Abstract
Islet β-cells express both insulin receptors and insulin-signaling proteins. Recent evidence from rodents in vivo and from islets isolated from rodents or humans suggests that the insulin signaling pathway is physiologically important for glucose sensing. We evaluated whether insulin regulates β-cell function in healthy humans in vivo. Glucose-induced insulin secretion was assessed in healthy humans following 4-h saline (low insulin/sham clamp) or isoglycemic-hyperinsulinemic (high insulin) clamps using B28-Asp insulin that could be immunologically distinguished from endogenous insulin. Insulin and C-peptide clearance were evaluated to understand the impact of hyperinsulinemia on estimates of β-cell function. Preexposure to exogenous insulin increased the endogenous insulin secretory response to glucose by ≈40%. C-peptide response also increased, although not to the level predicted by insulin. Insulin clearance was not saturated at hyperinsulinemia, but metabolic clearance of C-peptide, assessed by infusion of stable isotope–labeled C-peptide, increased modestly during hyperinsulinemic clamp. These studies demonstrate that insulin potentiates glucose-stimulated insulin secretion in vivo in healthy humans. In addition, hyperinsulinemia increases C-peptide clearance, which may lead to modest underestimation of β-cell secretory response when using these methods during prolonged dynamic testing.
Keywords: beta cell, type 2 diabetes mellitus, insulin resistance, insulin clearance, C-peptide
Insulin resistance and insulin secretory defects contribute to the pathogenesis of type 2 diabetes mellitus (T2D). These processes have largely been thought of as separate. Insulin and insulin-like growth factor–1 (IGF1) receptors and major insulin receptor substrates, IRS-1 through IRS-4, are present and functional in islets and/or β-cells (1, 2). Furthermore, β-cell–specific insulin receptor knockout (βIRKO) mice manifest defective glucose-stimulated insulin secretion and progressive glucose intolerance (3), leading to overt diabetes in some animals (1). Likewise, deletion of IRS-1, IRS-2, or Akt2 alters glucose sensing and β-cell growth (4–6). These murine models provide evidence implicating insulin signaling in the regulation of insulin secretion within β-cells and suggest a potential link between insulin resistance and the insulin secretory defect manifest in T2D.
In humans, insulin signaling proteins are more abundant in β-cells than in α-cells (7). Insulin-stimulated insulin secretion has been demonstrated to occur in isolated human β-cells (8) suggesting that cellular and rodent studies may be applicable to humans. However, although the presence of functional insulin receptors in β-cells is clearly established in rodents and humans (9, 10), it has previously been difficult to study the physiologic importance of this signaling pathway in humans in vivo. Insulin has largely been considered to have inhibitory effects on β-cells. Exogenous insulin infusion during euglycemia does not stimulate insulin secretion (11, 12). Yet effects in the basal, euglycemic state may differ from the stimulated, hyperglycemic condition. To date any effects of insulin to alter glucose stimulated insulin secretion in humans remains incompletely understood, and careful study of the feedback of insulin on insulin production has been limited by difficulty in distinguishing endogenous insulin from the infused product. To address this barrier, we developed a method of administering insulin analogs and using selective immunoassays to accurately discriminate endogenous from exogenous insulin.
Here, we evaluate whether β-cells are insulin responsive in healthy humans in vivo by determining whether preexposure to high physiologic insulin would alter the insulin secretory response to glucose. We performed paired isoglycemic-stepped hyperglycemic clamp studies in healthy persons, both with hyperinsulinemia and under sham clamp conditions using saline as a time and volume control. We administered an insulin analog that is biologically equivalent but can be distinguished immunogenically from endogenous insulin to permit a more direct assessment of β-cell function. We also assessed the impact of clamp conditions on insulin and C-peptide clearance. We found that, in healthy persons, preexposure to hyperinsulinemia potentiated glucose-stimulated insulin secretion.
Results
Validation of Differential Determination of Endogenous vs. Exogenous Insulin.
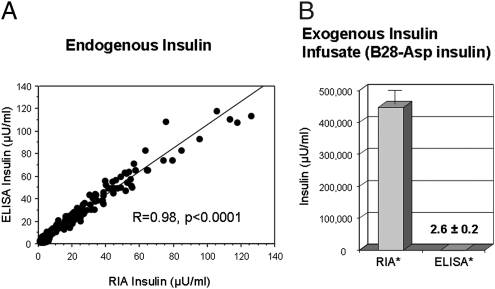
To directly assess endogenous insulin secretion in the setting of exogenous insulin infusion, we used a dual immunological approach with an RIA that detects total insulin (endogenous and exogenous) and an ELISA that detects only endogenous or native human insulin, not the B28-Asp exogenously administered insulin analog. Endogenous insulin alone was assessed across the physiologic range during glucose administration in the absence of exogenous insulin (during sham clamp graded glucose infusion) by both RIA and ELISAs, and insulin levels were compared. Fasting insulin concentrations were similar in both assays [4.7 ± 0.9 vs. 5.1 ± 0.7 μU/mL (32.8 ± 6.2 vs. 35.4 ± 4.9 pmol/L), RIA vs. ELISA, P = 0.3]. Likewise, the two assays were comparable for the assessment of endogenous insulin over a wide range of concentrations (R = 0.98, P < 0.0001; Fig. 1A). To demonstrate the ELISA would not detect the exogenous insulin infused during the clamp study, both ELISA and RIA assays were used to evaluate the insulin in the B28-Asp insulin infusate. By RIA, total insulin in the infusate was 447,000 ± 41,000 μU/mL (310 ± 28 × 104 pmol/L) but was essentially undetectable by ELISA [2.6 ± 0.2 μU/mL (18.1 ± 1.4 pmol/L); P < 0.10−11], demonstrating a greater than 100,000-fold selectivity of the ELISA for native insulin (Fig. 1B). In human serum samples, the ELISA was also effective in differentiation of exogenous and endogenous insulin. Mean insulin concentrations measured over 3 h at steady state (60–240 min) during B28-Asp insulin clamps differed using the two assays [5.4 ± 1.0 vs. 157.3 ± 17.0 μU/mL (37.5 ± 0.7 vs. 1092.4 ± 118.1 pmol/L), ELISA vs. RIA, P < 0.00004], consistent with ELISA detection of endogenous but not exogenous insulin. Together these data demonstrate the ELISA detects only endogenous or native human insulin and not the B28-Asp insulin analog, whereas the RIA will detect both.
