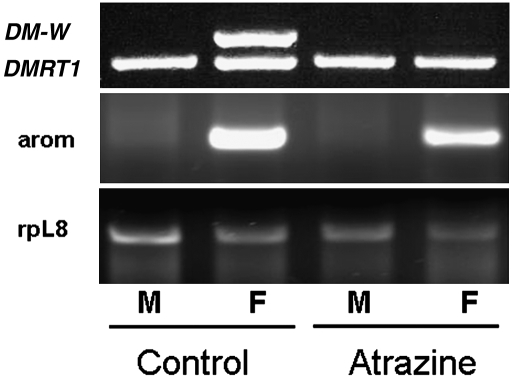
Fig. 2.
Atrazine-induced females expressed aromatase in their gonads. (Top) DMRT-1 and DM-W genes from a representative control and an atrazine-exposed adult male (M) and female (F). Morphologic sex was assigned on the basis of the presence of testes (males) or ovaries (females). (Middle and Bottom) Cyp-19 aromatase expression from gonads of the same animals genotyped at Top, along with the control gene, rpL8.
Demasculinization.
Morphologic evidence.
Atrazine-exposed males had reduced plasma testosterone levels, relative to control males (ANOVA: F = 6.647, df = 1, P < 0.025) when examined 2 years after metamorphosis. Consistent with diminished testosterone levels, atrazine-exposed males had a decrease in testosterone-dependent morphologies, as described below.
Nuptial pads and breeding glands.
The nuptial pads of control males were noticeably darker than in atrazine-exposed males (Fig. 3 A and B). Although color was not quantified, histologic analysis revealed that the size of the dermal breeding glands (determined by the cross-sectional area of the largest breeding gland) was reduced in atrazine-treated males (ANOVA: F = 11.589, df = 1, P < 0.005; Fig. 3 C–E). This effect was specific to the testosterone-dependent breeding glands (35), because the size of mucous glands and serous (poison) glands from the same histologic sections were not affected by atrazine (P > 0.05). Other features of the breeding gland that were examined were not significantly different between treatments (P > 0.05).
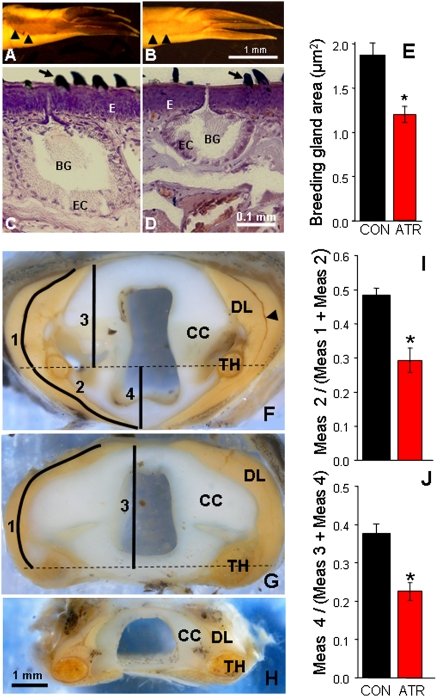
Fig. 3.
Atrazine-demasculinized male morphology as shown in the nuptial glands and the larynx. (A and B) Forearms, showing nuptial pads from control (A) and atrazine-exposed males (B). Note the reduced nuptial pads in the atrazine-exposed male (B). Black arrowheads in A and B show boundaries of nuptial pads. (C and D) Representative largest breeding gland (selected from the midpoint of the nuptial pad) from control (C) and atrazine-exposed (D) males. The area of the largest section of the largest gland was determined for each sample. Control males had significantly larger glands (E). (F–H) Transverse cross-sections through the dissected larynges of a representative sexually mature control male (F), atrazine-exposed male (G), and control female (H) X. laevis. Atrazine-exposed males had a laryngeal morphology intermediate between unexposed males and females. The dilater larynges (DL) extended well beyond the thiohyral (TH) in control males, but very little (or not at all, as in the example shown) in atrazine-exposed males. This measure was quantifiable and significantly different between controls and atrazine-exposed animals, regardless of whether the absolute length of the muscle was measured (I) or the straight-line distance (J). Black arrowhead in F indicates the slip of the dilator larynges. Horizontal dashed lines in F and G indicate the midpoint of the thiohyral. ATR, atrazine-exposed; BG, breeding gland; CC, cricoid cartilage; CON, control; E, epidermis; EC, epithelial cells. *P < 0.05; n = 14 for breeding glands, n = 11 for larynges. (Scale bar in B applies to A and B; in D applies to C and D; in H applies to F–H.)
Laryngeal morphology.
