Abstract
During natural schistosome infection, the induction of T helper type 2 (Th2) responses has been ascribed to parasite eggs, because exposure of the host to this life-cycle stage elicits a polarized Th2 response to egg antigens. In the present study, we show that schistosome worms also elicit systemic, antigen-specific type 2 responses during prepatent infection, before egg deposition begins. CD4+ T cells producing interleukin (IL)–4 were induced by both male and female worms during single-sex infections, demonstrating that this response is independent of exposure to eggs. The Th2 response was accompanied by production of immunoglobulin E and the sensitization of circulating basophils to produce additional IL-4 in response to schistosome antigens. Together, our data show that schistosome worms establish an immunologic milieu where CD4+ T cells and basophils are both primed to produce IL-4 before eggs are laid, suggesting that worms play a role in establishment of the Th2 response that is critical for host survival and parasite transmission.
Blood flukes of the genus Schistosoma infect >1200 million people globally, causing significant morbidity and mortality. As is the case with other helminths, schistosome infection ultimately results in establishment of a CD4+ T helper type 2 (Th2) response, both in human patients [1] and in laboratory animal models [2]. This response is characterized by production of immunoglobulin E (IgE) and eosinophilia, driven by the type 2 cytokines interleukin (IL)–4 [3] and IL-5 [4], respectively. Schistosome eggs are an important stimulus for type 2 responses during schistosome infection, because the onset of egg laying at 5–6 weeks after infection is accompanied by a robust Th2 response to egg antigens [5] that is critical for formation of granulomas around the eggs [6]. Indeed, schistosome eggs and egg antigens are potent, autonomous inducers of Th2 responses, even in the absence of schistosome worms, because Th2 responses are readily induced when intact eggs [7, 8] or egg antigen–pulsed dendritic cells [9] are introduced into naive animals. Ironically, egg-induced Th2 responses are an immunologic double-edged sword, participating in protection of host tissues from egg-induced injury [10] and in the development of the egg-induced pathology and fibrosis associated with chronic schistosome infection [11].
In contrast to schistosome eggs, the contribution of schistosome worms to the induction of adaptive responses and the subsequent development of pathology has received less consideration. Immune responses induced by schistosome worms are of biomedical significance for 3 reasons. First, acquired immunity to schistosome worms that results in reduced parasite burdens can be demonstrated in naturally exposed human subjects [12] and laboratory animal models of vaccine-induced immunity [13]. Although the mechanisms by which protection is mediated under these different circumstances are debated [12, 14], these observations suggest that induction of protective responses to schistosome worms, perhaps through vaccination, may be a feasible approach to controlling schistosomiasis [12]. Second, although schistosome eggs can induce Th2 responses independently of schistosome worms [7], naturally infected hosts are exposed to schistosome worms first, well before eggs are produced, providing ample opportunity for establishment and modulation of responses to cross-reactive antigenic epitopes that are common to both worms and eggs [15–19]. Indeed, there is evidence that worm-induced responses during prepatent infection do influence the subsequent response to eggs and can significantly alter egg-induced immunopathology [18, 20, 21]. Finally, we and others have provided evidence that the activities of CD4+ T cells during prepatent infection promote the development and reproductive fitness of schistosome worms [22, 23]. Together, these observations suggest that CD4+ T cell responses to schistosome worms have both positive and negative effects on the establishment of schistosome infection and the subsequent course of disease. This intimate relationship is presumably a reflection of extensive host-parasite coevolution, resulting in a relationship where schistosomes induce CD4+ T cell responses that facilitate establishment of infection while simultaneously avoiding excessive pathological damage to the host. An understanding of the CD4+ T cell responses induced by schistosome worms is therefore a prerequisite to identifying immune responses that can be modified to the benefit of the host and the detriment of the parasite.
In contrast to eggs, exposure to schistosome worms during the prepatent phase of infection was shown to induce modest production of interferon (IFN)–γ by CD4+ T cells [5, 8], leading to the conclusion that, unlike other helminths, schistosome worms induce an initial T helper type 1 (Th1) response that is subsequently down-modulated and replaced by an egg-induced T helper type 2 (Th2) response when egg deposition begins [24, 25]. However, there are reasons to suspect that responses to schistosome worms during prepatent infection are more complex. First, there is evidence of both circulating eosinophilia [26] and increased eosinopoiesis [27] during the first weeks of infection, responses typically mediated by the Th2 cytokine IL-5 [4]. Second, analyses of cutaneous responses to cercariae suggest that invading schistosome larvae stimulate a mixed T helper response in the skin, with evidence of both Th1 and Th2 polarization at the site of infection [28, 29].
In light of these findings, we tested the hypothesis that schistosome worms induce systemic type 2 responses before the onset of egg-laying, resulting in the development of worm antigen-specific IL-4–producing CD4+ T cells and IgE. Our data show that both these hallmarks of Th2 responses are readily detectable during prepatent infection, to the extent that basophils become sensitized to produce IL-4 in response to worm antigens well in advance of egg deposition. These data provide fresh insights into the phenotype of CD4+ T cell responses to schistosome worms during natural infection and have implications for understanding the role of worm-induced responses in the subsequent pathogenesis of egg-induced disease.
