Abstract
A fundamental question in nutritional biology is how distributed systems maintain an optimal supply of multiple nutrients essential for life and reproduction. In the case of animals, the nutritional requirements of the cells within the body are coordinated by the brain in neural and chemical dialogue with sensory systems and peripheral organs. At the level of an insect society, the requirements for the entire colony are met by the foraging efforts of a minority of workers responding to cues emanating from the brood. Both examples involve components specialized to deal with nutrient supply and demand (brains and peripheral organs, foragers and brood). However, some of the most species-rich, largest, and ecologically significant heterotrophic organisms on earth, such as the vast mycelial networks of fungi, comprise distributed networks without specialized centers: How do these organisms coordinate the search for multiple nutrients? We address this question in the acellular slime mold Physarum polycephalum and show that this extraordinary organism can make complex nutritional decisions, despite lacking a coordination center and comprising only a single vast multinucleate cell. We show that a single slime mold is able to grow to contact patches of different nutrient quality in the precise proportions necessary to compose an optimal diet. That such organisms have the capacity to maintain the balance of carbon- and nitrogen-based nutrients by selective foraging has considerable implications not only for our understanding of nutrient balancing in distributed systems but for the functional ecology of soils, nutrient cycling, and carbon sequestration.
Keywords: acellular slime mold, complexity, geometrical framework, nutrition, Physarum polycephalum
Plasmodia of Physarum polycephalum are single multinucleate cells extending up to hundreds of square centimeters. Cytoplasm streams rhythmically back and forth through a network of tubular elements, circulating nutrients and chemical signals and forming pseudopods that allow the organism to navigate around and respond to its environment. Plasmodia are distributed information processors, which, for example, can find the shortest route through a maze to locate food (1), anticipate the timing of periodic events (2), and solve multiobjective foraging problems (3).
Under adequate nutrition, P. polycephalum plasmodia are completely sedentary and grow steadily (4, 5), but on nonnutrient substrates, they migrate a few centimeters per hour (6), directed by external stimuli, including gradients of nutrients such as sugars and proteins (7–12). When two or more identical food sources are presented at various positions to a starved plasmodium, it optimizes the shape of the network to facilitate effective absorption of nutrients (1), and plasmodia select the higher concentration patch of two patches differing in nutrient concentration (3). Can it solve complex nutrient balancing problems by altering its growth form and movement to maintain an optimal ratio of macronutrients in the face of variation in the nutritional environment? We have used experimental designs based on recent advances in nutritional research (13) to show that P. polycephalum can indeed solve such challenges. The first stage was to establish the composition of an optimal diet by confining slime molds to 1 of 35 diets varying in the ratio and concentration of protein and carbohydrate in the food medium and measuring aspects of performance. Next, we challenged slime molds with foods of different nutritional compositions to discover whether plasmodia altered their growth patterns as required to maintain an optimal diet.