Fig. 1.
Endogenous and exogenous B28-Asp insulin (Novolog) are immunologically distinct. Regression plot of endogenous serum insulin assessed by RIA or ELISA during glucose induced insulin secretion in healthy persons demonstrates excellent agreement between assays (A). In contrast, B28-Asp insulin infusate can be detected by the RIA assay but not by the ELISA used (B). *RIA assay was performed at 1:100,000 dilution; ELISA was performed undiluted.
Effects of Insulin on Glucose-Stimulated Insulin Secretion.
Fasting insulin concentrations were similar on the two study days [5.1 ± 0.7 vs. 5.5 ± 1.0 μU/mL (33.3 ± 4.8 vs. 38.0 ± 6.9 pmol/L), ELISA, sham vs. hyperinsulinemic clamp, P = 0.6]. By design, total insulin levels achieved during the two clamps differed and remained low [3.5 ± 0.5 μU/mL (24.6 ± 3.4 pmol/L) by RIA (60–240 min) on the day of the sham clamp] but were markedly increased during the hyperinsulinemic clamp with steady-state concentrations of 157.3 ± 17.0 μU/mL (1,089.3 ± 117.7 pmol/L), providing a differing and high physiologic level of insulin preexposure of the β-cells before the glucose infusion (P < 0.00004; Fig. S2A). Subjects were insulin sensitive, demonstrated by steady-state whole-body glucose use rates (M) during the fourth hour of the hyperinsulinemic clamp of 11.1 ± 2.3 mg·kg−1·min−1 (61.5 ± 12.7 μmol·kg−1·min−1). The total volume infused on sham and insulin clamp days was similar (1,846 ± 96 vs. 1,942 ± 102 mL, sham vs. insulin clamp, respectively, P = 0.2). Importantly, glucose concentrations before the graded glucose infusion were similar during the sham and isoglycemic-hyperinsulinemia clamps (repeated-measures ANOVA, P = 0.4). Likewise, glucose concentrations achieved during graded glucose infusions (to promote insulin secretion) were highly similar (repeated-measures ANOVA, P = 0.6) (Fig. S2B). Glucose use was at steady state by 30 min and remained constant without decline over the 4 h of the isoglycemic clamp (Fig. S2C). During glucose-stimulated insulin secretion, while dextrose was administered at a rate of 8 and 10 mg/kg per min for 40 min each step on the day of sham clamp, glucose requirements to maintain similar glycemia were markedly higher following preexposure to insulin (an additional 12 ± 2 and 20 ± 3 mg·kg−1·min−1, first and second step respectively, hyperinsulinemic vs. sham clamp, P < 0.0001).
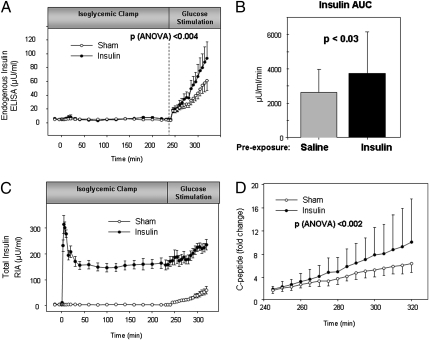
After glucose stimulation, endogenous insulin concentrations were significantly greater with preexposure to insulin than for the same individuals with preexposure to saline (Fig. 2 A and B). The glucose-induced insulin secretion rate, calculated using the ELISA insulin determination, was 40% higher following preexposure to exogenous insulin than following saline [0.60 ± 0.15 vs. 1.04 ± 0.29 μU/mL/min (4.1 ± 1.0 vs. 7.2 ± 2.0 pmol/L/min), sham vs. insulin, P = 0.04]. Although the difference in the insulin secretion rate following preexposure to insulin compared with saline was the primary prespecified study endpoint, the difference in glucose-stimulated insulin secretion (GSIS) was significant by the end of the first glucose step (time 280 min, repeated-measures ANOVA, P = 0.05) and more marked when considering all time points following GSIS (repeated-measures ANOVA, P < 0.004). Likewise, endogenous insulin calculated as area under the curve (AUC) increased 42% with insulin preexposure (2,617 ± 1,368 vs. 3,723 ± 2,428 μU/mL/min, sham vs. insulin clamp, P < 0.03). Similarly, total insulin (endogenous and exogenous assessed by RIA) showed greater increase during GSIS following insulin preexposure (Fig. 2C). Together, these data suggest that exogenous insulin does not suppress endogenous insulin production under isoglycemic conditions; in fact, preexposure to insulin augments glucose stimulated insulin secretion.