Materials and Methods
Animals and parasites
Wild-type C57BL/6 and C57BL/6 major histocompatibility complex class II–deficient (MHC II−/−) mice were purchased from the National Cancer Institute and Taconic, respectively, and were maintained in a specific pathogen-free environment. Mixed male and female cercariae of Schistosoma mansoni (Puerto Rican strain) were obtained from infected Biomphalaria glabrata snails provided by Fred Lewis (BRI). To obtain separate male and female cercariae, individual B. glabrata snails were exposed to single miracidia and tested for cercarial production 4–6 weeks later. Mice were infected by immersion of the tail for 40 min in water containing 35–150 S. mansoni cercariae, depending on the experiment, and were killed 1–8 weeks later. For preparation of schistosome worm antigen (SWAP), adult S. mansoni were perfused from the portal veins of infected mice and homogenized in phosphate-buffered saline on ice. Insoluble material was removed by centrifugation at 16,100 g for 30 min at 4°C, and the resulting supernatant was stored at −80°C, after filter sterilization and determination of protein concentration by Bradford assay. In all experiments, experimental groups of mice were exposed at the same time to parasites from the same cercarial pool. All studies involving mice were performed in accordance with protocols approved by the institutional animal care and use committee of the Uniformed Services University of the Health Sciences and included 5–10 mice in each group.
CD4+ T cell isolation and quantification of cytokine production
Single-cell suspensions of leukocytes were prepared from the spleens and livers of mice by dissociating tissues through nylon cell strainers and lysing erythrocytes with ACK lysing buffer (BioWhittaker). For livers, hepatocytes were also removed by Percoll density gradient centrifugation. CD4+ T cells were isolated using magnetic anti-CD4 microbeads and magnetically activated cell sorting separation columns (Miltenyi Biotech), in accordance with the instructions provided by the manufacturer, and they were cultured at a final concentration of 1 × 106 cells/mL. Antigen-presenting cells (APCs) were then added to the cocultures at a ratio of 1 APC:10 T cells. Either irradiated syngeneic spleen cells or splenic dendritic cells isolated using magnetic anti-CD11c microbeads from noninfected mice were used as APCs. SWAP was added at a final concentration of 50 μg/mL, whereas positive and negative control cultures received 1 μg/mL anti-CD3 antibody or no antigen, respectively. After incubation in 5% carbon dioxide at 37°C for 72 h, supernatants were collected, and the concentrations of IFN-γ, IL-4, and IL-10 were determined by sandwich enzyme-linked immunosorbent assay (ELISA; BD Biosciences), in accordance with the manufacturer's recommendations.
Determination of plasma IgE concentrations
For plasma isolation, blood was obtained by cardiac puncture at the time that euthanasia was done, collected into heparinized tubes, and centrifuged at 3300 g to remove cells. The plasma concentrations of total IgE were then determined using a sandwich ELISA kit (BD Biosciences), in accordance with the manufacturer's instructions.
Basophil activation assay
Basophil activation was analyzed as described elsewhere [30]. In brief, 100 μL of whole blood was collected from each mouse and incubated in the presence of medium alone, SWAP (20 μg/mL), or anti–mouse IgE (0.125 μg/mL). Monensin (BD GolgiStop protein transport inhibitor; BD Biosciences) was added after 1 h of incubation, and the tubes were incubated for 2 more hours. Cells were washed, lysed with Immuno-Lyse (Beckman Coulter), and immediately fixed. After 1 h of incubation in 1% bovine serum albumin/phosphate-buffered saline, cells were stained with antibodies to surface markers (anti-IgE fluorescein isothiocyanate, anti-CD4 peridinin chlorophyll A protein, and anti-B220 peridinin chlorophyll A protein [BD Biosciences]). Permeabilization was then performed with BD Perm/Wash buffer (BD Biosciences). Finally, the cells were stained with APC-conjugated anti–mouse IL-4 (BD-Pharmingen) and analyzed using an LSR II Optical Bench flow cytometer and Diva software (BD Biosciences). Basophils were identified by the presence of membrane-bound IgE and cell surface CD200R, together with the absence of CD4 and B220 [30]. The cutoff gate for IL-4–APC positivity was established using the fluorescence-minusone (FMO) technique [31]. All antibodies were individually titrated before use, to determine the optimal working concentration for the assay [30].
Statistical analyses
Because unequal variances between experimental groups were frequently encountered in some experiments, stringent nonparametric tests were used in all experiments to test the significance of differences between experimental groups. For comparison of 2 groups, significance was tested using Mann-Whitney tests, and for experiments involving ≥3 groups, the significance of differences was tested using Kruskal-Wallis tests followed by Dunn's multiple comparison tests. Statistical analyses were performed using Prism software (version 4.0; GraphPad). P < .05 was considered to denote statistical significance. Data are expressed as the mean value ± standard error (SE). All data are representative of ≥2 independent experiments.