Results
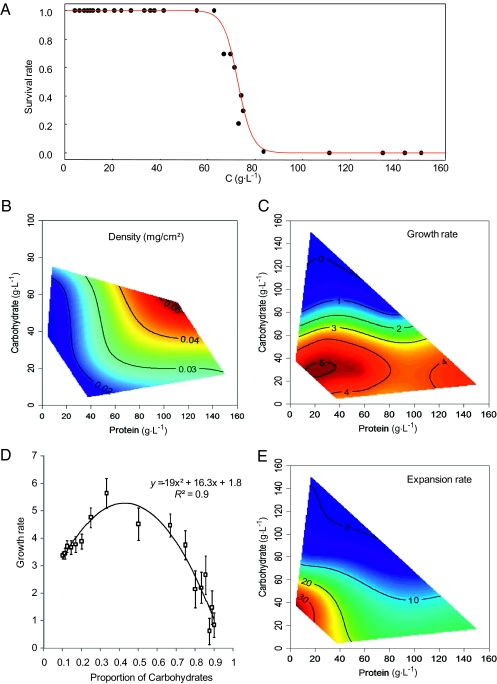
To establish the optimal diet composition in terms of the two major macronutrients, we individually confined 350 plasmodial fragments to 1 of 35 diets varying in both the ratio of protein to carbohydrate and in the total concentration of protein and carbohydrate combined (40, 80, or 160 g·L−1) (Fig. 1A). The area and the mass of the slime molds were measured after 60 h. Response surfaces for variables related to slime mold performance were fitted over the protein–carbohydrate concentration array. Survival depended only on carbohydrate concentration and seemed independent of protein concentration (Fig. 2A and Table S1). Slime molds grew most densely on diets comprising two times more protein than carbohydrate (Fig. 2B, Fig. S1, and Table S2). This resulted from a combination of two factors. First, the mass grown (measured as a rate) was strongly influenced by the carbohydrate content of the diet, falling sharply at concentrations above 60 g·L−1 (Fig. 2C and Table S1), and also by the ratio of protein to carbohydrate in the diet (Fig. 2D). Second, slime molds expanded across a greater area on more diluted diets, thus increasing the surface in contact with the food and compensating for nutrient dilution (Fig. 2E, Fig. S2, and Table S1). Plasmodia grew in a sparser manner on diets with a low density of nutrients, especially protein (Fig. S3A and Table S2). These diets generated plasmodia with directed structures, which migrated (Fig. S4 and Table S2). On substrates with a higher nutrient concentration, the plasmodia were more compact (Fig. S3B and Fig. S3C) and sedentary (Fig. S3B, Fig. S4B, and Table S2). On extremely protein-biased diets, slime molds split into pieces (Fig. S5 and Table S2).
Fig. 1.
Experimental setup. (A) No-choice experiment, diet 1:2, with a total concentration of 40 g·L−1. (B) Choice experiment, diet 6:1 vs. diet 1:2. (C) Multiple-choice experiment. The slime mold was initially placed at the center of the petri dish.
Fig. 2.
Performance responses. Data were recorded for individual slime molds confined for 60 h to 1 of 35 diets varying in both the ratio and total amount of protein and carbohydrate (40 g·L−1, 9 ratios; 80 g·L−1, 17 ratios; and 160 g·L−1, 9 ratios). Response surfaces were visualized using nonparametric thin-plate splines, which were fitted using the fields package (National Center for Atmospheric Research, Boulder, CO) in the statistical software R (36). Red indicates the highest values for the experimental variable on a given response surface, with values descending to lowest values in dark blue regions. (A) Effect of carbohydrate (C) concentration on proportion of slime that survived. The logistic regression analysis yielded a significant relationship between survival and carbohydrate concentration (χ2 = 299.81, P < 0.001; z = 18.91, P < 0.001). (B) Effects of diet composition on slime mold density [final mass (mg)/final area (cm2)]. The slime molds that did not survive were not included. (C) Effects of diet composition on growth rate [initial mass (mg)/final mass (mg)]. (D) Mean growth rate on a mass basis ± SD as a function of the proportion of carbohydrate in the diet: C/(P + C). The polynomial regression analysis yielded a significant relationship between growth rate and proportion of carbohydrate in the diet (R2 = 0.90, F2,14 = 63.46, P < 0.001). (E) Effects of diet composition on expansion rate [(initial area (cm2)/final area (cm2)]. The response surface regression analyses yielded significant relationships as follows: R2 = 0.65, F5,344 = 125.54, P < 0.001 for survival (surface not plotted, Table S1); R2 = 0.50, F5,294 = 57.74, P < 0.001 for density (Fig. 2B and Table S2); R2 = 0.50, F5,344 = 69.80, P < 0.001 for growth rate (Fig. 2C and Table S1), and R2 = 0.54, F5,344 = 80.44, P < 0.001 for expansion rate (Fig. 2E and Table S1).
