Abstract
Objective
To compare the risk-adjusted incidence of death or neuro-developmental impairment at 18–22 months corrected age, between twin and singleton extremely low birth weight infants.
Hypothesis
Twin gestation is independently associated with increased risk of death or adverse neuro-developmental outcomes at 18–22 months corrected age in extremely low birth weight infants.
Design/Methods
Retrospective study of inborn extremely low birth weight infants (BW 401– 1000g) admitted to NICHD Neonatal Research Network units between 1997 and 2005, who either died or had follow-up data available at 18–22 months corrected age. Neuro-developmental impairment (NDI), the primary outcome variable, was defined as the presence of any one of the following: moderate or severe cerebral palsy, severe bilateral hearing loss needing amplification, bilateral blindness, Bayley Mental Developmental Index or Psychomotor Developmental Index of less than 70. Death was included with NDI as a composite outcome since it is a competing variable. Results were compared for both twins, twin A, twin B, same sex twins, unlike sex twins and singleton infants. Logistic regression analysis was done to control for demographic and clinical factors that were different among the groups.
Results
The cohort of infants who either died or were assessed for NDI consisted of 7,630 singleton infants and 1,376 twins. Logistic regression adjusting for clinical and socio-demographic risk factors showed an increased risk of death or NDI for twins as a group when compared with the singletons (OR-1.39, 95% CI- 1.19–1.63). On analyzing twin A and B separately as well, risk of death or NDI was increased in both twin A (OR-1.32, 95% CI- 1.09–1.59) and for twin B (OR-1.47, 95% CI- 1.21–1.78), when compared with singleton infants.
Conclusions
Twin gestation in ELBW infants is associated with an independent increased risk of death or NDI at 18–22 months corrected age, compared to ELBW singleton gestation infants. Both first and second born twins are at increased risk of death or NDI when compared to singleton ELBW infants.
Keywords: twins, neuro-developmental impairment, extremely low birth weight infants
Introduction
Population based studies from large databases have shown a five-fold higher risk of cerebral palsy in twins as compared to cerebral palsy rates in singletons(1). Comparison of term twins to singleton pregnancies has been shown to be associated with a higher perinatal mortality and morbidity(2). The relationship between birth order of twins and adverse outcomes, with more adverse outcomes among second born twins, has been proposed by some authors(3) but refuted by others(4–6). Previous studies have proposed that there is a slowing of in-utero growth in twins after 30 weeks gestation and the increased rate of cerebral palsy in twins may apply only to infants closer to term gestation(7). An NICHD network study reported that the short term neonatal outcomes of very low birth weight twins (birth weight < 1,500 g) are similar to singleton infants in the same birth weight category(8). Wolf et al(9) showed similar results for short term morbidities in very low birth weight twins and singletons. Others have studied growth restricted twins and singletons and shown that the neonatal outcomes, although worse as compared to non-growth restricted infants, were similar in the two groups(10). To our knowledge, there has been no report on the neuro-developmental outcome of extremely low birth weight infants with reference to twin pregnancy. This retrospective cohort study was designed to compare the short-term neonatal outcomes during hospital stay and 18–22 month neuro-developmental outcomes of twin in comparison to the singleton extremely low birth weight infants.
Methods
This is a retrospective cohort study of ELBW infants admitted to 16 Neonatal Intensive Care Units in the NICHD Neonatal Research Network during calendar years 1997–2005. The primary hypothesis was that in ELBW infants, twin gestation is associated with an increased risk of death or adverse neuro-developmental outcome at 18–22 months corrected age.
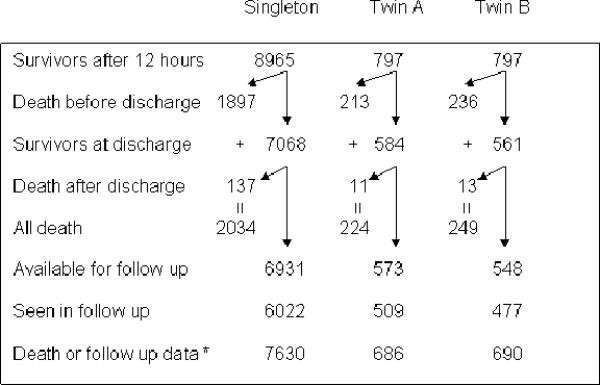
Infants with a birth weight between 401 and 1000 g were included in the study. The cohort consisted of 10,559 singleton and twin infants admitted to the Network Centers during the study period, who were inborn, met the weight criteria and survived beyond 12 hours after birth. Of these, 9,006 infants had data on survival and follow up at 18–22 months corrected age available for analysis (Figure 1). Triplets and higher order multiple births were excluded from the study. Infants were analyzed as twin and singleton cohorts. Within the twin category, the infants were further analyzed as first born twins (twin A) versus singletons and second born twins (twin B) versus singletons. Separate analyses were also conducted on same sex twins and unlike sex twins. Data on maternal and infant demographic and clinical characteristics were abstracted from medical records. All centers participating in the Neonatal Research Network received local IRB approval for data collection. Trained research coordinators obtained the data based on the specific definitions listed in the Manual of Operations.
