Abstract
Plants use light as a source of energy for photosynthesis and as a source of environmental information perceived by photoreceptors. Testing whether plants can complete their cycle if light provides energy but no information about the environment requires a plant devoid of phytochromes because all photosynthetically active wavelengths activate phytochromes. Producing such a quintuple mutant of Arabidopsis thaliana has been challenging, but we were able to obtain it in the flowering locus T (ft) mutant background. The quintuple phytochrome mutant does not germinate in the FT background, but it germinates to some extent in the ft background. If germination problems are bypassed by the addition of gibberellins, the seedlings of the quintuple phytochrome mutant exposed to red light produce chlorophyll, indicating that phytochromes are not the sole red-light photoreceptors, but they become developmentally arrested shortly after the cotyledon stage. Blue light bypasses this blockage, rejecting the long-standing idea that the blue-light receptors cryptochromes cannot operate without phytochromes. After growth under white light, returning the quintuple phytochrome mutant to red light resulted in rapid senescence of already expanded leaves and severely impaired expansion of new leaves. We conclude that Arabidopsis development is stalled at several points in the presence of light suitable for photosynthesis but providing no photomorphogenic signal.
Keywords: Clock, cryptochrome, germination, photosynthesis
Plants use light as a source of energy for photosynthesis and as a source of information about their surrounding environment. Phytochromes, cryptochromes, phototropins, and the zeitlupe family of photoreceptors capture the signals of the environment that provide spatial and temporal information and control growth and development (1). Phytochromes have absorbance maxima in the red (660 nm) and far-red light (730 nm). They are synthesized as Pr (the inactive form) that is converted by red light to the active form, Pfr. This reaction is reversible by far-red light, which converts Pfr back to Pr. Five phytochrome apoprotein genes are present in the reference plant Arabidopsis thaliana (PHYA–PHYE) (1), each with partially overlapping functions (2). Conversely, only three phytochrome genes are present in rice (3). Phytochromes bear a covalently attached linear tetrapyrrol chromophore, the phytochromobilin, which undergoes a cis-trans isomerization when photoconverted (4).
Phytochromes promote germination of sensitized Arabidopsis seeds even after a brief exposure to very low fluence of light, which may occur during disturbance of the soil surface (5). After this transient exposure to light, the seed germinates in darkness, under the soil surface, and uses seed reserves to grow against the gravitropic vector; the cotyledons remain closed and folded down to prevent damage to the apical meristem. Once the seedling reaches the soil surface it undergoes a light-triggered developmental transition, termed de-etiolation; where hypocotyl growth is arrested, the cotyledons unfold, open and turn green, establishing the photomorphogenic pattern of development (6). The triple phyA phyB phyC mutant of rice lacks inhibition of coleoptile growth, detectable synthesis of chlorophyll, and changes in gene expression under continuous red light, indicating that in grasses, phytochromes are the sole photoreceptors for red and far-red light during de-etiolation (3).
De-etiolation is also promoted by the UV-A and blue-light photoreceptors cryptochromes (7), which interact with phytochromes in the control of this transition (8). Based on classic photobiological experiments, Hans Mohr (9) had proposed that the sole action of blue light perceived by specific photoreceptors (now identified as cryptochromes) was to amplify the response to Pfr (i.e., cryptochrome action requires Pfr). Under suboptimal light input conditions, cryptochrome action requires phyB activity (10), and under those conditions, phyB Pfr has recently been shown to act downstream of cryptochrome as predicted by Mohr's model (11). Under prolonged exposures to blue light, cryptochromes operate independently of phyA and phyB (12), but they could depend on other members of the phytochrome family (8, 12). Because phytochromes also absorb blue light, a definitive test for this classic proposition is impossible without the quintuple phytochrome mutant (8, 12).
Green vegetation canopies lower the red to far-red ratio of the light. This reduces the activity of light-stable phytochromes such as phyB but enhances the activity of phyA (13). The hypocotyl-growth response to red/far-red ratio represents a balance between these actions. Photosystem I and even photosystem II extend their activity to the far-red region of the spectrum (14). The latter could be negligible under a strong background of light between 400 and 700 nm, but it could be important for the responses to shade when the proportion of far-red light is high (14). Because different phytochromes have opposing effects, the only way to test the actual contribution of photosynthetic reactions to the growth response to far-red light is to use plants without phytochrome.
Most organisms, from bacteria to humans, have an internal clock that allows them to synchronize daily and seasonal rhythms in physiological processes with periodic environmental changes. To maintain an anticipatory function throughout the year, circadian clocks must be adjusted daily. Such entrainment is effected, in part, through pathways that signal information from light–dark transitions to the clock. Genetic evidence indicates that phytochromes, cryptochromes, and members of the zeitlupe family of photoreceptors control the circadian oscillator in Arabidopsis plants (15). In mammals, cryptochromes are required to sustain circadian rhythms even in complete darkness (16). In contrast to what is observed in mammals, cryptochromes are not essential for clock function in plants (17, 18). Interestingly, most circadian clock mutants show defective developmental responses to red light (15). It is unknown whether the latter is just a consequence of circadian modulation of phytochrome signaling or reflects an involvement of phytochromes in the core mechanism underlying circadian rhythms in plants.
