Abstract
Some of the most successful gene therapy results have been obtained using recombinant viral vectors to treat animal models of inherited and acquired ocular diseases. Clinical trials using adenovirus vector systems have been initiated for two ocular diseases. Adeno-associated viruses (AAVs) represent an attractive alternative to adenoviral vector systems as they enable stable and long-term expression and can target a variety of different ocular cell types depending on the capsid serotype; recently clinical trails for congenital blindness was initiated with a vector-based AAV serotype 2. High levels of retinal gene transfer have been achieved using vectors based on AAV serotypes 1, 2, 4 and 5. This report compares the gene transfer efficacy and stability of expression of vector systems based on three novel AAV serotypes: AAV7, 8, 9, with the established vectors AAV1, 2, 5. We show here that AAV7 and 8 enable superior long-term transduction of retinal and also anterior chamber structures.
Keywords: adeno-associated virus, novel serotypes, ocular gene transfer
Introduction
There has been good progress over the past decade in demonstrating proof-of-principle for gene-based strategies to treat inherited and acquired blinding diseases in animal models. These diseases, which include retinitis pigmentosa, Leber congenital amaurosis, diabetic retinopathy, choroidal neovascularization and retinopathy of prematurity [1–6], currently either have no treatment or the treatment is imperfect.
The eye is an ideal target organ for viral gene transfer as it is small and compartmentalized and thus requires only a small amount of vector. In addition, the eye has favorable immunological properties with respect to virus-mediated gene transfer [7]. Finally, since many blinding diseases are bilaterally symmetrical and there are two eyes, it is possible to perform controlled experiments targeting ocular disease by using one eye as the experimental eye and the contralateral eye as the control. Adeno-associated virus (AAV) vectors have gained much favor in experimental systems over the past few years because they are able to transduce a variety of retinal cell types in a stable fashion [8–10]. Further, they do not cause significant toxicity in small or large animal models.
Over 100 different AAV serotypes have been described and nine of these have been tested already in vivo in animals [11–16]. Since the capsid protein of AAV is responsible for its tropism (and thus efficacy), a pseudotyping strategy was developed enabling the packaging of an AAV2 genome into the capsid of another serotype [9,14]. The hybrid vectors generated in this fashion have the combined advantage of safety and long-term expression of AAV2 and the improved in vivo efficacy and tropism of the novel serotypes. In the eye, AAV vectors based on the serotypes 1, 2, 4 and 5 have been evaluated after intravitreal and subretinal injection [8–10]. Of these, AAV2/2 emerged as the only vector able to efficiently transduce cells in the inner retinal layers after intravitreal injection [8]. In contrast, AAV1, 4 and 5-based vectors delivered transgenes efficiently to the neural retina and pigment epithelium after subretinal injection.
In general, the criteria for the ideal vector differ depending on the target disease. Inherited diseases caused by a single mutation in a specific gene call for a vector which is able to replace the non-functional gene in that specific cell type. In contrast, acquired diseases, such as diabetic retinopathy which are caused by complex pathological mechanisms and affect cells in all retinal layers, require vectors which are able to target a wide variety of cells or induce high levels of a secreted therapeutic protein.
In this study, we evaluated the transduction characteristics of the novel AAV serotypes 7, 8 and 9 in the eye and compared these to the already well-studied transduction patterns of AAV serotypes 1, 2 and 5. For this, we created hybrid AAV2/1, 2/2, 2/5, 2/7, 2/8 and 2/9 vectors which carried the enhanced green fluorescent protein (eGFP). The latter was used to evaluate onset and strength of expression through non-invasive means and also to evaluate cellular specificity and efficiency of transduction over time. We also used the secreted protein, rhesus erythropoietin (rhEpo), as the transgene in order to obtain quantitative data on secretion levels.
Methods
Vector production
The vectors carried transgene cassettes encoding eGFP or rhEpo under control of a cytomegalovirus (CMV) promoter. The AAV2 genome was packaged into an AAV1, 2, 5, 7, 8 or 9 capsid using triple transfection as described [8]. Virus was concentrated via heparin column (AAV2) or cesium purification (AAV1, 5, 7, 8, 9) [17]. Titers were determined by RealTime polymerase chain reaction (PCR) as described [19].
Animal studies
Adult (2 months old) C57BL/6 mice were used for all studies. All experiments were performed in compliance with institutional regulations and were approved by the Institutional Animal Care and Use Committee. Animals were anesthetized and intravitreal or subretinal injections were performed as described previously [19,20].