Results
Induction of IL-4–producing CD4+ T cells by prepatent schistosome infection
To examine the pattern of cytokine production by CD4+ T cells during prepatent schistosome infection, wild-type mice were infected with either a low (35 cercariae per mouse) or a high (150 cercariae per mouse) dose of parasites, and CD4+ T cells were isolated from spleens and livers at 4 weeks after infection, a time point that is 1–2 weeks before the usual onset of egg laying. CD4+ T cells were then restimulated in vitro with SWAP and APCs, and cytokines secreted into the culture supernatants were quantified using ELI-SAs. Consistent with studies reported elsewhere [8], splenic CD4+ T cells produced IFN-γ in response to SWAP (Figure 1A and 1B), and this response was more pronounced in heavily infected mice (Figure 1B). Likewise, hepatic CD4+ T cells, isolated from the site of antigen deposition during prepatent infection, produced modest levels of IFN-γ in response to SWAP, and production was similar irrespective of intensity of infection (Figure 1A and 1B). However, CD4+ T cells from infected mice also produced IL-4 (Figure 1C and 1D) and IL-10 (Figure 1E and 1F) in response to SWAP. Production of IL-4 and IL-10 by hepatic CD4+ T cells was more pronounced in animals exposed to low doses of cercariae (Figure 1C and 1E), and production of IL-10 by splenic CD4+ T cells was highest in heavily infected mice (Figure 1F). Thus, CD4+ T cells produce IL-4 (Figure 1C and 1D) and IL-10 (Figure 1E and 1F) in addition to IFN-γ during prepatent schistosome infection, suggesting that there is a Th2 and/or regulatory component to the CD4+ T cell response to schistosome worms before the onset of egg deposition. Furthermore, this Th2/regulatory response is more apparent when mice harbor lighter infections (Figure 1C and 1E).
Figure 1.
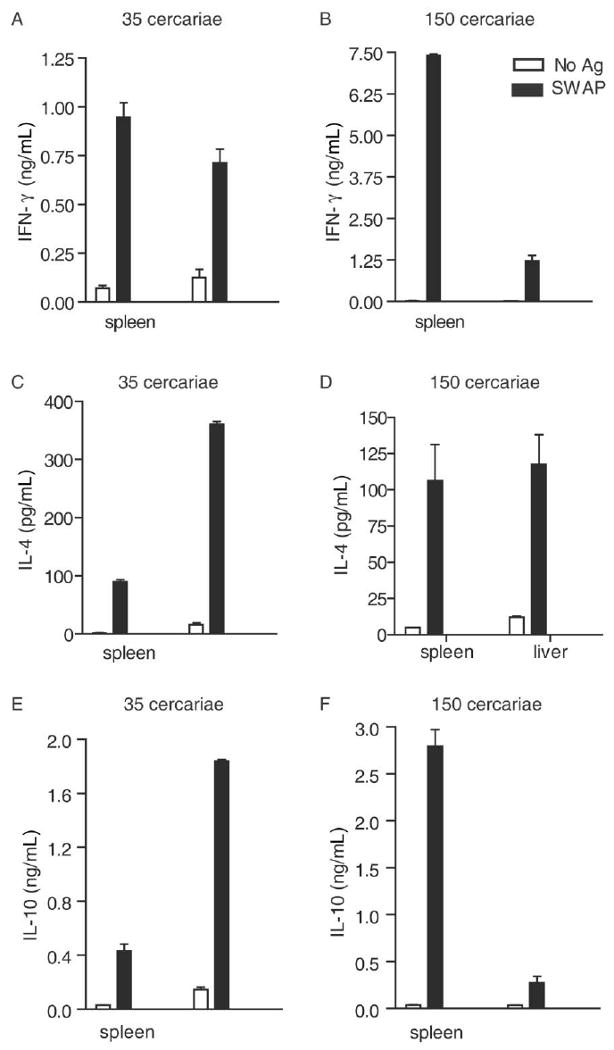
Induction of systemic type 2 CD4+ T cell responses by schistosome worms. Groups of 5 wild-type mice were exposed to 35 (A, C, and E) or 150 cercariae (B, D, and F) each, and infection was allowed to proceed for 4 weeks. A total of 1 × 106 CD4+ T cells were isolated from the livers and spleens and were then cocultured with irradiated syngeneic spleen cells in the presence or absence of soluble worm antigen preparation (SWAP). Culture supernatant was collected 72 h after incubation, and concentrations of interferon (IFN)–γ (A, B), interleukin (IL)–4 (C, D), and IL-10 (E, F) were determined by enzyme-linked immunosorbent assay. Results denote the mean ± standard error. Data are representative of 2 independent experiments. Ag, antigen.
Egg-independent induction of worm antigen–specific IL-4–producing CD4+ T cells
Detection of IL-4 production by CD4+ T cells in response to SWAP at 4 weeks after infection suggested that a Th2 response is elicited by schistosome worms during prepatent infection. However, because schistosome eggs are potent and independent inducers of Th2 responses [7], it was possible that unexpectedly early onset of undetected oviposition, rather than exposure to worms, might cause initial skewing of concomitant CD4+ T cell responses to worm antigens toward type 2 responses. To confirm that worms were the life-cycle stage inducing IL-4-producing CD4+ T cells, we examined CD4+ T cells from mice infected with only male or female worms, thus precluding any possibility that the animals were exposed to eggs. Infection with either male or female worms alone induced significant antigen-specific IL-4 production by splenic (Figure 2A) and hepatic (Figure 2B) CD4+ T cells by 4 weeks after infection. Thus, both male and female worms, in the absence of eggs, are independently sufficient for induction of IL-4–producing CD4+ T cells.
