Abstract
Aims: We have found consistent and significant sex differences in recovery from the increased seizure susceptibility observed during ethanol withdrawal (EW) in our rat model system. The main objective of the present study was to determine if sex differences in EW generalized to an additional behavioral measure startle reactivity. Methods: Acoustic startle or seizure threshold responses were measured in separate groups of rats at 1 day or 3 days of EW. Results: Both pair-fed control and EW males showed greater increases in acoustic startle responses than either the female or ovariectomized female (OVX) counterparts. There was a selective effect of pregnanolone on acoustic startle in that it reduced peak force of response only at 3 days EW in male rats. Unexpectedly, it modestly increased startle reactivity in control female and OVX rats. Acute treatment with low-dose ethanol trended toward reducing startle responses in control animals, as expected, while generally enhancing startle responses during EW. All sex conditions showed an enhanced startle response during EW following administration of the higher dose of estradiol compared to control animals. Estradiol did not alter seizure thresholds in control animals. However, it was anticonvulsant for males at 3 days EW, females and OVX at 1 day EW. Conclusions: Observed sex differences in the startle reactivity during EW were consistent with earlier findings comparing EW seizure risk in male and female rats. Responses of OVX suggested that both hormones and differences in brain structures between males and females have a role in these sex differences. Our findings add weight to recommendations that treatment of alcohol withdrawal in humans should consider hormonal status as well as withdrawal time.
INTRODUCTION
Alcohol (ethanol) withdrawal (EW) is a major health concern because of the symptomology that manifests when alcoholics stop drinking. Withdrawal symptoms typically include anxiety, agitation and general dysphoria (Ballenger and Post, 1978; Goldstein and Pal, 1971). Tremors may be present and, in severe withdrawal, may lead to intractable seizures. These symptoms are believed to arise from unmasking of the ‘hyper excitable’ state of the brain engendered by prolonged intake of ethanol. We have been using an animal model of ethanol dependence and withdrawal to study withdrawal behaviors and recovery progression. Several key behavioral assessments used in animal models reflect the hyper excitable state of the brain during EW, including elevated seizure risk and anxiety. For a number of years, we have used the determination of increased seizure risk as a major sign of EW (see Alele and Devaud (2007), Devaud and Chadda (2001) and Koirala et al. (2008)). Reactivity to an abrupt noise (acoustic startle) is believed to represent anxiety-like behaviors and sensorimotor processing (Koch, 1999) and has been used by several laboratories to assess EW (Chester et al., 2004, 2005; Macey et al., 1996). The increased startle reactivity generally observed during EW in various animal models is in agreement with reported enhanced startle reactivity in human alcoholics undergoing withdrawal (Krystal et al., 1997).
Of particular interest to us is the possibility that the hormonal environment impacts behavioral responses during EW, especially as we now know that several ovarian steroids and their derivatives are neuroactive. We find a consistent and robust difference in the recovery time between male and female rats, with females recovered to pre-EW seizure risk by 3 days of EW whereas males continue to display increased seizure risk at this time (Alele and Devaud, 2007; Devaud and Chadda, 2001). More recently, another study found that male mice demonstrated increased seizure risk following multiple EW, whereas females did not (Veatch et al., 2007), providing further evidence for significant sex differences of EW. The intent of the present set of studies was to further explore hormonal influences on EW behaviors by determining if sex differences in seizure risk generalized to the startle response. To address the role of hormones on behavioral responses, we included ovariectomized female rats as the third sex condition.
METHODS
SD rats (Simonson Labs, Gilroy, CA, USA) were ∼50 days old at the start of each experiment. Rats were given a nutritionally complete liquid diet for 14 days (MP Biomedical, Solon, OH, USA). Half of the rats had 6% ethanol incorporated into their liquid diet, while the remaining half had the ethanol replaced with equivalent calories from dextrose (Alele and Devaud, 2007; Koirala et al., 2008). The amount of diet consumed was recorded daily, and daily consumption averaged 10–13 g/kg of ethanol, enough to engender ethanol dependence. Animals were handled regularly to habituate before behavioral testing. Following the 14 days of ethanol exposure, the liquid diet was replaced with chow for all animals to initiate EW.
Bilateral ovariectomies were performed 5–7 days before the start of liquid diet administration in half of the female rats. The estrus cycle of intact females was monitored by daily collection of vaginal smears and histological examination of epithelial cell types. Near confluence of estrus cycles was achieved by the end of each experiment. Most females displayed a prolonged estrus stage, lasting 2–3 days.