In study I, animals were injected with AAV2/2, 2/1, 2/5, 2/7, 2/8 or 2/9.CMV.eGFP (2 μl/eye, giving a dose of 1 × 1010 gc/eye, n = 10/group). Each animal received an intravitreal injection in one eye and a subretinal injection in the contralateral eye. eGFP expression was followed via serial indirect ophthalmoscopic examinations at designated timepoints over the course of the study period. Evaluations were performed daily over the first week, at weekly intervals through 2 months and at monthly intervals through the 180 day timepoint thereafter. The extent of eGFP-specific fluorescence was assessed with a numerical grading system ranging from 0 to 4 [19]. Briefly, grade 0 represents no visible fluorescence; grade 1, isolated fluorescing cells; grade 2, patches of fluorescing cells 1–2 disc areas in size; grade 3, confluent patches of fluorescing cells several disc areas in size, but requiring a condensing lens for visualization; grade 4, same as grade 3, but fluorescence is visible through the pupil without use of a condensing lens. In order to correlate the pattern of eGFP expression observed in vivo with specific cell types expressing eGFP, cohorts of animals were killed at days 14 and 180 after injection, and eyes were enucleated for histology.
In study II, animals were injected with AAV.CMV.rhEpo. A pilot study was performed in which each animal received a unilateral subretinal or intravitreal injection of AAV2/7 or AAV2/8 vectors (2 μl/eye, dose 1 × 1010 gc/eye, n = 5/vector and injection route). After we were able to rule out potential cross-contamination of eyes of each individual animal with rhEpo, a larger study was carried out where bilateral injections were performed. Similar to the eGFP study described above, each animal received an intravitreal injection in one eye and a subretinal injection in the contralateral eye. Vectors used were AAV2/2, 2/1, 2/5, 2/7, 2/8 or 2/9.CMV.rhEpo (2 μl/eye, dose 1 × 1010 gc/eye, n = 10/group). Animals were killed and eyes were enucleated 28 days after injection. The eyes were then homogenized for detection of protein/transgene expression.
In study III, a dose response study was performed for AAV2/7 and 2/8. Vectors were injected bilaterally either subretinally (n = 5/group) or intravitreally (n = 5/group) at a dose of 1 × 109 gc, 3 × 109 gc or 1 × 1010 gc/eye, with 2 μl virus injected/eye. Twenty-four days after injection, the animals were killed, the eyes enucleated and intraocular Epo levels were determined.
Histology
Eyes injected with the various AAVs were enucleated and immediately fixed in 4% paraformaldehyde/phosphate-buffered saline (PBS). They were cryoprotected in 30% sucrose/PBS overnight and frozen in optimal cutting temperature compound (Fisher Scientific, Pittsburgh, PA, USA). For each eye, 150–200 10 μm serial sections were cut with a cryostat (Reichert Jung model 822; Leica Microsystems, Inc., Wetzlar, Germany). The sections were progressively distributed on 10 slides so that each slide contained 15–20 sections representative of different planes of the entire eye. For each eye, a minimum of three slides was analyzed. Sections were counterstained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI, Vectashield mounting medium; Vector Labs, Burlingame, CA, USA). All were mounted and analyzed with a Leica DME microscope (Leica Microsystems, Inc.) equipped with epifluorescence and images were captured with a Hamamatsu digital camera and Openlab 2.2 image analysis software (Improvision, Inc., Boston, MA, USA).
Detection of rhEpo expression
Eyes injected with rhEpo were enucleated and homogenized in radio-immunoprecipitation assay (RIPA) buffer. Total protein content of the homogenate was determined using a bicinchoninic acid (BCA) protein assay (Pierce Biotechnology, Rockford, IL) and rhEpo levels were evaluated using an enzyme-linked immunosorbent assay (ELISA) for human Epo (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
Results
Transduction characteristics of different AAV serotypes encoding eGFP
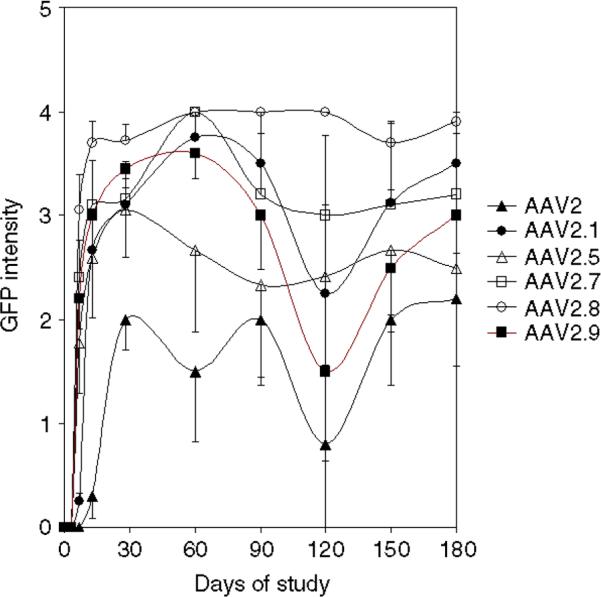
In study I, onset and intensity of eGFP expression were evaluated non-invasively via serial in vivo indirect evaluations. eGFP was detectable ophthalmoscopic indirect only in animals that had been injected subretinally, but not in those that had been injected intravitreally. The time of onset of eGFP expression differed for the various serotypes, with AAV2/1, 2/5, 2/7, 2/8 and 2/9 being apparent earliest (first apparent between 5–7 days after injection), and AAV2/2 being the latest (first appearing 2 weeks after injection). Levels of eGFP increased with time in all animals, but reached different intensities which stabilized at different timepoints (Figure 1). eGFP levels from all animals except for those injected with AAV2/2 reached a plateau at the 2 week timepoint after injection (Figure 1); AAV2/2 plateaued at 3 weeks after injection (Figure 1). Levels of eGFP fluorescence remained stable through the 180 day timepoint. Substantial animal-to-animal variation precluded a definitive assessment of relative expression using the in vivo evaluation that measures global GFP expression.