Figure 1.
Number of study infants
A comprehensive 18–22 month corrected age evaluation for survivors consisted of the following evaluations: neurologic, hearing, vision and development. A neurological examination based on the Amiel-Tison assessment was administered(11). The assessment was performed by certified examiners and included an evaluation of tone, strength, reflexes, angles, and posture. Cerebral palsy was defined as a nonprogressive central nervous system disorder characterized by abnormal muscle tone in at least one extremity and abnormal control of movement and posture.
Hearing status was obtained by parental history or from audiologic test results, when available. Deafness was defined as the need for bilateral amplification. A history of eye examinations and procedures since initial discharge was obtained and a standard eye examination was completed. Blindness was defined as bilateral corrected vision of less than 20/200.
The Bayley Scales of Infant Development –II [BSID-II] was administered and a Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI) were derived. Scores of 100+/− 15 represent the mean +/− 1 standard deviation (SD) with a score < 70 (≥ 2 standard deviations below the mean) indicating significant delay. Children who could not be assessed due to severe developmental delay were assigned MDI and PDI scores of 49.
Outcomes were analyzed in relation to specific maternal and neonatal demographic and clinical variables. The primary outcome was death or neuro-developmental impairment (NDI) at 18–22 months corrected age. NDI was defined as the presence of any one of the following: cerebral palsy, bilateral blindness, bilateral hearing loss needing amplification, Bayley Mental Developmental Index (MDI) less than 70 or Bayley Psychomotor Developmental index (PDI) less than 70. Death occurring after 12 hours of age and before follow up assessment was included in our composite primary outcome measure because it is a competing outcome for NDI in this high-risk ELBW population.
Statistical analyses
The rates of death or NDI, death and NDI among survivors were compared between singletons, twins as a group, twin A, twin B, same sex twins and unlike sex twins. Maternal and infant demographic and clinical characteristics were similarly compared between these groups. Logistic regressions were used to test for risk-adjusted differences between these groups. For our primary hypothesis, we performed adjusted logistic regression to determine the association of twin status with death or NDI, controlling for maternal and neonatal factors and short-term outcomes that were significantly different between the two groups. Birth weight, gestation, small for gestation (SGA) status (weight less than 10th percentile for gestation), male gender, race, antenatal steroids, early or late onset sepsis, respiratory distress, early indomethacin therapy, bronchopulmonary dysplasia, steroids for bronchopulmonary dysplasia and severe intra-ventricular hemorrhage/periventricular leukomalacia (IVH3/4 or PVL) were included in the model. Birth weight in grams was divided by 100, so the odds ratio represents a 100 g increase in birth weight. Similar analysis was done for twin A and B status and same and unlike sex in separate logistic regression models. All the other variables included were the same. The data were analyzed in clusters to account for correlated outcomes from the same twin pair, using Generalized Estimating Equations (GEE). These adjusted logistic regression analyses, presented in figures 2–4, were done using SUDAAN software, version 9.0.3. RTI International performed the statistical analyses. A p-value <0.05 was considered significant.
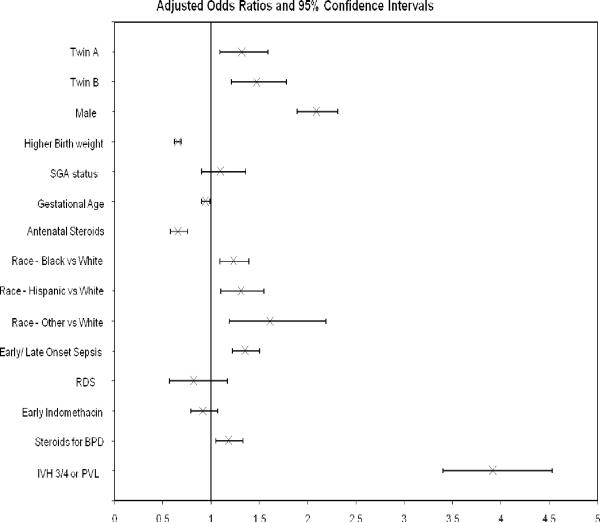
Figure 2.
Logistic regression analysis- Twins: death or NDI
Figure 4.