Figure 2.
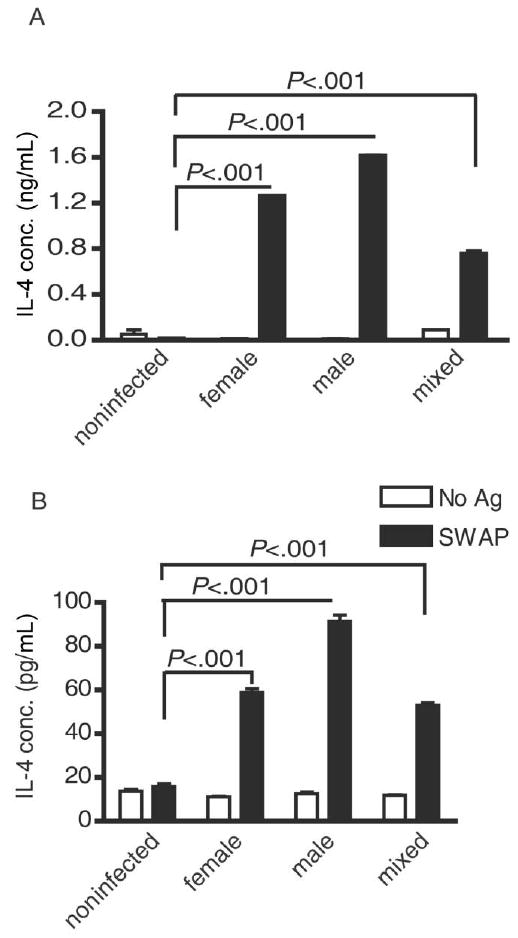
Type 2 CD4+ T cell responses to worm antigens, independent of schistosome eggs. The in vitro interleukin (IL)–4 recall responses of splenic (A) and hepatic (B) CD4+ T cells isolated from mice infected with 100 female, male, or mixed-sex cercariae 4 weeks previously were determined as outlined in Figure 1. Isolated CD4+ T cells were cocultured with dendritic cells in the presence or absence of soluble worm antigen preparation (SWAP). Five mice were included in each group. Data are representative of 2 independent experiments. The significance of all differences was tested using Kruskal-Wallis tests, followed by Dunn's multiple comparison posttests. Ag, antigen; conc., concentration.
Specific sensitization of basophils to worm antigens during prepatent schistosome infection
An important effector function of IL-4 is to stimulate germ-line transcription of the immunoglobulin ε heavy chain, leading to induction of B cell isotype switching and promotion of IgE production [3, 32, 33]. We therefore hypothesized that if physiologically relevant quantities of IL-4 were produced during prepatent schistosome infection, this response would be accompanied by significant IgE production. Consistent with this hypothesis, examination of plasma from infected wild-type mice at weekly intervals revealed an ∼10-fold increase in concentrations of circulating IgE by 4 weeks after infection (Figure 3A).
Figure 3.
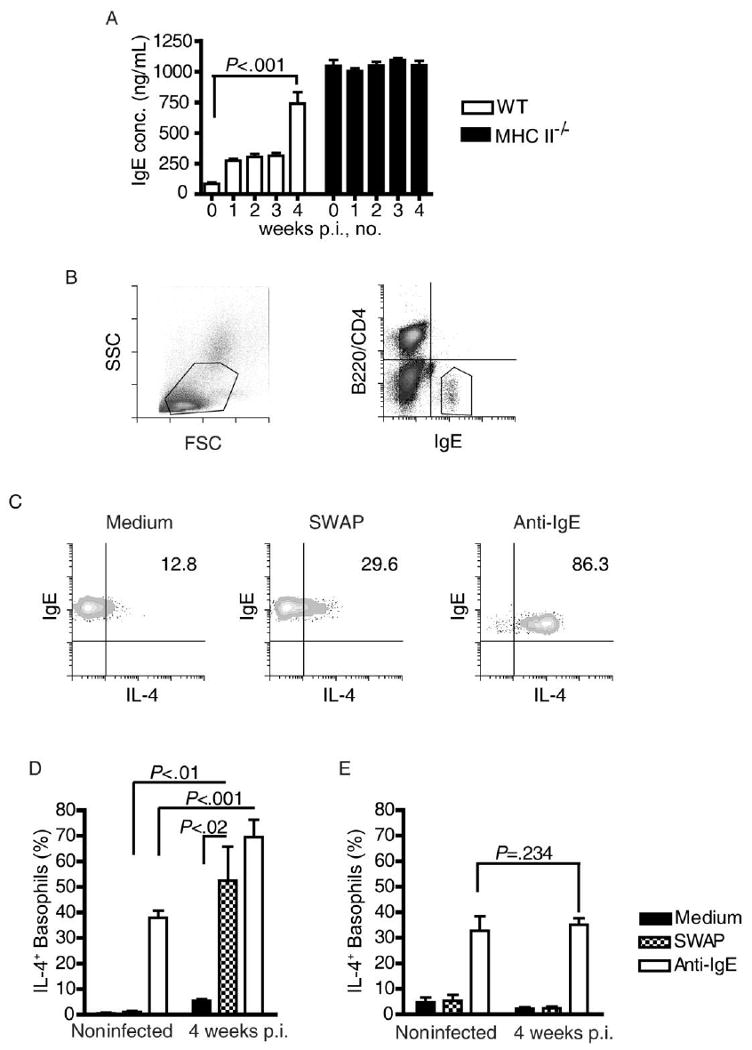
Prepatent schistosome infection and licensing of basophils to produce interleukin (IL)–4 in response to worm antigens. A, Concentrations of total immuoglobulin E (IgE) in the plasma of wild-type (WT) and major histocompatibility complex class II–deficient (MHC II−/−) mice during prepatent schistosome infection were measured by enzyme-linked immunosorbent assay. B, Flow cytometric identification of basophils by light-scattering properties (left) and presence of cell-surface IgE in the absence of B220 and CD4 expression (right). C, Flow cytometric quantification of IL-4 production by circulating basophils from a representative infected WT mouse when stimulated with soluble worm antigen preparation (SWAP) or anti-IgE antibody. D, IL-4 production by basophils from noninfected WT mice and WT mice infected for 4 weeks, in response to medium alone, SWAP, or anti-IgE antibody. E, IL-4 production by basophils from noninfected MHC−/− mice and MHC−/− mice infected for 4 weeks, in response to medium alone, SWAP, or anti-IgE antibody. Five mice were included in each group. The significance of all differences was tested using Kruskal-Wallis tests, followed by Dunn's multiple comparison posttests. All data are representative of at least 2 independent experiments. conc., concentration; FSC, forward scatter; IL-4+, IL-4 positive; p.i., post infection; SSC, side scatter.