Acoustic startle procedure
Testing was performed 1 day (1d) and 3 days (3d) after removal of the liquid diet. Animals were tested at both the 1d and 3d time points. The drugs used for the acoustic startle studies (pregnanolone, ethanol and estradiol) were injected IP 25 min prior to acoustic startle challenges. Different groups of animals were studied for each drug and each drug dose. Pregnanolone was tested at 4 mg/kg or 8 mg/kg (diluted in 20% cylclodextrin). Ethanol was tested at 0.62 g/kg (diluted to 20% v/v in saline). For estradiol, 120 μg/kg or 300 μg/kg doses were tested (dissolved at 100× in ethanol, then diluted with saline). Control animals were given vehicle-only injections. At 20 min after the injection, rats were placed in sound-attenuated chambers (Coulbourn Instruments, Allentown, PA, USA). A 5-min habituation period (no sound) initiated the session, followed by varying intensities (70 dB, 90 dB, 110 dB and 120 dB) of white noise in a randomized order and after randomized delays ranging from 8 to 20 s. Each tone was presented 10 times, taking ∼12 min in total. Wstart4 software (Coulbourn Instruments) was used for data collection.
Seizure threshold procedure
Seizure threshold determinations were made by constant tail vein infusion of pentylenetetrazol (PTZ). Animals were gently restrained for insertion of a 25 g butterfly needle into a lateral tail vein. The needle was taped in place, the animal removed from the hold and held lightly by the tip of the tail while allowed free movement. PTZ (5 mg/mL) was infused at a rate of 1.6 mL/min. The endpoint was taken as time to the first myoclonic twitch of the face and or neck. The estradiol or vehicle was administered IP 20 min before seizure threshold determinations. Seizure thresholds were calculated from the time of infusion × concentration of PTZ/body weight (kg). Estradiol was tested at 40 or 120 μg/kg. Lower doses were used than for acoustic startle testing because seizure threshold determination is a more sensitive measure. Different sets of animals were used for each dose of estradiol and animals were used once only at either 1d or 3d EW. Acute drug challenges were included in the experimental design to extend our previous studies and to look for the potential of drugs to reduce EW symptomology severity (Alele and Devaud, 2007; Koirala et al., 2008).
All studies were the summation of at least two independent experiments to increase sample size and verify reproducibility of results. Control animals were run with each sex and treatment condition but did not vary across experiments or EW time points; therefore, control data for each sex condition were pooled for analysis. Data were analyzed by three-way ANOVA (SPSS, Inc., Chicago, IL, USA) comparing the sex condition by diet (6% ethanol v/v or control) by acute drug treatment. Post hoc analyses (LSD) were conducted when an overall main effect was observed.
RESULTS
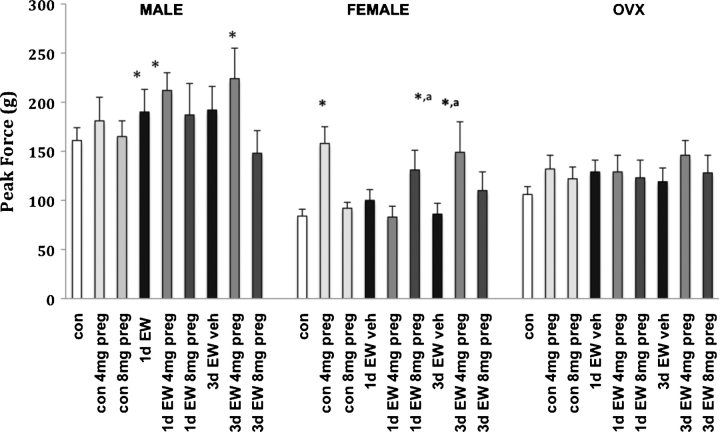
The initial set of experiments explored acoustic startle responses by EW rats, with animals presenting a difference in the degree of response that varied by tone intensity. However, relative changes within groups were similar across the four tones tested. Therefore, only data for responses to the 120 dB tone are presented, as it is representative of relationships between treatments across the four tones tested. As shown in Fig. 1, control male rats presented with an increased startle response compared to females or OVX, a consistent finding across all tones and sets of experiments, although the magnitude of this difference varied somewhat across experiments. Additionally, EW male rats displayed a significant increase in the startle response magnitude at both 1d and 3d EW compared to pair-fed control males (P < 0.05). There was a major difference in the EW acoustic startle response by sex condition as neither females nor OVX displayed increased startle reactivity during EW (except for a trend toward increased reactivity by OVX at 1d EW in the pregnanolone challenge experiments). In these same experiments, we found that neither dose of the GABAergic neuroactive steroid, pregnanolone, altered responses in pair-fed control males. However, administration of 4 mg/kg pregnanolone resulted in an additional increase in the startle response at both 1d and 3d EW whereas the higher dose either had no effect (1d) or reduced (3d) startle responses.