Here we report the isolation of the Arabidopsis quintuple phytochrome mutant. We show that in contrast to the rice triple mutant devoid of phytochrome, the quintuple phytochrome mutant of Arabidopsis partially greens under red light. We also demonstrate that both blue light-induced photomorphogenesis and phototropism and the circadian clock operate in the absence of phytochromes. However, the photomorphogenic signal is crucial, and in its absence photosynthetically active radiation is not sufficient to sustain development.
Results
Isolation of the Quintuple Phytochrome Mutant in the ft Background.
Two alternative strategies could be used to obtain plants without active phytochromes. One is to combine mutations to block phytochrome chromophore biosynthesis. This approach has not been successful because of the close linkage of some gene family members (19) and the leakiness of mutants at some loci (20). The second strategy is to combine alleles of the five PHY apoprotein genes. Our initial attempts to isolate the quintuple phytochrome apoprotein mutant from segregating populations were unsuccessful. However, in the progeny of a phyA phyC phyD phyE ft mutant that was heterozygous for phyB, we noted that a few plants (<0.5%) were unable to de-etiolate under red light, which is perceived mainly by phyB (2). We transferred these seedlings to white light and confirmed that they were indeed the quintuple phytochrome mutants in the ft background.
The Quintuple Phytochrome Mutant Depends on Exogenous GAs for Seed Germination.
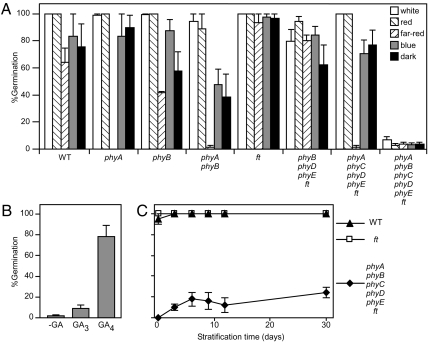
The involvement of individual phytochromes in seed germination is well established (21), so we tested the ability of the quintuple phytochrome mutant to germinate under different light conditions. Seeds were stratified for 3 days and then incubated at 23 °C under white, red, far-red, or blue light, or kept in darkness after sowing. The germination rate of the quintuple phytochrome mutant was very low compared to the other control genotypes because none of the light treatments increased its germination rates above dark controls (Fig. 1A), even at high fluencies of red or blue light (Fig. S1). All of the other genotypes used here showed high percentages of germination at least under some light conditions (Fig. 1A and Fig. S1).
Fig. 1.
Germination of the quintuple phytochrome mutant is not light responsive and requires exogenous GA. (A) Seeds were plated on Murashige and Skoog (MS) salts agar, stratified for 3 days at 4 °C, and incubated for 5 days under different light regimes at 23 °C before counting germinated seeds (radicle emergence). Light conditions: white light, 80 μmol m−2 s−1; red light, 10 μmol m−2 s−1; far-red light, 60 μmol m−2 s−1; blue light, 10 μmol m−2 s−1; and darkness. Data are averages ± SE of six or three (far-red) independent experiments with 50 seeds each. None of the light treatments promoted germination of the quintuple phytochrome mutant (one-way ANOVA, P = 0.43). (B) Seeds of the quintuple phytochrome mutant were sown on moistened filter paper containing 100 μM GA3, GA4 (Sigma), or no hormone and stratified for 3 days at 4 °C before the induction of germination at 23 °C under red light. Data scored 5 days later are averages ± SE of five independently collected seed pools (50 seeds each). One-way ANOVA followed by Bonferroni tests indicated significant differences between the control and +GA4 (P < 0.01). (C) Seeds of the WT, ft, and phyA phyB phyC phyD phyE ft mutants were sown on agar containing MS salts and stratified at 4 °C for the times indicated on the abscissa. Germination was induced and scored as in (B). Data are averages ± SE of five independently collected seed pools for the quintuple phytochrome mutant and two for the WT and ft controls. A t test indicates that the quintuple phytochrome mutant responded to stratification (P < 0.001).
The low germination frequency of the quintuple phytochrome mutant could be the consequence of irreversible defects on seed or embryo development or the induction of a dormant state that could not be reversed because of the absence of phytochrome. Because phytochromes induce germination by increasing GA biosynthesis (22, 23), we tested the ability of GA to restore germination. Despite the fact that the germination rate of the quintuple phytochrome mutant was very low, we could induce normal germination levels by adding GA4, but not GA3 (Fig. 1B and Fig. S2), showing that seeds are viable but remain dormant in the absence of the light signal provided by phytochromes.