Next, we tested whether this IgE response resulted in production of antigen-specific IgE that licensed basophils expressing the high-affinity IgE receptor FcεRI to respond to worm antigens. Using a flow cytometric assay, the accumulation of intracytoplasmic IL-4 in basophils from infected mice was assessed at the time of in vitro stimulation with worm antigens. To control for potential nonspecific interactions between schistosome antigens, IgE, and basophils [34], we also analyzed basophils from naive and infected MHC II−/− mice, which express constitutively high levels of natural IgE (Figure 3A) [35] but cannot mount antigen-specific IgE responses because of ablation of CD4+ T cell help for humoral responses [36]. Figure 3C shows the response to stimulation with SWAP among basophils from a representative wild-type mouse that had been infected for 4 weeks. Even without stimulation, ∼5% of the basophils from wild-type mice that had been infected for 4 weeks spontaneously produced IL-4, whereas basophils from noninfected wild-type mice did not (Figure 3D). When basophils from infected mice had brief in vitro exposure to SWAP, a significant increase in the number of IL-4–producing cells was induced (Figure 3D), whereas basophils from noninfected control mice were uniformly nonresponsive to SWAP (Figure 3D). Basophils from infected wild-type mice also responded more robustly to activation with a cross-linking anti-IgE antibody than did basophils from noninfected control mice (Figure 3D). These data show that prepatent schistosome infection licenses basophils to respond to worm antigens, presumably by inducing antigen-specific IgE, and induces a generalized increase in basophil sensitivity to IgE receptor–mediated activation. In contrast, basophils from MHC II−/− mice were not responsive to schistosome worm antigens, regardless of whether they were isolated from noninfected mice (Figure 3E) or infected mice (Figure 3E), suggesting that abundant, nonspecific natural IgE was not sufficient for basophil sensitization to schistosome worm antigens. There was no intrinsic defect in the competency of MHC II−/− basophils to produce IL-4 in response to IgE cross-linking, because their responses to anti-IgE were similar to those of basophils from naive wild-type mice (Figure 3D and 3E). However, there was no evidence for increased sensitivity to IgE cross-linking in response to prepatent infection (Figure 3E). Taken together, these data argue that prepatent schistosome infection sensitizes basophils to respond to worm antigens, most likely through induction of antigen-specific IgE responses.