Figure 1.
Presence and intensity of eGFP in eyes injected subretinally with AAVs. Serial average eGFP intensities after subretinal injection of novel AAVs over a 6 month timecourse
Fundus photographs were taken of representative eyes at selected timepoints during the course of the study (Figure 2) and also at the end of the study (Figure 3). These show presence of eGFP localized to the region of the retina which had received subretinal injection.
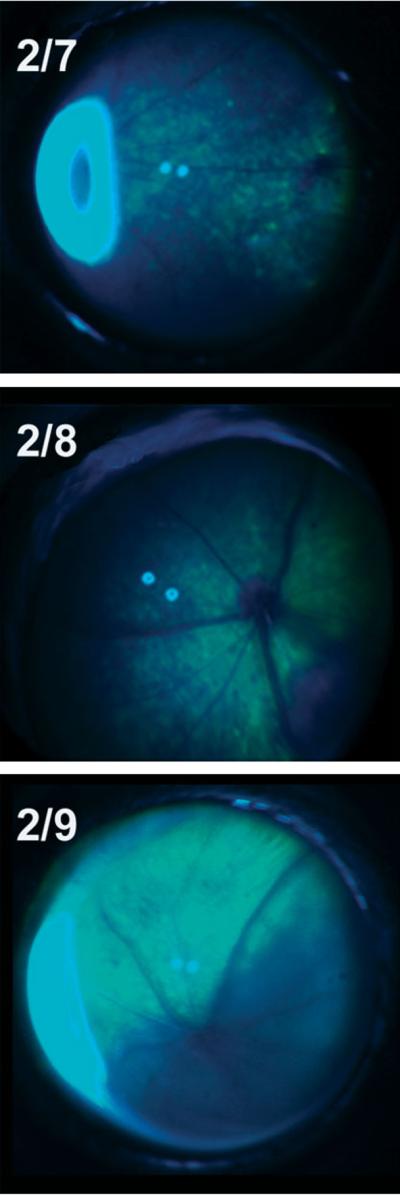
Figure 2.
Representative Fundus photos showing eGFP-positive regions of the retina 2 weeks after injection for the novel AAVs, AA2/7, 2/8 and 2/9. High levels of eGFP were observed with each of the novel vectors at this timepoint. Variability from injection to injection accounts for the differences in the area of each retina that was exposed to the vector. Inner retinal blood vessels (which appear dark against the eGFP-positive retina) are apparent in all three panels
Figure 3.
Representative Fundus photographs of eGFP-positive regions of retina taken 6 months after subretinal injection of (a) AAV2/1, (b) AAV2/2, (c) AAV2/5, (d) AAV2/7, (e) AAV2/8, or (f) AAV2/9. High levels of eGFP were observed in retinas injected with each of the AAV serotypes except for AAV2/2 (b). eGFP levels were generally lower in eyes treated with AAV2/9 (f) than with the other novel serotypes (d, e)
At the end of the study, eGFP was faint in retinas injected with AAV2/2 (Figure 4) and AAV2/9; levels were high in retinas injected with AAV2/1, 2/5, 2/7 and 2/8. Histology was performed to further delineate the transduction characteristics of the different serotypes.
