Abstract
There is increasing appreciation of the important role of B cells in many autoimmune diseases and consequently, increasing interest in treating these disorders through B cell-depletion therapy with rituximab, an anti-CD20 monoclonal antibody. Yet, precisely how this and related drugs exert their therapeutic effects remains controversial. In particular, it is unclear how, in a number of contexts, rituximab can greatly reduce the titer of serum autoantibodies without substantially altering the overall antibody titer. We have studied the action of this drug in the K/BxN mouse model of inflammatory arthritis after first crossing in a human CD20 transgene. Rituximab treatment of these mice led to a decrease in the titer of serum antibodies targeting glucose-6-phosphate isomerase, the relevant autoantigen, but not in the total antibody titer. Glucose-6-phosphate isomerase-specific plasma cells did not reside primarily in the bone marrow as expected but rather in the spleen and lymph nodes, where they had short lives, expressed CD20, and were rapidly depleted by rituximab. These data support a model whereby autoreactive plasma cells (at least certain specificities thereof) are intrinsically different from protective antimicrobial plasma cells in their differentiation, migration, and survival properties. Rituximab targets the former and spares the latter.
Keywords: autoimmunity, B cell depletion therapy, CD20, plasma cell, autoantibody
Blymphocytes are central players in the adaptive immune response, undergoing activation and further differentiation into plasma or memory cells in response to antigen encounter. They are often major drivers of autoimmunity, the autoantibodies (autoAbs) they produce being a feature of many autoimmune diseases, functioning directly or indirectly in disease pathogenesis (1). The lengthy persistence of autoAbs in autoimmune disorders can be attributed either to the activity of long-lived plasma cells or a continuing generation of short-lived “plasmablasts” which reflects the chronic nature of the immune response (reviewed in refs. 2 and 3). In the New Zealand Black/New Zealand White mouse model of systemic lupus erythematosus, for example, long- and short-lived antibody-secreting cells (ASCs) account for about 40% and 60%, respectively, of the autoAbs generated (4). Besides producing pathogenic autoAbs, B lymphocytes may promote autoimmune disease through one or more of their many other activities (reviewed in ref. 1): antigen presentation, cytokine or chemokine production, facilitation of T cell priming/expansion (5), contribution to the development of secondary lymphoid tissue, etc.
B cell-depletion therapy through rituximab has already been shown to be effective in rheumatoid arthritis, multiple sclerosis, and several other autoimmune diseases (6, 7). Originally developed for the treatment of B cell lymphomas, rituximab is a chimeric monoclonal antibody (mAb) that binds to human CD20, a B lymphocyte-specific cell-surface marker (8). However, precisely how the depletion of B cells by this drug is able to dampen autoimmunity remains poorly understood. Interestingly, in several disease contexts, a positive clinical response correlated with a substantial drop in the titer of autoAbs, whereas concentrations of protective antimicrobial Abs did not really change (reviewed in ref. 1). It is often stated that plasma cells do not express CD20 (1, 2, 9); therefore, it has been generally inferred that rituximab cannot target plasma cells, and instead, it blocks the generation of new ones by depleting B cells. One possible explanation for the differential sensitivity of autoAb and protective-Ab titers lies in the hypothesis that the former is produced by short-lived plasmablasts, whereas the latter is produced by long-lived plasma cells (1). Alternative possibilities are that autoAb-producing plasma cells may, for some reason, express CD20 and are, thereby, direct targets of rituximab or that B cell depletion may compromise the survival niches of long-lived plasma cells in inflamed tissues (2).
It has been difficult to decide between the various explanations for rituximab's mechanism of action in human patients given the limited access to relevant organs, the inability to track autoreactive plasma cells, and the unknown identity of the pathogenic antigen(s) in most disease contexts. Here, we model the action of rituximab in K/BxN mice (10, 11) carrying a human CD20 transgene (12). K/BxN mice are a well-studied model of inflammatory arthritis wherein the roles of B cells and autoAbs are both important and clearly defined. Breakdown of T and B cell tolerance leads to the production of high-titer autoAbs against glucose-6-phosphate isomerase (GPI), which can directly induce joint pathology. We show that serum titers of anti-GPI autoAbs, but not of other Abs, decrease substantially after rituximab treatment, recapitulating what often happens in human patients. Autoreactive anti-GPI plasma cells reside largely in the spleen and lymph nodes, are short-lived, express CD20, and are targeted by rituximab. These findings provide an explanation for the potent and specific action of rituximab in certain autoAb-dependent autoimmune diseases and provide a scenario whereby rituximab would be particularly effective.
Results
Modeling the Action of Rituximab in K/BxN Mice.
It has been reported that mice harboring a human CD20 (hCD20) bacterial artificial chromosome (BAC) transgene express murine and human CD20 in a parallel fashion (12). Rituximab depletes various B lymphocyte populations in such animals, the degree of loss depending on the particular B cell microenvironment.