Atrazine exposure altered the structure but not the size (P > 0.05) of the larynx (Fig. 3 F–H). The portion of the dilator laryngis that extended ventral to the thiohyrals was greater in control males than in atrazine-treated males, regardless of whether distances were determined by straight-line measurements (ANOVA: F = 11.974, df = 1, P < 0.01; Fig. 3I) or by the actual length of the muscle tracing the division between the slip and the dilator laryngis proper (ANOVA: F = 11.217, df = 1, P < 0.01; Fig. 3J). In fact, the shape of the larynx in atrazine-exposed males resembled the morphology typical of normal (ZW) females maintained in our stock colony (Fig. 3H).
Testes.
Atrazine exposure resulted in a significant reduction in the relative number of testicular tubules with mature sperm bundles in 2007 (n = 18; ANOVA: F = 8.65, df = 1, P < 0.01); that is, atrazine decreased the frequency of tubules with mature spermatozoa (G test: GH = 13545.2, df = 15, P < 0.001). Similar effects were not observed (P > 0.05) in animals (n = 10) 1 year later at 3 years after metamorphosis, in 2008. Other features of the gonads that were examined were not significantly different (P > 0.05).
Behavioral evidence.
Mating choice studies.
In experiments in which control males and atrazine-treated males competed for females, control males out-competed atrazine males (achieved amplexus) in three out of four trials examined, and only two atrazine-treated males (in a single trial) obtained amplexus (G test: GT = 61.82, df = 4, P < 0.001; Fig. 4A). Male size was not different between treatments and had no effect on the ability of males to achieve amlpexus (P > 0.05; Fig. 4B). Control males had significantly higher testosterone levels in the presence of females, when compared with atrazine-treated males when analyzed by ANOVA (F = 14.65, df = 1, P < 0.001; Fig. 4C) or by Kruskal-Wallis test (χ2 = 9.304, df = 1, P < 0.002).
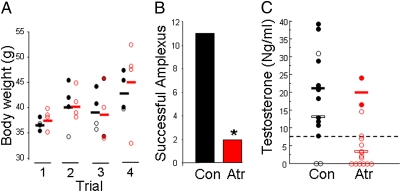
Fig. 4.
Control males out-competed atrazine-exposed males to copulate with females. Amplexus data from four mate choice trials for control (Con) and atrazine-treated (Atr) males (A). Eleven of 16 control males out-competed atrazine-exposed males for amplexus with females. Only two atrazine-exposed males in a single trial achieved amplexus. Male size did not affect breeding success (B). In all four trials, there was no difference (P > 0.05) in size between control (black symbols and bars) and atrazine-exposed males (red symbols and bars). Furthermore, in all trials smaller individuals from controls out-competed larger atrazine-exposed individuals. Filled circles show successful males, open circles show unsuccessful males, and horizontal bars show group means. (C) Testosterone levels for control and atrazine-treated males for all four trials. Filled symbols show successful (amplectant) males, and open symbols show unsuccessful males. Solid horizontal bars show mean testosterone levels for successful males, and open bars show the mean for unsuccessful males.
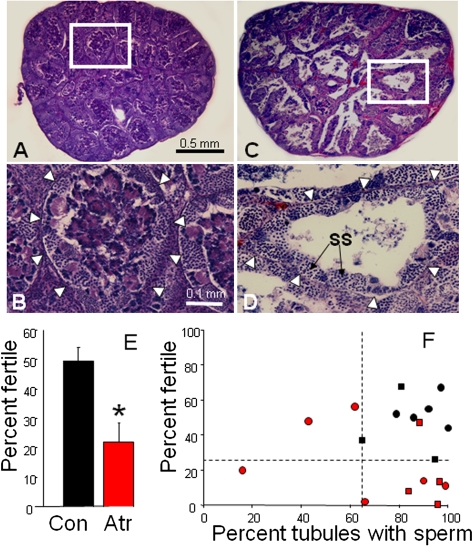
Fertility.
Representative testis for control and atrazine-treated males from 2007 are shown in Figs. 5 A–D. Atrazine-treated males had significantly lower fertility rates (proportion of eggs fertilized) when examined by ANOVA (F = 8.026, df = 1, P < 0.01; Fig. 5E) or when examined using a G test with the mean fertility for controls used as the expected frequency (GP = 10,434, df = 1, P < 0.001). Even atrazine-treated males with relatively high sperm content (e.g., animals from the 2008 study) had low fertility (Fig. 5F).
Fig. 5.
Atrazine decreased androgen-dependent sperm production, mating behavior, and fertility. (A and C) Largest testicular cross-sections for representative control (A) and atrazine-exposed males (C) from 2007. (B and D) Magnification of individual tubules for control (B) and atrazine-exposed (D) males. Arrowheads in B and D show outline of tubules. Control tubules are typically filled with mature spermatozoa bundles, whereas the majority of tubules in atrazine-exposed males lack mature sperm bundles and are nearly empty, with only secondary spermatocytes (SS) along the periphery of the tubule. (E) Fertility for control (Con) and atrazine-exposed (Atr) males. Pooled data from both 2007 and 2008 study are shown. *P < 0.005 (ANOVA). (F) Fertility plotted against sperm content (percentage of tubules with mature sperm bundles) for control males (black symbols) and atrazine-exposed males (red symbols) for the 2007 (circles) and the 2008 (squares) studies. Dashed lines indicate the lower limit for controls for fertility and sperm content. Sample size differs from the number of trials because no data are available from females that did not lay eggs. (Bar in A applies to A and C; in B applies to B and D.)