The promotion of seed germination by low temperatures occurs at least partially by inducing GA biosynthesis during seed stratification (imbibition at low temperatures) (24). Low temperatures can substitute for light exposure in germination assays (24), but even after a far-red light treatment it is technically impossible to remove all active phytochrome (i.e., to convert all Pfr back to Pr), because far-red light can produce low levels of Pfr due to the overlapping Pr and Pfr spectra (1). Therefore, we tested whether low temperatures could induce germination in the quintuple phytochrome mutant. Seeds were incubated in the dark at 4 °C for periods of variable duration and then moved to 23 °C to score germination percentages 5 days later (Fig. 1C). Stratification improved germination but did not restore the germination potential of the quintuple phytochrome mutants to those observed with added GA (Fig. 1 B and C, and Fig. S2). GA addition to the nongerminating seeds after ending the experiment was still effective in inducing germination.
Cryptochromes Promote De-Etiolation in the Absence of Phytochromes.
The model proposed by Hans Mohr (9) states that cryptochromes require at least some level of Pfr to effectively induce a response. Therefore, we decided to investigate the hypocotyl elongation response to blue light—a cryptochrome mediated response (1)—in the phytochrome-less mutant. The phyA phyB phyC phyD phyE ft mutant responded well to blue light, showing that cryptochromes do not require phytochromes to trigger the inhibition of hypocotyl elongation under blue light (Fig. 2A). Conversely, the quintuple phytochrome mutant was not responsive to red light at this stage as expected based on previous observations showing that inhibition of hypocotyl growth by red light is already absent in the phyA phyB double mutant (25). The latter is also consistent with a recent report showing that the triple phyA phyB phyC mutant of rice, a species that only has these three phytochromes, fails to respond to red light (3). Cotyledon opening and greening were also triggered by blue light (Fig. 2B). The hypocotyl phototropic response was present in the quintuple phytochrome mutant, indicating that phototropins can still function in the absence of phytochrome (Fig. S3).
Fig. 2.
The quintuple phytochrome mutant does not develop under red light and requires blue light for developmental progression. (A) Seedlings were grown on MS agar plates under blue- or red-light photoperiods (10 μmol m−2 s−1; 16 h light/8 h dark) for 4 days at 23 °C. Hypocotyl length was measured at the end of the treatment. Data are averages ± SE of at least 15 seedlings in three independent experiments. One-way anova followed by Bonferroni posttests indicated that the effect of blue light was significant in the quadruple and quintuple phytochrome mutants (P < 0.01), and the effect of red light was significant in the quadruple mutant (P < 0.05) but not in the quintuple phytochrome mutant (P > 0.05). (B) phyA phyB phyC phyD phyE ft quintuple phytochrome mutant grown for 4 days under blue light (Upper Left), 9 days under red light (Upper Right) or 9 days in the dark (Lower Right). (B Lower Left) phyA phyC phyD phyE ft quadruple phytochrome mutant grown for 9 days under red light. Light and growth conditions were as in (A). (C) Seedlings were grown under continuous fluorescent white light (30 μmol m−2 s−1) for 20 h to allow germination and then moved to continuous white light (30 μmol m−2 s−1) with or without the addition of far-red light (24 μmol m−2 s−1; λ max 735 nm) for 7 days. Data are averages ± SE of four independent replicate dishes with 10–15 seedlings each. One-way ANOVA followed by Bonferroni posttests indicate significant differences (P < 0.001) between high and low red/far-red ratios for the quadruple phytochrome mutants but not for the quintuple phytochrome mutant. (D) Detail of the apical portion of the phyA phyB phyC phyD phyE ft sextuple mutant grown on MS salts agar plus 2% sucrose under 50 μmol m−2 s−1 continuous red light for 36 days (three Left), or 60 days (Right). (E Left) A flowering phyA phyB phyC phyD phyE ft sextuple mutant grown on MS salts agar plus 2% sucrose, under 50 μmol m−2 s−1 continuous blue light for 40 days. (E Center and Right) Flowering phyB phyC phyD phyE ft mutant and phyA phyB double mutant grown under 50 μmol m−2 s−1 continuous red light. Arrows indicate flower buds. (F) Flowering plants of the ft (Upper Right), phyA phyB phyC phyD phyE ft mutant (Upper Left), and phyA phyB phyC phyD phyE quintuple mutant in the FT background (Lower) grown in long days (100 μmol m−2 s−1 cool white light). The stem-length of the quintuple mutant in the FT background is 1.5 cm; note the yellow micropipette tip for reference. (G) Chlorophyll accumulation in the absence of phytochrome. Quintuple phytochrome mutants were grown for 8 days at 23 °C in the dark or under red light (50 μmol m−2 s−1). See Fig. S5 for controls and full details. (H) Chlorophyll synthesis in the absence of phytochrome. Seedlings were grown on MS agar plates in the dark for 4 days at 23 °C and either treated with 15 min of red light (50 μmol m−2 s−1; Right) or kept in darkness (Left) before harvest. Protochlorophyllide was extracted as described in ref. 27, and emission spectra were recorded every 0.5 nm with excitation with light of 433 nm. The data are averages of three independent plates with 20 seedlings each. For clarity only the SE of the peaks are presented. (I) Sextuple mutants phyA phyB phyC phyD phyE ft and ft controls were grown in white-light photoperiods on MS plus 2% sucrose for 14 days and then moved to either 50 μmol m−2 s−1 blue light or 50 μmol m−2 s−1 red light for the other 13 days. These plants are representative of six plants. The arrow points to an older leaf that turned yellow during the treatment despite the fact that it was green before moving the plants to red-light conditions. (Scale bars: B and D, 1 mm; E, F, and I, 1 cm.)