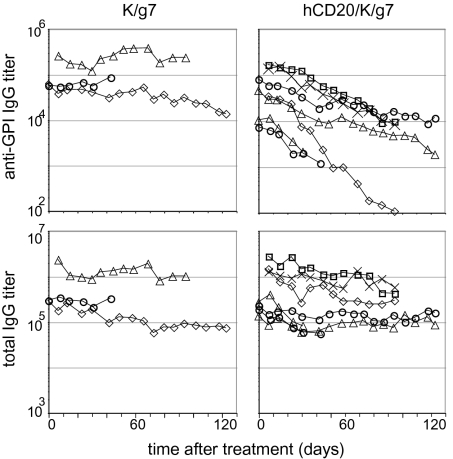
To model the effect of rituximab on autoimmunity in K/BxN mice, we crossed hCD20 transgenic onto KRN T cell receptor (TCR) transgenic and B6.H-2g7 congenic animals. The minimum genetic requirements for development of arthritis in the K/BxN model are the KRN TCR and H-2g7 MHC loci. Littermates that had both of these elements with or without the hCD20 transgene (designated hCD20/K/g7 and K/g7, respectively) developed full-blown arthritis around 4 weeks of age with the same kinetics and characteristics. hCD20/K/g7 and K/g7 mice were injected i.p. with 1 mg of rituximab weekly beginning at 5–8 weeks of age (i.e., when already arthritic and serum anti-GPI IgG titers have already reached the maximal levels). Ankle thickness and clinical index were monitored, and tail blood samples were collected each week. The serum anti-GPI IgG titer decreased substantially with time after treatment of hCD20/K/g7 mice (on average, 10-fold after 8 weeks), whereas no such change was detected in the K/g7 negative controls treated in parallel (Fig. 1 Upper). Total IgG levels also did not change in K/g7 mice as expected (Fig. 1 Lower Left). In hCD20/K/g7 mice, there was an initial drop in total IgG levels. This is not surprising given that the concentration of anti-GPI Abs is so high in such animals (up to 10 mg/mL) that they constitute the bulk of total serum IgG. As anti-GPI Abs disappeared, their contribution to the total IgG decreased, and eventually, the total IgG levels ceased to change (Fig. 1 Lower Right).
Fig. 1.
Ab titers after rituximab treatment. hCD20/K/g7 and K/g7 arthritic mice beginning at 5–8 weeks of age were injected with 1 mg rituximab weekly for up to 18 weeks; some mice were killed early for other experiments). Tail blood samples were collected each week to assay for serum-Ab titers by ELISA. The titer was determined as described in Materials and Methods. (Upper) Anti-GPI IgG titers. (Lower) Total IgG titers.
Despite the decrease in anti-GPI titers in rituximab-injected hCD20/K/g7 mice, these mice did not show any improvement compared with K/g7 negative controls injected in parallel when ankle thickness, clinical index, and joint histology were examined at the end of the treatment. This finding was not really surprising, because the anti-GPI IgG titers in untreated K/g7 mice are so high that we routinely transfer only 100 μl of serum from arthritic mice to induce full-blown disease in healthy recipients. Because the average total blood volume of a 25-g mouse is about 1.8–2.0 mL, this amount would be equivalent to a 20-fold dilution. Therefore, the anti-GPI titers in treated mice are probably not low enough and the decrease is not quick enough to reverse the disease when cartilage erosion has been too extensive to repair.
Localization of the Autoreactive Ab-Secreting Cells.
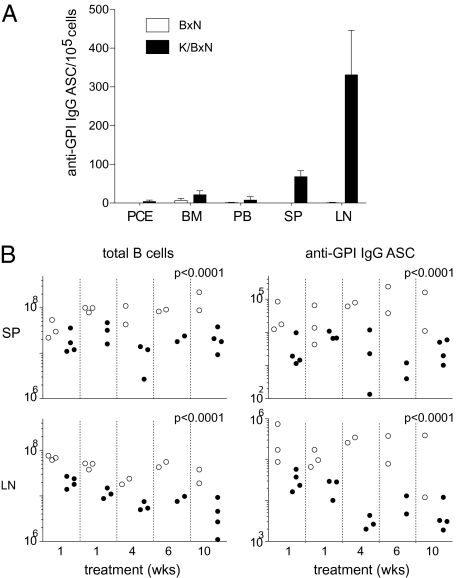
The rapid decrease in anti-GPI IgG, but not total IgG, titers in rituximab-treated hCD20-expressing mice suggested that this drug might specifically affect the generation and/or maintenance of B lineage cells producing autoAbs against GPI but not those producing bulk IgG. To track such cells after drug administration, we first examined their localization. Cells prepared from the bone marrow (BM), spleen (SP), lymph nodes (LNs; only inguinal, axillary, and brachial nodes were collected), peripheral blood (PB), and peritoneal cavity (PEC) of 8-week-old arthritic K/BxN mice were assayed for anti-GPI IgG ASCs by enzyme-linked immunosorbent spot (Fig. 2A). Interestingly, the SP and especially, the LNs had by far the highest number of anti-GPI ASCs, whereas the BM, where long-lived plasma cells are known to reside (13, 14), had very few. Although the ELISPOT assay measures only the percentage of ASCs of the total cells, this pattern of distribution reflects the absolute cell numbers as well given that the total numbers of cells in each organ were not very different. Therefore, plasma cells in the SP and LNs are the major source of anti-GPI autoantibodies, and we focused our further analyses on these cells.