Fig. 2.
Preexposure to isoglycemic hyperinslinemia potentiates glucose-induced insulin secretion. Following preexposure to insulin, similar glucose stimulation promotes a greater increase in insulin secretion (A), also demonstrated by an increased insulin area-under-the-curve β-cell response to glucose following preexposure to insulin in the isoglycemic-hyperinsulinemic clamp than to saline in the sham clamp (B). Total insulin (endogenous and exogenous) was higher by trial design during isoglycemic hyperinsulinemic clamp compared with sham, and showed greater increase during GSIS following insulin preexposure compared with sham (C). Likewise, the fold increase in C-peptide is greater following preexposure to higher compared with lower insulin conditions (D). Isoglycemic-hyperinsulinemic clamp (•), sham clamp (○).
Exogenous insulin has previously been suggested to suppress insulin secretion, based on reduced C-peptide levels following insulin (11, 12). Of note, at the end of the 4 h of sham compared with isoglycemic-hyperinsulinemic clamp, endogenous insulin concentrations (ELISA) were similar. Consistent with all other reports, we too demonstrated reduced C-peptide concentrations over the isoglycemic period of insulin administration, C-peptide concentrations were lower at the end of the 4 h of isoglycemic-hyperinsulinemic clamp [1.2 ± 0.1 vs. 0.87 ± 0.1 ng/mL (0.41 ± 0.03 vs. 0.29 ± 0.04 nmol/L) sham vs. insulin clamp, P = 0.05]. However consistent with our hypothesis that insulin preexposure may augment glucose-stimulated insulin secretion, during hyperglycemic stimulation the fold increase in C-peptide was 38% greater following exposure to high compared with low physiologic insulin concentrations (Fig. 2D). Differences between concentrations of endogenous insulin and C-peptide could occur because of altered secretion (13) or because of altered clearance rates of either insulin or C-peptide under the hyperinsulinemic clamp conditions. Therefore, we directly assessed both insulin clearance and C-peptide clearance at both low (sham clamp) and high physiologic insulinemia (discussed below).
Although the two experimental conditions were associated with closely matched glycemia, the hyperinsulinemic clamp induced changes in additional hormones and metabolites that could participate in the altered β-cell secretory response. Concentrations of glucagon, free fatty acids (FFA), potassium (K), cortisol, and catecholamines, epinephrine, and norepinephrine were all similar at baseline on the 2 study days (Fig. S3). However, glucagon (45.1 ± 2.7 vs. 29.1 ± 3.5 pg/mL, sham vs. insulin clamp respectively, P < 0.0002), FFA (0.51 ± 0.10 vs. 0.03 ± 0.002 mEq/L, P = 0.0002), and potassium (4.1 ± 0.1 vs. 3.6 ± 0.1 mg/dL, P = 0.0001) were significantly lower and norepinephrine (0.11 ± 0.02 vs. 0.17 ± 0.02 ng/mL, P = 0.005) higher at the end of the 4-h isoglycemic hyperinsulinemic clamp (time 240 min) compared with sham clamp.
Insulin Clearance.
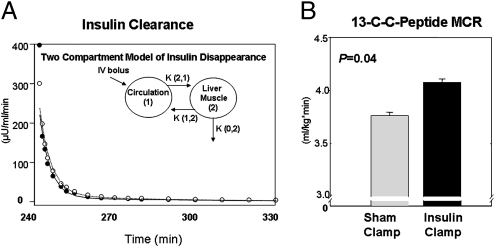
Higher insulin concentrations achieved during GSIS following preexposure to exogenous insulin might be a function of increased secretion or decreased insulin clearance. The latter might occur if clearance mechanisms had been saturated by the exogenous insulin infusion. Exogenous insulin, calculated by total (RIA) minus endogenous (ELISA) levels did not rise over the interval of GSIS 151.5 ± 13.7 μU/mL (1,052.2 ± 95.1 pmol/L; Fig. S2A), suggesting that insulin clearance is not altered. However, to measure insulin clearance directly, we performed paired studies again with low insulin (saline/sham clamp) or high insulin (isoglycemic hyperinsulinemic clamp using B28-Asp insulin). In these studies, Regular human insulin, indistinguishable from endogenous insulin by the ELISA, was administered at 240 min. Again glucose levels were similar on the 2 study days, both before and during the assessment of insulin clearance (Fig. S4A). Whole-body (including hepatic) insulin clearance fit a two-compartment model and was cleared at a similar rate under both low and high insulin preexposure conditions (Fig. 3A and Fig. S4B). Likewise, analysis revealed no significant difference in any of the fractional transfer rates between the two groups. Thus, we conclude that there was no difference in kinetics of insulin disappearance between subjects under low and high insulin conditions.
Fig. 3.
Insulin clearance was not different following preexposure to isoglycemic-hyperinsulinemic conditions, while C-peptide clearance increased during isoglycemic-hyperinsulinemic clamp. Insulin clearance rates were similar following preexposure to high physiologic insulin concentrations during isoglycemic clamp or sham clamp and fit a two-compartment model of disappearance. (A) Isoglycemic-hyperinsulinemic clamp (•), sham clamp (○). Isoglycemic-hyperinsulinemic clamp conditions induce increased 13C-C-peptide metabolic clearance (P = 0.04) (B) Insulin (dark filled bar) or sham (light filled bar) clamp.
