Abstract
Aims: Considerable laboratory research indicates that moderate doses of alcohol impair a broad range of skilled activities related to driving performance in young adults. Although laboratory studies show that the intensity of impairment is generally dependent on the blood alcohol concentration, some reviews of this literature suggest that women might be more sensitive to the impairing effects of alcohol than men. The present study tested this hypothesis. Methods: Drawing on data from previous experiments in our laboratory, we compared men and women in terms of the degree to which a challenge dose of alcohol (0.65 g/kg) impaired their simulated driving performance and measures of three separate behavioral and cognitive functions important to driving performance: motor coordination, speed of information processing and information-processing capacity. Results: Alcohol significantly impaired all aspects of performance. Moreover, women displayed greater impairment than men on all behavioral tests and also reported higher levels of subjective intoxication compared with men. Conclusions: Both biological and social–cultural factors have been implicated in gender differences in the behavioral responses to alcohol. The current evidence of heightened sensitivity to alcohol in women highlights the need for better understanding the biological and environmental factors underlying this gender difference.
INTRODUCTION
Accident reports suggest that up to 40% of fatal accidents in the United States involve alcohol (e.g. Evans, 2004). During the year 2002, over 17,000 motor vehicle fatalities in the United States involved alcohol, representing an average of one alcohol-related fatality every 30 min (NHTSA, 2003). Survey results have also highlighted the widespread extent of this problem. For example, 3 in 10 college students have reported driving after drinking any amount of alcohol and 1 in 10 students reported driving after consuming five or more drinks during the past 30 days (Wechsler et al., 2003). Other surveys have estimated that in any given year, over 2 million of the 8 million college students in the United States drive under the influence of alcohol (Hingson et al., 2002).
In the United States, drivers are considered to be legally intoxicated at a blood alcohol concentration (BAC) of 80 mg/ 100 mL (0.08%). Epidemiological studies of automobile accidents show a substantially elevated accident risk at this BAC (Linnoila et al., 1986; NIAAA, 1996; Evans, 2004). In terms of relative risk, drivers with a BAC of 80 mg/100 mL are three times more likely to be involved in a traffic accident than drivers with a zero BAC (e.g. Hurst et al., 1994). Considerable laboratory research indicates that moderate doses of alcohol impair a broad range of skilled activities related to driving performance in young adults (Mitchell, 1985; Stapleton et al., 1986; Moskowitz and Robinson, 1987; Holloway, 1995). Alcohol slows simple and complex reaction time (e.g. Holloway, 1995), decreases hand steadiness (Laberg and Loberg, 1989), reduces inhibitory control (Fillmore, 2003) and impairs pursuit rotor tracking (Harrison and Fillmore, 2005). One general determinant of the degree of alcohol-induced impairment is the drinker's BAC at the time of testing. Most laboratory studies measure subjects’ BACs at the time of testing in order to determine relationships between levels of impairment and BAC. Impaired functioning is reliably observed at BACs of 50 mg/100 mL, with higher BACs generally resulting in greater behavioral impairment (Holloway, 1995). Such research has been important for policy development, such as determining legal maximal BACs for automobile operation and for setting FAA regulations for operating aircraft (for a review, see Holloway (1995)).
Although laboratory studies show that the intensity of impairment is generally dependent on the BAC, some reviews of this literature suggest that gender may also be a factor; specifically, women may be more impaired by alcohol than men (Mumenthaler et al., 1999; Nolen-Hoeksema, 2004; Witt, 2007). For example, one study reported that alcohol increased the detection thresholds for visual stimuli to a greater degree in women compared with men (e.g. Avant, 1990). Studies of alcohol effects on short- and long-term memory also report that women display greater alcohol-induced impairments of immediate and delayed recall compared with their male counterparts (Jones and Jones, 1976, 1977; Niaura et al., 1987; Haut et al., 1989). Compared with men, women have also shown greater sensitivity to alcohol-induced impairment in tests of divided attention (Mills and Bisgrove, 1983) and Stroop task performance (e.g. Wang et al., 2003). Studies also have reported that compared with men, women display greater alcohol-induced impairment on manual dexterity tests (e.g. Price et al., 1986), smooth pursuit tracking tasks (Dougherty et al., 1998) and in measures of gross motor control, such as standing steadiness and gait (e.g. Wang et al., 2003).