Fig. 1.
Acoustic startle responses at 120 dB averaged across 10 trials per treatment condition at 1d and 3d EW. Values presented are the mean ± S.E.M. from 11 to 14 control animals and 6 to 8 EW animals per sex condition. Preg = pregnanolone, which was given as an acute injection at either 4 mg/kg or 8 mg/kg. *P < 0.05 compared to the same sex condition con; aP < 0.05 compared to same sex condition EW.
Pair-fed control female rats were much more sensitive to the 4 mg/kg dose of pregnanolone than males, evident as a dramatic 88% increase in response (P < 0.01). This enhancement of startle response was not seen at 1d EW but was again observed at the 3d EW time point. The 8 mg/kg dose of pregnanolone did not significantly alter startle responses during EW in females. Acute treatment with pregnanolone elicited a marked response only in pair-fed control OVX and at 3d EW for the 4 mg/kg dose, similar to responses by females. Statistical analysis found an overall main effect of pregnanolone (F2, 227 = 6.9, P < 0.01) and sex (F2, 227 = 21.8, P < 0.001). There were no significant interactions between EW, sex and pregnanolone. Post hoc analysis by LSD showed significance (P < 0.025) between vehicle and the 4 mg/kg dose of pregnanolone. Significant sex differences occurred between males versus females (P < 0.01) and versus OVX (P < 0.05) for control acoustic startle responses as well as EW startle responses. A comparison of responses across all test tones and experiments found that males consistently displayed enhanced startle responses at both 1d and 3d EW compared to control values, whereas female and OVX rats did not.
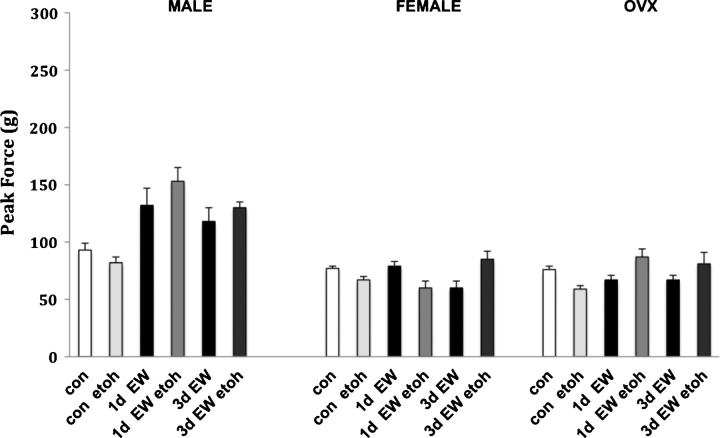
The second study tested the effects of low dose (0.62 g/kg) acute ethanol administration on startle responses during EW (Fig. 2). Across all sex conditions, pair-fed control animals displayed slight, but not significant, decreases in startle responses, suggesting sensitivity to the anxiolytic effects of ethanol in this measure. In contrast, EW males showed increased startle reactivity following acute ethanol administration, more suggestive of sensitivity to the disinhibiting actions of ethanol, likely due to tolerance. This effect persisted through 3d EW. In contrast, females showed a reduction in the startle response (24%) at 1d EW but a 42% enhancement at 3d EW (P < 0.05). OVX animals were more similar to males in that they presented with marked increases in startle responses at both 1d and 3d EW after the acute ethanol treatment. Statistical analysis found an overall main effect of sex (F2, 60 = 6.9, P < 0.01) in this set of experiments with a trend toward an effect of EW (F1, 60 = 3.8, P < 0.07). There was no significant overall sex × EW interaction, although males again presented with a 42% and 26% increase in the startle response at 1d and 3d EW, respectively. In contrast, there was no EW-induced increase in acoustic startle responses for either females or OVX, consistent with our other acoustic startle experiments.
Fig. 2.