Discussion
Previous studies showed that atrazine demasculinizes (chemically castrates) and feminizes exposed amphibian larvae, resulting in hermaphrodites (8, 10) or males with testicular oocytes (7, 9) at metamorphosis. Since our initial publications (7, 9, 10), the effects of atrazine on amphibian development and the significance of these effects to amphibian declines have been a subject of debate (30, 35, 36). Although some investigators, including Carr et al. (6), reported statistically significant effects of atrazine on gonadal morphology in X. laevis (P < 0.0003 for multiple testes and P = 0.0042 for hermaphrodites), others, using different experimental conditions and different populations of the same species, suggested that atrazine had no effect (29). Essential to this debate, however, is (i) the terminology used to describe gonadal abnormalities; (ii) the expertise and ability of other researchers to recognize abnormalities; (iii) the possibility of natural variation in sex differentiation processes between species and even between populations (or strains) within a species (37); and (iv) the long-term consequences and significance of the observed abnormalities to amphibian reproductive fitness. Here we describe complete and functional female development in genetic (ZZ) males exposed to atrazine, not the production of hermaphrodites or males with testicular oocytes. Thus, there is no confusion in the present study regarding proper terminology or proper identification. Furthermore, because we used an all genetic (ZZ) male colony and genotyped the atrazine-induced ZZ females, there is no question that atrazine completely sex-reversed genetic (ZZ) males, resulting in reproductively functional females.
The present study thoroughly examines the long-term effects of atrazine on reproductive function in amphibians. Although a single published study attempted to examine long-term reproductive effects of atrazine in amphibians (38), the authors did not report examinations of morphology. Furthermore, their examination of fertility and breeding of atrazine-exposed males was conducted after animals were injected with reproductive hormones (human chorionic gonadotropin, hCG), effectively providing “hormone replacement therapy” and reversing the effects of atrazine. The present study represents a more thorough examination of the effects of atrazine on sex hormone production, testosterone-dependent development and morphology, male reproductive behavior, and fertility.
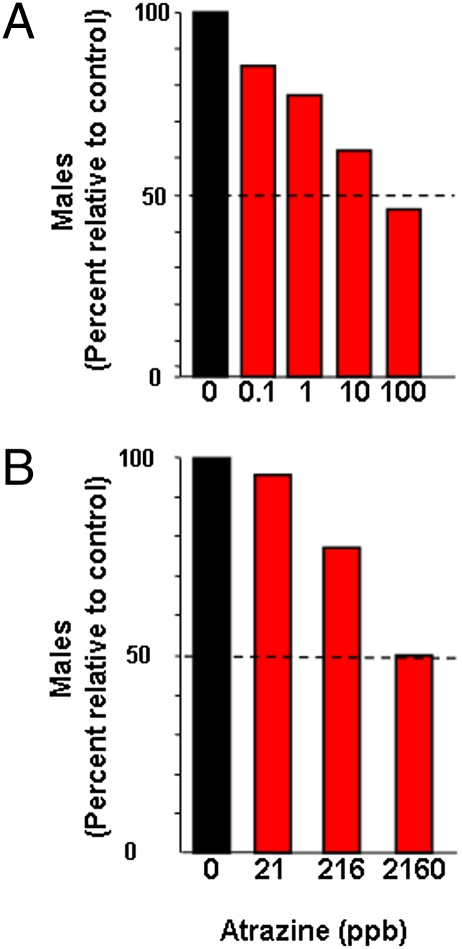
Perhaps the most dramatic finding here is that hermaphroditism observed at metamorphosis in animals exposed to atrazine (6, 10) can ultimately result in complete feminization. The complete feminization of males exposed to atrazine is consistent with two previous studies that showed that atrazine feminizes zebra fish (Danio rerio) (5) and Xenopus laevis (39) (Fig. 6) and a more recent study that showed that atrazine exposure feminizes leopard frogs, Rana pipiens (40). These previous reports based their findings on shifts in the sex ratio, however; our study showed that atrazine-induced females are indeed genetic males. Furthermore, we showed that feminization is persistent and complete, resulting in reproductively functional females capable of producing viable eggs. Together, the present data and these three similar reports (5, 39, 40) suggest that sex-reversal by atrazine (complete feminization of genetic males) is not a species-specific effect but rather one that occurs across nonamniote vertebrate classes.
Fig. 6.