C-Peptide Clearance.
As endogenous insulin concentrations were higher during glucose-stimulated insulin secretion following preexposure to hyperinsulinemia, and as insulin clearance was not saturated, we assessed the effects of hyperinsulinemia on C-peptide clearance in another cohort of healthy subjects. Glucose concentrations were again similar on the sham and insulin clamp days, 85.6 ± 1.3 vs. 85.2 ± 1.8 mg/dL (4.75 ± 0.07 vs. 4.73 ± 0.10 mmol/L; 0–240 min, respectively; repeated-measures ANOVA, P = 0.4). Serum insulin concentrations achieved during steady state of the hyperinsulinemic clamp were 163 ± 11 μU/mL (981 ± 68 pmol/L) compared with 3.6 ± 0.6 μU/mL (25 ± 4 pmol/L) on the saline sham day (P < 0.0001). Free fatty acid levels, as expected, were suppressed under hyperinsulinemia, 0.40 ± 2.0 μmol/l vs. 0.02 ± 0.02 (P < 0.0001) sham compared with insulin clamp conditions. Whole body glucose use (M) during the last 60 min of the isoglycemic-hyperinsulinemic clamp was 9.6 ± 2.5 mg·kg−1·min−1 (53.2 ± 14.0 μmol·kg−1·min−1), consistent with normal insulin sensitivity. The total volume of fluid infused on the sham and insulin clamp days were similar, although slightly higher on the sham day, 1,172 ± 103 vs. 1,071 ± 191 mL (P = 0.09).
Endogenous C-peptide levels measured by mass spectrometry decreased 31% during the hyperinsulinemic clamp [0.80 ± 0.06 vs. 0.55 ± 0.08 ng/mL (0.27 ± 0.02 vs. 0.18 ± 0.03 nmol/L), time 230–240 min, sham vs. insulin clamp, P < 0.002; Fig. S4C]. Serum tracer 13C-C-peptide concentrations were also significantly reduced during hyperinsulinemia [1.20 ± 0.01 vs. 1.11 ± 0.080 ng/mL (0.40 ± 0.02 vs. 0.37 ± 0.01 nmol/L) sham vs. insulin clamp (time 180–240 min), P < 0.03]. The calculated metabolic clearance rate of 13C-C-peptide increased 7% during hyperinsulinemic clamp (4.08 ± 0.08 vs. 3.76 ± 0.05 mL·kg−1·min−1 at steady state, P < 0.05; Fig. 3B). These findings demonstrate, under isoglycemic-hyperinsulinemic conditions in healthy humans, that C-peptide clearance is increased.
Discussion
Insulin resistance precedes T2D. β-Cell dysfunction also predicts T2D, and many genes that confer diabetes risk have physiologic importance for β-cell function. Insulin secretion and action are coupled; with increasing insulin resistance, there is a proportional greater secretory response to maintain similar glycemia (14). Diminished insulin secretion and action have been considered separate processes; yet the presence and functional activity of the insulin receptor and its signaling proteins within the β-cell suggest that insulin could regulate its own secretion.
We used combined isoglycemic-hyperinsulinemic and hyperglycemic clamps compared with sham clamps to study effects of preexposure to insulin on glucose-stimulated insulin secretion (GSIS). Using immunoassays to differentiate endogenous (secreted) from exogenous (administered) insulin, we demonstrated that preexposure to insulin augments the β-cell secretory response to glucose in healthy humans. In vivo, preexposure over 4 h to insulin under isoglycemic conditions increases the β-cell secretory response to glucose by ≈40%. We postulate that effects may be altered in persons with insulin resistance, providing a mechanism contributing to the coupling between insulin secretion and action.
Although higher circulating insulin levels during hyperinsulinemia might have been due to saturation of insulin clearance, this was not observed in parallel studies evaluating the metabolic clearance rate of insulin at hyperinsulinemia. Our measures of insulin clearance differ from normal physiology, as β-cells release insulin into the portal circulation and we administered insulin to the peripheral circulation. Whereas in vivo hepatic extraction occurs largely during the first pass through the liver, effects of the liver to clear insulin would be seen in the whole-body model of elimination that we used to calculate insulin clearance. Consistent with the physiologic relevance of our findings, the increased insulin concentrations achieved in the plasma during the chronic infusions in our studies are unlikely to exceed intraislet concentrations anticipated to occur near β-cells in vivo, which could be substantially higher because of the local release of insulin.
Although whole-body insulin clearance is not saturated at high physiologic insulin concentrations achieved during hyperinsulinemic clamp, we demonstrated using stable isotope labeled C-peptide techniques that C-peptide clearance increases modestly during isoglycemic hyperinsulinemia, accounting for more than 20% of the decreased C-peptide levels. These findings suggest that β-cell function may be modestly underestimated using methods based on C-peptide measured during sustained hyperinsulinemia or high insulin flux.