Acoustic startle responses at 120 dB averaged across 10 trials per treatment condition at 1d and 3d EW. Values presented are the mean ± S.E.M. from 9 to 10 control animals and 5 to 7 EW animals per sex condition. Etoh = ethanol, which was given as an acute injection at 0.62 g/kg (as a 20% solution). There was an overall main effect of the sex condition (P < 0.02) for males versus females and males versus OVX but no overall main effect of EW or acute ethanol.
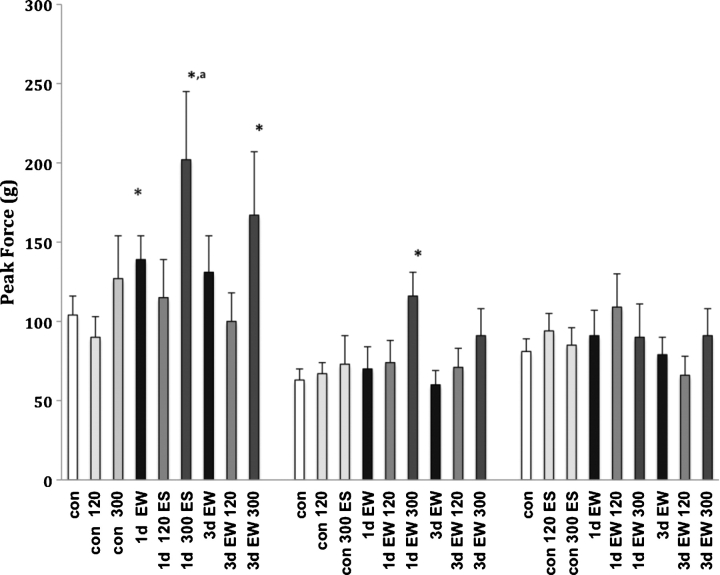
We next tested the acute effects of two supra-physiological doses of 17β-estradiol (Fig. 3). We again noted an enhanced startle response at both 1d and 3d EW in male, but not female or OVX rats. Neither dose of estradiol tested had a significant effect on the startle response in pair-fed control animals across the three sex conditions. During EW, startle responses were enhanced in males at both 1d (45%) and 3d (27%) EW (P <0.01), suggesting an increased anxiogenic activity of this steroid during EW. EW females followed a similar pattern, with the greatest increase in the startle response occurring at the higher dose at 1d EW (66%; P < 0.05). OVX animals did not present with any alterations in the startle response during EW following administration of estradiol.
Fig. 3.
Acoustic startle responses at 120 dB averaged across 10 trials per treatment condition at 1d and 3d EW. Values presented are the mean ± S.E.M. from 10 to 12 control animals and 6 to 7 EW animals per sex condition. ES = 17β-estradiol, which was given as an acute injection at 120 or 300 μg/kg. *P < 0.05 compared to the same sex condition con; aP < 0.05 compared to same sex condition EW.
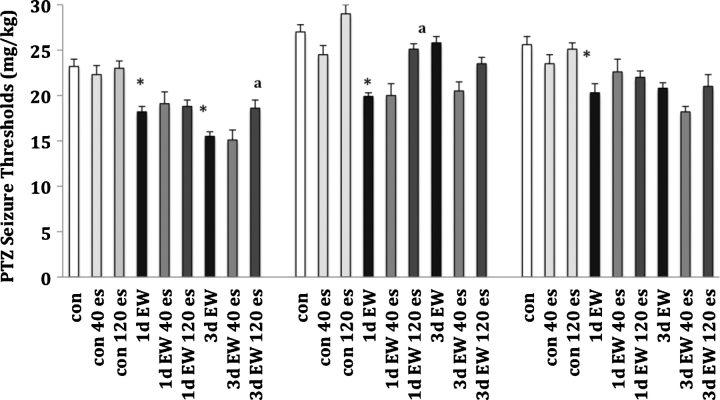
As these results tended to complement our earlier findings of the acute effects of the GABAergic modulators, pregnanolone and ethanol, on seizure risk, we next explored the effects of acute estradiol administration on seizure thresholds at 1d or 3d EW (Fig. 4). We used lower doses of estradiol than in the acoustic startle experiments because seizure threshold measurements are more sensitive to pharmacological manipulations. Consistent with our previous reports, we found that male rats showed an increased seizure risk (decreased seizure threshold) through 3d EW whereas females returned to control level seizure thresholds by the 3d time point. This is the first study where we directly compared seizure thresholds for OVX with males and females; OVX showed a reduction in seizure threshold that persisted through 3d EW, more similar to males than females. Acute administration of 120 μg estradiol provided a selective anticonvulsant effect, seen as increased seizure thresholds in 3d EW male rats and in 1d EW female rats. Effects of estradiol on seizure thresholds in OVX animals were more varied and of a smaller magnitude. For this measure, statistical analysis revealed an overall main effect of sex (F2, 154 = 39, P < 0.001), EW (F2, 154 = 52.8, P < 0.001) and estradiol treatment (F2, 154 = 8.7, P < 0.01) as well as an EW × estradiol interaction (F2, 154 = 2.6, P < 0.05).
