Abstract
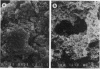
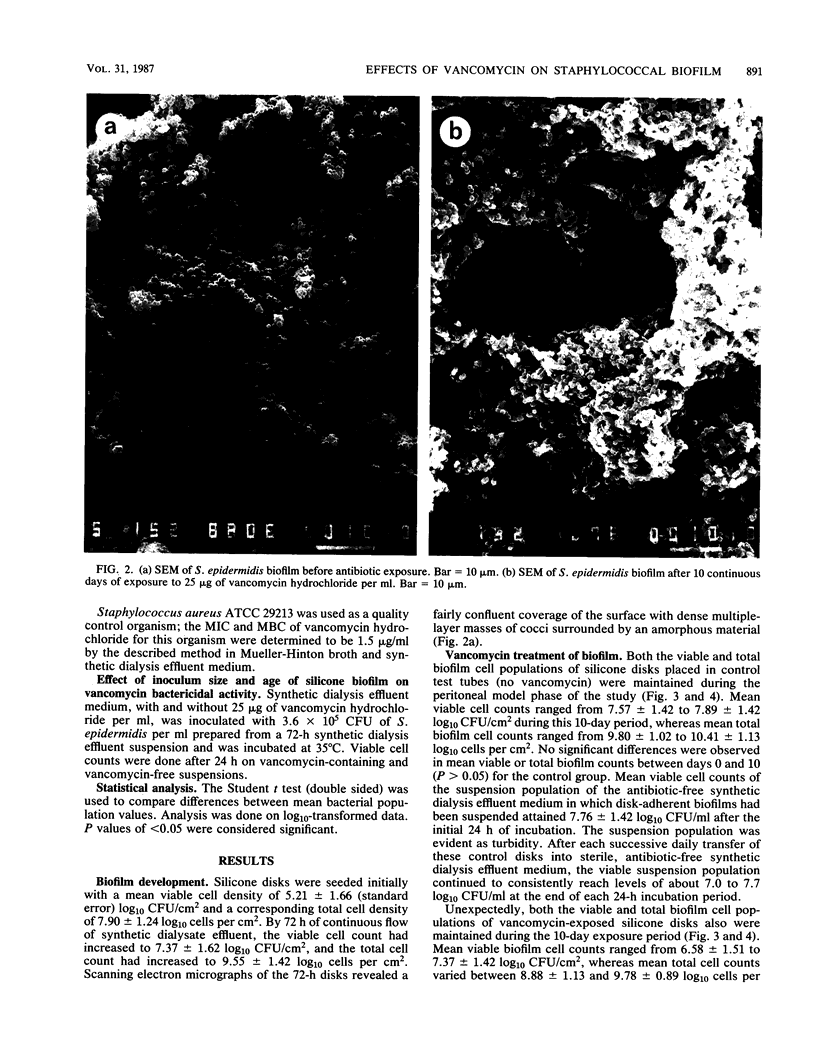
Peritonitis is a major complication of continuous ambulatory peritoneal dialysis. Relapsing peritonitis after the cessation of antimicrobial therapy is frequently reported and often involves Staphylococcus epidermidis. To investigate the potential role of catheter-associated biofilm in the pathogenesis of relapsing peritonitis, we describe an in vitro model permitting the development of an S. epidermidis biofilm on silicone elastomer biomaterial. This model has been used to investigate the ability of vancomycin hydrochloride to kill biofilm-encased organisms by using an antibiotic regimen typical of peritonitis therapy. No significant differences were seen between vancomycin-exposed and control groups in biofilm viable and total cell counts after 10 days. Vancomycin-exposed silicone-associated biofilm populations decreased by only 0.5 log10 CFU/cm2 over the study period. MICs and MBCs for the original S. epidermidis suspension were 3.125 and 6.25 micrograms/ml, respectively. For biofilm homogenate suspensions, MICs were 3.125 micrograms/ml, but MBCs were greater than 400 micrograms/ml. These data indicate that the biofilm organisms associated with an indwelling peritoneal catheter may display a form of tolerance, thereby suggesting one possible mechanism behind relapsing peritonitis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayus J. C., Eneas J. F., Tong T. G., Benowitz N. L., Schoenfeld P. Y., Hadley K. L., Becker C. E., Humphreys M. H. Peritoneal clearance and total body elimination of vancomycin during chronic intermittent peritoneal dialysis. Clin Nephrol. 1979 Mar;11(3):129–132. [PubMed] [Google Scholar]
- Bayston R., Penny S. R. Excessive production of mucoid substance in staphylococcus SIIA: a possible factor in colonisation of Holter shunts. Dev Med Child Neurol Suppl. 1972;27:25–28. doi: 10.1111/j.1469-8749.1972.tb09769.x. [DOI] [PubMed] [Google Scholar]
- Brauner L., Kahlmeter G., Lindholm T., Simonsen O. Vancomycin and netilmicin as first line treatment of peritonitis in CAPD patients. J Antimicrob Chemother. 1985 Jun;15(6):751–758. doi: 10.1093/jac/15.6.751. [DOI] [PubMed] [Google Scholar]
- Bunke C. M., Aronoff G. R., Brier M. E., Sloan R. S., Luft F. C. Vancomycin kinetics during continuous ambulatory peritoneal dialysis. Clin Pharmacol Ther. 1983 Nov;34(5):631–637. doi: 10.1038/clpt.1983.225. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982 Jul;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens R. M., Tuomanen E., Tosch W., Zak O., Suter J., Tomasz A. Evaluation of the bactericidal activity of beta-lactam antibiotics on slowly growing bacteria cultured in the chemostat. Antimicrob Agents Chemother. 