Fig. 2.
(A) Anti-GPI IgG ASCs in different organs. Cells prepared from the indicated organs of 8-week-old K/BxN arthritic mice and BxN negative-control littermates were assayed by ELISPOT. The mean and SD are shown (n = 3). Cells were from peritoneal cavity exudates (PCE), bone marrow (BM), peripheral blood (PB), spleen (SP), and lymph nodes (LN). (B) Depletion of B cells and ASCs in SP and LNs of hCD20/K/g7 mice after rituximab treatment. Results from five independent experiments with the indicated lengths of drug administration to hCD20-positive and -negative littermates are shown. Each dot represents an individual mouse. The significance of the differences between data on hCD20/K/g7 (filled circles) and K/g7 (open circles) mice was calculated by one-way ANOVA using Stata.
Depletion of GPI-Specific ASCs by Rituximab Treatment.
Next, we examined the extent of depletion of B lineage cells by rituximab treatment in this system. Arthritic hCD20/K/g7 and K/g7 mice were treated with rituximab for different periods of time. Residual B cells were quantified by flow cytometry, and the number of ASCs was determined by ELISPOT. There was a moderate, approximately 4-fold on average, reduction in numbers of B cells in both the SP and LNs of hCD20/K/g7 compared with K/g7 mice (Fig. 2B Left). A parallel reduction in anti-GPI IgG ASCs was observed for these two organs, and it was more profound, averaging approximately 15-fold (Fig. 2B Right). Rituximab treatments as short as 1 week in duration provoked substantial cell depletion in hCD20-expressing mice; longer drug administration did not augment the extent of depletion in the SP but proved more effective than the shorter protocols in the LNs. To provide data parallel to those obtained from studies on human patients, we also determined the ratio of CD19+ B cells to CD45+ hematopoietic cells in PB of the same mice. On average, there were 48.9% and 11.1% B cells in rituximab-treated K/g7 mice and hCD20/K/g7 animals, respectively (i.e., an 87% reduction in the latter compared with the near-complete B cell depletion in rituximab-treated humans).
Detection and Characterization of GPI-Specific Plasma Cells.
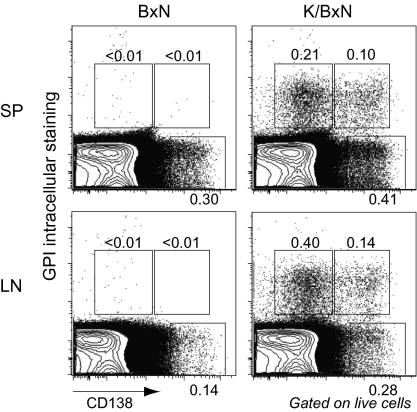
To further characterize anti-GPI ASCs, we performed a flow cytometric analysis. Plasma cells are characterized by expression of CD138 (syndecan-1) and the very high intracellular levels of the Abs that they produce, which can be revealed by staining with labeled cognate antigen. Thus, GPI-specific plasma cells were identified by anti-CD138 cell-surface positivity and intracellular staining with fluorochrome-labeled GPI at a concentration 10 times lower than that used for detecting cell-surface anti-GPI Igs. SPs from arthritic K/BxN and control BxN mice harbored similar populations of polyclonal plasma cells (GPI-CD138+) as did the LNs of arthritic K/BxN mice (Fig. 3). These organs from BxN mice contained few if any GPI-specific plasma cells, whereas K/BxN SP and LNs had two populations: the majority of GPI-positive cells expressed low levels of CD138, and a distinct, smaller population expressed CD138hi (Fig. 3).
Fig. 3.
Detection of GPI-specific plasma cells by flow cytometry. Cells prepared from SP or LNs of K/BxN or BxN mice at 8 weeks of age were first stained with anti-CD138 mAb and then stained intracellularly with Alexa Fluor 647-labeled GPI. Three gates and the percentages of cells within them are shown after gating on live cells. Representative of five independent experiments.