We recognize that our human in vivo experiments are limited, as we cannot demonstrate insulin signaling in β-cells directly. Reduced glucagon levels during hyperinsulinemic clamp suggest physiologic insulin action within the islet, albeit via the α-cell (15). Our findings that insulin can augment GSIS in healthy humans in vivo are consistent with a growing body of work. In humans and in rodent models of diabetes and obesity, β-cells compensate for insulin resistance with both increased mass and function (16, 17). Recent studies demonstrate the presence and importance of insulin signaling in the β-cell, and insulin and insulinlike growth factor–1 (IGF1) receptors participate in β-cell development and function (1, 5). Genetic engineering techniques to knockout or knockdown IRS proteins in the insulin signaling pathway either at the whole body level or in β-cells demonstrate their significance in glucose homeostasis (4, 6, 18, 19).
Mice with a β-cell–specific insulin receptor knockout (βIRKO), an example of β-cell insulin resistance, manifest defective glucose-stimulated insulin secretion, progressive glucose intolerance, and increased rates of diabetes (1, 3). Insulin exerts a positive effect on its own synthesis (20), and stimulation of β-cells with exogenous insulin leads to increased intracellular Ca2+ suggesting insulin also stimulates its own secretion by mobilizing Ca2+ from the endoplasmic reticulum (19). Insulin modifies intracellular insulin processing, leading to altered secretion of prohormone and metabolites (21). Consistent results are seen using the insulin-mimetic compound L-783281 (22). Insulin appears to act differentially via type A and type B insulin receptor isoforms to modulate insulin and glucokinase gene expression, respectively, in murine β-cells (23). Higher insulin concentrations may induce β-cell glucokinase expression, potentiating glucose-stimulated insulin release. Finally, at euglycemia, glucose effects on β-cell growth and survival require activation of insulin signaling proteins (24), and hyperglycemia-induced reduction in expression of insulin receptor and activation of the proapoptotic cascade is physiologically antagonized by insulin signaling through the IRS-PI 3kinase-AKT-Bad cascade (25, 26). Together these studies show an important role for the insulin signaling pathway in β-cell development and function, and support a link between insulin action and secretion.
Multiple studies support the relevance of insulin signaling in human β-cells. Oligonucleotide microarray analysis and real-time PCR on isolated pancreatic islets from humans with T2D show reduced insulin receptor, IRS-2, and Akt2, and increased phosphatidylinositol phosphatase SH2 domain containing inositol 5-phosphatase 2 (SHIP2) mRNA expression, which would reduce insulin signal transduction (27). Insulin also stimulates Ca2+ mobilization and promotes de novo insulin synthesis in human transplantable islets (9). Islets with a polymorphism in IRS-1 associated with human T2D (28) have reduced insulin content, fewer mature insulin secretory granules, and impaired insulin secretion to glucose (18, 29).
Our findings that preexposure to insulin enhances GSIS differ from those of Del Prato et al. (30), who demonstrated euglycemic hyperinsulinemia of 72–96 h did not augment β-cell secretory response to glucose. There are several differences in study design. First, the dose of insulin was 0.25 mU/kg/min, ∼10% of the dose that we studied, leading to differences in β-cell insulin exposure (mean ∼48 vs. 1089 pmol/L). Second, in their study, the duration of preexposure was considerably longer and was sufficient to induce insulin resistance manifest as decreased whole-body glucose disposal, apparent after 48 h of sustained insulin exposure. This effect could be due to down-regulation of insulin receptors and insulin signaling following prolonged exposure to hyperinsulinemia (31), a process that we speculate may also occur within the islet. As such, constitutive-basal secretion of insulin by β-cells would permit down-regulation of insulin receptors and signaling proteins, as demonstrated in islets from persons with T2D (27), and might confound the effects of additional ligand. In addition, β-cell function was estimated using C-peptide, which could modestly underestimate the secretory response. The shorter duration of exposure to hyperinsulinemia in our studies was not associated with decreasing glucose disposal, and might be more similar to fluctuations in vivo following meals, although we did not perform studies with insulin preexposure of less than 4 h.
We also demonstrate increased metabolic clearance of C-peptide during isoglycemic hyperinsulinemia, which is important, as C-peptide concentrations are frequently used to estimate β-cell function, assuming that clearance is stable over the dynamic range of insulin occurring during provocative studies. Metabolic clearance rates for C-peptide are similar following peripheral or intraportal infusions of biosynthetic C-peptide (32). The kidney participates in clearance by glomerular filtration and peritubular extraction, removing 30–70% of C-peptide compared with 10–30% of insulin (33). Insulin induces systemic and renal vasodilation and increases glomerular filtration (34, 35), which could underlie effects of hyperinsulinemia to induce C-peptide clearance. Consistent with our findings, renal metabolic clearance of C-peptide was increased in the postprandial state (36). Previous human studies have shown that C-peptide metabolic clearance decreases ∼7% after elevation of circulating FFA concentrations induced with intralipid/heparin infusion (37), an amount comparable but opposite in direction to the magnitude of change in our studies, which were associated with a drop in FFA concentrations.
Yet the increase in C-peptide metabolic clearance rate does not fully explain the reduction in circulating concentrations following hyperinsulinemia. Insulin is crystallized within the secretory granule, whereas C-peptide remains soluble, contributing to differential intracellular degradation of the two proteins (38). Neerman-Arbez and Halban (13) evaluated cellular degradation of C-peptide in INS cells using a pulse-chase experimental approach. The intracellular insulin to C-peptide ratio (I/CP) began to rise within 2–4 h of stimulation, which is within the time frame of our investigations. This ratio was inverted in the medium without extracellular degradation of insulin or C-peptide. Their findings are consistent with progressive intracellular degradation of C-peptide from functionally competent granules, and suggest that I/CP ratios may be variable under some physiologic conditions. Thus, secretory and clearance mechanisms might both contribute to reduced circulating C-peptide concentrations.