Chlorophyll Synthesis in the Absence of Phytochrome.
One of the last steps in chlorophyll synthesis, the conversion of protochlorophyllide into chlorophyllide, is catalyzed by the light-driven enzyme protochlorophyllide oxidoreductase. Although this enzyme is directly activated by light (26), the phyA phyB phyC rice mutant lacks detectable chlorophyll levels. Conversely, we were able to measure the conversion of protochlorophyllide into chlorophyllide after a red light pulse, detected as a decrease of emission at 635 nm (protochlorophyllide) and increase of emission at 670 nm (chlorophyllide), in the quintuple phytochrome mutant of Arabidopsis (Fig. 2H). These results show that Arabidopsis plants devoid of phytochromes are not totally blind to red light.
Isolation of the Quintuple phyA phyB phyC phyD phyE Mutant in the FT Background.
With the knowledge acquired about the quintuple phytochrome mutant, we tried to isolate the corresponding mutant in the FT (WT) background. We used a phyA phyD phyE mutant population segregating for phyB and phyC, induced to germinate with GA. Etiolated seedlings were selected under red light, allowed to de-etiolate in white light, transplanted to soil, and genotyped for phyB and phyC. Under white light, the phyA phyB phyC phyD phyE mutants were tiny plants as compared with the isogenic line in the ft background and only formed a couple of small siliques (Fig. 2F). None of the seeds obtained germinated in the absence of GA (at least 200 seeds were tested). These results underscore the advantages of having used the ft background, which was recently shown to improve germination (28). The ft mutation caused a delay in flowering time in the quintuple phytochrome mutant background (Fig. 2F) allowing sufficient seed production. Therefore, we decided to continue our work with the genotypes in the ft mutant background that also allow a longer vegetative phase, useful for the subsequent experiments.
The Quintuple Phytochrome Mutant Lacks Growth Responses to Red/Far-Red Ratio.
Green leaves reflect and transmit far-red light more efficiently than red light and, therefore, neighbor plants lower the level of active phytochrome and induce shade-avoidance reactions, which typically include accelerated stem growth (29). In our conditions, the addition of far-red light lowered the red/far-red ratio and promoted hypocotyl growth in the phyA phyC phyD phyE ft mutant, where only phyB is present (Fig. 2C). Conversely, in the phyB phyC phyD phyE ft mutant, where phyA is the only remaining phytochrome, supplementary far-red light reduced hypocotyl growth because of the high-irradiance response mediated by phyA (Fig. 2C). In the WT and ft mutant, supplementary far-red light caused some reduction of hypocotyl growth indicating that the high-irradiance response (30) dominates over the shade-avoidance response at this early stage of the life of the seedling (Fig. 2C; see also ref. 31). Red/far-red reversibility is the classic signature of phytochrome activity, and no response to the red/far-red ratio was observed in the quintuple phytochrome mutant (Fig. 2C).
Developmental Arrest of the Quintuple Phytochrome Mutant Under Red Light.
To test whether photosynthetic light unable to provide photomorphogenic signals is enough to sustain plant development, we cultivated the phyA phyB phyC phyD phyE mutant in the ft background under continuous red light. All of the quintuple phytochrome mutants that germinated under red light (at least 10 plants in two independent experiments) did not develop beyond the cotyledon stage. In subsequent experiments we decided to include sucrose in the media, to increase the lifetime of the plant and the possibility of a light-triggered development. We avoided the contact of sucrose with the aerial tissues (Fig. S4) because the latter provides a morphogenic signal sufficient to complete the life cycle in the dark (32). In some seedlings, sucrose promoted root growth and some additional development of aerial tissues. However, most seedlings (at least 20 plants in two independent experiments) did not develop more than a long hypocotyl and a barely expanded pair of cotyledons; some seedlings showed stem extension above the cotyledonary node and some rudimentary unexpanded leaves (Fig. 2D). We were able to detect chlorophyll in the quintuple mutant even after 8 days under red light (Fig. 2G and Fig. S5). However, seedlings ended up turning brownish, and development became arrested after 6 to 8 weeks (Fig. 2 B and D). Blue light bypassed this block, because the quintuple phytochrome mutant grown under blue light was capable of vegetative development and flowering (Fig. 2E). As controls, the phyA phyB double mutant or the phyB phyC phyD phyE ft quadruple mutant bearing only active phyA were able to develop and flower under red light alone (Fig. 2E). Because under red light Arabidopsis phyA has a half-life of only 30 min (33), the latter demonstrates how little phytochrome is enough but indispensable to complete the life cycle under red light. In rice, phyC alone is not able to promote photomorphogenesis (3). In Arabidopsis, PHYC abundance is reduced in the phyB background, but phyC alone was able to promote leaf growth (phyA phyB phyD phyE ft compared to the quintuple phytochrome mutant in Fig. S6) (34, 35).