One of the reasons for this apparent heightened sensitivity to alcohol among female drinkers could be elevated BACs during the time of behavioral testing under the dose. Despite basing alcohol doses on individual body weights so as to yield comparable BACs among subjects, women as a group might still achieve higher BACs than men. Indeed, studies of alcohol pharmacokinetics have now established that women can achieve higher peak BACs from a given dose of alcohol compared with men (Frezza et al., 1990). Moreover, this gender difference might be most evident at alcohol doses that are sufficient to produce reliable impairments in most behavioral and cognitive functions (e.g. 1.0 g/kg). Reasons why women might achieve higher BACs than men are not entirely clear but appear to involve gender differences in body-water volume and first-pass metabolism (Frezza et al., 1990). In terms of body-water volume, women generally have less body water available for the distribution of alcohol, resulting in higher concentrations. With regard to metabolism, studies find that women have lower levels of gastric alcohol dehydrogenase, a critical enzyme that metabolizes alcohol (e.g. Frezza et al., 1990). There is also evidence suggesting that menstrual cycle might influence BAC and behavioral sensitivity in women. Some studies report elevated BACs and increased behavioral sensitivity to alcohol during mid-luteal and ovulatory phases, as compared with follicular phases (Brick et al., 1986; Cole-Harding and Wilson, 1987; Sutker et al., 1987). However, others have failed to demonstrate any influence of menstrual cycle on BACs or behavioral sensitivity (Jones and Jones, 1976; Cole-Harding and Wilson 1987; Lammers, 1995).
In much of the past research on alcohol impairment, BACs at the time of behavioral testing were not routinely measured, making it difficult to discern whether the greater behavioral impairment seen among women could be attributed to elevated BACs. However, some studies that found greater impairment in women also reported comparable BACs between men and women at the time of behavioral testing. Some of these studies achieved comparable BACs by adjusting the doses, such that women received a lower dose than men in order to prevent elevated BACs (Burns and Moskowitz, 1978; Linnoila et al., 1978; Hindmarch et al., 1992). Other studies obtained comparable BACs between men and women by testing them during the ascending limb of the blood alcohol curve when both genders show a comparable, rapid rise in BACs (e.g. Weafer and Fillmore, 2008). Taken together, the findings from this research provide intriguing evidence to suggest that women might be more sensitive to some of the behaviorally impairing effects of alcohol.
However, evidence remains somewhat equivocal as some recent studies have failed to observe gender differences in behavioral impairment of alcohol (e.g. Nyberg et al., 2004). One major reason for the inability to draw any definitive conclusions about gender differences in alcohol impairment is that there has been little systematic laboratory investigation of the issue. Most of the evidence is based on individual studies that differ in alcohol doses, behavioral measures and testing procedures, making it difficult to compare findings across studies. Moreover, many studies are simply not designed to examine gender differences or are under-powered to detect differences that might be of modest effect size. In some cases, women may be more impaired than men, but such differences may not reach conventional levels of statistical significance and therefore could go unreported. Finally, with regard to behavioral measures, there has been little systematic investigation of gender differences in alcohol impairment of skills specifically related to driving. Driving involves efficient motor control, the ability to quickly process and respond to ever-changing stimuli and the ability to divide attention in a multi-task situation (Mitchell, 1985). It is important for research on gender differences to examine these functions on an individual basis, using specific measures (e.g. tests of motor coordination and reaction time) to examine the complex situation of driving, in which the functions are assumed to operate in concert.