Fig. 4.
Pentylenetetrazol seizure thresholds. Values presented are the mean ± S.E.M. from 7 to 10 control animals and 6 to 8 EW animals per sex condition at 1d or 3d EW. ES = 17β-estradiol, which was given as an acute injection at 40 or 120 μg/kg. *P < 0.05 compared to the same sex condition con; aP < 0.05 compared to the same sex condition EW.
DISCUSSION
Findings from the present set of studies expand on previously observed sex differences in behavioral measures of EW with the inclusion of a third sex condition, ovariectomized female rats. A key finding was that basal (ethanol naïve) acoustic startle responses differed across the three sex conditions and across all tones tested, suggesting that there are innate differences between males and females in reactivity to the startle stimulus. A second major finding was that EW male rats showed a consistent increase in startle reactivity during EW whereas both EW females and EW OVX showed small to no changes in acoustic startle responses compared to ethanol naïve controls, with some variability between tones and across experiments. As all three sex conditions show other signs of withdrawal, including an increased seizure risk at 1d EW, the lack of an EW effect on acoustic startle responses for both groups of females suggest a separation between mechanisms underlying seizure risk from those underlying anxiety-like behaviors and/or sensorimotor gating. As development of ethanol dependence and ethanol withdrawal involve multiple brain areas and neurotransmitter systems (Grant and Lovinger, 1995; Olsen et al., 2007), it is possible that there are differential brain adaptations between the sexes, thus conferring observed differences between measures of EW. The hippocampus, substantia nigra, piriform cortex and temporal cortex appear to be predominant sites involved in seizure initiation and expression (Brevard et al., 2006; Gale, 1992) whereas nuclei in the amygdala have been suggested to play a major role in moderating startle responses (Koch, 1999; Toufexis, 2007). Previous studies have found basal sex differences in anxiety-like behaviors using the elevated plus maze and social interaction tests (Genn et al., 2005). Furthermore, there is evidence for modulation of both anxiety-like and seizure responses by the hormonal environment (Foldvary-Schaefer et al., 2004; Maguire et al., 2005), as well structural differences between male and female brains (see Schwarz and McCarthy (2008) for a review). Several groups of investigators have shown an association between sensitivity to ethanol and acoustic startle responses using animal models selected for differences in EW (Chester et al., 2004, 2005; Ponomarev and Crabbe, 1999), with studies conducted in male animals. Of interest was recent gene expression profiling of selected lines of mice, which found a greater effect of sex than of withdrawal sensitivities (Hashimoto and Wiren, 2008). Taken together with the present observations, it is likely that there are innate sex differences in behaviors that modulate EW responses involving modulation by the hormonal environment.
Use of several acute pharmacological challenges during EW revealed differential sensitivity across the sex conditions as well as the two time points of withdrawal assayed. These divergent responses may also reflect innate differences in behaviors, such as in basal sensorimotor gating or fear/freezing that may be modulated by the presence of ovarian steroids, and are somewhat similar to basal differences observed in measures of anxiety-like behaviors. This could partially explain the consistently observed reduced startle in ethanol naïve female and OVX animals compared to males. However, no studies have directly addressed this question. Future experiments employing pre-pulse inhibition on startle responses are needed to better characterize potential inherent differences between male and female rats.
Testing the effects of these challenge drugs also revealed shifting expression of tolerance during EW, particularly in response to the acute ethanol treatment. Acute administration of a low dose of ethanol slightly reduced startle reactivity across sex conditions in ethanol naïve animals, indicating sensitivity to its anxiolytic activity. This effect was lost during EW in male and OVX animals, suggestive of tolerance. In contrast, EW females showed increased sensitivity to its anxiolytic effects at 1d EW but a shift to disinhibition (increased startle), indicative of tolerance at 3d EW. These data suggest that females remain sensitive to ethanol during early withdrawal, with tolerance more evident at later time points.