1986 May;29(5):797–802. doi: 10.1128/aac.29.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport D. S., Massanari R. M., Pfaller M. A., Bale M. J., Streed S. A., Hierholzer W. J., Jr Usefulness of a test for slime production as a marker for clinically significant infections with coagulase-negative staphylococci. J Infect Dis. 1986 Feb;153(2):332–339. doi: 10.1093/infdis/153.2.332. [DOI] [PubMed] [Google Scholar]
- Fang L. S., Tolkoff-Rubin N. E., Rubin R. H. Efficacy of single-dose and conventional amoxicillin therapy in urinary-tract infection localized by the antibody-coated bacteria technic. N Engl J Med. 1978 Feb 23;298(8):413–416. doi: 10.1056/NEJM197802232980802. [DOI] [PubMed] [Google Scholar]
- Finch J. E., Brown M. R. The influence of nutrient limitation in a chemostat on the sensitivity of Pseudomonas aeruginosa to polymyxin and to EDTA. J Antimicrob Chemother. 1975 Dec;1(4):379–386. doi: 10.1093/jac/1.4.379. [DOI] [PubMed] [Google Scholar]
- Golper T. A., Hartstein A. I. Analysis of the causative pathogens in uncomplicated CAPD-associated peritonitis: duration of therapy, relapses, and prognosis. Am J Kidney Dis. 1986 Feb;7(2):141–145. doi: 10.1016/s0272-6386(86)80135-4. [DOI] [PubMed] [Google Scholar]
- Govan J. R., Fyfe J. A. Mucoid Pseudomonas aeruginosa and cystic fibrosis: resistance of the mucoid from to carbenicillin, flucloxacillin and tobramycin and the isolation of mucoid variants in vitro. J Antimicrob Chemother. 1978 May;4(3):233–240. doi: 10.1093/jac/4.3.233. [DOI] [PubMed] [Google Scholar]
- Gray E. D., Peters G., Verstegen M., Regelmann W. E. Effect of extracellular slime substance from Staphylococcus epidermidis on the human cellular immune response. Lancet. 1984 Feb 18;1(8373):365–367. doi: 10.1016/s0140-6736(84)90413-6. [DOI] [PubMed] [Google Scholar]
- Harford A. M., Sica D. A., Tartaglione T., Polk R. E., Dalton H. P., Poynor W. Vancomycin pharmacokinetics in continuous ambulatory peritoneal dialysis patients with peritonitis. Nephron. 1986;43(3):217–222. doi: 10.1159/000183833. [DOI] [PubMed] [Google Scholar]
- Johnson G. M., Lee D. A., Regelmann W. E., Gray E. D., Peters G., Quie P. G. Interference with granulocyte function by Staphylococcus epidermidis slime. Infect Immun. 1986 Oct;54(1):13–20. doi: 10.1128/iai.54.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristinsson K. G., Spencer R. C., Brown C. B. Clinical importance of production of slime by coagulase negative staphylococci in chronic ambulatory peritoneal dialysis. J Clin Pathol. 1986 Jan;39(1):117–118. doi: 10.1136/jcp.39.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy F. D., Hammer S. M. Staphylococcus epidermidis infections. Ann Intern Med. 1983 Dec;99(6):834–839. doi: 10.7326/0003-4819-99-6-834. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Nelligan J., Costerton J. W. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation. 1982 Dec;66(6):1339–1341. doi: 10.1161/01.cir.66.6.1339. [DOI] [PubMed] [Google Scholar]