One method of avoiding problems associated with cross-study inconsistencies is to examine gender differences in response to alcohol's effects on driving-related measures that are tested using a single common methodology. The present study was designed to test the hypothesis that women display greater sensitivity to alcohol impairment on driving-related skills by examining gender differences in response to a moderate dose of alcohol on a host of measures tested under a common methodology. Drawing on data from previous experiments in our laboratory, this study compares gender groups in terms of the degree to which alcohol impaired their performance on measures related to driving. Gender differences were examined in subjects’ simulated driving performance and measures of three separate behavioral and cognitive functions considered to be integral to driving performance: motor coordination, speed of information processing and information-processing capacity. Gender differences in levels of subjective intoxication were also examined.
METHOD
Overview
Data were examined from seven experiments previously conducted in our laboratory from 2002 to 2008 (see Table 1). All of the studies tested healthy young adults with no history of alcohol or other drug dependence. All studies tested the behavioral effects of a moderate dose of alcohol (0.65 g/kg) in a placebo-controlled design. The alcohol dose yields a target peak BAC of 80 mg/100 mL and was chosen on the basis of prior research showing that behavior is reliably impaired at this BAC (e.g. Fillmore and Vogel-Sprott, 1999, 2000). The alcohol and placebo test occurred on separate days and the test order was counterbalanced across subjects within each study. The experiments were approved by the University of Kentucky Medical Institutional Review Board and all volunteers were paid for their participation.
Table 1.
Comparisons of mean ages and drinking habits in men and women in each experiment
| N | Age | Drinks | Dose | Frequency | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female |
| Marczinski et al. (2008) | 20 | 20 | 22.5 (2.3) | 22.2 (1.8) | 5.2 (1.8) | 4.6 (2.0) | 1.2 (0.4) | 1.2 (0.6) | 2.1 (1.4) | 2.0 (1.1) |
| Marczinski and Fillmore (in press) | 16 | 12 | 22.0 (1.3) | 23.5 (3.1) | 6.5 (3.5) | 4.8 (2.0) | 1.4 (0.7) | 1.1 (0.6) | 2.5 (1.7) | 2.1 (1.2) |
| Harrison and Fillmore (2005) | 8 | 8 | 22.3 (1.2) | 22.4 (1.4) | 6.5 (3.4) | 4.4 (2.0) | 1.4 (0.7) | 1.2 (0.5) | 2.1 (1.4) | 2.5 (1.4) |
| Weafer and Fillmore (2008) | 14 | 12 | 21.9 (1.1) | 21.9 (1.7) | 6.9 (3.2) | 4.8 (2.4) | 1.6 (0.8) | 1.3 (0.7) | 1.9 (0.7) | 2.5 (1.5) |
| Marczinski and Fillmore (2006) | 6 | 6 | 25.5 (3.6) | 23.7 (2.6) | 4.5 (2.2) | 3.8 (1.3) | 1.1 (0.6) | 1.0 (0.4) | 2.3 (1.2) | 2.2 (1.5) |
| Fillmore and Van Selst (2002) | 7 | 5 | 24.3 (3.9) | 24.0 (2.1) | 4.8 (2.7) | 5.1 (1.8) | 0.9 (0.4) | 1.5 (0.4) | 2.9 (2.0) | 1.9 (0.9) |
| Fillmore et al. (2009)a | 10 | 10 | 23.8 (3.8) | 22.5 (2.1) | 11.7 (7.8) | 6.1 (4.5) | ||||
Standard deviations reported in parentheses.
Drinks = the number of standard drinks consumed per drinking occasion.
Dose = milliliters of absolute alcohol per kilogram of body weight typically consumed during a single drinking occasion.
Frequency = number of drinking occasions per week.
aMeasures of dose and frequency were not collected by Fillmore et al. (2009).