Other studies have shown that atrazine alters sex ratios. Data from Oka et al. (39) (A) and Suzawa and Ingraham (5) (B) showing a concentration-dependent decline in males due to atrazine exposure in African clawed frogs (A) and zebrafish (B). The dashed line shows the 50% mark in both cases.
In addition to feminization, individuals exposed to atrazine that appeared male were demasculinized in the present study. The decline in testosterone in atrazine-exposed males, also shown in previous studies (10), is consistent with the decline in all testosterone-dependent morphologies examined here, including demasculinized/feminized laryngeal morphology and decreased breeding gland size. The decreased testosterone and absence of increased testosterone in atrazine-exposed males in the presence of females is further consistent with the inability of atrazine-exposed males to compete with unexposed males for access to females and consistent with the decline in sperm production and severely impaired fertility observed in atrazine-exposed males. The decreased frequency of tubules containing mature sperm suggests that the previously reported decline in germ cells and nursing cells after only 48 h exposure to atrazine in X. laevis (11) persists through adulthood. Likewise, the demasculinized larynges suggest that the smaller laryngeal size observed at metamorphosis in previous studies (10, 41) results in persistent effects through sexual maturity. The low fertility rate of atrazine-treated males (regardless of sperm content) suggests that even atrazine-exposed males with adequate sperm do not show the copulatory behavior necessary for successful reproduction.
The present results are also consistent with other studies that examined long-term behavioral effects of atrazine in fish (salmon, Salmo salar) (4). Salmon exposed to atrazine (≥6 ppb) showed a dose-dependent decrease in androgens. Atrazine-exposure (≥6 ppb) resulted in a significant decline in sperm production (milt), and exposed males lost the ability to respond to the attractant female pheromone. Furthermore, atrazine reduced sperm content in a reptile (caiman, Caiman latirostris), producing a morphology nearly identical to what we report here (18). The similarities between these previous findings in fish (4) and in reptiles (18) and the present findings in an amphibian suggest that the demasculinizing effects of atrazine are also not species, genera, family, or even order specific but occur across vertebrate classes. Indeed, declining androgens (22, 26) and decreased sperm production have been shown in laboratory rodents exposed to atrazine as well (22, 26, 42), albeit at higher doses. Furthermore, atrazine exposure is highly correlated (P < 0.009) with low sperm count, poor semen quality, and impaired fertility in humans (43).
Although atrazine reportedly affects vertebrates through a number of mechanisms, the reported mechanism most consistent with the effects observed on amphibian reproduction here is the induction of aromatase, which has been shown in several vertebrate classes (5, 15, 16). The induction of aromatase is consistent with the natural sex differentiation process in X. laevis, in which the sex-determining gene, DM-W, is a transcription factor (44) that induces aromatase expression in the developing undifferentiated gonad of genetic (ZW) females (44). Transcription and subsequent translation of aromatase leads to estrogen production, which in turn directs differentiation of the ovary from the undifferentiated gonad. Just as exogenous estrogen results in the differentiation of ovaries in exposed genetic (ZZ) male X. laevis (45), induction of aromatase and subsequent estrogen production likely explain the complete feminization of genetic male X. laevis by atrazine. Although ideally one needs to show that atrazine induces aromatase in genetic males before the transformation into females to support this hypothesis, it is not clear how such a study can be conducted here. Animals euthanized to measure aromatase expression do not have the opportunity to develop further, and thus it cannot be shown that the individuals that expressed aromatase were destined to become females. Furthermore, why only some males (10% in the present population) are completely feminized, whereas their siblings are merely demasculinized, remains to be explored.
Regardless of the mechanism, the impacts of atrazine on amphibians and on wildlife in general are potentially devastating. The negative impacts on wild amphibians is especially concerning given that the dose examined here (2.5 ppb) is in the range that animals experience year-round in areas where atrazine is used (1, 32, 46), well within levels found in rainfall (47), in which levels can exceed 100 ppb in the midwestern United States (48), and below the current US Environmental Protection Agency drinking water standard of 3 ppb (49). Furthermore, recent studies have shown that frog skin absorbs atrazine at much higher rates than the skin of mammals (50), and even semiterrestrial frog species take up significant amounts of atrazine (51). Thus, the exposure level examined in the present study is relevant even to semiterrestrial amphibians.