Logistic regression analysis-Same sex or unlike sex twin :death or NDI
Results
A total of 9,006 infants had data on survival or follow up available and were enrolled in the study (Figure 1). The cohort consisted of 7,630 singleton infants and 1,376 twins, of whom 686 were twin A and 690 were twin B. Survival to hospital discharge was higher for singleton infants as compared to twins. 7,068 out of 8,965 singletons (78.8%) as compared to 584 out of 797 twin A (73.3%) and 561 out of 797 twin B (70.4%) survived to hospital discharge (Figure 1).Compliance rates for those available for follow up were 87% for singleton and 89% and 87% for twin A and B respectively. Table 1 depicts the clinical and demographic characteristics of the study groups. There were about twice as many same sex twins as compared to unlike sex twins A greater proportion of twins analyzed as the twin, twin A or twin B and same sex twin were male as compared to singleton infants. The mean birth weight and gestational age for twins(including same xex or unlike sex) were lower than for singleton infants. Twins were less likely to be SGA as compared to singleton infants. A higher proportion of white infants were likely to be born as a result of twin gestation as compared to black or Hispanic infants.. Pre-eclampsia/hypertension was more common as a pregnancy complication in mothers of singletons. As compared to singletons, twins were more likely to develop sepsis, respiratory distress syndrome (RDS) and patent ductus arteriosus (PDA). They were more likely to receive surfactant and early indomethacin therapy. There was no difference in administration of antenatal steroids. Mothers of twins were more likely to have received less than high school education.
Table 1.
Characteristics of study infants
| Variables | Singleton (n=8965) | Both twins (n=1594) | Twin A (n=797) | Twin B (n=797) | Same sex twins (n=1050) | Unlike sex twins (n=544) |
|---|---|---|---|---|---|---|
| Male | 4323 (48.2%) | 856 (53.7%)*** | 427(53.6%)** | 429 (53.8%)** | 584 (55.6%)*** | 272 (50.0%) |
|
| ||||||
| Birth weight | 760 ± 145 | 738 ± 140*** | 741 ± 140*** | 735 ± 140*** | 737 ± 140*** | 740 ± 140** |
|
| ||||||
| Gestation | 25.9 ± 2.0 | 25.3 ± 1.5*** | 25.3 ± 1.5*** | 25.3 ± 1.5*** | 25.4 ± 1.6*** | 25.2 ± 1.5*** |
|
| ||||||
| SGA | 1698 (18.9%) | 144 (9.0%)*** | 60 (7.5%)*** | 84 (10.5%)*** | 110 (10.5%)*** | 34 (6.3%)*** |
|
| ||||||
| Race | ||||||
| Black | 4137 (46.2%) | 628(39.5%)*** | 315(39.6%)*** | 313 (39.4%)*** | 412 (39.4%)*** | 216 (39.7%)*** |
| White | 2995 (33.5%) | 715 (45.0%)*** | 357 (44.9%)*** | 358 (45.0%)*** | 450 (43.0%)*** | 265 (48.7%)*** |
| Hispanic | 1509 (16.9%) | 197 (12.4%)*** | 98 (12.3%)*** | 99 (12.5%)*** | 152 (14.5%)*** | 45 (8.3%)*** |
| Other | 314 (3.5%) | 50 (3.1%)*** | 25 (3.1%)*** | 25 (3.1%)*** | 32 (3.1%)*** | 18 (3.3%)*** |
|
| ||||||
| Pre-eclampsia/Hypertension | 2799 (31.2%) | 191 (12.0%)*** | 96 (12.1%)*** | 95 (11.9%)*** | 130 (12.4%)*** | 61 (11.2%)*** |
|
| ||||||
| Mother < HS Graduate | 1737 (26.7%) | 236 (22.4%)** | 122 (22.7%)* | 114 (22.0%)* | 170 (24.9%) | 66 (17.7%)*** |
|
| ||||||
| Any ANS | 7389 (82.5%) | 1340 (84.1%) | 668 (83.8%) | 672 (84.3%) | 883 (84.1%) | 457 (84.0%) |
|
| ||||||
| Early/late onset sepsis | 3507 (39.2%) | 673 (42.3%)* | 337 (42.3%) | 336 (42.3%) | 435 (41.5%) | 238 (43.8%)* |
|
| ||||||
| RDS | 8739 (97.6%) | 1581 (99.3%)*** | 790 (99.3%)** | 791 (99.3%)** | 1042 (99.3%)*** | 539 (99.1%)* |
|
| ||||||
| Surfactant therapy | 7214 (80.7%) | 1450 (91.2%)*** | 715 (89.9%)*** | 735 (92.5%)*** | 951 (90.8%)*** | 499 (91.9%)*** |
|
| ||||||
| Early Indomethacin | 3110 (35.2%) | 683 (43.4%)*** | 338 (43.0%)*** | 345 (43.8%)*** | 430 (41.4%)*** | 253 (47.2%)*** |
|
| ||||||
| PDA | 3883 (43.4%) | 860 (54.0%)*** | 412 (51.7%)*** | 448 (56.2%)*** | 550 (52.4%)*** | 310 (57.0%)*** |
|
| ||||||
| Steroids for BPD | 2362 (26.4%) | 478 (30.0%)** | 229 (28.8%) | 249 (31.3%)** | 292 (27.8%) | 186 (34.3%)*** |
p value less than 0.05, as compared to singletons
p value less than 0.01, as compared to singletons
p value less than 0.001 as compared to singletons
Table 2 depicts short term outcomes of the study infants. The incidence of broncho-pulmonary dysplasia (BPD), defined as need for supplemental oxygen at 36 post conceptual weeks of gestation was higher in twins (p<0.001). Similarly, the incidence of grade 3/4 IVH and PVL was higher in twins (p<0.001) as compared to singleton infants . Incidence of severe ROP was also higher among twins (p<0.001). Twins had a higher rate of these morbidities as compared to singleton infants, even when they were analyzed separately as twin A, twin B, same sex or unlike sex twins.