Participants
Each experiment included between 12 and 40 participants from 21 to 35 years of age. Participants completed the Personal Drinking Habits Questionnaire (Vogel-Sprott, 1992) to provide information regarding their current, typical drinking habits. Those with a self-reported psychiatric disorder, substance abuse disorder, head trauma or other injury of the central nervous system were excluded from participating. Volunteers with a Short Michigan Alcoholism Screening Test (Selzer et al., 1975) score of 5 or higher were excluded from the studies. No female volunteers who were pregnant or breast-feeding, as determined by self-report and urine analysis, participated in any of the studies.
Procedure
Screening/familiarization
Participants were recruited by notices posted on community bulletin boards and by local newspaper advertisements. Volunteers provided informed consent prior to participating. Individuals who responded to the advertisements called the laboratory and participated in a telephone-screen interview. They were told that the purpose of the experiment was to study the effects of alcohol on driving performance and performance on computer tasks. Sessions were conducted in the Human Behavioral Pharmacology Laboratory of the Department of Psychology at the University of Kentucky.
Prior to alcohol testing, subjects were familiarized with the behavioral tasks during a familiarization session. The primary purpose of the familiarization session was to introduce participants to the tasks and laboratory procedures. They were given instructions and standardized practice for all tasks. They also completed questionnaires providing demographic information, drug and alcohol use history, and physical and mental health status information.
Tests of alcohol effects on performance
Behavioral performance was assessed in response to two doses of alcohol: 0.0 g/kg (placebo) or 0.65 g/kg. Doses were administered on different days and the dose order was counterbalanced across subjects. Before each test session, participants were instructed to fast for 4 h and to abstain from alcohol for 24 h. Urine samples were tested for the presence of drug metabolites (On Trak TesTstiks; Roche Diagnostics Corporation, Indianapolis, IN, USA) and pregnancy in the female participants (Mainline Confirms HGL; Mainline Technology, Ann Arbor, MI, USA). In addition, a zero BAC was verified for each participant at the start of all sessions. BACs were determined from breath samples measured by an Intoxilyzer, Model 400 (CMI, Owensboro, KY). Testing was conducted in a small room consisting of a chair and a desk with a computer that operated the tasks.
The alcohol dose was calculated on the basis of body weight. A dose was administered as absolute alcohol divided equally into two drinks containing one part alcohol and three parts carbonated mix. Participants had 1 min to finish each drink, and the second drink was served 5 min after the first. This dosing procedure produced a mean rate of rise in BAC of 1.0 mg/ 100 mL per minute (Fillmore et al., 1998). Thus, the peak BAC occurred ∼75 min after drinking began. Once peak BAC was achieved, it remained at a steady state for ∼10 min (Fillmore et al., 1998).
The placebo consisted of a volume of carbonated mix that matched the total volume of the 0.65 g/kg alcohol drink. A small amount (3 mL) of alcohol was floated on the surface of the beverage. It was served in two glasses that had been sprayed with an alcohol mist that resembled condensation and provided a strong alcoholic scent as the beverages were consumed. Participants drank each drink within 1 min. Research indicates that individuals report that this beverage contains alcohol (e.g. Fillmore et al., 1998). After the beverage was consumed, participants relaxed and read magazines in the waiting room.
Task performance and subjective effects were assessed ∼30 min after drinking. The behavioral tasks required ∼10–20 min to complete, which coincided with the ascending limb of the BAC curve in the active dose condition. BAC was measured just prior to testing during both the alcohol and placebo sessions.
After testing, participants relaxed in a waiting room within the laboratory. They each received a meal and remained at leisure to read magazines or watch TV. Their BACs were measured every 20 min until they fell below 20 mg/100 mL at which point they were released from the laboratory. Upon completion of the final session, participants were paid and debriefed.
Dependent measures
Simulated driving. Driving performance was measured by a computerized driving simulation task (STISIM Drive, Systems Technology, Inc., Hawthorne, CA, USA). This task is sensitive to the impairing effects of alcohol and is described in detail elsewhere (Marczinski et al., 2008) and summarized here. Participants sat in front of the computer display that presented the driving simulation. They controlled the vehicle by moving a steering wheel and manipulating accelerator and brake pedals. Participants were instructed to maintain a constant speed of 55 mph and to maintain their vehicle position in the center of the right lane.