Although many studies have focused on death from disease and its role in global amphibian declines and sudden enigmatic disappearances of populations, virtually no attention has been paid to the slow gradual loss of amphibian populations due to failed recruitment (52). The present study suggests several ways that exposure to endocrine disruptors such as atrazine may lead to population level effects in the wild and contribute to amphibian declines. Certainly, the inability to compete for females and the significant decline in fertility in exposed males, as reported in the present study, will have a direct impact on exposed populations. Furthermore, sex-reversed males (ZZ females) are only capable of producing genetic male (ZZ) offspring, so the sex ratio in exposed populations would be skewed both by the production of atrazine-induced ZZ females as well as by the fact that ZZ females can only produce ZZ (genetically male) offspring. In fact, mathematical models suggest that this very mechanism (the production of sex-reversed all male-producing animals) could drive populations to extinction (53). Additionally, it is not known whether the increased susceptibility in the ZZ females is heritable or whether the “resistance” apparently present in atrazine-exposed males that do not become females is heritable. In either case, clearly, selection for resistance or susceptibility will affect population genetics and perhaps even cause bottlenecking and loss of genetic diversity. Atrazine likely affects amphibian populations through any combination of these effects and, as such, is a likely contributor to global amphibian declines. It seems that the concerns of Sanderson et al. [“A logical concern would be that exposure of wildlife and humans to triazine herbicides, which are produced and used in large quantities, and are ubiquitous environmental contaminants, may similarly contribute to estrogen-mediated toxicities and inappropriate sexual differentiation.” (15)] may be borne out.
Materials and Methods
Atrazine Exposure.
For methods regarding generation of sex-reversed (F1) males and F2-ZZ larvae, as well as animal husbandry, see SI Materials and Methods. ZZ larvae (F2) were reared in atrazine (2.5 ppb dissolved in ethanol) from hatching, through metamorphosis [Nieuwkoop and Faber (NF) stage 66] and throughout postmetamorphic life for comparison with control (ethanol-treated) animals. Total ethanol concentration was 0.003%. Atrazine levels and the absence of atrazine in control tanks were monitored by ELISA (Abraxis BioScience).
Morphometric Analysis (Larynx, Breeding Glands, and Gonads) at Sexual Maturity.
These analyses were conducted on sexually mature animals (2 or 3 years after metamorphosis, as indicated). For larynges, the proportion of the dilator larynges that extended below the thiohyral was determined (Fig. 3 F–H). For nuptial pads, histologic sections (8 μm) were cut through the geometric center of the nuptial pad. The maximum cross-sectional area of breeding glands was determined and compared with the maximum cross-sectional area of mucous and serous glands. For testis, the stages of spermatogenesis from five random testicular tubules and from the largest tubule from each cross-section were analyzed, as well as the proportion of testicular tubules from the largest cross-sections with and without spermatozoa bundles. For other measurements and corrections conducted, see SI Materials and Methods.
Molecular Markers for Sex.
Genomic DNA was isolated from toe tips prepared by tissue lysis and proteinase k protein digestion. The ZW genotype was determined by multiplex PCR amplification (37 cycles) of DM-W (W specific) (44). The four animals determined to be female, on the basis of external morphology, were analyzed for comparison with four males. For conditions and primers, see SI Materials and Methods.
RT-PCR for cyp19 Aromatase.
RT-PCR for cyp19 aromatase was conducted using RNA extracted from gonads of the two atrazine-treated females that were euthanized, along with four control males and four stock ZW females as controls. For conditions and primers, see SI Materials and Methods.
Mate Choice.
To compare the ability of control and atrazine-exposed males to attract females and achieve amplexus, we conducted the following behavioral studies. Males and females were marked for identification with unique single black or white stitches placed (without anesthesia) in the dorsal skin using silk thread. Stock ZW females maintained for this purpose (SI Materials and Methods) were then injected with hCG (1,000 IU) at 1500 hours. Four stock females (ZW), four control males (no hCG injection), and four atrazine-exposed males (no hCG injection) were all placed in a circular pool (diameter = 168 cm, height = 41 cm) filled with 264 L of fresh dechloraminated water. Animals were left overnight. At 0600 hours the next day (1 h before lights on), amplectant pairs were observed and animals identified under red light. Pairs and single males were removed from the pool and blood samples immediately taken from all males via cardiac puncture without anesthesia, as described previously (10). Sampling was alternated between controls and atrazine-treated males and the time of capture and time of blood draw recorded for each. Blood plasma was collected after centrifugation at 209 × g at 4 °C and stored at −20 °C. Plasma testosterone was extracted and measured by RIA as described by Hayes et al. (10). These behavioral trials were replicated five times, but data were obtained for only four owing to a failure in lighting during one trial. In all cases, all control and atrazine-treated males were virgins and had never been exposed to females. Stock females were also virgins and had never been exposed to males. In each replicate, control and atrazine-treated males were matched for size (snout–vent length and body weight) so that there were no significant differences (ANOVA: P > 0.05) in male size between groups and size had no effect on mating success (SI Materials and Methods). To examine the frequency of successful copulations, a G test (52) was used with replicate (i.e., trial) nested within treatment. For testosterone analysis, an ANOVA was conducted to examine differences in testosterone levels between control and atrazine-treated males (SYSTAT; SPSS). Data were also examined using a Kruskal-Wallis test.
Fertility Analysis.