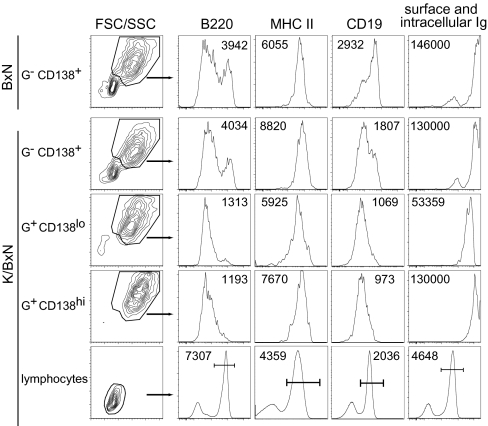
To phenotype the GPI-specific plasma cell populations and in particular, to determine their maturation status, we stained for various cell-surface markers as well as for surface and intracellular Ig (Fig. 4). For comparison purposes, the analysis included polyclonal plasma cells (GPI-CD138+) from both control, nonarthritic BxN mice and arthritic K/BxN animals. The splenic GPI-CD138+ populations from both types of mice were enriched in cells with the characteristics of early stage plasma cells: high forward-scatter and side-scatter, high B220loMHCII+CD19int, and high levels of intracellular Ig. Cells with lower forward-scatter and side-scatter and higher B220 probably represent contaminating B lymphocytes, because they clearly express surface Ig. The CD138lo and CD138hi populations of GPI-positive splenic cells from K/BxN mice also looked like typical early stage plasma cells by all of these phenotypic criteria; the former expressed about 3-fold less intracellular Ig. Similar results were obtained on LN cells from K/BxN and BxN mice as well as on SP and LN cells from K/g7 and hCD20/K/g7 mice. These results suggest that GPI-specific plasma cells are early stage and likely plasmablasts.
Fig. 4.
Phenotyping of GPI-specific plasma cells. Each population of cells from 7-week-old K/BxN or BxN mice was defined as in Fig. 3 [GPI intracellular staining (G)]. The number in each histogram is the mean fluorescence intensity (MFI) of all cells or gated cells when the gate is indicated by a bar. Representative of three independent experiments.
Depletion of GPI-Specific Plasma Cells by Rituximab Reflects Their Expression of CD20 and Short Half-Life.
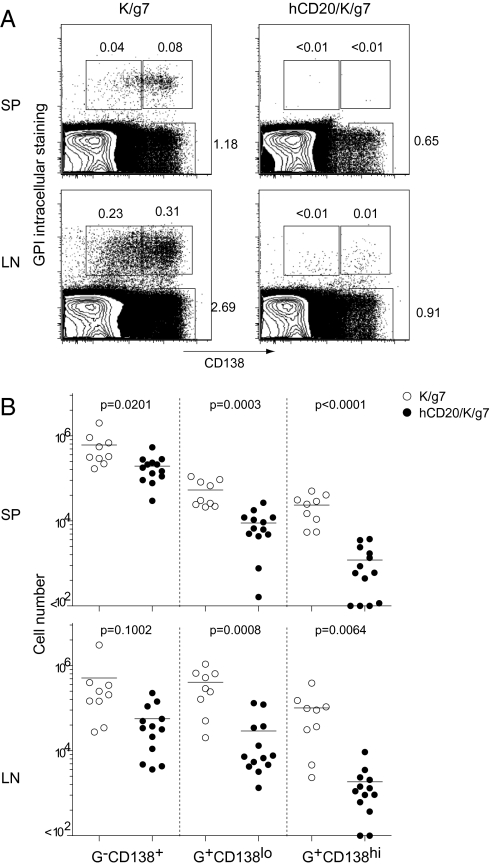
Rituximab treatment resulted in dramatic, preferential reduction of the CD138lo and CD138hi populations of GPI-specific plasma cells from hCD20/K/g7 mice compared with K/g7 negative controls (Fig. 5A). Weekly treatment beginning at 5–7 weeks of age resulted in dramatic reduction of these two cell types, particularly the CD138hi population. The polyclonal plasma cell population (GPI-CD138+) was also reduced but typically only severalfold, and the difference did not reach statistical significance. Absolute numbers of the various populations calculated from the flow analysis (Fig. 5B) were consistent with the numbers of ASCs measured by ELISPOT (Fig. 2B).
Fig. 5.
Depletion of GPI-specific plasma cells after rituximab treatment. (A) Representative flow cytometrical detection of GPI-specific plasma cells. K/g7 and hCD20/K/g7 mice were treated for 3.5 weeks beginning at 7 weeks of age. Cells prepared from SP and LNs are shown. (B) Absolute numbers of the three populations of cells in SP and LNs as defined in A after treatment [GPI intracellular staining (G)]. Lymphocytes were excluded from the G-CD138+ population by forward-scatter and side-scatter. Plotted are results from four independent experiments with 4- to 7-week-old mice treated with rituximab for 1–10 weeks. Each dot represents one individual mouse. Unpaired t test was performed on each population.