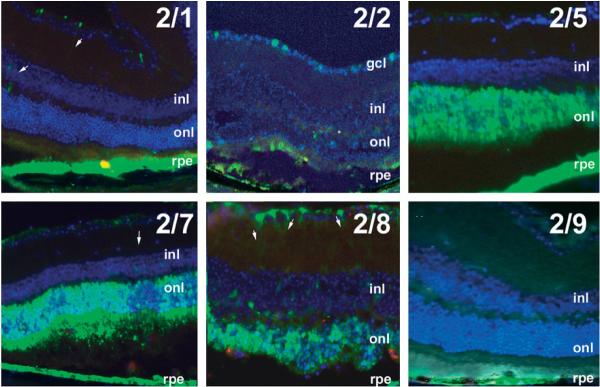
Figure 4.
Representative fluorescence patterns in cryosections 6 months after subretinal injection with AAV2/1, 2/2, 2/5, 2/7, 2/8 or 2/9. Arrows indicate eGFP-labeled Muller cells. gcl, ganglion cell layer; inl, inner nuclear layer; onl, outer nuclear layer; rpe, retinal pigment epithelium
After subretinal injection, the retinal pigment epithelium (RPE) was transduced by all AAV serotypes (Figure 4), although eGFP fluorescence was more intense after exposure to certain serotypes (2/1, 2/5, 2/7, 2/8) than others (2/9 > 2/2; Table 1). Photoreceptors, which have their cell bodies located in the outer nuclear layer (onl), were transduced efficiently by AAV2/5, 2/7 and 2/8. Ganglion cells (with cell bodies in the ganglion cell layer) were transduced by AAV2/2 and 2/8. Occasional Muller cells were transduced by AAV2/1, 2/5, 2/8 and 2/9 (see arrows in Figure 4).
Table 1.
Summary of cellular specificity of hybrid AAV vectors after subretinal injection. Relative expression from highest (+++) to lowest (−) is shown
| Capsid | RPE | PRs | Muller | GCL |
|---|---|---|---|---|
| 1 | +++ | − | rare | − |
| 2 | + | + | + | + |
| 5 | +++ | +++ | + | − |
| 7 | +++ | +++ | − | |
| 8 | +++ | +++ | + | +++ |
| 9 | +++ | − | ++ | − |
After intravitreal injection only AAV2/2 and AAV2/8 were able to transduce retinal ganglion cells (Figure 5g, arrow, data not shown). Occasional Muller cells were transduced by AAV2/2, 2/8 and 2/9 (Figure 5g and data not shown). None of the other vectors showed significant retinal expression after intravitreal injection. Some of these transduced anterior segment structures, however. The novel AAV vectors (AAV2/7, 2/8, and 2/9) targeted trabecular meshwork (tm, Figures 5a–5c), Schlemm's canal and the uveo-scleral outflow tract (arrow in Figure 5c) and corneal stroma (see Figures 5d–5f), iris (Figure 5h and not shown) and lens epithelium (Figure 5i and not shown). AAVs 2/2, 2/7 and 2/8 transduced occasional cells of the ciliary body (Ci, Figure 5g and not shown). Neither AAV2/1 nor 2/2 nor 2/5 transduced corneal cells (not shown).
Figure 5.
Anterior segment structures targeted by novel AAVs. (a) Intravitreal injection of novel AAV vectors results in transduction of a variety of tissues including trabecular meshwork and Schlemm's canal (a–c), stromal cells of the cornea (d–f) and lens epithelium (i) and iris (h), but not cells of the ciliary body (Ci). The latter are transduced by AAV2/2 (g). Arrow in (c) indicates uveo-scleral outflow tract
Expression levels of different AAV serotypes encoding rhEpo
To evaluate the ability of the novel AAV vectors to deliver a secreted protein to the eye, further studies were performed using rhesus erythropoietin (rhEpo) as a reporter gene. In an initial pilot study we determined whether there was cross-contamination of the secreted protein from one eye to the other in individual animals. For this study, mice were injected either subretinally or intravitreally with the AAV.rhEpo vector unilaterally. Contralateral eyes received a surgical sham injection. Eyes were harvested 28 days later and intraocular Epo levels were determined. Epo was detectable only in those eyes injected with AAV.rhEpo (Figures 6a and 6b). Having established that no leakage occurred, bilateral injections were performed in the succeeding experiments.
Figure 6.