Developmental Distortion of the Quintuple Phytochrome Mutant Transferred from White Light to Red Light.
We investigated whether phytochromes are required for normal development after the establishment of the photomorphogenic program. The quintuple phytochrome mutants were grown under white light photoperiods for 14 days (five visible leaves) and then moved to continuous red or blue light as a control. After another 13 days under red light, the leaves that were already expanded, and green turned yellow and the newly emerging leaves failed to expand, whereas the controls under blue light retained green leaves and expanded new leaves (Fig. 2I).
The Endogenous Clock Shows Rhythmic Behavior in the Absence of Phytochromes.
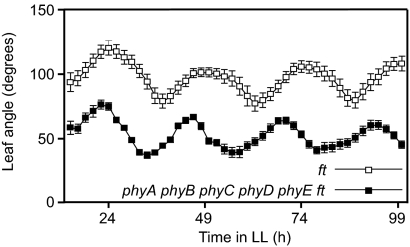
In mammals, cryptochromes are integral components of the circadian oscillator (16), but this is not the case in Arabidopsis (17, 18). Because all of the genes involved in the core transcriptional feedback loops of the Arabidopsis circadian oscillator have been implicated in red-light signaling (36), we speculated that whereas cryptochromes are required for clock function in mammals, phytochromes could be necessary in plants. Plants of the quintuple phytochrome mutant entrained under 12 h white light/12 h dark photoperiods failed to show rhythmic leaf movement when transferred to free running conditions under red light (Fig. S7). Some plants retained leaves in the upward (epinastic) position and others in the downward (hyponastic) position, and this accounts for the large variation in the data. However, this arrhythmic behavior could also result from defects in the input to the clock and/or in the gating of the output. To check for specific defects in the clock itself, the plants were transferred to free-running conditions under white light, where other photoreceptors are active and could satisfy input and output requirements. Despite a shorter period (Fig. S8), absence of phytochromes did not abolish rhythmicity and, indeed, leaf rhythms were still strong, indicating that phytochromes are not required for the oscillations of the endogenous clock (Fig. 3).
Fig. 3.
The circadian clock still functions in the absence of phytochromes. Seeds of the ft and phyA phyB phyC phyD phyE ft mutants were sown on soil and entrained in 12-h white light/12-h dark photoperiods at 23 °C for 10 days. After entrainment, lightning was set to continuous mode (LL) and the angle between primary leaves was measured every 2 h for 3 days. The data represent the average ± SE of four independent experiments with three seedlings each.
Discussion
Despite individual mutations in all five Arabidopsis PHY genes and even the quadruple phytochrome mutants being reported several years ago (34, 35, 37, 38), the isolation of the quintuple mutant has remained elusive. We have obtained the phyA phyB phyC phyD phyE mutant of Arabidopsis. Previous failure could be due to the fact that the seeds of the quintuple mutant do not germinate if GA is not added to the substrate. Here, we initially obtained the quintuple phytochrome mutant in the ft mutant background, which has recently been shown to improve germination (28).
Seed germination of the quintuple phytochrome mutants failed to respond to light, indicating that no other photoreceptors are able to break seed dormancy (Fig. 1A and Fig. S1). GAs were enough to promote high germination levels (Fig. 1B and Fig. S2), implying that phytochrome promotion of GA synthesis is essential for Arabidopsis seed germination (22). Under certain conditions, stratification may substitute for light treatments in the promotion of germination—even if a far-red light pulse is given before stratification to lower Pfr levels (24). However, given the overlapping absorption spectra of Pr and Pfr, some Pfr remains after far-red light. In the quintuple phytochrome mutant in the ft background, stratification produced only a modest increase in germination rates (Fig. 1C). We were not able to germinate the quintuple phytochrome mutant in the FT background without adding GA, which demonstrates the strict requirement of phytochrome activity for Arabidopsis seed germination. Thus, seed germination is the first developmental transition blocked by phytochrome absence in Arabidopsis. In contrast, modern rice cultivars might have lost alleles that are important for seed dormancy (39), and the rice phyA phyB phyC triple mutant does not require exogenous GA for seed germination (3).