The driving simulator yields two measures of driving performance that are typically impaired as a result of alcohol intoxication: deviation of lane position and steering rate. Deviation of lane position is an indicator of the degree of adjustment that a driver implements to maintain a desired position within the lane. Greater lane deviation indicates poorer driving precision. The lane position standard deviation (LPSD) score for a test was obtained by averaging deviation measures sampled at each foot of the driving test.
Steering rate (SR) is a measure of the average speed with which the participant turns the steering wheel to maintain position on the road. Intoxicated drivers tend to make abrupt, quick movements to the steering wheel, which is reflected by an increase in the rate of steering movement. Rate of steering movement was measured in terms of the average degree change in the steering wheel per second, with greater values indicating increased steering rate.
Capacity of information processing
Information-processing capacity was measured by a ‘dual task’ that required participants to respond to two different stimuli presented in close succession. Alcohol has been shown to impair performance on this task, which is described in greater detail in Marczinski and Fillmore (2006). The task requires a participant to inhibit or activate a response to task 1, a simple go/no-go task, which is immediately followed by a simple auditory discrimination task (task 2). The capacity constraint imposed by task 1 results in a refractory period that slows response time to task 2. The task 2 stimulus was presented after one of four delays (50, 200, 600 or 800 ms) following task 1. The dependent measure was the interference (i.e. slowing) on the RT to task 2. Interference is greatest when task 2 follows task 1 by the shortest delay (50 ms) and is least when task 2 follows task 1 by the longest delay (800 ms). The interference is expressed as a single value (RT2 shortest SOA − RT2 longest SOA), with larger scores indicating greater interference (less available capacity).
Speed of information processing
Information-processing speed was measured using the rapid information-processing (RIP) task. A fixed, pseudo random sequence of digits consisting of the digits 1–8 was presented to the participants. They were required to press a key whenever they saw a digit that represented the third digit of a three-digit sequence (a triad) consisting of even digits (e.g. 6, 2, 4) or of odd digits (e.g. 5, 1, 7). Each correct response to a triad increased the digit presentation rate, while a failure to respond to a triad or a response to a non-triad slowed the presentation rate. Thus, the task measured the rate of information processing by adjusting the presentation rate according to the subject's ability to constantly encode and update information in the working memory in order to detect triads. The dependent measure, information-processing speed, was measured by the average number of digits presented per minute on the test, with fewer digits per minute indicating slower information-processing speed. Previous studies that describe this task in detail find that RIP performance is reliably slowed by moderate doses of alcohol (Fillmore et al., 2009).
Motor coordination
A computerized pursuit rotor task was used to measure psychomotor performance. The task measured a participant's ability to track a moving visual target by the isomorphic manipulation of a computer mouse. Participants controlled a cross-hair sight (1.5 cm) by moving the mouse and were instructed to keep the sight on the red circle for as long as possible during a 60-s trial. The computer measures the percent of time on target (% TOT) during each trial, with greater values indicating more precise motor coordination. This task is fully described in Harrison and Fillmore (2005) and has been found to be sensitive to the impairing effects of alcohol.
Subjective effects
A visual analog scale (VAS) was used to measure subjective intoxication to alcohol. Participants rated their subjective level of intoxication on the scale by placing a vertical mark through a 100 mm line, with the left side (0 mm) indicating ‘not at all’, and the right side (100 mm) indicating ‘very much’. The scale has been used in previous research to demonstrate subjective ratings of intoxication (e.g. Chutuape et al., 1994; Fillmore, 2001).