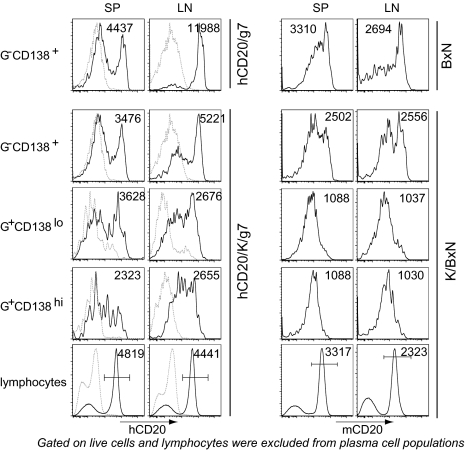
These results prompted us to question whether or not GPI-specific plasma cells in the SP and LNs might be a direct target of rituximab, previously considered unlikely because plasma cells have generally been thought to be devoid of CD20 (1, 2, 9). hCD20 was clearly detectable on both the CD138lo and CD138hi GPI-positive populations in the SP and LNs of untreated hCD20/K/g7 mice, although the expression levels varied over a broad range and were lower than on B lymphocytes (Fig. 6). This unanticipated expression may be related to the relatively immature status of plasmablasts, or it may be a property of the hCD20 BAC transgene. Therefore, we assessed the display of endogenous mouse CD20 on GPI-positive plasma cells in K/BxN vs. BxN mice with a well-characterized biotinylated anti-mouse CD20 antibody (clone 18B12 IgG2b; Biogen). Both of the GPI-positive plasma cell populations in SP and LNs expressed mouse CD20, although the expression levels were about one-third to one-half of that on standard B cells (Fig. 6).
Fig. 6.
Expression of human and murine CD20 on plasma cells. Each population of cells from 7-week-old hCD20/K/g7 vs. hCD20/g7 mice or K/BxN vs. BxN mice was defined as in Fig. 3 [GPI intracellular staining (G)]. The number in each histogram represents the MFI of all cells or gated cells when the gate is indicated by a bar. For hCD20 expression, K/g7 mice were used as negative control and indicated by the light dotted line in each histogram. Lymphocytes were excluded from the G-CD138+ population by forward-scatter and side-scatter.
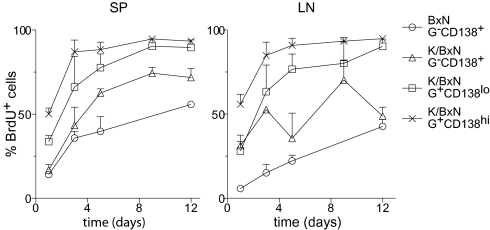
Reductions in the GPI-positive plasma cell populations after rituximab treatment might, in addition, reflect a short half-life coupled with a block in the generation of new plasma cells through ablation of their B cell precursors. We analyzed the turnover rates of SP and LN plasma cell populations from 7- to 8-week-old arthritic K/BxN and control BxN mice by continuous BrdU labeling through its inclusion in the drinking water for different amounts of time (Fig. 7). Both the CD138lo and CD138hi GPI-positive plasma cell populations contained a high proportion of BrdU-labeled cells, the latter having the greatest fraction. The 70–90% of GPI-specific plasma cells labeled by BrdU during just a few days suggested that they have a very fast turnover rate, characteristic of plasmablasts. Interestingly, both populations had much higher percentages of BrdU+ cells than did the polyclonal GPI-CD138+ populations from either K/BxN or BxN mice, suggesting that autoreactive plasma cells might have a faster turnover rate.
Fig. 7.
Half-life of GPI-specific plasma cells. Eight-week-old K/BxN and BxN mice (n = 2–5 for each time point) were fed BrdU (0.8 mg/mL) in the drinking water for the indicated amount of time. The percentage of BrdU+ cells (mean + SD) in each population defined as in Fig. 3 is plotted [GPI intracellular staining (G)]. Lymphocytes were excluded from the G-CD138+ population by forward-scatter and side-scatter.
Discussion
This study explored the mechanism of action of rituximab in the K/BxN model of inflammatory arthritis, wherein mice develop joint-specific auto-inflammatory disease because of T and B cell reactivity to the ubiquitously expressed self-antigen, GPI. Although rituximab treatment did not reverse ankle thickness and clinical index in K/BxN mice, the power of this model for these mechanistic explorations is that the autoantigen is known and the autoreactive B lineage cells are present in high proportions, together permitting us to track the behavior of specific, relevant cell populations subsequent to drug administration. As has been seen in several human-disease contexts (reviewed in ref. 1), rituximab treatment greatly reduced titers of serum autoAbs without substantially altering total Ab titers. The reduction in serum anti-GPI Abs was accompanied by a loss of B lineage cells, particularly SP and LN anti-GPI plasma cells. Two characteristics of these plasma cell populations show that rituximab's ability to clear autoAbs from the serum is likely to reflect their ablation: their expression of substantial levels of CD20, which renders them directly targetable by the drug, and their rapid turnover, which means that targeting them and their B lymphocyte precursors stands to have a significant impact on numbers of pathogenic ASCs. We have not assessed the relative importance of these two features in our model's response to rituximab but would like to suggest that the former might be the most important in this context. GPI-specific SP and LN B cells were reduced only about 4- to 5-fold, whereas GPI-specific ASCs were depleted 15-fold; this more impressive reduction almost certainly was a result of direct targeting of the antigen-specific plasmablasts. Importantly, SP and LN plasmablasts were found to be the major anti-GPI ASCs (i.e., the usual dominant population of long-lived, BM-residing antigen-specific plasma cells was not detected).