Continuous red light failed to inhibit hypocotyl growth in the quintuple phytochrome mutant, and this agrees with the lack of coleoptile responses to red light in the triple phytochrome mutant of rice (3). However, in contrast to the rice triple mutant, the Arabidopsis quintuple mutant does show detectable greening (Fig. 2 B, D, G, and H). This is consistent with an effect mediated by red-light absorption by protochlorophyllide oxidoreductase, which reduces protochlorophyllide to 3,8-divinyl chlorophyllide in one of the last steps of chlorophyll biosynthesis (26). Therefore, phytochromes are not the sole red-light photoreceptors in Arabidopsis. Despite chlorophyll synthesis, the quintuple phytochrome mutant was unable to develop under red light beyond some rudimentary leaves (Fig. 2D). Thus, seedling development is the second transition blocked by phytochrome absence in Arabidopsis, unless this restriction is bypassed by the activation of cryptochromes. Arabidopsis plants can even flower in full darkness if the leaves are in contact with sucrose, but the latter provides a morphogenic cue (32).
Blue light was sufficient to inhibit hypocotyl elongation and promote de-etiolation in the quintuple phytochrome mutant (Fig. 2 A and B). We therefore reject the hypothesis that cryptochromes do not operate in the absence of phytochromes (9). Although phytochromes modulate phototropic responses (40), the quintuple mutant also retained hypocotyl phototropism mediated by phototropins (Fig. S3). In white-light-grown plants, no response to red/far-red ratios was observed. This excludes a detectable role of the photosynthetic apparatus in the growth response to increased far-red levels and is consistent with phytochromes being the only sensor of red/far-red ratios. The robust rhythmic oscillations of leaf position observed in the quintuple phytochrome mutant (Fig. 3) indicates that phytochromes are not part of the core mechanism of the circadian clock, and that the defects observed in clock mutants under red light (15, 36) likely reflect interactions between the endogenous clock and phytochrome signaling.
Plants relay on external cues to adjust their growth and development to the environment that they have to face. The isolation of the quintuple phytochrome mutant allowed us to uncouple the role of light as a signal from the role of light as a source of energy. The known minimum list of inputs from the environment that a plant requires to sustain growth and development was water, essential mineral nutrients, air (carbon dioxide, oxygen), and photosynthetically active radiation. This list has to be expanded to include photomorphogenic cues because in the absence of phytochromes, photosynthetically active radiation is not enough to support plant development. This demonstrates that photomorphogenic signals are so deeply linked to the processes that control plant development that in the absence of these signals the developmental progress becomes arrested or severely impaired.
Materials and Methods
Plant Material and Growth Conditions.
To obtain the quintuple phytochrome mutant, we used phyA-211, a γ-induced deletion (25); phyB-9, a nonsense mutation that eliminates part of the GAF domain needed for phytochrome assembly (4, 41); phyC-2, a T-DNA insertion mutant that does not produce PHYC apoprotein (38); and phyD-201 and phyE-201, two T-DNA insertion mutants described recently (42) that bear insertions in the first exon that also produce deletions of 8 and 26 bp, respectively. All of these mutants are in the Columbia background and are considered null (4, 25, 38, 41, 42). The ft-1 allele was previously introgressed in the Col background (43).
Seeds were sterilized with chlorine in the vapor phase. For experiments with seedlings, sterilized seeds were suspended in 100 μM GA4+7 (Duchefa Biochemie), stratified for 3 days at 4 °C, and pipetted on plates or tubes with Murashige and Skoog (MS) salts media and 0.8% Plant Agar (Duchefa Biochemie); when stated, 2% sucrose was added to the media. The experiments were performed on dedicated growth chambers (Model I30BLL; Percival Scientific). For low fluency red and blue light, we used fluorescent tubes in combination with red and blue filters. For far-red and high fluency blue or red light, we used light-emitting diodes.
Analysis of Segregating Populations.
phyA-211 and phyB-9 mutations were followed in the first instance by their long hypocotyls in far-red and red light, respectively. In subsequent generations, mutations were repeatedly confirmed by genotyping. phyB-9 was genotyped as described in ref. 44. phyC-2, phyD-201, and phyE-201 were genotyped as described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We are grateful to the Arabidopsis Biological Resource Center (Ohio State University) and Peter Quail (University of California, Berkeley) for seed stocks; G. Auge, V. Arana, S. Sánchez, A. Villamil Giraldo, and E. Trejo for technical advice; and other laboratory members for their support. This work was supported by Agencia Nacional de Promoción Científica y Tecnológica Grant PICT-2006-01593, International Centre for Genetic Engineering and Biotechnology Grant CRP/ARG05-02 (to P.D.C.), and Consejo Nacional de Investigaciones Científicas y Técnicas Grant PIP5958. B.S. and M.S.-L. were supported by pre-doctoral fellowships from the YPF foundation and Consejo Nacional de Investigaciones Científicas y Técnicas, respectively.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910446107/DCSupplemental.