RESULTS
Drinking habits
Table 1 presents the average age and typical drinking habits of the men and women in each study. t-tests revealed no significant gender differences in the age of participants in any experiment. The PDHQ (Vogel-Sprott, 1992) yielded three measures of current drinking habits. Two of these measures concern the actual quantity of alcohol consumed: drinks and dose. Drinks refer to the number of standard drinks typically consumed per drinking occasion. t-tests revealed no gender differences in the number of drinks consumed by males and females in any experiment. Dose refers to the milliliters of absolute alcohol per kilogram body weight typically consumed during a single drinking occasion. This measure provides an assessment of alcohol consumption that takes into account differences in the body weight as well as the amount of alcohol found in different alcoholic beverages. t-tests revealed a significant gender difference in only one study (Fillmore and Van Selst, 2002). In that study, women reported consuming a significantly higher dose of alcohol than men (P = 0.019). The third PDHQ drinking measure, frequency, refers to the typical number of drinking occasions per week. There were no significant gender differences in the frequency of drinking in any study.
Baseline functioning
Table 2 presents the mean performance scores for men and women in response to placebo, which provided the measure of baseline, sober performance in every test. t-tests revealed no gender difference on the baseline performance for any test, indicating no significant differences in the skill level between men and women prior to alcohol administration.
Table 2.
Baseline performance of men and women for each behavioral test
| Baseline score (placebo) | |||||
|---|---|---|---|---|---|
| Males | Females | ||||
| Tasks and studies | Measures | M | (SD) | M | (SD) |
| Simulated drive | |||||
| Marczinski et al. (2008) | LPSD | 1.2 | (0.5) | 1.3 | (0.3) |
| SR | 6.9 | (1.9) | 7.5 | (1.7) | |
| Marczinski and Fillmore (in press) | LPSD | 1.5 | (0.4) | 1.2 | (0.3) |
| SR | 7.8 | (2.6) | 7.3 | (1.7) | |
| Pursuit rotor task | |||||
| Harrison and Fillmore (2005) | %TOT | 52.9 | (7.3) | 43.9 | (14.4) |
| Weafer and Fillmore (2008) | %TOT | 51.4 | (11.8) | 50.1 | (13.6) |
| Dual task | |||||
| Marczinski and Fillmore (2006) | Interference | 202.8 | (48.7) | 188.7 | (69.4) |
| Fillmore and Van Selst (2002) | Interference | 196.1 | (35.0) | 198.8 | (40.9) |
| RIP task | |||||
| Fillmore et al. (2009) | Rate | 103.2 | (22.2) | 112.2 | (19.6) |
Standard deviations reported in parentheses.
LPSD = lane position standard deviation (feet).
SR = steering rate (degrees).
%TOT = percent time on target.
Interference = interference effect (milliseconds).
Rate = rate of information processing (digits per minute).
Alcohol effects
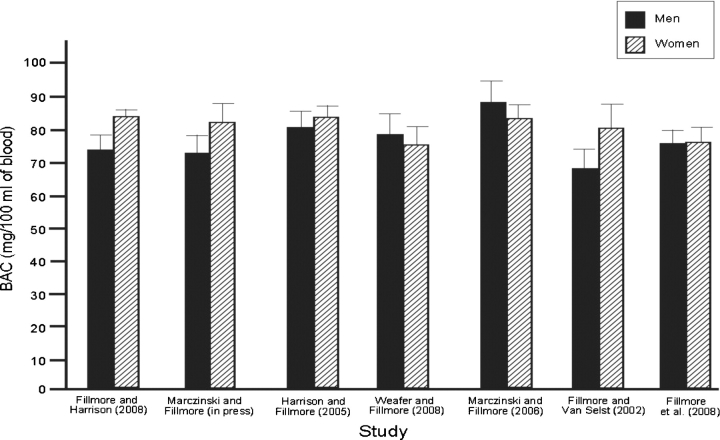
Figure 1 presents the mean BACs for men and women at the time of testing in each study. Average BACs were generally comparable across studies, with participants obtaining BACs between 67 and 88 mg/100mL at the time of testing. The mean BACs of men and women were compared by t-tests in each experiment and no significant gender differences were observed.
Fig. 1.
Mean blood alcohol concentration (mg/100 mL of blood) for men and women in each experiment.