Innocular Epo levels. Levels of rhEpo 28 days after (a) subretinal or (b) intravitreal injection of AAV2/7 or AAV2/8 in the respective eye. (T = treated; M = mock-injected; n = 5/group). Comparison of intraocular Epo levels after (c) subretinal injection (n = 10/group, p < 0.05 AAV2/8 vs. AAV2/2, 2/5, 2/9; p < 0.01 AAV2/7 vs. AAV2/2, 2/1, 2/9) or (d) intravitreous injection (n = 10/group, p < 0.05 AAV2/2 vs. AAV2/1 and 2/9 with AAV2/2, 2/1, 2/7, 2/8 or 2/9 vectors). Effect of vectors (AAV2/7 and AAV2/8) on subretinal (e) or intravitreal (f) injection
For the direct comparison of the different serotypes 10 animals per group were injected with either AAV2/1, 2/2, 2/5, 2/7, 2/8 or 2/9. Each individual animal received the same vector dose bilaterally; however, an intravitreal injection was performed in one eye and a subretinal injection in the other. The studies revealed that subretinal injection results in 5–10-fold higher expression levels than intravitreal injection (cf. Figures 6a, 6b and 6c, 6d). AAV2/7 resulted in the highest levels of Epo after subretinal injection, followed by AAV2/1 and 2/8 (Figure 6c). After intravitreal injection, AAV2/2 resulted in similar levels as AAV2/7 and was closely followed only by AAV2/5 (Figure 6d).
To further analyze the potential of the novel AAV2/7 and 2/8 vectors, a dose-response study was performed. There was a clear dose response noted after subretinal injection, with highest levels (0.17 mlU/μg protein) observed after injection of 1 × 1010 vector genomes (Figure 6e). In contrast, after intravitreal injection, only the highest dose of AAV2/7 led to significant Epo levels (Figure 6f).
Discussion
Ocular gene therapy has great potential because of the unique features of the eye. First, the eye is easily accessible using standard minor surgical procedures thereby facilitating delivery of the vector; second, its small size limits the vector dose needed; and, finally, the eye enjoys a relative immune-privileged status, due to a low abundance of antigen-presenting cells and the presence of factors which actively suppress T-cell activation thus enabling stable expression of the transgene [21,22].
In this study, we compared the efficacy of ocular gene transfer using vectors based on the three novel AAV serotypes: 7, 8 and 9, to those already described, AAV2, 1 and 5 [8,9]. One set of vectors encoded eGFP so that evaluation of cellular transduction characteristics could be appreciated non-invasively in vivo and then histologically. The other set encoded the secreted protein, rhEpo, which we have previously demonstrated to be a reliable biological marker protein that is secreted [23,24]. Gene transfer efficacy was determined after intravitreal as well as subretinal injection.
Depending on the disease to be treated and the specific ocular tissue affected, it is desirable to select a vector system which is able to induce high transgene levels in the target cell type(s). So far, the main focus of ocular gene therapy remains on retinal diseases and the first clinical trials for a complication of macular degeneration, choroidal neovascularization and another trial targeting retinoblastoma are in progress [25,26]. These trials use adenoviral vectors. Other trials, using AAVs to test the safety of treatment of congenital blindness, were recently initiated.
In rodents, several vector systems based on adeno-, adeno-associated or lentiviruses have been shown to induce sufficient levels of expression in various retinal layers to demonstrate proof-of-principle for gene therapy. However, retinal transduction characteristics and duration of transgene expression vary immensely between these vectors. Adenoviral vectors result in transient expression and this can be further limited by immune response [20]. In contrast, lentiviral and adeno-associated viral vectors enabled stable expression thereby representing the more favorable tool for gene delivery [1,8]. In a side-by-side comparison study of lentiviral and AAV vectors based on serotypes 1, 2 and 5, it was shown that expression levels of these vectors – except AAV2 – were similar, yet expression pattern differed [8]. Expression was limited to the RPE with AAV1 and Lenti-Mokola vectors after subretinal injection, whereas AAV5 and lenti-VSVG vectors also transduced photoreceptors. Transgene expression after treatment with AAV2/2 was lower compared to the other serotypes. Nonetheless, AAV2/2 emerged as the only serotype able to transduce retinal ganglion cells and optic nerve fibers after intravitreal delivery [8].
We were able to show in this report that the novel serotypes AAV2/7 and 2/8 lead to an improved retinal gene transfer by efficiently and stably transducing cells in the neural retina. In addition, there was a rapid onset of transgene expression after delivery of these vectors. The overall eGFP intensity as determined using indirect ophthalmoscopy was in both cases higher than after treatment with AAV2, 2/1, 2/5, and 2/9.
Preclinical gene therapy studies for the most common blinding retinal disorders, adult macular degeneration and diabetic retinopathy, have generally made use of secretable antiangiogenic growth factors [27–29]. Additional studies aiming to develop a universal treatment for retinitis pigmentosa have utilized secreted neurotrophic factors [6,30,31]. Therefore, we evaluated a second vector system which expressed the secreted protein rhEpo. The results confirmed those seen with the eGFP vectors. Intraocular Epo levels were highest after subretinal injection with AAV2/7, followed by treatment with AAV2/8 and 2/1.
