Biological systems consist of a myriad of highly interactive and complex interconnections and pathways across multiple orders of length and time scales. Natural neuronal networks are valuable examples of such systems because they are important for understanding how the brain functions. Monitoring activities of a large number of neurons and intercommunication among them is critical for studying neuronal networks, for which passive microfabricated multielectrode arrays (MEAs) (1) and active planar silicon field effect transistor (FET) arrays (2) have been the two popular techniques. Now, a unique and more powerful technique has been developed, as demonstrated by Qing et al. in PNAS (3). The authors show that neuronal networks can now be studied with much higher spatial and temporal resolution while obtaining higher sensitivity of extracellular recording.
In the control center of the body, billions of neurons are linked together in a highly organized network for communication and control. A typical neuron consists of a cell body (soma), dendrites, and an axon (4). Neurons conduct information in two ways. One method is by electrical impulses traveling along the axon from one end of a neuron to the other; these impulses are known as action potentials. The second method is chemical transmission across the minute gap between neurons, the synapse, and is usually triggered by action potentials. Action potentials are based on excited movements of ions between the outside and inside of the cell membrane. Therefore, to study neuronal network behavior, the ability to monitor the electrochemical potential with high spatial and temporal resolution is essential.
Planar neuronal network systems, such as cultured neurons and brain slices, have been studied by MEAs (1). Extracellular field potentials could be recorded, but the spatial resolution is not enough to achieve single-cell level detection and signals are smaller than those detected by conventional micropipette electrodes. To overcome the problems associated with MEAs, planar FET arrays were developed. Because of the local amplification power of transistors, the weak signals do not have to travel through long leads and wires before being amplified (2). Thus signal amplitude and spatial resolution are greatly improved. However, because of the intrinsic size of the transistor and the limited capacitive coupling between the planar-gate electrode and neurons, it is necessary to have a fairly large gate-electrode area to obtain enough charge flow. This necessity limits further improvements of the spatial and temporal resolution of transistor arrays. In addition, a cleft filled by electrolyte is usually maintained between the neurons and transistors because of the flat substrate surface. This electrolyte layer causes a large leakage of potential signal. Therefore, a protruding device is preferred to achieve more efficient coupling.
Semiconductor nanowires (NWs) afford a unique, powerful chemical and biological sensing platform that has been developed in the past decade. Semiconductor NWs with diameters less than ∼100 nm have been synthesized with good control over composition, shape, and size (5 –7). A variety of NW-based electronic devices (6), including FETs (8), have been fabricated, which have laid out a solid foundation for high performance sensors. Since the first demonstration that silicon NW FETs can be converted into sensors in 2001 (9), they have been shown to be able to detect charged chemicals, biomolecules, and viruses (9 –15); they have also been used to study individual cultured neuron cells (16) and cardiac tissues (17). Nanowires have also been used to deliver biological molecules into cells (18, 19). In the present study (3), Qing et al. demonstrate that NW arrays on transparent substrates can be reliably used to study acute brain slices. The authors characterize the NW devices by simultaneously combining NW FET and patch clamp techniques to identify potential signals (Fig. 1). With NW devices, the authors not only identify action potential signals but also find additional features at earlier times. Using synaptic and ion-channel blockers during NW device sensing, the authors are able to assign these potential signals to presynaptic firing and postsynaptic depolarization. Interestingly, NW devices can access and identify different regions of neuron cells, which can either be close to somata or abundant in dendritic projections. With two-dimensional NW device array sensors interfacing with the olfactory cortex, the authors demonstrate multiplexed mapping and reveal spatially heterogeneous functional connectivity.
Fig. 1.
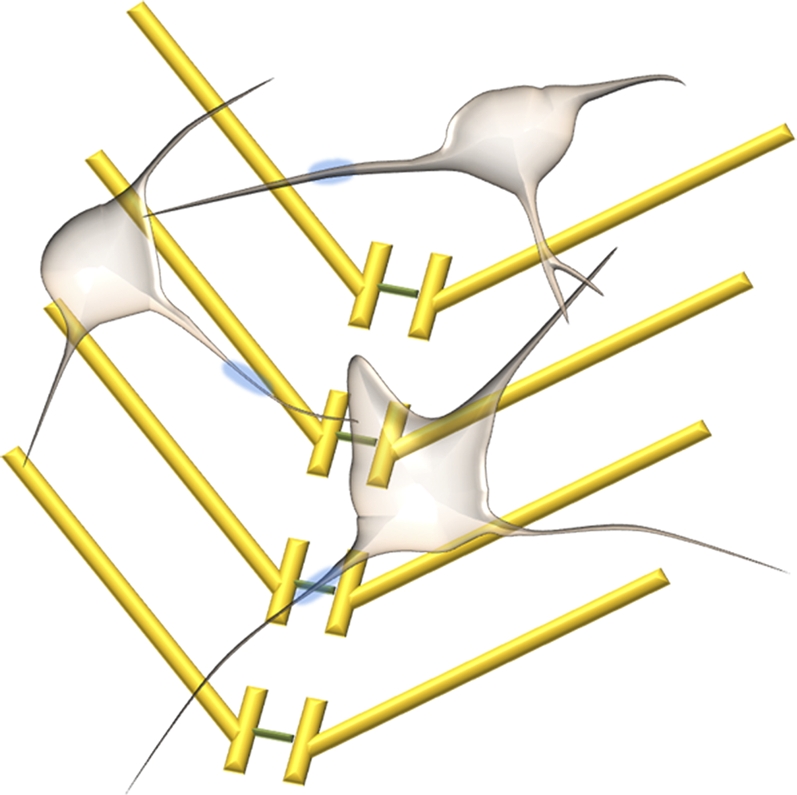
Schematic of interconnected neurons and nanowire FET arrays.