To examine fertility, two studies were conducted. In the first, conducted on December 8, 2007, nine control and nine atrazine-treated males (both without hCG injections) were paired with stock ZW females (hCG-injected). Females (from the same San Diego colony) were injected and paired with individual males in aquaria (46 × 25 × 20 cm) with 10 L of fresh 10% Holtfreter’s solution and left overnight at 22 °C. At 900 hours, eggs were collected. Eggs were aerated and allowed to develop for 72 h, after which time they were fixed in Bouin’s fixative for 48 h and then preserved in 70% ethanol (two changes over 48 h). Fertility was determined by counting the number of undeveloped eggs and the number of developed embryos (NF stages 14–34). Fertility data (proportions) were arcsine transformed before ANOVA. In addition, mate choice data were subjected to analysis by G test. All males and females used in this study were virgins and had no previous exposure to the opposite sex. The animals used in this study were distinct from those used in the breeding studies above. This study was repeated on December 12, 2008 with five control and five atrazine-exposed virgin males. In both cases, the control males and the atrazine-treated males were tested in separate rooms, so that vocalizations from one group did not affect the results in the other. The animals in both of these studies were the same ones used for the histologic analyses and morphometric studies described above.
Supplementary Material
Acknowledgments
We thank Roger Liu for generating the ZZ colony. This work was supported by grants from the Park Water Company, the Mitchell Kapor Foundation, the David Foundation, the Cornell-Douglas Foundation, the Wallace Foundation, and the Class of ‘43 Endowed Chair (to T.B.H.). R.L. was supported by a grant from the University of California Toxic Substances Research and Teaching Program, and the colony was generated under funding from the National Science Foundation. T.B. was supported by a Hewlett Packard Fellowship. V.K. and A.N. were supported by Biology Fellows Program grants from the Howard Hughes Medical Institute. S.G. was supported by a Mentored Research fellowship from the University of California, Berkeley. Animals were collected from the field under a permit from the California Department of Fish and Game (to T.B.H.), with the permission of Tecalote Regional Park. All animal studies were conducted in accordance with Animal Use Protocol R209-011BRC (to T.B.H.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909519107/DCSupplemental.
References
- 1.Solomon K, et al. Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem. 1996;15:31–76. doi: 10.1002/etc.2050. [DOI] [PubMed] [Google Scholar]
- 2.Thurman E, Cromwell A. Atmospheric transport, deposition, and fate of triazine herbicides and their metabolites in pristine areas at Isle Royale National Park. Environ Sci Technol. 2000;34:3079–3085. [Google Scholar]
- 3.Mast MA, Foreman WT, Skaates SV. Current-use pesticides and organochlorine compounds in precipitation and lake sediment from two high-elevation national parks in the Western United States. Arch Environ Contam Toxicol. 2007;52:294–305. doi: 10.1007/s00244-006-0096-1. [DOI] [PubMed] [Google Scholar]
- 4.Moore A, Waring C. Mechanistic effects of a triazine pesticide on reproductive endocrine function in mature male Atlantic salmon (Salmo salar L.) parr. Pestic Biochem Physiol. 1998;62:41–50. [Google Scholar]
- 5.Suzawa M, Ingraham H. The herbicide atrazine activates endocrine gene networks via non-steroidal NR5A nuclear receptors in fish and mammalian cells. PLoS One. 2008;3:e2117. doi: 10.1371/journal.pone.0002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr J, et al. Response of larval Xenopus laevis to atrazine: Assessment of growth, metamorphosis, and gonadal and laryngeal morphology. Environ Toxicol Chem. 2003;22:396–405. [PubMed] [Google Scholar]
- 7.Hayes T, et al. Atrazine-induced hermaphroditism at 0.1 ppb in American leopard frogs (Rana pipiens): Laboratory and field evidence. Environ Health Perspect. 2002;111:568–575. doi: 10.1289/ehp.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes T, et al. Characterization of atrazine-induced gonadal malformations and effects of an androgen antagonist (cyproterone acetate) and exogenous estrogen (estradiol 17β): Support for the demasculinization/feminization hypothesis. Environ Health Perspect. 2006;114:134–141. doi: 10.1289/ehp.8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes T, et al. Feminization of male frogs in the wild. Nature. 2002;419:895–896. doi: 10.1038/419895a. [DOI] [PubMed] [Google Scholar]