After intravitreal injection, AAV2/2 was the only vector which induced significant levels of eGFP expression in the retinal layers. AAV2/7 was able to transduce anterior chamber structures such as the trabecular meshwork, the iris and the cornea, however. Correlating with these results, AAV2/2 and AAV2/7 induced similar intraocular Epo levels after intravitreal injection.
Besides macular degeneration, glaucoma is a common cause of blindness, affecting more than 67 million people worldwide [32]. Glaucoma is often associated with an elevated ocular pressure which leads to a progressive destruction of retinal cells. In this form of the disease, open angle glaucoma, the aqueous humor, which fills the anterior and posterior chamber of the eye, is produced by the ciliary body and drained by the trabecular meshwork in the anterior segment of the eye. Since the complex pathophysiological mechanisms resulting in glaucoma are not completely elucidated, current therapies focus predominantly on decreasing fluid production or increasing drainage to decrease the intraocular pressure. There are other forms of glaucoma (normal-tension glaucoma) that develop without increased eye pressure. The recent identification of potential causal genes [33,34] provides fuel for new approaches for gene therapy strategies. A major limitation in the treatment of open-angle glaucoma is the lack of vectors which allow stable transduction of the aqueous outflow tract (the trabecular meshwork and Schlemm's canal) in vivo. Adenoviral vectors have been shown to enable transduction of ciliary cells and trabecular meshwork in mice and monkeys [35–38], but expression generally does not persist more than 2 weeks [38]. In contrast, we have shown here that intravitreal delivery of the novel AAV vectors 2/7 and 2/8 allows stable transduction of trabecular meshwork and Schlemm's canal over a period of 6 months. This finding provides a new therapeutic option for treatment of glaucoma through the delivery of molecules directly to the outflow tract of the eye. The ability to deliver molecules which are secreted into intraocular compartments will also allow evaluation of potential ameliorating effects of neurotrophic factors on the course of the retinal degenerative processes associated with glaucoma.
An additional feature of the novel AAV serotypes 2/7, 2/8 and 2/9 is their ability to transduce corneal stromal cells. Genetic modification of corneal cells can be useful for a variety of paradigms including: (1) treatment of inherited lysosomal storage diseases such as mucopolysaccharidosis VII, which leads to corneal clouding [39]; (2) inhibition of haze formation following refractive laser stromal ablation; (3) treatment of corneal neovascularization (or of neovascularization affecting other portions of the eye); or (4) the prevention of graft rejection after transplantation. Gene transfer has been achieved in cultured corneal cells, ex vivo corneas and also in vivo in animal models using lenti- [40] and adenoviral vectors [41]. Despite this, long-term therapeutic studies using these vectors have not been published to date. The novel AAVs therefore might provide a means of treating acquired and inherited blinding diseases by enabling stable and long-term expression in the cornea.
In conclusion, we characterized ocular expression patterns of three novel AAV vectors. These vectors expand the possibilities for development of ocular gene therapy due to their unique ocular transduction properties. All three vectors have a rapid onset of transgene expression. AAV2/7 and 2/8 result in extremely high levels of transgene expression in retinal cells. All three vectors expand the possibilities of developing treatment using the anterior segment as a gene delivery platform. These vectors are also promising as humans are not commonly exposed to antigens of these vectors. There is, therefore, less possibility that delivery of these vectors to the human eye will result in immune-mediated clearance (unlike the situation for AAV2/2 [42]). Further studies will be needed to evaluate effects of these novel serotypes in larger animal models, as well as examine their biodistribution after intraocular gene transfer.
Acknowledgements
This study was supported by NIH R01 EY10820 (JB) and 5-P30-DK-47747-10 (Vector Core Facility), the Foundation Fighting Blindness (JB), the William and Mary Greve International Research Scholar Award (JB), Research to Prevent Blindness, Inc., the Paul and Evanina Mackall Trust, and the F.M. Kirby Foundation. We thank Zhangyong Wei and Aatish Patel (University of Pennsylvania) for technical assistance. This work was also supported by GlaxoSmithKline Pharmaceuticals (JMW). Dr. James M. Wilson is an inventor on patents licensed to various commercial entities.