The silicon NW arrays demonstrated by Qing et al. afford unique features for neuronal network study (3). First, NW devices exhibit very high sensitivity and good signal-to-noise ratio. In the present study, the NW devices show signal amplitudes from 0.3 to 3.0 mV, which are higher than MEAs and planar FETs. Such high sensitivity results from the small diameter and protruding nature of NWs. NW devices protrude from the substrate surface, which reduces the separation between NWs and cell membrane; in turn, this promotes nanostructure-cell interaction and decreases the leakage of potential signal. This is a significant advantage over planar devices. Second, the NW devices have high spatial resolution for detection. The NW diameters are typically less than 100 nm and the active junction area of typical NW devices is fairly small (∼0.06 μm2, which is two orders of magnitude smaller than MEA and planar FETs). NW arrays can be fabricated with several microns or less between wires, which defines the spatial resolution. Finally, the NW devices also have submillisecond temporal resolution. The NW array platform demonstrated by Qing et al. (3) opens up exciting opportunities for understanding neural circuitry with a higher level of sensitivity and spatial and temporal resolution, which could eventually help reveal the secrets of the brain.
Footnotes
The authors declare no conflict of interest.
See companion article on page 1882 in issue 5 of volume 107.
References
- 1.Taketani M, Baudry M. Advances in Network Electrophysiology Using Multi- Electrode Arrays. New York: Springer Science and Business Media, Inc.; 2006. in. [Google Scholar]
- 2.Hutzler M, et al. High-resolution multitransistor array recording of electrical field potentials in cultured brain slices. J Neurophysiol. 2006;96:1638–1645. doi: 10.1152/jn.00347.2006. [DOI] [PubMed] [Google Scholar]
- 3.Qing Q, et al. Nanowire transistor arrays for mapping neural circuits in acute brain slices. Proc Natl Acad Sci USA. 2009;107:1882–1887. doi: 10.1073/pnas.0914737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levitan IB, Kaczmarek LK. The Neuron: Cell and Molecular Biology. 3rd Ed. Oxford: Oxford University Press; 2002. [Google Scholar]
- 5.Cui Y, Lauhon LJ, Gudiksen MS, Wang J, Lieber CM. Diameter-controlled synthesis of single crystal silicon nanowires. Appl Phys Lett. 2001;78:2214–2216. [Google Scholar]
- 6.Lieber CM. Nanoscale science and technology: Building a big future from small things. MRS Bull. 2003;28:486–491. [Google Scholar]
- 7.Xia Y, et al. One-dimensional nanostructures: synthesis, characterization and applications. Adv Mater. 2003;15:353–389. [Google Scholar]
- 8.Cui Y, Duan X, Hu J, Lieber CM. Doping and electrical transport in silicon nanowires. J Phys Chem B. 2000;104:5213–5216. [Google Scholar]
- 9.Cui Y, Wei Q, Park H, Lieber CM. Nanowire nanosensors for highly-sensitive, selective and integrated detection of biological and chemical species. Science. 2001;293:1289–1292. doi: 10.1126/science.1062711. [DOI] [PubMed] [Google Scholar]
- 10.Zheng GF, et al. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat Biotechnol. 2005;23:1294–1301. doi: 10.1038/nbt1138. [DOI] [PubMed] [Google Scholar]
- 11.Stern E, et al. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature. 2007;445:519–522. doi: 10.1038/nature05498. [DOI] [PubMed] [Google Scholar]
- 12.Patolsky F, et al. Electrical detection of single viruses. Proc Natl Acad Sci USA. 2004;101:14017–14022. doi: 10.1073/pnas.0406159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patolsky F, Timko BP, Zheng GF, Lieber CM. Nanowire-based nanoelectronic devices in the life sciences. MRS Bull. 2007;32:142–149. [Google Scholar]
- 14.Li Z, et al. Sequence-specific label-free DNA sensors based on silicon nanowires. Nano Lett. 2004;4:245–247. [Google Scholar]
- 15.Bunimovich YL. Quantitative real-time measurements of DNA hybridization with alkylated nonoxidized silicon nanowires in electrolyte solution. J Am Chem Soc. 2006;128:16323–16331. doi: 10.1021/ja065923u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patolsky F, et al. Detection, stimulation, and inhibition of neuronal signals with high density nanowire transistor arrays. Science. 2006;313:1100–1104. doi: 10.1126/science.1128640. [DOI] [PubMed] [Google Scholar]
- 17.Timko BP, et al. Electrical recording from hearts with flexible nanowire device arrays. Nano Lett. 2009;9:914–918. doi: 10.1021/nl900096z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim W, Ng JK, Kunitake ME, Conklin BR, Yang P. Interfacing silicon nanowires with mammalian cells. J Am Chem Soc. 2007;129:7228–7229. doi: 10.1021/ja071456k. [DOI] [PubMed] [Google Scholar]
- 19.Shalek AK, et al. Vertical silicon nanowires as a universal platform for delivering biomolecules into living cells. Proc Natl Acad Sci USA. 2010;107:1870–1875. doi: 10.1073/pnas.0909350107. [DOI] [PMC free article] [PubMed] [Google Scholar]