Table 2.
Short term outcomes of study infants
| Variables | Singleton (n=8965) | Both twins (n=1594) | Twin A (n=797) | Twin B (n=797) | Same sex twins (n=1050) | Unlike sex twins (n=544) |
|---|---|---|---|---|---|---|
| BPD | 3428 (46.9%) | 653 (55.0%)*** | 324 (53.6%)** | 329 (56.3%)*** | 417 (55.2%)*** | 236 (54.5%)** |
| IVH Grade 3/4 or PVL1 | 1504 (17.7%) | 347 (23.5%)*** | 170 (23.1%)*** | 177 (24.0%)*** | 237 (24.6%)*** | 110 (21.4%)* |
| ROP Grade 3 or higher2 | 1591 (22.5%) | 359 (30.4%)*** | 175 (29.3%)*** | 184 (31.5%)*** | 233 (31.0%)*** | 126 (29.3%)** |
The denominator for BPD was the number of infants who had BPD defined, i.e. - who survived at least until 36 weeks post-conceptional age
The denominator for IVH/PVL and ROP was the number of infants who survived long enough to have these conditions defined
p value less than 0.05, as compared to singletons
p value less than 0.01, as compared to singletons
p value less than 0.001 as compared to singletons
Table 3 shows the 18–22 month follow up outcomes of study infants. The incidence rates for death or NDI and NDI among survivors were higher in twins as compared to singleton infants. The rates of low MDI and PDI were also higher in twins. The rate of moderate to severe CP was however higher in twin B but did not attain a statistically significant difference in twin A as compared to singletons.
Table 3.
18–22 months outcomes of study infants
| Variables | Singleton (n=6022) | Both twins (n=986) | Twin A (n=509) | Twin B (n=477) | Same sex twins (n=633) | Unlike sex twins (n=353) |
|---|---|---|---|---|---|---|
| NDI | 2013 (36.0%) | 407 (45.1%)*** | 206 (44.6)*** | 201 (45.6)*** | 258 (44.6%)*** | 149 (45.9%)*** |
| Mod/Severe CP | 372 (6.3%) | 81 (8.4%)* | 37 (7.4%) | 44 (9.4%)** | 58 (9.3%)** | 23 (6.6%) |
| Blind | 46 (0.8%) | 11 (1.1%) | 6 (1.2%) | 5 (1.1%) | 8 (1.3%) | 3 (0.9%) |
| Deaf | 93 (1.6%) | 19 (2.0%) | 11 (2.2%) | 8 (1.7%) | 12 (1.9%) | 7 (2.0%) |
| MDI <70 | 1657 (29.9%) | 350 (39.0%)*** | 179 (38.8%)*** | 171 (39.2%)*** | 218 (38.3%)*** | 132 (40.4%)*** |
| PDI <70 | 1115 (20.3%) | 227 (25.7%)*** | 118 (26.0%)** | 109 (25.5%)* | 147 (26.3%)** | 80 (24.8%) |
| Any Bayley < 70 | 1951 (35.0%) | 397 (43.8%)*** | 200 (43.0%)*** | 197 (44.6%)*** | 250 (43.3%)*** | 147 (44.7%)*** |
| Death or NDI1 | 4047 (53.0%) | 880 (64.0%)*** | 430 (62.7%)*** | 450 (65.2%)*** | 597 (65.1%)*** | 283 (61.7%)*** |
The denominator used for death/NDI was the total cohort on which this information was available, i.e. - 7630 infants for singleton, 686 infants for twin A and 690 for twin B. The denominator for other outcomes was the number of infants seen at follow up
p value less than 0.05, as compared to singletons
p value less than 0.01, as compared to singletons
p value less than 0.001 as compared to singletons
On adjusted logistic regression analysis (Figure 2), the risk for death or NDI in twins was increased as compared to singleton infants (OR-1.39, 95% CI- 1.19–1.63). When twins were analyzed separately as twin A or twin B (Figure 3), risk of death or NDI was increased in both twin A (OR-1.32, 95% CI- 1.09–1.59) and for twin B (OR-1.47, 95% CI- 1.21–1.78). On analyzing twins separately as same sex (OR-1.41, 95% CI-1.17 to 1.71) or unlike sex twins (OR-1.36, 95% CI- 1.06–1.74), risk for death or NDI was similarly increased in twins (Figure 4).