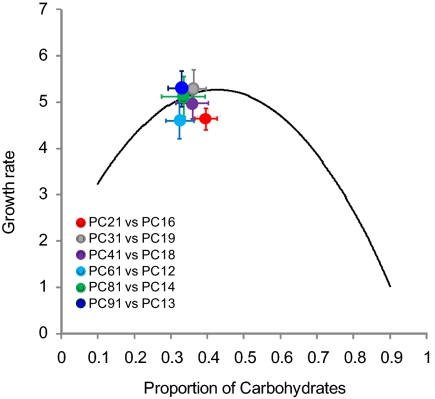
Having established that slime molds grow and survive better on certain concentrations and ratios of protein to carbohydrate, we next aimed to establish whether plasmodia could grow to contact food patches of different nutrient ratios to mix an optimal diet. We conducted two experiments to test this. In the first experiment, slime molds were offered various food pairings (Fig. 1B), and in the second, they were provided with a clock face of 11 foods varying in their ratio of protein to carbohydrate (Fig. 1C). Sixty fragments of slime mold were offered a total of six different two-food choices, varying in the ratio of protein to carbohydrate (Fig. 1B). Nutrient intake was assumed to be proportional to the product of the area covered of the two different food sources and the concentration of nutrients within the foods (e.g., Fig. 3). The six combinations were (i) 2:1 vs. 1:6 protein to carbohydrate (80 g·L−1), (ii) 3:1 vs. 1:9 (80 g·L−1), (iii) 4:1 vs. 1:8 (80 g·L−1), (iv) 6:1 vs. 1:2 (80 g·L−1), (v) 8:1 vs. 1:4 (80 g·L−1), and (vi) 9:1 vs. 1:3 (80 g·L−1). Achieving the same optimal intake of protein and carbohydrate in the face of these six different complementary food pairings would require that slime molds grow to contact the two foods in a precise ratio and area for each food pairing. They were able to do so, in each case, differentially covering the two-food patches to achieve the same diet composition that best supported growth in the no-choice experiment (Fig. 3, Figs. S6 and S7, and Table S3). The slime molds’ growth rate was not significantly different between the six choice experiments and corresponded to the optimal growth rate found in the first experiment (one-way ANOVA: F5,54 = 0.82, P = 0.541; Fig. 3).
Fig. 3.
“Intake” regulation when faced with two foods varying in protein (P) and carbohydrate (C) content. Mean growth rate [initial mass (mg)/final mass (mg)] ± SD as a function of the mean proportion of carbohydrates ingested ± SD [C intake/(P intake + C intake)], measured for the binary choice experiment (SI Text describes method used to estimate intake) (n = 10 slime mold fragments per choice treatment, total dietary concentration of P and C in each food was 80 g·L−1). The black curve comes from the no-choice experiments (Fig. 2C).
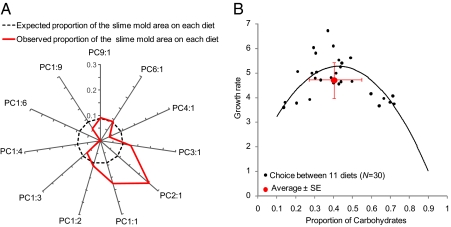
When offered a choice between 11 food patches, plasmodia grew preferentially to cover patches that were of the same composition that supported best performance in the no-choice experiment (binomial test: P < 0.05 for 1:1 and 2:1) (Fig. 4A) and, in so doing, attained optimal growth rates (Fig. 4B).
Fig. 4.
“Intake” regulation when faced with multiple foods varying in protein and carbohydrate content. (A) Observed proportional coverage by slime molds of the different foods in the 11-food array compared with the expected proportion if slime molds had grown to cover foods indiscriminately (n = 30 slime molds). To test whether slime molds preferred particular nutrient ratios over others, we used a binomial test on the number of slime molds covering each diet. The null hypothesis was that slime molds could cover each food with equal probability (we computed the probability to cover a particular food knowing that one slime mold could cover between 1 and 3 foods at a time but not more). Slime molds significantly avoided extremely carbohydrate (C)-biased diets (binomial test: P < 0.05 for 1:4, 1:6, and 1:9 ratios) and preferred diets that contained equal proportions of protein (P) and carbohydrate or were slightly protein-biased (binomial test: P < 0.05 for 1:1 and 2:1 ratios). (B) Mean growth rate in term of mass ± SD as a function of the mean proportion of carbohydrates ingested ± SD [C intake/(P intake + C intake)], measured for the multiple-choice experiment (n = 30 slime molds, 11 foods all with a macronutrient concentration of 80 g·L−1). The black curve comes from the no-choice experiments (Fig. 2C).
