Abstract
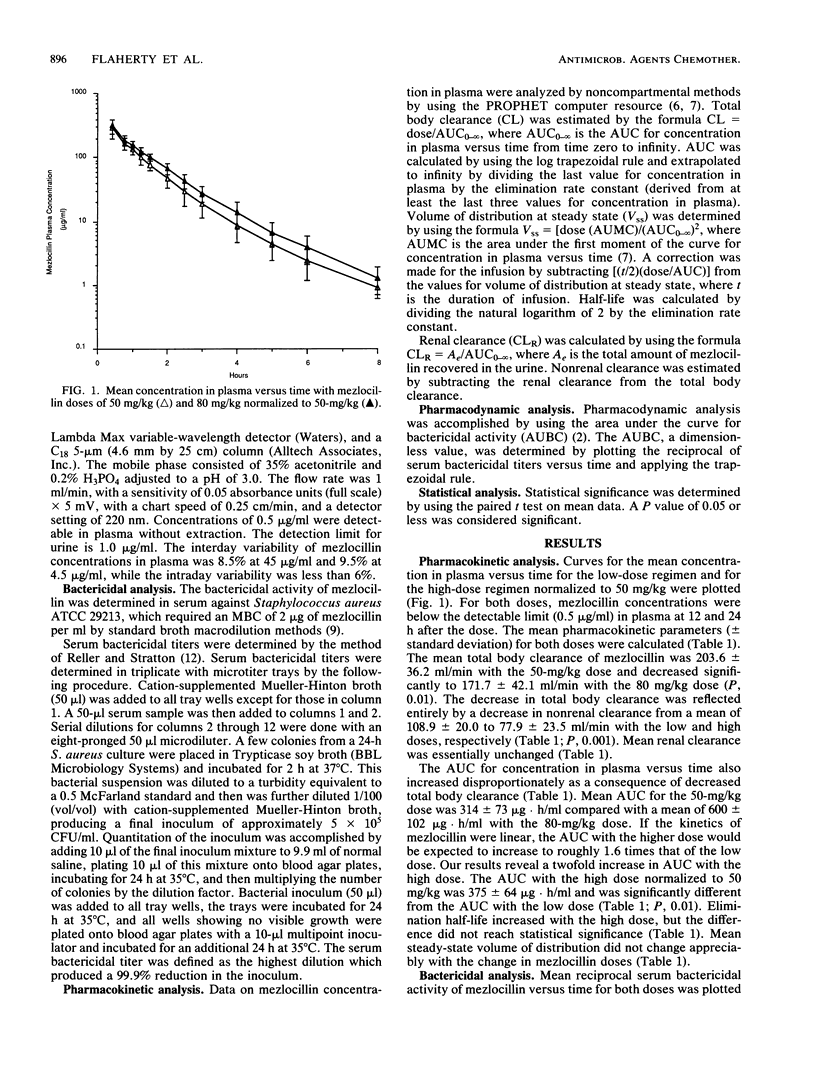
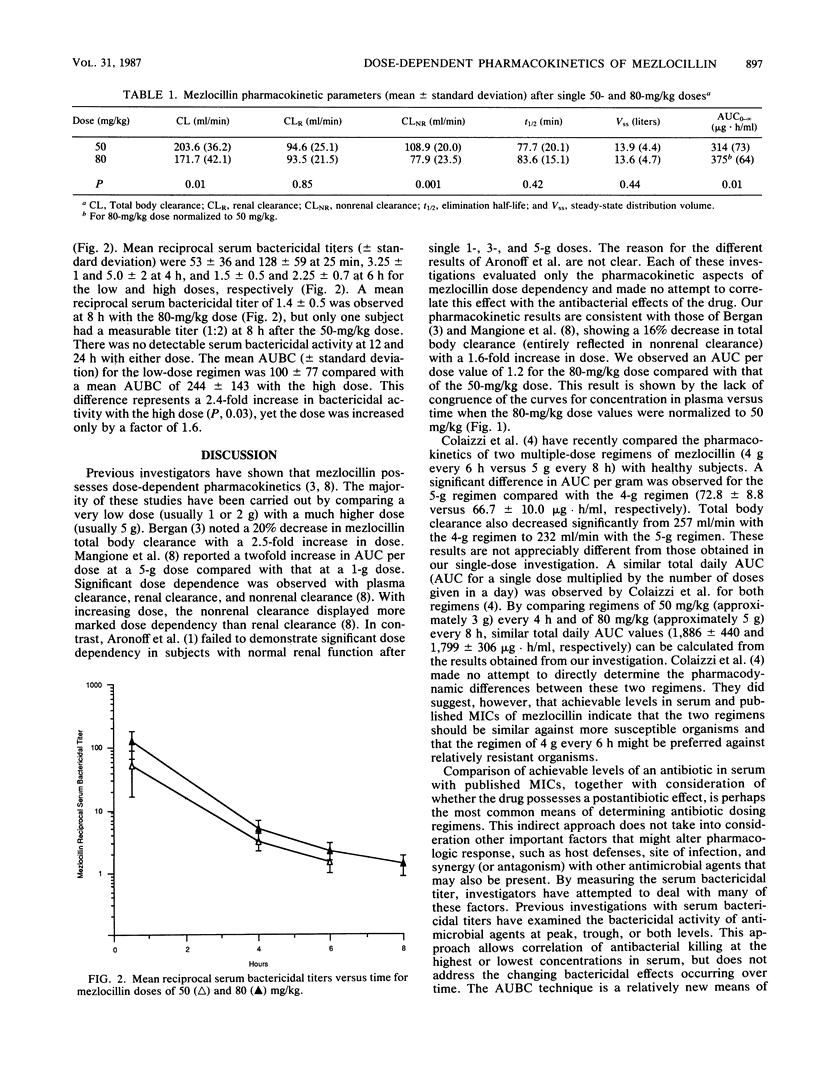
Mezlocillin is subject to dose-dependent pharmacokinetics. Previous studies have examined the pharmacokinetic but not the pharmacodynamic aspects of this effect. The pharmacokinetic disposition of mezlocillin was determined in eight healthy volunteers in a randomized, crossover fashion after single infusions of 50 and 80 mg of mezlocillin per kg of body weight. Plasma and urine were assayed with a specific high-pressure liquid chromatography assay and analyzed by noncompartmental methods. Pharmacodynamic (bactericidal) effects were evaluated from serial serum bactericidal titers obtained after each dose by using the area under the bactericidal activity curve method. The mean mezlocillin total body clearance decreased from 203.6 +/- 36.2 ml/min after the 50-mg/kg dose to 171.7 +/- 42.1 ml/min after the 80-mg/kg dose (P, 0.01). The decreased clearance was reflected by a decrease in nonrenal clearance only (108.9 +/- 20.0 to 77.9 +/- 23.5 ml/min, respectively; P, 0.001). Mean areas under the curve for concentration in plasma versus time normalized to the 50-mg/kg dose were 314 +/- 73 and 375 +/- 64 micrograms X h/ml for the low and high doses, respectively (P, 0.01). No significant changes were observed in the steady-state volume of distribution or elimination half-life. Mean areas under the bactericidal activity curve were 100 +/- 77 and 244 +/- 143 for the 50- and 80-mg/kg doses, respectively. The decrease in mezlocillin clearance and the disproportionate increase in the area under the curve for concentration in plasma versus time, coupled with the observed prolonged bactericidal effects of the 80-mg/kg dose, lend support for administration of mezlocillin at a higher dose less frequently (e.g., 5 g every 8 h). Clinical trials with the higher-dose regimen are warranted to validate these observations.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronoff G. R., Sloan R. S., Stanish R. A., Fineberg N. S. Mezlocillin dose dependent elimination kinetics in renal impairment. Eur J Clin Pharmacol. 1982;21(6):505–509. doi: 10.1007/BF00542046. [DOI] [PubMed] [Google Scholar]