We cannot differentiate direct from indirect effects of insulin on β-cell function. Additional hormones and metabolites that might indirectly alter β-cell function were assessed, including glucagon, cortisol, adrenergic hormones, FFA, and potassium. FFA differed most. Chronic elevation of FFA has been associated with β-cell lipotoxicity and decreased GSIS in humans (39). Hence, FFA suppression might improve GSIS, and raises the question of whether the differences that we observed were indirect effects of hyperinsulinemia mediated by suppression of FFA. Alternatively, maintenance and acute elevation of circulating FFA are associated with enhanced GSIS (39, 40). Based on these data and the fact that healthy subjects in our study had not been chronically exposed to elevated concentrations of FFA, we do not postulate that the observed improved GSIS is a result of decreased FFA. Finally, while FFA concentrations are reduced, it is possible that FFA flux is increased, contributing to the effect of insulin to potentiate GSIS. Thus, we cannot exclude the possibility that indirect effects of FFA or other hormones or metabolites contribute to the effects of insulin to regulate β-cell function.
Furthermore, in the pancreatic islet, α-adrenergic activity predominates over β-adrenergic activity. α-Adrenergic stimulation inhibits insulin secretion such that insulin stimulation is suppressed during sympathetic stimulation associated with stress (41). Glucagon can promote insulin secretion independent of the effects on glucose (42) and is a potent nonglucose secretagogue. Likewise, low serum potassium is associated with attenuated insulin secretion (43). Thus the increase in norepinephrine and/or decrease in glucagon or potassium observed during hyperinsulinemic compared with sham clamp might each be expected to decrease the β-cell secretory response. Despite these changes in hormones and metabolites observed during the insulin clamp, enhanced GSIS was demonstrated. Careful control of any or all of these variables might enhance the observed effect of insulin to potentiate β-cell function.
At isoglycemia, requirements for insulin levels to be maintained are not altered. During administration of exogenous insulin, we administered dextrose to maintain glycemia, and endogenous insulin concentrations did not fall. It is interesting to consider the physiologic relevance of a positive feedback loop of insulin on β-cell function, also seen in cellular studies (8). Insulin secretion is biphasic. Early insulin secretion is important to maintain normal glucose tolerance for its role to suppress endogenous glucose production and prime insulin sensitive tissues. In patients at risk for or with T2D, abnormalities occur in both the relative amount and pattern of insulin release. First-phase insulin secretion is diminished (44, 45). Although our 4-h preexposure to isoglycemic hyperinsulinemia was longer in duration compared with the early insulin secretory response, the acute response may play an additional role in β-cell priming. Notably, whereas insulin release in response to i.v. glucose is diminished in individuals with T2D, insulin response to the nonglucose stimulant, arginine, is preserved (44). This differential β-cell response to the different secretagogues is paralleled in the βIRKO model (3), which also showed reduced glucokinase expression in the β-cell (46). Together, these data suggest that the effects of insulin on the β-cell may be specific for regulation of the physiologic response to glucose. Location of receptors on the apical and/or basolateral surface of human β-cells relative to the release of secretory granule content remains incompletely understood and is difficult to assess using immunohistochemistry. Cellular architecture might modulate the feedback loop between local and systemic insulin concentrations and effects on secretion. Finally, one cannot exclude the possibility that the effect of insulin preexposure to augment GSIS is mediated by β-cell rest provided by the 4 h of insulin administration at isoglycemia. These findings may have relevance in the pathogenesis of T2D, or in therapy whereby insulin or other oral therapies such as insulin sensitizers may protect β-cell function.
In conclusion, our findings demonstrate that insulin potentiates the β-cell insulin secretory response to glucose in healthy, insulin-sensitive persons. These findings are consistent with our hypothesis that the human β-cell is an insulin-responsive tissue. Finally, we hypothesize that insulin regulation of glucose-stimulated insulin secretion might be altered in persons with insulin resistance or T2D and might contribute to the progressive loss of β-cell function in individuals with T2D.
Materials and Methods
To test whether insulin could enhance the β-cell insulin secretory response to glucose, subjects were evaluated during paired studies conducted in a single-mask design, of either 4 h of saline (sham clamp/low insulin) or isoglycemic hyperinsulinemia (high insulin) immediately followed by glucose administration. Additional details and insulin and C-peptide clearance methods are described in SI Text, along with subject characteristics.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health Grants K23-DK02795, R01 DK070648, DK67536, DK061644, DK33201, P30-DK36836, M01-RR001032, and RR12248; American Diabetes Association 06-CD-07; the Harvard 50th Anniversary Scholars in Medicine: Priscilla White Fellowship; a Lilly Fellowship Grant; and the Clinical Investigator Training Program of Harvard–Massachusetts Institute of Technology Health Sciences and Technology–Beth Israel Deaconess Medical Center, in collaboration with Pfizer Inc. and Merck & Company, Inc.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1000002107/DCSupplemental.