Discussion
Distributed control of nutritional regulation has been reported for social insects (14–18) but involves specialized foragers collecting nutrients for the entire colony and responding to nutritional cues from within the nest. Here, we have shown that another distributed system, the plasmodium of P. polycephalum, is able to solve complex nutritional challenges without possessing a centralized processing center or specialized foraging agents. Plasmodia responded to the concentration and balance of macronutrients in their environment and were able to alter their growth form and movement to exploit complementary food resources and regulate the supply of carbohydrate and protein to a target ratio that maximized performance.
How nutritional cues are integrated within the cellular matrix of a slime mold plasmodium is not known, but they seem likely to be fully distributed, involving local nutrient sensing mechanisms, movement, and growth responses (19). Growth of the slime molds involves two different processes: extending the area of network available to collect nutrients and increasing mass. These processes are comparable to what has been called explorative and exploitative growth, respectively, in fungal colonies (20). Increasing the area of substrate covered occurs both when exploiting multiple complementary food resources and when responding to dilution (6, 21) and the ratio of nutrients in the medium. We observed that the plasmodia tended to migrate and develop in a sparser and more extensive manner on substrates with a lower concentration of nutrients. A recent study showed that P. polycephalum increases allocation toward exploratory growth when its current food resource is diluted (22). On substrates with higher nutrient concentrations, the plasmodia in the present study grew more compactly, with slime molds on high-protein diets producing a veinless structure, indicating that media able to support rapid growth depress migration, allowing the organism to remain at a site until nutrients are exhausted (11, 23).This result has strong parallels with observations in other distributed systems, such as bacterial and fungal colonies, in which the overall pattern of growth is influenced by varying the concentration of nutrient (20, 24). The ratio of protein and carbohydrate in the diet also had a strong influence on migration distance. Excluding those diets in which slime molds typically died, migration distance was least when the protein-to-carbohydrate ratio in the diet was near optimal and increased markedly as the ratio shifted away from the optimum (Fig. S4C). Similar effects of macronutritional imbalance on migration have been reported in animals, sometimes with profound effects at the population level (25, 26).
Our study revealed two other effects of elevated nutrient concentrations. First, we observed that plasmodia split when protein concentration was high. Fragmentation is a form of morphogenesis that brings about an increase in the number of independent organisms. It can occur as a result of exposure to UV irradiation (27) or low temperatures (28). In another more primitive acellular slime mold, Echinostelium minutum, the plasmodium divides itself when nuclei reach a certain number. Thus, the fragmentation we observed might be an expression of plasmodial division under conditions in which nitrogenous resources for growth are abundant (29). A second consequence of high nutrient concentrations was that the higher the concentration of carbohydrate in the medium, the higher was the incidence of mortality. This may be related to the sensitivity of slime molds to high osmotic pressure (11, 30, 31). The osmotic effects of high sugar concentrations have been suggested to cause both reduced migration rate (31, 32) and negative chemotaxis (11, 33). Glucose has been shown to be repellent at high concentration (56 g·L−1) (33); here, we found that none of the slime molds survived when glucose concentration exceeded 60 g·L−1.
Our demonstration that slime mold foraging and performance are determined by multiple nutrient currencies has implications beyond the behavior of these strange organisms. Understanding the nutritional currencies that shape exploitation of food resources by organisms such as slime molds and fungi, and the consequences of such nutritional decisions for growth, form, and function, is centrally important to developing models of soil and litter community ecology, biodiversity, and remediation and have broader significance for local and global nutrient cycling and carbon sequestration (34, 35).