References
- 1.O'Reilly M, et al. RNA interference-mediated suppression and replacement of human rhodopsin in vivo. Am J Hum Genet. 2007;81:127–135. doi: 10.1086/519025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acland GM, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dejneka NS, Surace EM, Aleman TS, et al. In utero gene therapy rescues vision in a murine model of congenital blindness. Mol Ther. 2004;9:182–188. doi: 10.1016/j.ymthe.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Reich SJ, Bennett J. Gene therapy for ocular neovascularization: a cure in sight. Curr Opin Genet Dev. 2003;13:317–322. doi: 10.1016/s0959-437x(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 5.Tschernutter M, Schlichtenbrede FC, Howe S, et al. Long-term preservation of retinal function in the RCS rat model of retinitis pigmentosa following lentivirus-mediated gene therapy. Gene Ther. 2005;12:694–701. doi: 10.1038/sj.gt.3302460. [DOI] [PubMed] [Google Scholar]
- 6.Liang F-Q, Dejneka NS, Cohen DR, et al. AAV-mediated delivery of ciliary neurotrophic factor prolongs photoreceptor survival in the rhodopsin knockout mouse. Mol Ther. 2001;3:241–248. doi: 10.1006/mthe.2000.0252. [DOI] [PubMed] [Google Scholar]
- 7.Bennett J. Immune response following intraocular delivery of recombinant viral vectors. Gene Ther. 2003;10:977–982. doi: 10.1038/sj.gt.3302030. [DOI] [PubMed] [Google Scholar]
- 8.Auricchio A, Kobinger G, Anand V, et al. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum Mol Genet. 2001;10:3075–3081. doi: 10.1093/hmg/10.26.3075. [DOI] [PubMed] [Google Scholar]
- 9.Rabinowitz JE, Rolling F, Li C, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surace EM, Auricchio A, Reich SJ, et al. Delivery of adeno-associated virus vectors to the fetal retina: impact of viral capsid proteins on retinal neuronal progenitor transduction. J Virol. 2003;77:7957–7963. doi: 10.1128/JVI.77.14.7957-7963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiorini J, Kim F, Yang L, et al. Cloning and characterization of adeno-associated virus type 5. JVirol. 1999;73:1309–1319. doi: 10.1128/jvi.73.2.1309-1319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiorini JA, Yang L, Liu Y, et al. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. J Virol. 1997;71:6823–6833. doi: 10.1128/jvi.71.9.6823-6833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao G, Vandenberghe LH, Alvira MR, et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao G-P, Alvira M, Wang L, et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handa A, Muramatsu S, Qiu J, et al. Adeno-associated virus (AAV)-3-based vectors transduce haematopoietic cells not susceptible to transduction with AAV-2-based vectors. J Gen Virol. 2000;81:2077–2084. doi: 10.1099/0022-1317-81-8-2077. [DOI] [PubMed] [Google Scholar]
- 16.Rutledge EA, Halbert CL, Russell DW. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auricchio A, O'Connor E, Hildinger M, et al. A single-step affinity column for purification of serotype-5 based adeno-associated viral vectors. Mol Ther. 2001;4:372–374. doi: 10.1006/mthe.2001.0462. [DOI] [PubMed] [Google Scholar]
- 18.Gao G, Wilson P. Purification of Recombinant Adeno-Associated virus Vectorsby Column Chromatography and its performance in vivo. Hum Gene Ther. 2000;11:2079–2091. doi: 10.1089/104303400750001390. [DOI] [PubMed] [Google Scholar]
- 19.Bennett J, Duan D, Engelhardt JF, et al. Real-time, noninvasive in vivo assessment of adeno-associated virus-mediated retinal transduction. Invest Ophthalmol Vis Sci. 1997;38:2857–2863. [PubMed] [Google Scholar]
- 20.Hoffman LM, Maguire AM, Bennett J. Cell-mediated immune response and stability of intraocular transgene expression after adenovirus-mediated delivery. Invest Ophthalmol Vis Sci. 1997;38:2224–2233. [PubMed] [Google Scholar]
- 21.Bennett J, Maguire AM. Gene therapy for ocular disease. Mol Ther. 2000;1:501–505. doi: 10.1006/mthe.2000.0080. [DOI] [PubMed] [Google Scholar]
- 22.Dejneka NS, Bennett J. Gene therapy and retinitis pigmentosa: advances and future challenges. Bioessays. 2001;23:662–668. doi: 10.1002/bies.1092. [DOI] [PubMed] [Google Scholar]