References
- 1.Chen M, Chory J, Fankhauser C. Light signal transduction in higher plants. Annu Rev Genet. 2004;38:87–117. doi: 10.1146/annurev.genet.38.072902.092259. [DOI] [PubMed] [Google Scholar]
- 2.Quail PH, et al. Phytochromes: photosensory perception and signal transduction. Science. 1995;268:675–680. doi: 10.1126/science.7732376. [DOI] [PubMed] [Google Scholar]
- 3.Takano M, et al. Phytochromes are the sole photoreceptors for perceiving red/far-red light in rice. Proc Natl Acad Sci USA. 2009;106:14705–14710. doi: 10.1073/pnas.0907378106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rockwell NC, Su YS, Lagarias JC. Phytochrome structure and signaling mechanisms. Annu Rev Plant Biol. 2006;57:837–858. doi: 10.1146/annurev.arplant.56.032604.144208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botto JF, Sanchez RA, Whitelam GC, Casal JJ. Phytochrome A mediates the promotion of seed germination by very low fluences of light and canopy shade light in Arabidopsis. Plant Physiol. 1996;110:439–444. doi: 10.1104/pp.110.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Von Arnim A, Deng XW. Light control of seedling development. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:215–243. doi: 10.1146/annurev.arplant.47.1.215. [DOI] [PubMed] [Google Scholar]
- 7.Cashmore AR. In: Photomorphogenesis in Plants and Bacteria. 3rd Ed. Schafer E, Nagy F, editors. Dordrecht, The Netherlands: Springer; 2006. pp. 199–221. [Google Scholar]
- 8.Casal JJ. In: Photomorphogenesis in Plants and Bacteria. 3rd Ed. Schafer E, Nagy F, editors. Dordrecht, The Netherlands: Springer; 2006. pp. 407–437. [Google Scholar]
- 9.Mohr H. In: Photomorphogenesis in Plants. 2nd Ed. Kendrick RE, Kronenberg GHM, editors. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 353–373. [Google Scholar]
- 10.Casal JJ, Mazzella MA. Conditional synergism between cryptochrome 1 and phytochrome B is shown by the analysis of phyA, phyB, and hy4 simple, double, and triple mutants in Arabidopsis. Plant Physiol. 1998;118:19–25. doi: 10.1104/pp.118.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sellaro R, Hoecker U, Yanovsky M, Chory J, Casal JJ. Synergism of red and blue light in the control of Arabidopsis gene expression and development. Curr Biol. 2009;19:1216–1220. doi: 10.1016/j.cub.2009.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poppe C, Sweere U, Drumm-Herrel H, Schafer E. The blue light receptor cryptochrome 1 can act independently of phytochrome A and B in Arabidopsis thaliana . Plant J. 1998;16:465–471. doi: 10.1046/j.1365-313x.1998.00322.x. [DOI] [PubMed] [Google Scholar]
- 13.Yanovsky M, Casal JJ, Whitelam GC. Phytochrome A, phytochrome B and HY4 are involved in hypocotyl growth responses to natural radiation in Arabidopsis: weak de-etiolation of the phyA mutant under dense canopies. Plant Cell Environ. 1995;18:788–794. [Google Scholar]
- 14.Thapper A, Mamedov F, Mokvist F, Hammarstrom L, Styring S. Defining the Far-Red Limit of Photosystem II in Spinach. Plant Cell. 2009;21:2391–2401. doi: 10.1105/tpc.108.064154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harmer SL. The circadian system in higher plants. Annu Rev Plant Biol. 2009;60:357–377. doi: 10.1146/annurev.arplant.043008.092054. [DOI] [PubMed] [Google Scholar]
- 16.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 17.Devlin PF, Kay SA. Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity. Plant Cell. 2000;12:2499–2510. doi: 10.1105/tpc.12.12.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanovsky MJ, Mazzella MA, Casal JJ. A quadruple photoreceptor mutant still keeps track of time. Curr Biol. 2000;10:1013–1015. doi: 10.1016/s0960-9822(00)00651-5. [DOI] [PubMed] [Google Scholar]
- 19.Emborg TJ, Walker JM, Noh B, Vierstra RD. Multiple heme oxygenase family members contribute to the biosynthesis of the phytochrome chromophore in Arabidopsis. Plant Physiol. 2006;140:856–868. doi: 10.1104/pp.105.074211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohchi T, et al. The Arabidopsis hy2 gene encodes phytochromobilin synthase, a ferredoxin-dependent biliverdin reductase. Plant Cell. 2001;13:425–436. doi: 10.1105/tpc.13.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo M, Nambara E, Choi G, Yamaguchi S. Interaction of light and hormone signals in germinating seeds. Plant Mol Biol. 2009;69:463–472. doi: 10.1007/s11103-008-9429-y. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi S, Smith MW, Brown RG, Kamiya Y, Sun T. Phytochrome regulation and differential expression of gibberellin 3beta-hydroxylase genes in germinating Arabidopsis seeds. Plant Cell. 1998;10:2115–2126. doi: 10.1105/tpc.10.12.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi S, Kamiya Y, Sun T. Distinct cell-specific expression patterns of early and late gibberellin biosynthetic genes during Arabidopsis seed germination. Plant J. 2001;28:443–453. doi: 10.1046/j.1365-313x.2001.01168.x. [DOI] [PubMed] [Google Scholar]