- Barriere S. L., Ely E., Kapusnik J. E., Gambertoglio J. G. Analysis of a new method for assessing activity of combinations of antimicrobials: area under the bactericidal activity curve. J Antimicrob Chemother. 1985 Jul;16(1):49–59. doi: 10.1093/jac/16.1.49. [DOI] [PubMed] [Google Scholar]
- Bergan T. Pharmacokinetics of mezlocillin in healthy volunteers. Antimicrob Agents Chemother. 1978 Dec;14(6):801–806. doi: 10.1128/aac.14.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaizzi P. A., Coniglio A. A., Poynor W. J., Vishniavsky N., Karnes H. T., Polk R. E. Comparative pharmacokinetics of two multiple-dose mezlocillin regimens in normal volunteers. Antimicrob Agents Chemother. 1986 Nov;30(5):675–678. doi: 10.1128/aac.30.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey B. M., Frey F. J., Holford N. H., Lozada F., Benet L. Z. Prednisolone pharmacodynamics assessed by inhibition of the mixed lymphocyte reaction. Transplantation. 1982 Jun;33(6):578–584. doi: 10.1097/00007890-198206000-00002. [DOI] [PubMed] [Google Scholar]
- Mangione A., Janicke D. M., Boudinot F. D., Schultz R. M., Jusko W. J. Dose-dependent pharmacokinetics of mezlocillin in subjects with normal and impaired renal function. J Antimicrob Chemother. 1982 Jan;9 (Suppl A):81–85. doi: 10.1093/jac/9.suppl_a.81. [DOI] [PubMed] [Google Scholar]
- O'Reilly R. A. Dynamic interaction between disulfiram and separated enantiomorphs of racemic warfarin. Clin Pharmacol Ther. 1981 Mar;29(3):332–336. doi: 10.1038/clpt.1981.45. [DOI] [PubMed] [Google Scholar]
- Pancoast S. J., Jahre J. A., Neu H. C. Mezlocillin in the therapy of serious infections. Am J Med. 1979 Nov;67(5):747–752. doi: 10.1016/0002-9343(79)90729-0. [DOI] [PubMed] [Google Scholar]
- Reller L. B., Stratton C. W. Serum dilution test for bactericidal activity. II. Standardization and correlation with antimicrobial assays and susceptibility tests. J Infect Dis. 1977 Aug;136(2):196–204. doi: 10.1093/infdis/136.2.196. [DOI] [PubMed] [Google Scholar]
- Thadepalli H., Roy I., Bach V. T., Webb D. In vitro activity of mezlocillin and its related compounds against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1979 Mar;15(3):487–490. doi: 10.1128/aac.15.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]