Figure 3.
Logistic regression analysis-Twin A or Twin B: death or NDI
Other variables found to be significant were higher birth weight and antenatal steroids which reduced the risk of death or NDI while male gender, early or late onset sepsis and presence of grade 3/4 IVH or PVL increased the risk of death or NDI.
Discussion
There has been a large increase in the number of multiple births over the past two decades(12). This is associated with an increasing concern that twin gestation may be associated with a higher long-term morbidity rate. Multiple gestation is associated with a higher prematurity rate and a higher incidence of low birth weight. Infants born as a result of multiple gestation have been shown to have a higher incidence of adverse outcomes, including a higher mortality rate (13, 14). Other studies have shown an increase in prevalence of cerebral palsy (CP) in twins, as compared to the overall population rate(15). This is consistent with our data, which identified a higher rate of moderate to severe CP in twin B survivors. Whether this increased adverse outcome rate is simply a result of prematurity and a lower birth weight or a result of an independent influence of multiple gestation or being the second born twin is not clear. The fact that our association was adjusted for gestation and birth weight suggests that this association is more likely a result of being born as multiple gestation rather than due to prematurity. Luke et al(16) performed a case-control study comparing twins to gestation matched controls. They showed no difference in morbidity, length of stay or cost of care between twins and gestation- matched singletons, less than 34 weeks gestation.. Their study however evaluated a more mature cohort of infants as compared to our study, which included only ELBW infants.-Rajan: I checked this reference and they did not do long term ourcome. It was designed to look at cost of care.
A higher neonatal adverse outcome rate was not seen when VLBW twin and singleton infants were studied adjusting for birth weight using the NICHD database(8). In our study, however, we did find a higher rate of grade 3/4 IVH and PVL in twins compared to ELBW singletons The two studies differ in population (Birth weight <1,000 g for our series and <1,500 g for Donovan's study) and time era (11997–2005 in ours and 1991–1993 in Donovan's study) which may account for the discrepancy in findings. In this retrospective study, we compared the incidence of death or neuro-developmental impairment between ELBW twins and singletons and found a higher risk of this composite outcome in twin ELBW infants. Analysis of twin A and B separately showed that there was a higher risk of the composite outcome of death or NDI in both twins as compared to singleton infants. This finding adds to the controversy regarding the outcomes of twins with reference to birth order (3–6). In our study, twin B survivors had the highest rates of low Bayley PDI and moderate to severe CP. Twin A survivors on the other hand did not have a higher incidence of moderate to severe CP when compared to ELBW singleton infants. These differences may be explained by the differences in the characteristics of infants, as the combined risk of death or NDI after controlling for confounding factors, was higher in twins overall as compared to singleton infants.
We restricted our analysis to only inborn ELBW infants to achieve a more homogenous group of study infants and to exclude the possible influence of varying resuscitation practices in referring institutions of outborn infants. There were however differences in several characteristics among the study groups. We controlled for these differences by adjusting for these factors in the logistic regression analysis. The retrospective nature of this study does not allow further exploration of this issue.
Infants included in this analysis had a good follow up rate. Of the infants who were eligible for follow up, approximately 87% were followed up at 18–22 months corrected age. We also compared short-term morbidities of infants who were followed up and those who were lost to follow up . There were no consistent differences in these short term morbidities in the two groups, which makes it unlikely that the results of this study were influenced by the infants who were lost to follow up (data not shwn).
Using the Israeli national VLBW registry, Shinwell et al(17) reported increased rate of adverse neonatal neurological outcomes in twins compared to singletons. They defined adverse neurological outcome as severe IVH, periventricular leukomalacia and post-hemorrhagic hydrocephalus; they did not report post-discharge neuro-developmental outcomes. Our study finding of higher rates of IVH grade 3–4 and PVL is in agreement with their results. In addition, we found higher rates of death or NDI in ELBW twins compared to ELBW singletons. We also found a higher survival to discharge for ELBW singletons as compared to twins. Infants dying within the first 12 hours of birth were excluded to control for the possible influence of varying attitudes towards resuscitation among different centers.