Methods
Species.
P. polycephalum is an acellular slime mold that is typically yellow in color and inhabits shady, cool, and moist areas. In the wild, P. polycephalum eats bacteria and dead organic matter. For the experiment, we used synthetic foods varying in the ratio and concentration of protein and digestible carbohydrate (37).
No-Choice Diet Experiment.
We confined 350 fragments of slime mold (mean weight ± SD: 15.6 ± 4.4 mg, mean area ± SD: 0.16 ± 0.4 cm2) to 1 of 35 diets varying in both the protein-to-carbohydrate ratio and total concentration of protein + carbohydrate. We tested 17 ratios (9:1, 8:1, 7:1, 6:1, 5:1, 4:1, 3:1, 2:1, 1:1, 1:2, 1:3, 1:4, 1:5, 1:6, 1:7, 1:8, and 1:9) at a concentration of 80 g·L−1 and 9 ratios (9:1, 6:1, 4:1, 2:1, 1:1, 1:2, 1:4, 1:6, and 1:9) at two other concentrations of 40 g·L−1 and 160 g·L−1. Each slime mold was placed in the center of a Petri dish directly on the food (diameter, 100 mm; height, 15 mm) (Fig. 1A).
Choice Diet Experiment.
We allowed 60 fragments of slime mold (mean weight ± SD: 14.03 ± 3.9 mg, mean area ± SD: 0.15 ± 0.3 cm2) to select between foods differing in their content of protein and carbohydrate. The experiment consisted of six binary choices using 12 protein-to-carbohydrate ratios with a protein + carbohydrate concentration of 80 g·L−1 (9:1 vs. 1:3, 8:1 vs. 1:4, 6:1 vs. 1:2, 4:1 vs. 1:8, 3:1 vs. 1:9, and 2:1 vs. 1:6). Each slime mold was placed in the center of the Petri dish (diameter, 100 mm; height, 15 m) 5 mm away from the two food sources (diameter, 20 mm) (Fig. 1B).
Multiple-Choice Diet Experiment.
We allowed 30 fragments of slime mold (mean weight ± SD: 16.03 ± 4.6 mg, mean area ± SD: 0.17 ± 0.5 cm2) to select between 11 foods differing in their content of protein and carbohydrate (9:1, 6:1, 4:1, 3:1, 2:1, 1:1, 1:2, 1:3, 1:4, 1:6, and 1:9), all with a total protein + carbohydrate concentration of 80 g·L−1. The food sources were placed from the most protein-biased food to the most carbohydrate-biased food in a clockwise manner. Each slime mold was placed in the center of the Petri dish (diameter, 150 mm; height, 25 mm) 40 mm away from each food source (diameter, 20 mm) (Fig. 1C).
Measures.
Pictures of the slime mold were taken at different times (0, 5, 19, 24, 29, 43, 48, and 60 h) after introduction. The slime molds were weighed before they were placed in the center of the Petri dish and again at the end of the experiment at 60 h. The area of each slime mold was measured for all the images.
Supplementary Material
Acknowledgments
We thank Professor John Bonner for his comments on a previous version of the manuscript. A.D. was supported by a postdoctoral grant from the University of Sydney, by a Fyssen Foundation research grant, and by the Centre National de la Recherche Scientifique. S.J.S. was supported by the Australian Research Council Federation and Laureate fellowships. M.B. was supported by the Australian Research Council (Grant DP0878924), the Human Frontier Science Program, and the University of Sydney. T.L. was supported by the Human Frontier Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912198107/DCSupplemental.