IgG, being the most abundant serum Ig, has a half-life of 5–9 days in mice and 21–24 days in humans (reviewed in ref. 15). The levels of IgG can be maintained over a long period by continuous production of antibody by either short-lived or long-lived plasma cells. Our data showed that most of the anti-GPI IgGs made in these mice are from short-lived plasma cells. When this population is absent after rituximab treatment, the IgG present is presumably made by long-lived plasma cells. This sustained production resulting in prolonged IgG half-life could explain the lack of change in total IgG level. Thus, titers of specific autoantibodies can decline without an overall decrease in serum IgG.
At first glance, the observation that SP and LN anti-GPI plasma cells in the K/BxN model expressed substantial amounts of CD20 seems unexpected. It is frequently said that plasma cells do not display this cell-surface marker at a significant level, at least nothing like B cell levels (1, 2, 9). However, this contention is based mostly on older studies on lymphoma and leukemia cells, B cells activated in vitro, or ex vivo plasma cells primarily of BM origin (16, 17). More recent studies—cognizant of the clear distinction, for both humans and rodents, between short-lived plasmablasts residing mostly in the secondary lymphoid organs and long-lived plasma cells localized mainly in the BM (13, 14, 18)—have taken a closer look. Indeed, human tonsillar plasma cells were found to express substantial levels of CD20 and proapoptotic molecules (e.g., CD95), whereas BM plasma cells had a much lower level of CD20 but high expression of anti-apoptotic molecules (e.g., Bcl-2) (18, 19). In addition, rituximab treatment of a xenochimeric system (human tonsils grafted into immunocompromised mice) resulted in partial depletion of the human tonsillar plasma cells (19). These findings are generally consistent with our observations. As for mice, DiLillo et al. (20) reported that both splenic and BM plasma cells expressed CD20 at levels 4- to 5-fold lower than those on B lymphocytes but that these cells resisted anti-CD20 treatment. These results differ from ours, but the discrepancy may be attributable to the use of a very different anti-CD20 reagent and/or to a different means of quantifying plasma cell depletion.
Our data are consistent with a scenario whereby rituximab would be particularly effective in those autoimmune diseases in which autoAbs (i) play some important role in pathogenesis and (ii) are produced primarily by CD20-expressing, short-lived plasmablasts. It has been proposed that autoAbs might be produced preferentially by plasma cells that rapidly turnover, but although such a preference proved to be true of some autoAb specificities, it was by no means a general phenomenon (1, 3). It is not currently known what elements determine if plasma cells of a given specificity populate mostly the short-lived pool in secondary lymphoid organs or the long-lived BM pool, but likely possibilities include the site and levels of autoantigen expression, the form of the autoantigen (i.e., if it is soluble, membrane-bound, or intracellular and how well it can cross-link surface Ig receptors), and the factors that influence the availability of BM plasma cell survival niches (e.g., the age of the animal; reviewed in ref. 3). Another consideration that is likely to be relevant is whether or not the autoAb response matures into a germinal center reaction or remains an extrafollicular process, which has been recently described for rheumatoid factor in a mouse model of lupus (21). The fact that GPI-specific plasma cells in the K/BxN arthritis model were essentially absent from the long-lived BM pool probably reflects the facts that GPI is expressed by all cells and also circulates in the blood and that anti-GPI autoAbs are generally of quite high affinity (22).
Perhaps this information will prove of use in deciding which autoimmune and other disorders are most likely to be responsive to rituximab and similar therapies. There is no currently available method to estimate what fraction of autoAbs derives from short- vs. long-lived plasma cells in a given human-disease context. For the time being, data from animal models might be a good place to start—for example, the finding that as many as 40% of autoAbs in the NZB/NZW lupus model derive from long-lived plasma cells (4) might have foretold rituximab's so far disappointing performance in systemic lupus erythematosus (23, 24). In the future, it may be possible to develop assays for human long-lived plasma cells, perhaps through small bone-marrow aspirations or by capturing the recently described (18) blood-borne precursors of BM plasma cells as a biomarker. The relative diversity of the autoAb repertoire might also be informative, and long-term affinity maturation favors more homogeneity.