References
- 1.Assmann A, Hinault C, Kulkarni RN. Growth factor control of pancreatic islet regeneration and function. Pediatr Diabetes. 2009;10:14–32. doi: 10.1111/j.1399-5448.2008.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lingohr MK, et al. IRS-3 inhibits IRS-2-mediated signaling in pancreatic beta-cells. Mol Cell Endocrinol. 2003;204:85–99. doi: 10.1016/s0303-7207(03)00124-2. [DOI] [PubMed] [Google Scholar]
- 3.Kulkarni RN, et al. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 4.Withers DJ, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 5.Cho H, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 6.Kulkarni RN, et al. Altered function of insulin receptor substrate-1-deficient mouse islets and cultured beta-cell lines. J Clin Invest. 1999;104:R69–R75. doi: 10.1172/JCI8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller D, Huang GC, Amiel S, Jones PM, Persaud SJ. Gene expression heterogeneity in human islet endocrine cells in vitro: The insulin signalling cascade. Diabetologia. 2007;50:1239–1242. doi: 10.1007/s00125-007-0671-7. [DOI] [PubMed] [Google Scholar]
- 8.Aspinwall CA, Lakey JR, Kennedy RT. Insulin-stimulated insulin secretion in single pancreatic beta cells. J Biol Chem. 1999;274:6360–6365. doi: 10.1074/jbc.274.10.6360. [DOI] [PubMed] [Google Scholar]
- 9.Luciani DS, Johnson JD. Acute effects of insulin on beta-cells from transplantable human islets. Mol Cell Endocrinol. 2005;241:88–98. doi: 10.1016/j.mce.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Muller D, Huang GC, Amiel S, Jones PM, Persaud SJ. Identification of insulin signaling elements in human beta-cells: Autocrine regulation of insulin gene expression. Diabetes. 2006;55:2835–2842. doi: 10.2337/db06-0532. [DOI] [PubMed] [Google Scholar]
- 11.Elahi D, et al. Feedback inhibition of insulin secretion by insulin: Relation to the hyperinsulinemia of obesity. N Engl J Med. 1982;306:1196–1202. doi: 10.1056/NEJM198205203062002. [DOI] [PubMed] [Google Scholar]
- 12.Kraegen EW, Lazarus L, Campbell LV. Failure of insulin infusion during euglycemia to influence endogenous basal insulin secretion. Metabolism. 1983;32:622–627. doi: 10.1016/0026-0495(83)90034-3. [DOI] [PubMed] [Google Scholar]
- 13.Neerman-Arbez M, Halban PA. Novel, non-crinophagic, degradation of connecting peptide in transformed pancreatic beta cells. J Biol Chem. 1993;268:16248–16252. [PubMed] [Google Scholar]
- 14.Kahn SE, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 15.Kawamori D, et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 2009;9:350–361. doi: 10.1016/j.cmet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell GI, Polonsky KS. Diabetes mellitus and genetically programmed defects in beta-cell function. Nature. 2001;414:788–791. doi: 10.1038/414788a. [DOI] [PubMed] [Google Scholar]
- 17.Accili D. Lilly lecture 2003: The struggle for mastery in insulin action: From triumvirate to republic. Diabetes. 2004;53:1633–1642. doi: 10.2337/diabetes.53.7.1633. [DOI] [PubMed] [Google Scholar]
- 18.Porzio O, et al. The Gly972→Arg amino acid polymorphism in IRS-1 impairs insulin secretion in pancreatic beta cells. J Clin Invest. 1999;104:357–364. doi: 10.1172/JCI5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aspinwall CA, et al. Roles of insulin receptor substrate-1, phosphatidylinositol 3-kinase, and release of intracellular Ca2+ stores in insulin-stimulated insulin secretion in beta-cells. J Biol Chem. 2000;275:22331–22338. doi: 10.1074/jbc.M909647199. [DOI] [PubMed] [Google Scholar]
- 20.Leibiger B, Wahlander K, Berggren PO, Leibiger IB. Glucose-stimulated insulin biosynthesis depends on insulin-stimulated insulin gene transcription. J Biol Chem. 2000;275:30153–30156. doi: 10.1074/jbc.M005216200. [DOI] [PubMed] [Google Scholar]
- 21.Assmann A, et al. Insulin signaling regulates insulin processing in pancreatic beta-cells. Diabetes. 2008;57:A64. [Google Scholar]
- 22.Westerlund J, Wolf BA, Bergsten P. Glucose-dependent promotion of insulin release from mouse pancreatic islets by the insulin-mimetic compound L-783,281. Diabetes. 2002;51(Suppl 1):S50–S52. doi: 10.2337/diabetes.51.2007.s50. [DOI] [PubMed] [Google Scholar]
- 23.Leibiger B, et al. Selective insulin signaling through A and B insulin receptors regulates transcription of insulin and glucokinase genes in pancreatic beta cells. Mol Cell. 2001;7:559–570. doi: 10.1016/s1097-2765(01)00203-9. [DOI] [PubMed] [Google Scholar]
- 24.Assmann A, Ueki K, Winnay JN, Kadowaki T, Kulkarni RN. Glucose effects on beta-cell growth and survival require activation of insulin receptors and insulin receptor substrate 2. Mol Cell Biol. 2009;29:3219–3228. doi: 10.1128/MCB.01489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Federici M, et al. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: A potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes. 2001;50:1290–1301. doi: 10.2337/diabetes.50.6.1290. [DOI] [PubMed] [Google Scholar]