Fetal zygosity has been proposed to have an influence on outcomes of twins, with monozygous twins suffering more adverse outcomes. Of the monozygous twins, monochorionic twins are proposed to have outcomes that are worse than dichorionic twins (18). Since this is a retrospective study, the information on chorionicity of twins is lacking. We did however compare same sex with unlike sex twins as a surrogate of chorionicity, based on the premise that a significant percentage of same sex twins may have had monochorionic placentation, whereas that will never be the case in unlike sex twins. We found that the risk of death or NDI was increased in both same sex twins and unlike sex twins when compared to singleton infants in our study (Figure 4). Thus, although using our surrogate parameter is not ideal to indicate chorionicity, the lack of difference may suggest the chorionicity is not a factor in the neuro-developmental outcome in twin pregnancyl
Preterm higher order multiples have been shown to have short term outcomes that are worse than preterm twins (17, 19). We however excluded triplets and higher order multiples form our study because of the potential of making the study very confusing with multiple comparisons.
Our study has the following limitations: it is a retrospective analysis and important variables that might affect outcomes such as zygosity, mode of conception (7, 20, 21), and twin-twin transfusion syndrome (22, 23), were not available. While some studies have shown a higher incidence of neuro-developmental impairment in twins conceived by ART as compared to naturally conceived twins (24), others have shown the opposite to be true (25). There was no data available on the acuity of illness of these infants that may have had an impact on their long-term outcomes. The strength of this study is a large cohort of ELBW infants with both neonatal and 18–22 month follow-up data.
Our data indicate a higher risk of death or NDI in ELBW twin infants, independent of the influence of prematurity and birth weight. This fact should be included in discussions when counseling families of multiple gestation ELBW infants about long term outcomes.
Acknowledgments
Supported by grants from the National Institute of Child Health and Human Development (U10 HD53124, U10 HD53119, U10 HD53109, U10 HD53089, U10 HD40689, U10 HD40521, U10 HD40498, U10 HD40492, U10 HD40461, U10 HD34216, U10 HD34167, U10 HD27904, U10 HD27881, U10 HD27880, U10 HD27871, U10 HD27856, U10 HD27853, U10 HD27851, U10 HD21415, U10 HD21397, U10 HD21385, U10 HD21373, U10 HD21364, and U01 HD36790) and from the National Institutes of Health (CCTS UL1 RR24128, CCTS UL1 RR24148, GCRC M01 RR30, GCRC M01 RR32, GCRC M01 RR39, GCRC M01 RR44, GCRC M01 RR54, GCRC M01 RR59, GCRC M01 RR64, GCRC M01 RR70, GCRC M01 RR80, GCRC M01 RR633, GCRC M01 RR750, GCRC M01 RR997, GCRC M01 RR1032, GCRC M01 RR2172, GCRC M01 RR2588, GCRC M01 RR2635, GCRC M01 RR6022, GCRC M01 RR7122, GCRC M01 RR8084, and GCRC M01 RR16587).
Appendix
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators participated in the NICHD Neonatal Research Network's Generic Database (1997–2001) and Follow-up Studies (1998–2003): Chair: Alan Jobe, MD PhD, University of Cincinnati; Brown University Women & Infants Hospital of Rhode Island – William Oh, MD; Betty R. Vohr, MD; Angelita Hensman, BSN RNC; Lucy Noel RN. Case Western Reserve University Rainbow Babies & Children's Hospital – Avroy A. Fanaroff, MB BCh; Deanne Wilson-Costello, MD; Nancy S. Newman, BA RN; Bonnie S. Siner, RN. Duke University University Hospital, Alamance Regional Medical Center, Duke Raleigh Hospital, and Durham Regional Hospital – Ronald N. Goldberg, MD; Ricki Goldstein, MD; Kathy Auten, BS; Melody Lohmeyer, RN. Emory University Grady Memorial Hospital, Emory Crawford Long Hospital, and Children's Healthcare of Atlanta – Barbara J. Stoll, MD; Ira Adams-Chapman, MD; Ellen Hale, RN BS. Harvard Medical School Brigham and Women's Hospital – Ann R. Stark, MD; Kimberly Gronsman Lee, MD; Kerri Fournier, RN; Colleen Driscoll. Indiana University Indiana University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services – James A. Lemons, MD; Anna M. Dusick, MD; Diana D. Appel, RN BSN; Dianne Herron, RN; Lucy Miller, RN BSN CCRC; Leslie Dawn Wilson, RN BSN. National Institute of Child Health and Human Development – Linda L. Wright, MD; Elizabeth M. McClure, MEd. Research Triangle Institute – W. Kenneth Poole, PhD; Betty Hastings. Stanford University Dominican Hospital, El Camino Hospital, and Lucile Packard Children's Hospital – David K. Stevenson, MD; Susan R. Hintz, MD MS; Barry E. Fleisher, MD; M. Bethany Ball, BS CCRC. University of Alabama at Birmingham Health System and Children's Hospital of Alabama – Waldemar A. Carlo, MD; Myriam Peralta-Carcelen, MD; Monica V. Collins, RN BSN; Shirley S. Cosby, RN BSN; Vivien Phillips, RN BSN. University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women – Neil N. Finer, MD; Yvonne E. Vaucher, MD MPH; Maynard R. Rasmussen MD; Kathy Arnell, RN; Clarence Demetrio, RN; Martha G. Fuller, RN MSN; Chris Henderson, RCP CRTT. University of Cincinnati University Hospital, Cincinnati Children's Hospital Medical Center, and Good Samaritan Hospital – Edward F. Donovan, MD; Jean Steichen, MD; Barb Alexander, RN; Cathy Grisby, BSN CCRC; Marcia Mersmann, RN; Holly Mincey, RN; Jody Shively, RN; Teresa Gratton, PA. University of Miami Holtz Children's Hospital – Shahnaz Duara, MD; Charles R. Bauer, MD; Ruth Everett, RN BSN. University of New Mexico Health Sciences Center – Lu-Ann Papile, MD; Conra Backstrom Lacy, RN. University of Rochester Golisano Children's Hospital at Strong – Dale L. Phelps, MD; Gary Myers, MD; Linda Reubens, RN; Diane Hust, RN PNP; Rosemary Jensen; Erica Burnell, RN. University of Tennessee – Sheldon B. Korones, MD; Henrietta S. Bada, MD; Tina Hudson, RN BSN; Kim Yolton, PhD; Marilyn Williams, LCSW. University of Texas Southwestern Medical Center at Dallas Parkland Health & Hospital System and Children's Medical Center Dallas – Abbot R. Laptook, MD; R. Sue Broyles, MD; Roy J. Heyne, MD; Susie Madison, RN; Jackie Hickman, RN; Sally Adams, PNP; Linda Madden, PNP; Elizabeth Heyne, PA. University of Texas at Houston Health Science Center and Children's Memorial Hermann Hospital – Jon E. Tyson, MD MPH; Brenda H. Morris, MD; Pamela J. Bradt, MD MPH; Esther G. Akpa, RN BSN; Patty A. Cluff, RN; Anna E. Lis, RN BSN; Georgia McDavid, RN. Wake Forest University Baptist Medical Center, Forsyth Medical Center, and Brenner Children's Hospital – T. Michael O'Shea, MD MPH; Robert Dillard, MD; Nancy Peters, RN; Barbara Jackson, RN BSN. Wayne State University Hutzel Women's Hospital and Children's Hospital of Michigan – Seetha Shankaran, MD; Yvette Johnson, MD; Rebecca Bara, RN BSN; Geraldine Muran, RN BSN; Debbie Kennedy, RN. Yale University Yale-New Haven Children's Hospital – Richard A. Ehrenkranz, MD; Patricia Gettner, RN; Monica Konstantino, RN; Elaine Romano, RN.
Footnotes
There are no relevant financial disclosures for any of the authors.
References
- 1.Pharoah PO. Neurological outcome in twins. Semin Neonatol. 2002;7(3):3–223. doi: 10.1053/siny.2002.0109. [DOI] [PubMed] [Google Scholar]
- 2.Barrett JF. Delivery of the term twin. Best Pract Res Clin Obstet Gynaecol. 2004;18(4):4–625. doi: 10.1016/j.bpobgyn.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Prins RP. The second-born twin: can we improve outcomes? Am J Obstet Gynecol. 1994;170(6):6–1649. discussion 56–7. [PubMed] [Google Scholar]
- 4.el-Jallad MF, Abu-Heija AT, Ziadeh S, Obeidat A. Is the second-born twin at high risk? Clin Exp Obstet Gynecol. 1997;24(4):4–226. [PubMed] [Google Scholar]
- 5.Rettwitz-Volk W, Tran TM, Veldman A. Cerebral morbidity in preterm twins. J Matern Fetal Neonatal Med. 2003;13(4):4–218. doi: 10.1080/jmf.13.4.218.223. [DOI] [PubMed] [Google Scholar]
- 6.Sheay W, Ananth CV, Kinzler WL. Perinatal mortality in first- and second-born twins in the United States. Obstet Gynecol. 2004;103(1):1–63. doi: 10.1097/01.AOG.0000101291.14773.F0. [DOI] [PubMed] [Google Scholar]
- 7.Hall JG. Twinning. Lancet. 2003;362(9385):9385–735. doi: 10.1016/S0140-6736(03)14237-7. [DOI] [PubMed] [Google Scholar]