Materials and Methods
Mice.
K/BxN animals were generated by crossing KRN TCR transgenic mice on the B6 genetic background with non-obese diabetic mice (10). hCD20/K/g7 animals were generated by first crossing hCD20 transgenic mice on the B6 background (12) to B6.H-2g7 congenic mice and then, crossing the offspring with KRN TCR transgenic mice. hCD20/K/g7 mice or K/g7 control mice were injected intraperitoneally with 1 mg of rituximab weekly beginning at 5–8 weeks of age. Ankle thickness and clinical index were monitored, and tail blood samples were collected each week.
ELISA.
Serum titers of anti-GPI IgG and total IgG were determined by ELISA. Plates were coated with 5 μg/mL of recombinant mouse GPI and goat anti-mouse IgG(H+L) (Jackson ImmunoResearch Laboratories Inc.), respectively. After incubating with serial dilutions of serum samples, bound antibodies were detected by adding biotinylated goat anti-mouse IgG (subclasses 1+2a+2b+3) Fcγ fragment-specific antibody (Jackson ImmunoResearch Laboratories Inc.) followed by alkaline phosphatase-conjugated streptavidin. The data were fitted by a four-parameter curve using DeltaSOFT 3 (Biometallics, Inc.). Titer is defined as the serum dilution that gave an optical density of 50% maximum (inflection point) of the curve.
Abs and Flow Cytometric Analyses.
Rituximab was a gift from Genentech. Commercially obtained mAbs used in these studies included anti-CD138, anti-MHCII, anti-B220, and anti-CD19 (BD Pharmingen). Expression of hCD20 was determined by staining with anti-hCD20 (BD Pharmingen) or by first incubating the cells with a saturating amount of rituximab and then staining with a biotinylated antihuman IgG-Fc Ab (Caltag) followed by fluorochrome-conjugated streptavidin. Expression of mouse CD20 was assessed by staining with a biotinylated anti-mouse CD20 mAb clone 18B12, (Marilyn Kehry and Robert Dunn at Biogen Idec).
For detection of GPI-specific plasma cells, dispersed cells (usually from the SP or LNs) were first stained with various surface markers and then fixed in Perm/Fix (BD Pharmingen) and stained in Perm/Wash (BD Pharmingen) with AlexaFluor-647–labeled GPI at 1 μg/mL. For detection of surface and intracellular Ig, cells were stained in Perm/Wash with an anti-Ig kappa mAb (187.1; BD Pharmingen).
Samples were run on a LSR II or a FACSCanto analyzer (BD Biosciences); 1–2 × 106 events were collected for each sample. Flow cytometry data were analyzed by FlowJo (Tree Star, Inc.).
ELISPOT Assay for Quantification of GPI-Specific ASCs.
MultiScreen IP plates (Millipore) were prewet following the manufacturer's instructions and were coated with recombinant GPI in PBS (10 μg/mL) at 4 °C overnight. Plates were washed and blocked with 2% BSA in PBS or cell medium (RPMI-1640, 10% FBS, pencillin, and streptomycin) for 2 h at 37 °C. Cells prepared from various organs were plated at multiple cell numbers starting with no more than 106 cells per well. After incubation at 37 °C for 4–6 h, plates were washed with PBS/0.01% Tween-20 and incubated with biotin-labeled goat–anti-mouse IgG(1+2a+2b+3)-Fcγ specific Ab followed by alkaline–phosphate-conjugated streptavidin (Jackson Immunoresearch Laboratories Inc.). Spots were developed by adding 5-Bromo-4-Chloro-3′-Indolyphosphate p-Toluidine salt/nitro-blue tetrazolium chloride (BCIP/NBT) substrate solution (Pierce) and were counted on an ImmunoSpot counter (Cellular Technology Ltd).
BrdU Labeling and Detection.
Adult mice were given 0.8 mg/mL BrdU (Sigma) in the drinking water for the specified times. Cells were harvested and stained for surface expression of various markers; detection of BrdU was by flow cytometry after anti-BrdU mAb staining following the manufacturer's protocol (BD Pharmingen). AlexaFluor488 anti-BrdU mAb and AlexaFluor 647-GPI were incubated with permeabilized cells at the same time.