- 26.Hribal ML, et al. Chronic hyperglycemia impairs insulin secretion by affecting insulin receptor expression, splicing, and signaling in RIN beta cell line and human islets of Langerhans. FASEB J. 2003;17:1340–1342. doi: 10.1096/fj.02-0685fje. [DOI] [PubMed] [Google Scholar]
- 27.Gunton JE, et al. Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005;122:337–349. doi: 10.1016/j.cell.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 28.Almind K, et al. Aminoacid polymorphisms of insulin receptor substrate-1 in non-insulin-dependent diabetes mellitus. Lancet. 1993;342:828–832. doi: 10.1016/0140-6736(93)92694-o. [DOI] [PubMed] [Google Scholar]
- 29.Marchetti P, et al. Insulin secretory function is impaired in isolated human islets carrying the Gly(972)→Arg IRS-1 polymorphism. Diabetes. 2002;51:1419–1424. doi: 10.2337/diabetes.51.5.1419. [DOI] [PubMed] [Google Scholar]
- 30.Del Prato S, et al. Effect of sustained physiologic hyperinsulinaemia and hyperglycaemia on insulin secretion and insulin sensitivity in man. Diabetologia. 1994;37:1025–1035. doi: 10.1007/BF00400466. [DOI] [PubMed] [Google Scholar]
- 31.Gavin JR, 3rd, Roth J, Neville DM, Jr, de Meyts P, Buell DN. Insulin-dependent regulation of insulin receptor concentrations: A direct demonstration in cell culture. Proc Natl Acad Sci USA. 1974;71:84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polonsky KS, et al. C-peptide and insulin secretion. Relationship between peripheral concentrations of C-peptide and insulin and their secretion rates in the dog. J Clin Invest. 1984;74:1821–1829. doi: 10.1172/JCI111601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zavaroni I, et al. Renal metabolism of C-peptide in man. J Clin Endocrinol Metab. 1987;65:494–498. doi: 10.1210/jcem-65-3-494. [DOI] [PubMed] [Google Scholar]
- 34.Creager MA, Liang CS, Coffman JD. Beta adrenergic-mediated vasodilator response to insulin in the human forearm. J Pharmacol Exp Ther. 1985;235:709–714. [PubMed] [Google Scholar]
- 35.Cohen AJ, McCarthy DM, Stoff JS. Direct hemodynamic effect of insulin in the isolated perfused kidney. Am J Physiol. 1989;257:F580–F585. doi: 10.1152/ajprenal.1989.257.4.F580. [DOI] [PubMed] [Google Scholar]
- 36.Samnegård B, Brundin T. Renal extraction of insulin and C-peptide in man before and after a glucose meal. Clin Physiol. 2001;21:164–171. doi: 10.1046/j.1365-2281.2001.00316.x. [DOI] [PubMed] [Google Scholar]
- 37.Goswami G, et al. The metabolic clearance rate of C-peptide but not insulin is decreased by hyperlipidemia. Diabetes. 2005;54:A372. [Google Scholar]
- 38.Verchere CB, et al. Des-(27-31)C-peptide. A novel secretory product of the rat pancreatic beta cell produced by truncation of proinsulin connecting peptide in secretory granules. J Biol Chem. 1996;271:27475–27481. doi: 10.1074/jbc.271.44.27475. [DOI] [PubMed] [Google Scholar]
- 39.Carpentier A, et al. Acute enhancement of insulin secretion by FFA in humans is lost with prolonged FFA elevation. Am J Physiol. 1999;276:E1055–E1066. doi: 10.1152/ajpendo.1999.276.6.E1055. [DOI] [PubMed] [Google Scholar]
- 40.Dobbins RL, Chester MW, Daniels MB, McGarry JD, Stein DT. Circulating fatty acids are essential for efficient glucose-stimulated insulin secretion after prolonged fasting in humans. Diabetes. 1998;47:1613–1618. doi: 10.2337/diabetes.47.10.1613. [DOI] [PubMed] [Google Scholar]
- 41.Porte D, Jr, Robertson RP. Control of insulin secretion by catecholamines, stress, and the sympathetic nervous system. Fed Proc. 1973;32:1792–1796. [PubMed] [Google Scholar]
- 42.Samols E, Marri G, Marks V. Promotion of insulin secretion by glucagon. Lancet. 1965;2:415–416. doi: 10.1016/s0140-6736(65)90761-0. [DOI] [PubMed] [Google Scholar]
- 43.Helderman JH, et al. Prevention of the glucose intolerance of thiazide diuretics by maintenance of body potassium. Diabetes. 1983;32:106–111. doi: 10.2337/diab.32.2.106. [DOI] [PubMed] [Google Scholar]
- 44.Pfeifer MA, Halter JB, Porte D., Jr Insulin secretion in diabetes mellitus. Am J Med. 1981;70:579–588. doi: 10.1016/0002-9343(81)90579-9. [DOI] [PubMed] [Google Scholar]
- 45.Eriksson J, et al. Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. N Engl J Med. 1989;321:337–343. doi: 10.1056/NEJM198908103210601. [DOI] [PubMed] [Google Scholar]
- 46.Otani K, et al. Reduced beta-cell mass and altered glucose sensing impair insulin-secretory function in betaIRKO mice. Am J Physiol Endocrinol Metab. 2004;286:E41–E49. doi: 10.1152/ajpendo.00533.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.