Discussion
Pivotal studies by Pearce, Grzych, and colleagues [5, 8] uncovered the central importance of schistosome eggs in the induction of highly polarized type 2 CD4+ T cell responses during schistosome infection. Other studies have since uncovered the critical role of Th2 responses during acute schistosome infection. For example, mice with defects in IL-4 production [10, 37] or IL-4 receptor signal transduction [38] succumb to schistosome infection soon after the onset of oviposition, because IL-4 plays an essential role in regulating inflammatory processes initiated by eggs [10, 38, 39]. Macrophages appear to be the critical targets of IL-4 in this regard, because specific loss of IL-4 receptor expression on macrophages alone still results in mortality during acute infection, because of the inability of macrophages to acquire an alternatively activated phenotype when deprived of IL-4 and IL-13 signals [38]. Complete deficiency of IL-4/IL-13 signaling results in profound defects in the formation of granulomas around eggs [6, 40], illustrating the central role of type 2 cytokines in orchestrating the cellular response to schistosome eggs. Ironically, Th2 responses and alternative activation of macrophages are also of benefit to the parasite. These responses not only increase the likelihood of parasite transmission by prolonging host survival, but they also facilitate transit of schistosome eggs across the bowel wall and into the feces [38]. Given the central importance of Th2 responses to both host and parasite, it is therefore perhaps not surprising that schistosomes and their hosts have evolved redundant mechanisms for the induction of Th2 responses in the face of a schistosome infection. Although previously attributed only to schistosome eggs, our data clearly show that worms also induce expression of type 2 cytokines by host CD4+ T cells, both systemically and at the site of worm antigen deposition in the liver.
One of the primary functions of Th2 responses is to direct synthesis of IgE, thus sensitizing cells expressing FcεRI, such as basophils, and licensing them to perform critical effector functions in response to foreign antigens [41]. That schistosome worms induce physiologically significant type 2 responses during prepatent infection is made evident by the increase in plasma concentrations of IgE and subsequent licensing of basophils to produce IL-4 in response to worm antigens. Previous studies have suggested a role for basophils in amplifying type 2 responses, by directing further Th2 differentiation of CD4+ T cells [42] and stimulating IgE synthesis by B cells [43]. Another recent study provided evidence that basophils significantly augment the effector mechanisms of type 2 responses by promoting eosinophilia and differentiation of alternatively activated macrophages [44]. Before the onset of egg production, schistosome worms therefore establish an immunologic environment that is primed for the amplification of type 2 responses to parasite antigens. Considering the importance of type 2 responses both to host survival and parasite transmission, this immune priming may represent an adaptation on the part of the parasite to ensure that the subsequent response to schistosome eggs adopts a type 2 phenotype. This redundancy in Th2-inducing mechanisms may be particularly relevant in schistosome infection in humans, in whom type 2 polarization of CD4+ T cell responses to schistosome eggs is less predictable than it is in genetically inbred mice [45].
For schistosome worms to significantly modulate the subsequent response to eggs, there would need to be antigenic cross-reactivity between these 2 life-cycle stages. The first classic study of cross-reacting antigens by Warren and Domingo [46] suggested that preexisting responses to worm antigens did not significantly alter responses to eggs. However, several more-recent studies have provided evidence for significant priming of anti-egg responses by worm antigens. Lukacs and Boros [18] found that prepatent infection with S. mansoni primed granuloma formation in response to injected eggs, and studies by Cheever et al [20] and Leptak and McKerrow [21] produced similar results by priming with single-sex S. mansoni infections. A similar priming effect by worms has also been found with S. japonicum [47]. The data we present in this study suggest that priming of anti-egg type 2 responses may occur at 2 levels—by induction of CD4+ T cells producing IL-4 with specificity for schistosome antigens and by priming basophils to produce IL-4 in response to worm antigens. Of these 2 sources of IL-4, basophils may be the most significant, because studies comparing the ability of these 2 populations to release IL-4 indicate that basophils produce this cytokine in greater amounts, at a higher frequency, and in response to lower levels of antigen than do CD4+ T cells [48]. Basophils may therefore play a role in initiating Th2 responses to eggs during natural infection, by releasing IL-4 that directly promotes Th2 cell differentiation [49] and stimulates the Th2 priming capacity of dendritic cells [50].
In summary, our data suggest that, during natural schistosome infection, when eggs are produced at 5–6 weeks after infection, they are introduced into an environment where CD4+ T cells and basophils are already primed to produce IL-4 in response to schistosome antigens. These findings argue for a reappraisal of the mechanisms by which type 2 responses are initiated during schistosomiasis.
Acknowledgments
We thank Mitali Chatterjee and Sean Maynard for invaluable technical assistance. Schistosoma mansoni–infected and –noninfected Biomphalaria glabrata snails were provided by Fred Lewis through the National Institutes of Health/National Institute of Allergy and Infectious Diseases (contract N01 AI30026).