- 10.Hayes T, et al. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc Natl Acad Sci USA. 2002;99:5476–5480. doi: 10.1073/pnas.082121499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tavera-Mendoza L, et al. Response of the amphibian tadpole (Xenopus laevis) to atrazine during sexual differentiation of the testis. Environ Toxicol Chem. 2002;21:527–531. doi: 10.1897/1551-5028(2002)021<0527:rotatx>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Tavera-Mendoza L, et al. Response of the amphibian tadpole Xenopus laevis to atrazine during sexual differentiation of the ovary. Environ Toxicol Chem. 2002;21:1264–1267. [PubMed] [Google Scholar]
- 13.Fan W, et al. Herbicide atrazine activates SF-1 by direct affinity and concomitant co-activators recruitments to induce aromatase expression via promoter II. Biochem Biophys Res Commun. 2007;355:1012–1018. doi: 10.1016/j.bbrc.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 14.Fan W, et al. Atrazine-induced aromatase expression is SF-1-dependent: Implications for endocrine disruption in wildlife and reproductive cancers in humans. Environ Health Perspect. 2007;115:720–727. doi: 10.1289/ehp.9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanderson JT, Seinen W, Giesy JP, van den Berg M. 2-chloro-triazine herbicides induce aromatase (CYP19) activity in H295R human adrenocortical carcinoma cells: A novel mechanism for estrogenicity? Toxicol Sci. 2000;54:121–127. doi: 10.1093/toxsci/54.1.121. [DOI] [PubMed] [Google Scholar]
- 16.Crain D, Guillette LJ, Rooney AA, Pickford D. Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ Health Perspect. 1997;105:528–533. doi: 10.1289/ehp.97105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kettles MA, Browning SR, Prince TS, Hostman SW. Triazine exposure and breast cancer incidence: An ecologic study of Kentucky counties. Environ Health Perspect. 1997;105:1222–1227. doi: 10.1289/ehp.971051222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rey F, et al. Prenatal exposure to pesticides disrupts testicular histoarchitecture and alters testosterone levels in male Caiman latirostris. Gen Comp Endocrinol. 2009;162:286–292. doi: 10.1016/j.ygcen.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 19.Matsushita S, et al. Effects of in ovo exposure to imazalil and atrazine on sexual differentiation in chick gonads. Poult Sci. 2006;85:1641–1647. doi: 10.1093/ps/85.9.1641. [DOI] [PubMed] [Google Scholar]
- 20.Babic-Gojmerac T, Kniewald Z, Kniewald J. Testosterone metabolism in neuroendocrine organs in male rats under atrazine and deethylatrazine influence. J Steroid Biochem. 1989;33:141–146. doi: 10.1016/0022-4731(89)90369-5. [DOI] [PubMed] [Google Scholar]
- 21.Eldridge J, Wetzel L, Tyrey L. Estrous cycle patterns of Sprague-Dawley rats during acute and chronic atrazine administration. Reprod Toxicol. 1999;13:491–499. doi: 10.1016/s0890-6238(99)00056-8. [DOI] [PubMed] [Google Scholar]
- 22.Trentacoste S, et al. Atrazine effects on testosterone levels and androgen-dependent reproductive organs in peripubertal male rats. J Androl. 2001;22:142–148. [PubMed] [Google Scholar]
- 23.Ueda M, et al. Possible enhancing effects of atrazine on growth of 7,12-dimethylbenz(a) anthracene induced mammary tumors in ovariectomized Sprague–Dawley rats. Cancer Sci. 2005;96:19–25. doi: 10.1111/j.1349-7006.2005.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoker T, Laws S, Guidici D, Cooper R. The effect of atrazine on puberty in male Wistar rats: An evaluation in the protocol for the assessment of pubertal development and thyroid function. Toxicol Sci. 2000;58:50–59. doi: 10.1093/toxsci/58.1.50. [DOI] [PubMed] [Google Scholar]
- 25.Rayner J, Enoch R, Fenton S. Adverse effects of prenatal exposure to atrazine during a critical period of mammary gland growth. Toxicol Sci. 2005;87:255–266. doi: 10.1093/toxsci/kfi213. [DOI] [PubMed] [Google Scholar]
- 26.Friedmann A. Atrazine inhibition of testosterone production in rat males following peripubertal exposure. Reprod Toxicol. 2002;16:275–279. doi: 10.1016/s0890-6238(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 27.Cooper RL, et al. Atrazine disrupts the hypothalamic control of pituitary-ovarian function. Toxicol Sci. 2000;53:297–307. doi: 10.1093/toxsci/53.2.297. [DOI] [PubMed] [Google Scholar]
- 28.Cummings A, Rhodes B, Cooper R. Effect of atrazine on implantation and early pregnancy in 4 strains of rats. Toxicol Sci. 2000;58:135–143. doi: 10.1093/toxsci/58.1.135. [DOI] [PubMed] [Google Scholar]
- 29.Kloas W, et al. Does atrazine influence larval development and sexual differentiation in Xenopus laevis? Toxicol Sci. 2009;107:376–384. doi: 10.1093/toxsci/kfn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayes T. There is no denying this: Defusing the confusion about atrazine. Bioscience. 2004;54:1138–1149. [Google Scholar]
- 31.Orton F, Carr J, Handy R. Effects of nitrate and atrazine on larval development and sexual differentiation in the northern leopard frog Rana pipiens. Environ Toxicol Chem. 2006;25:65–71. doi: 10.1897/05-136r.1. [DOI] [PubMed] [Google Scholar]