Modulation of startle responses by acute challenge with pregnanolone generally supported previous evidence for an anxiolytic action of the GABAergic neuroactive steroids (Baulieu, 1997; Paul and Purdy, 1992). During EW, females had a reduced response to pregnanolone, suggestive of tolerance, with the enhanced startle reactivity restored by 3d EW, indicating a quicker recovery for females compared to males. In this measure, OVX animals responded more similar to females than males. Of particular interest was the apparent pregnanolone-induced disinhibition of startle seen in control females, adding further support to the suggestion that there are innate differences in startle reactivity between males and females. Responses to the acute estradiol challenge were generally more limited and unpredictable. 17β-Estradiol is a steroid with multiple actions that has generally been shown to be neuroprotective (Gerrits et al., 2006; Jung et al., 2004; Lunga and Herbert, 2004; Rewal et al., 2004), although some studies suggest that estradiol may enhance excitatory neurotransmission (Ruddick and Woolley, 2001). Our observations were more suggestive of a protective (anticonvulsant) effect, which predominated in EW males. Estradiol has been shown to have multiple effects on multiple brain systems, with its actions influenced by sex, stress, age and other factors, making it difficult to tie any response to a particular mechanism (see Maggi et al. (2004) for a review). One commonality appears to be the influence of estradiol on the formation of dendritic spines that varies by brain area (Cooke and Woolley, 2005) and by stress (Shors et al., 2001, 2004), which would influence behavioral expression of EW.
In summary, these findings extend previous investigations showing that sex condition modulates EW behaviors and recovery, likely involving influences by the hormonal milieu as well as innate structural differences in the brain. They support previous evidence for important sex differences in ethanol withdrawal symptomology (Deshmukh et al., 2003) and brain pathology (Pfefferbaum et al., 2001) in humans. The present results further highlight the need to be cognizant of the impact of gender when managing withdrawal in recovering alcoholics.
Acknowledgments
These studies were supported by PHS AA011877. The expert technical assistance of Emily Baergen and Praweg Koirala is greatly appreciated.
References
- Alele PE, Devaud LL. Sex differences in steroid modulation of ethanol withdrawal in male and female rats. J Pharmacol Exp Ther. 2007;320:427–36. doi: 10.1124/jpet.106.107896. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndrome. Br J Psych. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog Horm Res. 1997;52:1–32. [PubMed] [Google Scholar]
- Brevard ME, Kulkarni P, King JA, et al. Imaging the neural substrates involved in the genesis of pentylenetetrazol-induced seizures. Epilepsia. 2006;47:745–54. doi: 10.1111/j.1528-1167.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Froelich JC. Acoustic startle reactivity during acute alcohol withdrawal in rats that differ in genetic predisposition toward alcohol drinking: effect of stimulus characteristics. Alcohol Clin Exp Res. 2004;28:677–87. doi: 10.1097/01.alc.0000125345.19665.09. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Froehlich JC. Effects of chronic alcohol treatment on acoustic startle reactivity during withdrawal and subsequent alcohol intake in high and low alcohol drinking rats. Alcohol Alcohol. 2005;40:379–87. doi: 10.1093/alcalc/agh172. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64:34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- Deshmukh A, Rosenbloom MJ, Sassoon S, et al. Alcoholic men endorse more DSM-IV withdrawal symptoms than alcoholic women matched in drinking history. J Stud Alcohol. 2003;64:375–9. doi: 10.15288/jsa.2003.64.375. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Chadda R. Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcohol Clin Exp Res. 2001;25:1689–96. [PubMed] [Google Scholar]
- Foldvary-Schaefer N, Harden C, Herzog A, et al. Hormones and seizures. Cleve Clin J Med. 2004;71:S11–8. doi: 10.3949/ccjm.71.suppl_2.s11. [DOI] [PubMed] [Google Scholar]
- Gale K. Role of GABA in the genesis of chemoconvulsant seizures. Toxicol Lett. 1992;64/65:417–28. doi: 10.1016/0378-4274(92)90215-6. [DOI] [PubMed] [Google Scholar]
- Genn RF, Tucci SA, Thomas A, et al. Age-associated sex differences in response to food deprivation in two animal tests of anxiety. Neurosci Biobehav Rev. 2005;40:379–87. doi: 10.1016/s0149-7634(03)00017-4. [DOI] [PubMed] [Google Scholar]