Financial support: National Institutes of Health/National Institute of Allergy and Infectious Diseases (grant R01 AI066227 to S.J.D.); National Council for Scientific and Technical Development (fellowship 210320/2006-0 to L.A.O.F.).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Williams ME, Montenegro S, Domingues AL, et al. Leukocytes of patients with Schistosoma mansoni respond with a Th2 pattern of cytokine production to mitogen or egg antigens but with a Th0 pattern to worm antigens. J Infect Dis. 1994;170:946–54. doi: 10.1093/infdis/170.4.946. [DOI] [PubMed] [Google Scholar]
- 2.Scott P, Pearce E, Cheever AW, Coffman RL, Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989;112:161–82. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 3.Finkelman FD, Katona IM, Urban JF, Jr, Snapper CM, Ohara J, Paul WE. Suppression of in vivo polyclonal IgE responses by monoclonal antibody to the lymphokine B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986;83:9675–8. doi: 10.1073/pnas.83.24.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffman RL, Seymour BW, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989;245:308–10. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- 5.Grzych JM, Pearce E, Cheever A, et al. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991;146:1322–7. [PubMed] [Google Scholar]
- 6.Jankovic D, Kullberg MC, Noben-Trauth N, et al. Schistosome-infected IL-4 receptor knockout (KO) mice, in contrast to IL-4 KO mice, fail to develop granulomatous pathology while maintaining the same lymphokine expression profile. J Immunol. 1999;163:337–42. [PubMed] [Google Scholar]
- 7.Vella AT, Pearce EJ. CD4+ Th2 response induced by Schistosoma mansoni eggs develops rapidly, through an early, transient, Th0-like stage. J Immunol. 1992;148:2283–90. [PubMed] [Google Scholar]
- 8.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–66. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald AS, Straw AD, Bauman B, Pearce EJ. CD8− dendritic cell activation status plays an integral role in influencing Th2 response development. J Immunol. 2001;167:1982–8. doi: 10.4049/jimmunol.167.4.1982. [DOI] [PubMed] [Google Scholar]
- 10.Brunet LR, Finkelman FD, Cheever AW, Kopf MA, Pearce EJ. IL-4 protects against TNF-α–mediated cachexia and death during acute schistosomiasis. J Immunol. 1997;159:777–85. [PubMed] [Google Scholar]
- 11.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–94. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McManus DP, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev. 2008;21:225–42. doi: 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hewitson JP, Hamblin PA, Mountford AP. Immunity induced by the radiation-attenuated schistosome vaccine. Parasite Immunol. 2005;27:271–80. doi: 10.1111/j.1365-3024.2005.00764.x. [DOI] [PubMed] [Google Scholar]
- 14.Wynn TA, Hoffmann KF. Defining a schistosomiasis vaccination strategy—is it really Th1 versus Th2? Parasitol Today. 2000;16:497–501. doi: 10.1016/s0169-4758(00)01788-9. [DOI] [PubMed] [Google Scholar]
- 15.Dunne DW, Bickle QD, Butterworth AE, Richardson BA. The blocking of human antibody-dependent, eosinophil-mediated killing of Schistosoma mansoni schistosomula by monoclonal antibodies which cross-react with a polysaccharide-containing egg antigen. Parasitology. 1987;94:269–80. doi: 10.1017/s0031182000053944. [DOI] [PubMed] [Google Scholar]
- 16.Yi XY, Omer-Ali P, Kelly C, Simpson AJ, Smithers SR. IgM antibodies recognizing carbohydrate epitopes shared between schistosomula and miracidia of Schistosoma mansoni that block in vitro killing. J Immunol. 1986;137:3946–54. [PubMed] [Google Scholar]
- 17.Harn DA, Mitsuyama M, Huguenel ED, Oligino L, David JR. Identification by monoclonal antibody of a major (28 kDa) surface membrane antigen of Schistosoma mansoni. Mol Biochem Parasitol. 1985;16:345–54. doi: 10.1016/0166-6851(85)90075-1. [DOI] [PubMed] [Google Scholar]
- 18.Lukacs NW, Boros DL. Identification of larval cross-reactive and egg-specific antigens involved in granuloma formation in murine schistosomiasis mansoni. Infect Immun. 1991;59:3237–42. doi: 10.1128/iai.59.9.3237-3242.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salama MM, Aronstein WS, Weiss JB, Strand M. Monoclonal antibody identification of protein antigens in the liver of mice infected with Schistosoma mansoni. Am J Trop Med Hyg. 1984;33:608–20. doi: 10.4269/ajtmh.1984.33.608. [DOI] [PubMed] [Google Scholar]
- 20.Cheever AW, Lewis FA, Wynn TA. Schistosoma mansoni: unisexual infections sensitized mice for granuloma formation around intravenously injected eggs. Parasitol Res. 1997;83:57–9. doi: 10.1007/s004360050208. [DOI] [PubMed] [Google Scholar]
- 21.Leptak CL, McKerrow JH. Schistosome egg granulomas and hepatic expression of TNF-α are dependent on immune priming during parasite maturation. J Immunol. 1997;158:301–7. [PubMed] [Google Scholar]
- 22.Davies SJ, Grogan JL, Blank RB, Lim KC, Locksley RM, McKerrow JH. Modulation of blood fluke development in the liver by hepatic CD4+ lymphocytes. Science. 2001;294:1358–61. doi: 10.1126/science.1064462. [DOI] [PubMed] [Google Scholar]
- 23.Harrison RA, Doenhoff MJ. Retarded development of Schistosoma mansoni in immunosuppressed mice. Parasitology. 1983;86:429–38. doi: 10.1017/s0031182000050629. [DOI] [PubMed] [Google Scholar]