- 23.Auricchio A, Rivera VM, Clackson T, et al. Pharmacological regulation of protein expression from adeno-associated viral vectors in the eye. Mol Ther. 2002;6:238–242. doi: 10.1006/mthe.2002.0660. [DOI] [PubMed] [Google Scholar]
- 24.Lebherz C, Auricchio A, Maguire AM, et al. Long-term inducible gene expression in the eye via adeno-associated virus gene transfer in nonhuman primates. Hum Gene Ther. 2005;16:178–186. doi: 10.1089/hum.2005.16.178. [DOI] [PubMed] [Google Scholar]
- 25.Chevez-Barrios P, Leen A, Chai SJ, et al. Immune response of retinoblastoma treated with suicide gene therapy using adenoviral-mediated delivery of thymidine kinase followed by ganciclovir. Ft Lauderdale, FL: 2005. p. 5218. [Google Scholar]
- 26.Rasmussen H, Chu KW, Campochiaro P, et al. Clinical protocol. An open-label, phase I, single administration, dose-escalation study of ADGVPEDF.11D (ADPEDF) in neovascular age-related macular degeneration (AMD) Hum Gene Ther. 2001;12:2029–2032. [PubMed] [Google Scholar]
- 27.Behling K, Auricchio A, O'Connor E, et al. AAV-mediated retinal transfer of anti-angiogenic genes in a murine model of retinopathy of prematurity (ROP) Invest Ophthalmol Vis Sci. 2002 [Google Scholar]
- 28.Duh EJ, Yang HS, Suzuma I, et al. Pigment epithelium-derived factor suppresses ischemia-induced retinal neovascularization and VEGF-induced migration and growth. Invest Ophthalmol Vis Sci. 2002;43:821–829. [PubMed] [Google Scholar]
- 29.Mori K, Gehlbach P, Yamamoto S, et al. AAV-mediated gene transfer of pigment epithelium-derived factor inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci. 2002;43:1994–2000. [PubMed] [Google Scholar]
- 30.Lau D, McGee L, Zhou S, et al. Retinal degeneration is slowed in transgenic rats by AAV-mediated delivery of FGF-2. Invest Ophthalmol Vis Sci. 2000;41:3622–3633. [PubMed] [Google Scholar]
- 31.McGee Sanftner L, Abel H, Hauswirth W, et al. Glial cell line derived neurotrophic factor delays photoreceptor degeneration in a transgenic rat model of retinitis pigmentosa. Mol Ther. 2001;4:622–629. doi: 10.1006/mthe.2001.0498. [DOI] [PubMed] [Google Scholar]
- 32.Rouland JF, Berdeaux G, Lafuma A. The economic burden of glaucoma and ocular hypertension: implications for patient management: a review. Drugs Aging. 2005;22:315–321. doi: 10.2165/00002512-200522040-00004. [DOI] [PubMed] [Google Scholar]
- 33.Monemi S, Spaeth G, DaSilva A, et al. Identification of a novel adult-onset primary open-angle glaucoma (POAG) gene on 5q22.1. Hum Mol Genet. 2005;14:725–733. doi: 10.1093/hmg/ddi068. [DOI] [PubMed] [Google Scholar]
- 34.Stone EM, Fingert JH, Alward WLM, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 35.Borras T, Tamm ER, Zigler JS., Jr. Ocular adenovirus gene transfer varies in efficiency and inflammatory response. Invest Ophthalmol Vis Sci. 1996;37:1282–1293. [PubMed] [Google Scholar]
- 36.Borras T, Gabelt BA, Peterson J, et al. Non-invasive observation of repeated adenoviral GFP gene delivery to the anterior segment of the monkey eye in vivo. J Gene Med. 2001;3:437–449. doi: 10.1002/jgm.210. [DOI] [PubMed] [Google Scholar]
- 37.Borras T, Matsumoto Y, Epstein DL, et al. Gene transfer to the human trabecular meshwork by anterior segment perfusion. Invest Ophthalmol Vis Sci. 1998;39:1503–1507. [PubMed] [Google Scholar]
- 38.Budenz D, Bennett J, Alonso L, et al. In vivo gene transfer into murine trabecular meshwork and corneal endothelial cells. Invest Ophthalmol Vis Sci. 1995;36:2211–2215. [PubMed] [Google Scholar]
- 39.Mollard RJ, Telegan P, Haskins M, et al. The corneal endothelium in mucopolysaccharide storage disorders – morphological studies in animal models. Cornea. 1996;15:25–34. [PubMed] [Google Scholar]
- 40.Seitz B, Moreira L, Baktanian E, et al. Retroviral vector-mediated gene transfer into keratocytes in vitro and in vivo. Am J Ophthalmol. 1998;126:630–639. doi: 10.1016/s0002-9394(98)00205-0. [DOI] [PubMed] [Google Scholar]
- 41.Kamata Y, Okuyama T, Kosuga M, et al. Adenovirus-mediated gene therapy for corneal clouding in mice with mucopolysaccharidosis type VII. Mol Ther. 2001;4:307–312. doi: 10.1006/mthe.2001.0461. [DOI] [PubMed] [Google Scholar]
- 42.Vandenberghe LH, Wang L, Somanathan S, et al. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat Med. 2006;12:967–971. doi: 10.1038/nm1445. [DOI] [PubMed] [Google Scholar]