- Gerrits M, Bakker PL, Koch T, et al. Stress-induced sensitization of the limbic system in ovariectomized rats is partly restored by cyclic 17β-estradiol administration. Eur J Neurosci. 2006;23:1747–56. doi: 10.1111/j.1460-9568.2006.04701.x. [DOI] [PubMed] [Google Scholar]
- Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science. 1971;172:288–90. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- Grant KA, Lovinger DM. Cellular and behavioral neurobiology of alcohol: receptor-mediated neuronal processes. Clin Neurosci. 1995;3:155–64. [PubMed] [Google Scholar]
- Hashimoto JG, Wiren KM. Neurotoxic consequences of chronic alcohol withdrawal: expression profiling reveals the importance of gender over withdrawal severity. Neuropsychopharm. 2008;33:1084–96. doi: 10.1038/sj.npp.1301494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ME, Rewal M, Perez E, et al. Estrogen protects against brain lipid peroxidation in ethanol withdrawn rats. Pharmacol Biochem Behav. 2004;79:573–86. doi: 10.1016/j.pbb.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–28. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Koirala B, Alele PE, Devaud LL. Influence of hormonal status on behavioral responses to an acute ethanol challenge during ethanol withdrawal in male and female rats. Pharmacol Biochem Behav. 2008;90:691–700. doi: 10.1016/j.pbb.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Grillon C, et al. Evidence of acoustic startle hyperreflexia in recently detoxified early onset male alcoholics: modulation by yohimbine and m-chlorophenylpiperazine (mCPP) Psychopharmacol. 1997;131:207–15. doi: 10.1007/s002130050285. [DOI] [PubMed] [Google Scholar]
- Lunga P, Herbert J. 17β-oestradiol modulates glucocorticoid, neural and behavioral adaptations to repeated restraint stress in female rats. J Neuroendocrinol. 2004;16:776–85. doi: 10.1111/j.1365-2826.2004.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, et al. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol. 1996;13:163–70. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Maggi A, Ciani P, Belcredito S, et al. Estrogens in the nervous system: mechanisms and nonreproductive functions. Ann Rev Physiol. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, et al. Ovarian cycle-linked changes in GABA-A receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nature Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Hanchar HJ, Meera P, et al. GABAA receptor subtypes: the ‘one glass of wine’ receptors. Alcohol. 2007;41:201–9. doi: 10.1016/j.alcohol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–22. [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Deshmukh A, et al. Sex differences on the effects of alcohol on brain structure. Am J Psychiatry. 2001;158:188–97. doi: 10.1176/appi.ajp.158.2.188. [DOI] [PubMed] [Google Scholar]
- Ponomarev I, Crabbe JC. Genetic association between chronic ethanol withdrawal severity and acoustic startle parameters in WSP and WSR mice. Alcohol Clin Exp Res. 1999;23:1730–5. [PubMed] [Google Scholar]
- Rewal M, Jung ME, Simpkins JW. Role of the GABA-a system in estrogen-induced protection against brain lipid peroxidation in ethanol-withdrawn rats. Alcohol Clin Exp Res. 2004;28:1907–15. doi: 10.1097/01.alc.0000148100.78628.e7. [DOI] [PubMed] [Google Scholar]
- Ruddick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21:6532–43. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, McCarthy MM. Steroid-induced sexual differentiation of the developing brain: multiple pathways, one goal. J Neurochem. 2008;105:1561–72. doi: 10.1111/j.1471-4159.2008.05384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–7. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Falduto J, Leuner B. The opposite effect of stress on dendritic spines in male versus female rats are NMDA receptor-dependent. Eur J Neurosci. 2004;19:145–50. doi: 10.1046/j.1460-9568.2003.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufexis D. Region- and sex-specific modulation of anxiety behaviors in the rat. J Neuroendocrinol. 2007;19:461–73. doi: 10.1111/j.1365-2826.2007.01552.x. [DOI] [PubMed] [Google Scholar]
- Veatch LM, Wright TM, Randall CL. Only male mice show sensitization of handing-induced convulsions across repeated ethanol withdrawal cycles. Alcohol Clin Exp Ther. 2007;31:477–85. doi: 10.1111/j.1530-0277.2006.00328.x. [DOI] [PubMed] [Google Scholar]