References
- 1.Nakagaki T, Yamada H, Tóth A. Maze-solving by an amoeboid organism. Nature. 2000;407:470. doi: 10.1038/35035159. [DOI] [PubMed] [Google Scholar]
- 2.Saigusa T, Tero A, Nakagaki T, Kuramoto Y. Amoebae anticipate periodic events. Phys Rev Lett. 2008;100:018101–018104. doi: 10.1103/PhysRevLett.100.018101. [DOI] [PubMed] [Google Scholar]
- 3.Latty T, Beekman M. Going into the light: Food quality and the risk of light exposure affect patch choice decisions in the acellular slime mould Physarum polycephalum. Ecology. 2009 doi: 10.1890/09-0358.1. in press. [DOI] [PubMed] [Google Scholar]
- 4.Ashworth JM, Dee J. The Biology of Slime Moulds. London: Edward Arnold Ltd.; 1975. [Google Scholar]
- 5.Sauer HW. Developmental Biology of Physarum. Cambridge, UK: Cambridge Univ Press; 1982. [Google Scholar]
- 6.Halvorsrud R, Wagner G. Growth patterns of the slime mold Physarum on a nonuniform substrate. Phys Rev E. 1998;57:941–948. [Google Scholar]
- 7.Carlile MJ. Nutrition and chemotaxis in the myxomycete Physarum polycephalum: The effect of carbohydrates on the plasmodium. J Gen Microbiol. 1970;63:221–226. doi: 10.1099/00221287-63-2-221. [DOI] [PubMed] [Google Scholar]
- 8.Ueda T, Terayama K, Kurihara K, Kobatake Y. Threshold phenomena in chemoreception and taxis in slime mold Physarum polycephalum. J Gen Physiol. 1975;65:223–234. doi: 10.1085/jgp.65.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durham ACH, Ridgway EB. Control of chemotaxis in Physarum polycephalum. J Cell Biol. 1976;69:218–223. doi: 10.1083/jcb.69.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chet I, Naveh A, Henis Y. Chemotaxis of Physarum polycephalum towards carbohydrates, amino acids and nucleotides. J Gen Microbiol. 1977;102:145–148. [Google Scholar]
- 11.Knowles DJC, Carlile MJ. The chemotactic response of plasmodia of the myxomycete Physarum polycephalum to sugars and related compounds. J Gen Microbiol. 1978;108:17–25. doi: 10.1099/00221287-108-1-17. [DOI] [PubMed] [Google Scholar]
- 12.Kincaid RL, Mansour TE. Chemotaxis toward carbohydrates and amino acids in Physarum polycephalum. Exp Cell Res. 1978;116:377–385. doi: 10.1016/0014-4827(78)90461-5. [DOI] [PubMed] [Google Scholar]
- 13.Simpson SJ, Raubenheimer D. A multi-level analysis of feeding behaviour: The geometry of nutritional decisions. Phil Trans R Soc London B. 1993;342:381–402. [Google Scholar]
- 14.Page RE, Jr., Scheiner R, Erber J, Amdam GV. 8. The development and evolution of division of labor and foraging specialization in a social insect (Apis mellifera L.) Curr Top Dev Biol. 2006;74:253–286. doi: 10.1016/S0070-2153(06)74008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page RE, Linksvayer T, Amdam GV. Social life from solitary regulatory networks: A paradigm for insect sociality. In: Gadau G, Fewell JH, editors. Organization of Insect Societies. Cambridge, MA: Harvard Univ Press; 2009. [Google Scholar]
- 16.Cassill DL, Tschinkel WR. Information flow during social feeding in ant societies. In: Detrain C, Deneubourg JL, Pasteels JM, editors. Information Processing in Social Insects. Basel: Birkhäuser; 1999. pp. 69–81. [Google Scholar]
- 17.Dussutour A, Simpson SJ. Communal nutrition in ants. Curr Biol. 2009;19:740–744. doi: 10.1016/j.cub.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Dussutour A, Simpson SJ. Carbohydrate regulation in relation to colony growth in ants. J Exp Biol. 2008;211:2224–2232. doi: 10.1242/jeb.017509. [DOI] [PubMed] [Google Scholar]
- 19.Bonner J. The Social Amoebae: The Biology of Cellular Slime Molds. Princeton: Princeton Univ Press; 2009. [Google Scholar]
- 20.Ritz K, Crawford J. Quantification of the fractal nature of colonies of Trichoderma viride. Mycol Res. 1990;94:1138–1141. [Google Scholar]