- 24.Yamauchi Y, et al. Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell. 2004;16:367–378. doi: 10.1105/tpc.018143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka R, Tanaka A. Tetrapyrrole biosynthesis in higher plants. Annu Rev Plant Biol. 2007;58:321–346. doi: 10.1146/annurev.arplant.57.032905.105448. [DOI] [PubMed] [Google Scholar]
- 27.Huq E, et al. Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science. 2004;305:1937–1941. doi: 10.1126/science.1099728. [DOI] [PubMed] [Google Scholar]
- 28.Chiang GC, Barua D, Kramer EM, Amasino RM, Donohue K. Major flowering time gene, FLOWERING LOCUS C, regulates seed germination in Arabidopsis thaliana . Proc Natl Acad Sci USA. 2009;106:11661–11666. doi: 10.1073/pnas.0901367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franklin KA. Shade avoidance. New Phytol. 2008;179:930–944. doi: 10.1111/j.1469-8137.2008.02507.x. [DOI] [PubMed] [Google Scholar]
- 30.Staneloni RJ, et al. Bell-like homeodomain selectively regulates the high-irradiance response of phytochrome A. Proc Natl Acad Sci USA. 2009;106:13624–13629. doi: 10.1073/pnas.0906598106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson E, Bradley M, Harberd NP, Whitelam GC. Photoresponses of light-grown phyA mutants of Arabidopsis (phytochrome A is required for the perception of daylength extensions) Plant Physiol. 1994;105:141–149. doi: 10.1104/pp.105.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roldan M, Gomez-Mena C, Ruiz-Garcia L, Salinas J, Martinez-Zapater JM. Sucrose availability on the aerial part of the plant promotes morphogenesis and flowering of Arabidopsis in the dark. Plant J. 1999;20:581–590. doi: 10.1046/j.1365-313x.1999.00632.x. [DOI] [PubMed] [Google Scholar]
- 33.Hennig L, Buche C, Eichenberg K, Schafer E. Dynamic properties of endogenous phytochrome A in Arabidopsis seedlings. Plant Physiol. 1999;121:571–577. doi: 10.1104/pp.121.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franklin KA, Davis SJ, Stoddart WM, Vierstra RD, Whitelam GC. Mutant analyses define multiple roles for phytochrome C in Arabidopsis photomorphogenesis. Plant Cell. 2003;15:1981–1989. doi: 10.1105/tpc.015164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franklin KA, Allen T, Whitelam GC. Phytochrome A is an irradiance-dependent red light sensor. Plant J. 2007;50:108–117. doi: 10.1111/j.1365-313X.2007.03036.x. [DOI] [PubMed] [Google Scholar]
- 36.Ito S, et al. Genetic linkages between circadian clock-associated components and phytochrome-dependent red light signal transduction in Arabidopsis thaliana. Plant Cell Physiol. 2007;48:971–983. doi: 10.1093/pcp/pcm063. [DOI] [PubMed] [Google Scholar]
- 37.Halliday KJ, Salter MG, Thingnaes E, Whitelam GC. Phytochrome control of flowering is temperature sensitive and correlates with expression of the floral integrator FT . Plant J. 2003;33:875–885. doi: 10.1046/j.1365-313x.2003.01674.x. [DOI] [PubMed] [Google Scholar]
- 38.Monte E, et al. Isolation and characterization of phyC mutants in Arabidopsis reveals complex crosstalk between phytochrome signaling pathways. Plant Cell. 2003;15:1962–1980. doi: 10.1105/tpc.012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu XY, Kianian SF, Foley ME. Multiple loci and epistases control genetic variation for seed dormancy in weedy rice (Oryza sativa) Genetics. 2004;166:1503–1516. doi: 10.1534/genetics.166.3.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whippo CW, Hangarter RP. Phototropism: bending towards enlightenment. Plant Cell. 2006;18:1110–1119. doi: 10.1105/tpc.105.039669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wollenberg AC, Strasser B, Cerdan PD, Amasino RM. Acceleration of flowering during shade avoidance in Arabidopsis alters the balance between FLOWERING LOCUS C-mediated repression and photoperiodic induction of flowering. Plant Physiol. 2008;148:1681–1694. doi: 10.1104/pp.108.125468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee H, et al. The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis . Genes Dev. 2000;14:2366–2376. doi: 10.1101/gad.813600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strasser B, Alvarez MJ, Califano A, Cerdan PD. A complementary role for ELF3 and TFL1 in the regulation of flowering time by ambient temperature. Plant J. 2009;58:629–640. doi: 10.1111/j.1365-313X.2009.03811.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.