Acknowledgments
We thank Dr. Rudolf A. Manz for advice on plasma-cell detection, Vanesa Tran, Kimie Hattori, and Adriana Ortiz-Lopez for help with mice, Genentech for providing human CD20 transgenic mice and rituximab, Marilyn Kehry and Robert Dunn for anti-mouse CD20 mAb, and Dr. Martin Weigert for critical reading of the manuscript. This work was supported by Grant R01 AR046580 (to D.M. and C.B.) from the National Institutes of Health and by grants from Joslin's National Institute of Diabetes and Digestive and Kidney Diseases-supported Diabetes and Endocrinology Research Center cores. H.H. was supported by Grant DRG-1616 from the Damon Runyon Cancer Research Foundation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Martin F, Chan AC. B cell immunobiology in disease: Evolving concepts from the clinic. Annu Rev Immunol. 2006;24:467–496. doi: 10.1146/annurev.immunol.24.021605.090517. [DOI] [PubMed] [Google Scholar]
- 2.Hoyer BF, Manz RA, Radbruch A, Hiepe F. Long-lived plasma cells and their contribution to autoimmunity. Ann N Y Acad Sci. 2005;1050:124–133. doi: 10.1196/annals.1313.014. [DOI] [PubMed] [Google Scholar]
- 3.Radbruch A, et al. Competence and competition: The challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 4.Hoyer BF, et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199:1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouaziz JD, et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci USA. 2007;104:20878–20883. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leandro MJ, de la Torre I. Translational mini-review series on B cell-directed therapies: The pathogenic role of B cells in autoantibody-associated autoimmune diseases—lessons from B cell-depletion therapy. Clin Exp Immunol. 2009;157:191–197. doi: 10.1111/j.1365-2249.2009.03978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorner T, Radbruch A, Burmester GR. B-cell-directed therapies for autoimmune disease. Nat Rev Rheumatol. 2009;5:433–441. doi: 10.1038/nrrheum.2009.141. [DOI] [PubMed] [Google Scholar]
- 8.Grillo-López AJ, Hedrick E, Rashford M, Benyunes M. Rituximab: Ongoing and future clinical development. Semin Oncol. 2002;29(1 Suppl 2):105–112. [PubMed] [Google Scholar]
- 9.Bour-Jordan H, Bluestone JA. B cell depletion: A novel therapy for autoimmune diabetes? J Clin Invest. 2007;117:3642–3645. doi: 10.1172/JCI34236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kouskoff V, et al. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 11.Korganow AS, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 12.Gong Q, et al. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174:817–826. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 13.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 14.Manz RA, Löhning M, Cassese G, Thiel A, Radbruch A. Survival of long-lived plasma cells is independent of antigen. Int Immunol. 1998;10:1703–1711. doi: 10.1093/intimm/10.11.1703. [DOI] [PubMed] [Google Scholar]
- 15.Zuckier LS, Rodriguez LD, Scharff MD. Immunologic and pharmacologic concepts of monoclonal antibodies. Semin Nucl Med. 1989;19:166–186. doi: 10.1016/s0001-2998(89)80012-1. [DOI] [PubMed] [Google Scholar]
- 16.Anderson KC, et al. Expression of human B cell-associated antigens on leukemias and lymphomas: A model of human B cell differentiation. Blood. 1984;63:1424–1433. [PubMed] [Google Scholar]
- 17.Stashenko P, Nadler LM, Hardy R, Schlossman SF. Expression of cell surface markers after human B lymphocyte activation. Proc Natl Acad Sci USA. 1981;78:3848–3852. doi: 10.1073/pnas.78.6.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medina F, Segundo C, Campos-Caro A, González-García I, Brieva JA. The heterogeneity shown by human plasma cells from tonsil, blood, and bone marrow reveals graded stages of increasing maturity, but local profiles of adhesion molecule expression. Blood. 2002;99:2154–2161. doi: 10.1182/blood.v99.6.2154. [DOI] [PubMed] [Google Scholar]
- 19.Withers DR, et al. T cell-dependent survival of CD20+ and CD20- plasma cells in human secondary lymphoid tissue. Blood. 2007;109:4856–4864. doi: 10.1182/blood-2006-08-043414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiLillo DJ, et al. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008;180:361–371. doi: 10.4049/jimmunol.180.1.361. [DOI] [PubMed] [Google Scholar]
- 21.William J, Euler C, Shlomchik MJ. Short-lived plasmablasts dominate the early spontaneous rheumatoid factor response: Differentiation pathways, hypermutating cell types, and affinity maturation outside the germinal center. J Immunol. 2005;174:6879–6887. doi: 10.4049/jimmunol.174.11.6879. [DOI] [PubMed] [Google Scholar]
- 22.Maccioni M, et al. Arthritogenic monoclonal antibodies from K/BxN mice. J Exp Med. 2002;195:1071–1077. doi: 10.1084/jem.20011941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dörner T, Burmester GR. New approaches of B-cell-directed therapy: Beyond rituximab. Curr Opin Rheumatol. 2008;20:263–268. doi: 10.1097/BOR.0b013e3282f5e08d. [DOI] [PubMed] [Google Scholar]
- 24.Looney RJ, Anolik J, Sanz I. B cells as therapeutic targets for rheumatic diseases. Curr Opin Rheumatol. 2004;16:180–185. doi: 10.1097/00002281-200405000-00003. [DOI] [PubMed] [Google Scholar]