- 21.Takamatsu A, Takaba E, Takizawa G. Environment-dependent morphology in plasmodium of true slime mold Physarum polycephalum and a network growth model. J Theor Biol. 2009;256:29–44. doi: 10.1016/j.jtbi.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Latty T, Beekman M. Food quality affects search strategy in the acellular slime mould, Physarum polycephalum. Behav Ecol. 2009;20:1160–1167. [Google Scholar]
- 23.Daniel JW, Babcock KL, Sievert AH, Rusch HP. Organic requirements and synthetic media for growth of the myxomycete Physarum polycephalum. J Bacteriol. 1963;86:324–331. doi: 10.1128/jb.86.2.324-331.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Jacob E, et al. Generic modelling of cooperative growth patterns in bacterial colonies. Nature. 1994;368:46–49. doi: 10.1038/368046a0. [DOI] [PubMed] [Google Scholar]
- 25.Simpson SJ, Sword GA, Lorch PD, Couzin ID. Cannibal crickets on a forced march for protein and salt. Proc Natl Acad Sci USA. 2006;103:4152–4156. doi: 10.1073/pnas.0508915103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simpson SJ, Raubenheimer D, Charleston MA, Clissold FJ the ARC-NZ Vegetation Function Network Herbivory Working Group. Modelling nutritional interactions: From individuals to communities. Trends Ecol Evol. 2009;25:53–60. doi: 10.1016/j.tree.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Kakiuchi Y, Takahashi T, Murakami A, Ueda T. Light irradiation induces fragmentation of the plasmodium, a novel photomorphogenesis in the true slime mold Physarum polycephalum: Action spectra and evidence for involvement of the phytochrome. Photochem Photobiol. 2001;73:324–329. doi: 10.1562/0031-8655(2001)073<0324:liifot>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Kakiuchi Y, Ueda T. Fragmentation of the plasmodium into equally sized pieces by low temperatures in the true slime mold Physarum polycephalum: A new morphogenesis. Protoplasma. 1999;206:131–136. [Google Scholar]
- 29.Ueda T. An intelligent slime mold: A self-organizing system of cell shape and information. Lecture Notes in Complex System. 2005;3:221–253. [Google Scholar]
- 30.Hohl HR, Raper KB. Nutrition of cellular slime mold. II. Growth of Polysphondylium pallidum in axenic culture. J Bacteriol. 1963;85:200–206. doi: 10.1128/jb.85.1.199-206.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knowles DJC, Carlile MJ. Growth and migration of plasmodia of the myxomycete Physarum polycephalum: The effect of carbohydrates, including agar. J Gen Microbiol. 1978;108:9–15. doi: 10.1099/00221287-108-1-9. [DOI] [PubMed] [Google Scholar]
- 32.Taylor RL, Mallette MF. Growth of Physarum gyrosum on agar plates and in liquid culture. Antimicrob Agents Chemother. 1976;10:613–617. doi: 10.1128/aac.10.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueda T, Muratsugu M, Kurihara K, Kobatake Y. Chemotaxis in Physarum polycephalum. Effects of chemicals on isometric tension of the plasmodial strand in relation to chemotactic movement. Exp Cell Res. 1976;100:337–344. doi: 10.1016/0014-4827(76)90157-9. [DOI] [PubMed] [Google Scholar]
- 34.Crawford JW, Harris JA, Ritz K, Young IM. Towards an evolutionary ecology of life in soil. Trends Ecol Evol. 2005;20:81–87. doi: 10.1016/j.tree.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 35.De Deyn GB, Cornelissen JHC, Bardgett RD. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol Lett. 2008;11:516–531. doi: 10.1111/j.1461-0248.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee KP, et al. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc Natl Acad Sci USA. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dussutour A, Simpson SJ. Description of a simple synthetic diet for studying nutritional responses in ants. Insectes Soc. 2008;55:329–333. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.