- 32.Du Preez LH, et al. Population structure characterization of the clawed frog (Xenopus laevis) in corn-growing versus non-corn-growing areas in South Africa. Afr J Herpetol. 2005;54:61–68. [Google Scholar]
- 33.Reeder A, et al. Forms and prevalence of intersexuality and effects of environmental contaminants on sexuality in cricket frogs (Acris crepitans) Environ Health Perspect. 1998;106:261–266. doi: 10.1289/ehp.98106261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy MB, et al. Atrazine concentrations, gonadal gross morphology and histology in ranid frogs collected in Michigan agricultural areas. Aquat Toxicol. 2006;76:230–245. doi: 10.1016/j.aquatox.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Rohr JR, Mckoy KA. A qualitative meta-analysis reveals consistent effects of atrazine on freshwater fish and amphibians. Environ Health Perspect. 2009;118:20–32. doi: 10.1289/ehp.0901164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solomon KR, et al. Effects of atrazine on fish, amphibians, and aquatic reptiles: A critical review. Crit Rev Toxicol. 2008;38:721–772. doi: 10.1080/10408440802116496. [DOI] [PubMed] [Google Scholar]
- 37.Du Preez LH, et al. Population-specific incidence of testicular ovarian follicles in Xenopus laevis from South Africa: A potential issue in endocrine testing. Aquat Toxicol. 2009;95:10–16. doi: 10.1016/j.aquatox.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 38.Du Preez LH, et al. Reproduction, larval growth, and reproductive development in African clawed frogs (Xenopus laevis) exposed to atrazine. Chemosphere. 2008;71:546–552. doi: 10.1016/j.chemosphere.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 39.Oka T, et al. Effect of atrazine on metamorphosis and sexual differentiation in Xenopus laevis. Aquat Toxicol. 2008;87:215–226. doi: 10.1016/j.aquatox.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Langlois V, et al. Low levels of the herbicide atrazine alters sex ratios and reduces metamorphic success in Rana pipiens tadpoles raised in outdoor mesocosms. Environ Health Perspect. 2009 doi: 10.1289/ehp.0901418. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayes T. Welcome to the revolution: Integrative biology and assessing the impact of endocrine disruptors on environmental and public health. Integrative Comp Biol. 2005;45:321–329. doi: 10.1093/icb/45.2.321. [DOI] [PubMed] [Google Scholar]
- 42.Kniewald J, et al. Disorders of male rat reproductive tract under the influence of atrazine. J Appl Toxicol. 2000;20:61–68. [PubMed] [Google Scholar]
- 43.Swan S, et al. Semen quality in relation to biomarkers of pesticide exposure. Environ Health Perspect. 2003;111:1478–1484. doi: 10.1289/ehp.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshimoto S, et al. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proc Natl Acad Sci USA. 2008;105:2469–2474. doi: 10.1073/pnas.0712244105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayes T. Sex determination and primary sex differentiation in amphibians. J Exp Zool. 1998;281:373–399. [PubMed] [Google Scholar]
- 46.Du Preez LH, et al. Seasonal exposures to triazine and other pesticides in surface waters in the western Highveld corn-production region in South Africa. Environ Pollut. 2005;135:131–141. doi: 10.1016/j.envpol.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 47.Van Dijk H, Guicherit R. Atmospheric dispersion of current-use pesticides: A review of the evidence from monitoring studies. Water Air Soil Pollut. 1999;115:21–70. [Google Scholar]
- 48.Hatfield JL, Wesley CK, Prueger JH, Pfeiffer RL. Herbicide and nitrate distribution in central Iowa rainfall. J Environ Qual. 1996;25:259–264. [Google Scholar]
- 49.US Environmental Protection Agency . List of Contaminants and Their MCLs (EPA 816-F-02-013) Washington, DC: US EPA; 2002. [Google Scholar]
- 50.Quaranta A, Bellantuono V, Cassano G, Lippe C. Why amphibians are more sensitive than mammals to xenobiotics. PLoS One. 2009;4:e7699. doi: 10.1371/journal.pone.0007699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendez SIS, Tillitt DE, Rittenhouse TAG, Semlitsch RD. Behavioral response and kinetics of terrestrial atrazine exposure in American toads (Bufo americanus) Arch Environ Contam Toxicol. 2009;57:590–597. doi: 10.1007/s00244-009-9292-0. [DOI] [PubMed] [Google Scholar]
- 52.Hayes TB. The cause of global amphibian decline: A developmental endocrinologist’s perspective. J Exp Biol. 2010 doi: 10.1242/jeb.040865. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gutierres JB, Teem Jl. A model describing the effect of sex-reversed YY fish in an established wild population: The use of a Trojan Y chromosome to cause extinction of an introduced exotic species. J Theor Biol . 2006;241:333–341. doi: 10.1016/j.jtbi.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 54.Sokal R, Rohlf F. Biometry: The Principles and Practice of Statistics in Biological Research. New York: W.H. Freeman; 1981. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.