- McCoy W. F., Bryers J. D., Robbins J., Costerton J. W. Observations of fouling biofilm formation. Can J Microbiol. 1981 Sep;27(9):910–917. doi: 10.1139/m81-143. [DOI] [PubMed] [Google Scholar]
- McDonald W. A., Watts J., Bowmer M. I. Factors affecting Staphylococcus epidermidis growth in peritoneal dialysis solutions. J Clin Microbiol. 1986 Jul;24(1):104–107. doi: 10.1128/jcm.24.1.104-107.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minuth J. N., Musher D. M., Thorsteinsson S. B. Inhibition of the antibacterial activity of gentamicin by urine. J Infect Dis. 1976 Jan;133(1):14–21. doi: 10.1093/infdis/133.1.14. [DOI] [PubMed] [Google Scholar]
- Nickel J. C., Ruseska I., Wright J. B., Costerton J. W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985 Apr;27(4):619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982 Oct;146(4):479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- Pfaller M. A., Krogstad D. J., Granich G. G., Murray P. R. Laboratory evaluation of five assay methods for vancomycin: bioassay, high-pressure liquid chromatography, fluorescence polarization immunoassay, radioimmunoassay, and fluorescence immunoassay. J Clin Microbiol. 1984 Sep;20(3):311–316. doi: 10.1128/jcm.20.3.311-316.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogge M. C., Johnson C. A., Zimmerman S. W., Welling P. G. Vancomycin disposition during continuous ambulatory peritoneal dialysis: a pharmacokinetic analysis of peritoneal drug transport. Antimicrob Agents Chemother. 1985 Apr;27(4):578–582. doi: 10.1128/aac.27.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth N. K., Bartell C. A., Roth D. A. In vitro study of bacterial growth in continuous ambulatory peritoneal dialysis fluids. J Clin Microbiol. 1986 Jun;23(6):1096–1098. doi: 10.1128/jcm.23.6.1096-1098.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack M. P., Nichols W. W. The penetration of antibiotics through sodium alginate and through the exopolysaccharide of a mucoid strain of Pseudomonas aeruginosa. Lancet. 1981 Sep 5;2(8245):502–503. doi: 10.1016/s0140-6736(81)90885-0. [DOI] [PubMed] [Google Scholar]
- Verbrugh H. A., Keane W. F., Conroy W. E., Peterson P. K. Bacterial growth and killing in chronic ambulatory peritoneal dialysis fluids. J Clin Microbiol. 1984 Aug;20(2):199–203. doi: 10.1128/jcm.20.2.199-203.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]