- 24.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 25.Pearce EJ, Kane CM, Sun J, Taylor JJ, McKee AS, Cervi L. Th2 response polarization during infection with the helminth parasite Schistosoma mansoni. Immunol Rev. 2004;201:117–26. doi: 10.1111/j.0105-2896.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 26.Colley DG. Schistosoma mansoni: eosinophilia and the development of lymphocyte blastogenesis in response to soluble egg antigen in inbred mice. Exp Parasitol. 1972;32:520–6. doi: 10.1016/0014-4894(72)90070-7. [DOI] [PubMed] [Google Scholar]
- 27.Davies SJ, Smith SJ, Lim KC, et al. In vivo imaging of tissue eosinophilia and eosinopoietic responses to schistosome worms and eggs. Int J Parasitol. 2005;35:851–9. doi: 10.1016/j.ijpara.2005.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mountford AP, Trottein F. Schistosomes in the skin: a balance between immune priming and regulation. Trends Parasitol. 2004;20:221–6. doi: 10.1016/j.pt.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Hogg KG, Kumkate S, Anderson S, Mountford AP. Interleukin-12 p40 secretion by cutaneous CD11c+ and F4/80+ cells is a major feature of the innate immune response in mice that develop Th1-mediated protective immunity to Schistosoma mansoni. Infect Immun. 2003;71:3563–71. doi: 10.1128/IAI.71.6.3563-3571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torrero MN, Larson D, Hubner MP, Mitre E. CD200R surface expression as a marker of murine basophil activation. Clin Exp Allergy. 2009;39:361–9. doi: 10.1111/j.1365-2222.2008.03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumgarth N, Roederer M. A practical approach to multicolor flow cytometry for immunophenotyping. J Immunol Methods. 2000;243:77–97. doi: 10.1016/s0022-1759(00)00229-5. [DOI] [PubMed] [Google Scholar]
- 32.Coffman RL, Ohara J, Bond MW, Carty J, Zlotnik A, Paul WE. B cell stimulatory factor–1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986;136:4538–41. [PubMed] [Google Scholar]
- 33.Finkelman FD, Katona IM, Urban JF, Jr, et al. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988;141:2335–41. [PubMed] [Google Scholar]
- 34.Schramm G, Mohrs K, Wodrich M, et al. Cutting edge: IPSE/α-1, a glycoprotein from Schistosoma mansoni eggs, induces IgE-dependent, antigen-independent IL-4 production by murine basophils in vivo. J Immunol. 2007;178:6023–7. doi: 10.4049/jimmunol.178.10.6023. [DOI] [PubMed] [Google Scholar]
- 35.McCoy KD, Harris NL, Diener P, et al. Natural IgE production in the absence of MHC class II cognate help. Immunity. 2006;24:329–39. doi: 10.1016/j.immuni.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Grusby MJ, Johnson RS, Papaioannou VE, Glimcher LH. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991;253:1417–20. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 37.Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol. 2000;164:2585–91. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- 38.Herbert DR, Holscher C, Mohrs M, et al. Alternative macrophage activation is essential for survival during schistosomiasis and down-modulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–35. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 39.La Flamme AC, Patton EA, Bauman B, Pearce EJ. IL-4 plays a crucial role in regulating oxidative damage in the liver during schistosomiasis. J Immunol. 2001;166:1903–11. doi: 10.4049/jimmunol.166.3.1903. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan MH, Whitfield JR, Boros DL, Grusby MJ. Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J Immunol. 1998;160:1850–6. [PubMed] [Google Scholar]
- 41.Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol. 2002;2:773–86. doi: 10.1038/nri914. [DOI] [PubMed] [Google Scholar]
- 42.Oh K, Shen T, Le Gros G, Min B. Induction of Th2 type immunity in a mouse system reveals a novel immunoregulatory role of basophils. Blood. 2007;109:2921–7. doi: 10.1182/blood-2006-07-037739. [DOI] [PubMed] [Google Scholar]
- 43.Gauchat JF, Henchoz S, Mazzei G, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365:340–3. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 44.Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood. 2009;113:2816–25. doi: 10.1182/blood-2008-05-154773. [DOI] [PubMed] [Google Scholar]
- 45.Stadecker MJ, Asahi H, Finger E, Hernandez HJ, Rutitzky LI, Sun J. The immunobiology of Th1 polarization in high-pathology schistosomiasis. Immunol Rev. 2004;201:168–79. doi: 10.1111/j.0105-2896.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- 46.Warren KS, Domingo EO. Schistosoma mansoni: stage specificity of granuloma formation around eggs after exposure to irradiated cercariae, unisexual infections, or dead worms. Exp Parasitol. 1970;27:60–6. doi: 10.1016/s0014-4894(70)80010-8. [DOI] [PubMed] [Google Scholar]
- 47.Guan XH, Zhao WX. The sensitizing role of the gut-associated antigen of Schistosoma japonicum in granuloma formation around the ova [in Chinese] Zhonghua Yi Xue Za Zhi. 1986;66:479–83. [PubMed] [Google Scholar]
- 48.Mitre E, Taylor RT, Kubofcik J, Nutman TB. Parasite antigen-driven basophils are a major source of IL-4 in human filarial infections. J Immunol. 2004;172:2439–45. doi: 10.4049/jimmunol.172.4.2439. [DOI] [PubMed] [Google Scholar]
- 49.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–83. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 50.Lutz MB, Suri RM, Niimi M, et al. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30:1813–22. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]