- 8.Donovan EF, Ehrenkranz RA, Shankaran S, Stevenson DK, Wright LL, Younes N, et al. Outcomes of very low birth weight twins cared for in the National Institute of Child Health and Human Development Neonatal Research Network's intensive care units. Am J Obstet Gynecol. 1998;179(3 Pt 1):742–9. doi: 10.1016/s0002-9378(98)70075-4. [DOI] [PubMed] [Google Scholar]
- 9.Wolf EJ, Vintzileos AM, Rosenkrantz TS, Rodis JF, Lettieri L, Mallozzi A. A comparison of pre-discharge survival and morbidity in singleton and twin very low birth weight infants. Obstet Gynecol. 1992;80(3 Pt 1):436–9. [PubMed] [Google Scholar]
- 10.Baker ER, Beach ML, Craigo SD, Harvey-Wilkes KB, D'Alton ME. A comparison of neonatal outcomes of age-matched, growth-restricted twins and growth-restricted singletons. Am J Perinatol. 1997;14(8):8–499. doi: 10.1055/s-2007-994189. [DOI] [PubMed] [Google Scholar]
- 11.Amiel-Tison C. A method for neurologic evaluation within the first year of life. Curr Probl Pediatr. 1976;7(1):1–1. [PubMed] [Google Scholar]
- 12.Martin JA, Park MM. Trends in twin and triplet births: 1980–97. Natl Vital Stat Rep. 1999;47(24):24–1. [PubMed] [Google Scholar]
- 13.Doyle P. The outcome of multiple pregnancy. Hum Reprod. 1996;11(Suppl 4):110–7. doi: 10.1093/humrep/11.suppl_4.110. discussion 8–20. [DOI] [PubMed] [Google Scholar]
- 14.Mazhar SB, Peerzada A, Mahmud G. Maternal and perinatal complications in multiple versus singleton pregnancies: a prospective two years study. J Pak Med Assoc. 2002;52(4):4–143. [PubMed] [Google Scholar]
- 15.Pharoah PO, Adi Y. Consequences of in-utero death in a twin pregnancy. Lancet. 2000;355(9215):9215–1597. doi: 10.1016/s0140-6736(00)02215-7. [DOI] [PubMed] [Google Scholar]
- 16.Luke B, Bigger HR, Leurgans S, Sietsema D. The cost of prematurity: a case-control study of twins vs singletons. Am J Public Health. 1996;86(6):6–809. doi: 10.2105/ajph.86.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinwell ES, Blickstein I, Lusky A, Reichman B. Excess risk of mortality in very low birthweight triplets: a national, population based study. Arch Dis Child Fetal Neonatal Ed. 2003;88(1):F36–40. doi: 10.1136/fn.88.1.F36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carroll SG, Tyfield L, Reeve L, Porter H, Soothill P, Kyle PM. Is zygosity or chorionicity the main determinant of fetal outcome in twin pregnancies? Am J Obstet Gynecol. 2005;193(3 Pt 1):757–61. doi: 10.1016/j.ajog.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 19.Blickstein I. How and why are triplets disadvantaged compared to twins? Best Pract Res Clin Obstet Gynaecol. 2004;18(4):4–631. doi: 10.1016/j.bpobgyn.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Fitzsimmons BP, Bebbington MW, Fluker MR. Perinatal and neonatal outcomes in multiple gestations: assisted reproduction versus spontaneous conception. Am J Obstet Gynecol. 1998;179(5):5–1162. doi: 10.1016/s0002-9378(98)70125-5. [DOI] [PubMed] [Google Scholar]
- 21.Bower C, Hansen M. Assisted reproductive technologies and birth outcomes: overview of recent systematic reviews. Reprod Fertil Dev. 2005;17(3):3–329. doi: 10.1071/rd04095. [DOI] [PubMed] [Google Scholar]
- 22.Cordero L, Franco A, Joy SD. Monochorionic monoamniotic twins: neonatal outcome. J Perinatol. 2006;26(3):3–170. doi: 10.1038/sj.jp.7211457. [DOI] [PubMed] [Google Scholar]
- 23.Dickinson JE, Duncombe GJ, Evans SF, French NP, Hagan R. The long term neurologic outcome of children from pregnancies complicated by twin-to-twin transfusion syndrome. Bjog. 2005;112(1):1–63. doi: 10.1111/j.1471-0528.2004.00330.x. [DOI] [PubMed] [Google Scholar]
- 24.Ito A, Honma Y, Inamori E, Yada Y, Momoi MY, Nakamura Y. Developmental outcome of very low birth weight twins conceived by assisted reproduction techniques. J Perinatol. 2006;26(2):2–130. doi: 10.1038/sj.jp.7211433. [DOI] [PubMed] [Google Scholar]
- 25.Minakami H, Sayama M, Honma Y, Matsubara S, Koike T, Sato I, et al. Lower risks of adverse outcome in twins conceived by artificial reproductive techniques compared with spontaneously conceived twins. Hum Reprod. 1998;13(7):7–2005. doi: 10.1093/humrep/13.7.2005. [DOI] [PubMed] [Google Scholar]