Gender differences in alcohol impairment
With regard to the acute effects of alcohol, the studies showed robust impairment from alcohol on all tasks. Measures of simulated driving performance showed that alcohol resulted in as much as 40% decrements compared with placebo (Marczinski et al., 2008; Marczinski and Fillmore, in press). Studies of RIP and dual task performance reported that alcohol reduced speed and capacity of information processing by as much as 10% and 19%, respectively (Fillmore and Van Selst, 2002; Marczinski and Fillmore, 2006; Fillmore et al., 2009). With respect to motor coordination, the studies of pursuit rotor performance found that alcohol impaired psychomotor coordination by as much as 25% compared with placebo (Harrison and Fillmore, 2005; Weafer and Fillmore, 2008).
Table 3 presents the effect sizes of the impairing effects of alcohol separately for men and women. Effect sizes were calculated as the difference between the mean score under placebo and the mean score under alcohol for each of the nine individual tests examined in the seven experiments. The mean effect size averaged over tasks was 1.16 for women and 0.72 for men. Moreover, the table shows that women displayed effect sizes greater than one standard deviation for the majority of the tests. The magnitude of the effects resulted in an upper bound confidence interval of 1.65. By contrast, for men, the majority of effect sizes were modest with only one effect greater than one standard deviation.
Table 3.
Effect sizes comparing performance under alcohol and placebo
| Effect sizes | |||
|---|---|---|---|
| Tasks and studies | Measures | M | F |
| Simulated drive | |||
| Marczinski et al. (2008) | LPSD | 0.66 | 0.80 |
| STR | 0.44 | 0.55 | |
| Marczinski and Fillmore (in press) | LPSD | 0.54 | 0.66 |
| STR | 0.44 | 1.11 | |
| Pursuit rotor task | |||
| Harrison and Fillmore (2005) | %TOT | 1.34 | 2.59 |
| Weafer and Fillmore (2008) | %TOT | 0.75 | 1.02 |
| Dual task | |||
| Marczinski and Fillmore (2006) | Interference | 0.86 | 1.42 |
| Fillmore and Van Selst (2002) | Interference | 0.52 | 0.73 |
| RIP task | |||
| Fillmore et al. (2009) | Rate | 0.90 | 1.59 |
| Mean | 0.72 | 1.16 | |
| 0.44–1.34 | 0.55–2.59 | ||
| Range CI (95%) | (0.50–0.94) | (0.68–1.65) | |
| rMF | 0.933 | ||
LPSD = lane position standard deviation (feet).
SR = steering rate (degrees).
%TOT = percent time on target.
Interference = interference effect (milliseconds).
Rate = rate of information processing (digits per minute).
In addition to an effect size difference between men and women in terms of average magnitude, the gender difference was also evident in terms of its frequency of occurrence. There was a considerable range in effect sizes across tests from medium (e.g. 0.52) to fairly large sizes (e.g. 2.59) (Cohen, 1988) and a Pearson correlation showed effect sizes to be highly correlated between men and women over tests (r = 0.93, P < 0.01). However, despite the correlation between men and women, women displayed a greater effect size than men on all nine tests, indicating a greater response to alcohol regardless of measure. The frequency of this gender difference was confirmed statistically by a non-parametric sign test that tallied the differences between pairs of effect sizes for men and women across the tests. The sign test showed that the observed frequency of tests in which women displayed a greater effect size than men differed significantly from chance (P < 0.01).
Gender differences in subjective intoxication
Five of the experiments obtained measures of subjective intoxication and found that alcohol produced significant increases in levels of subjective intoxication compared with placebo. Table 4 presents the effect sizes of the subjective intoxication ratings for men and women based on the difference between the mean ratings under placebo and the alcohol dose. The effect sizes for women were greater than those for men in four of the five studies. For women, the mean effect size across studies was nearly three times the mean effect size observed in men (3.05 versus 1.33).
Table 4.
Effect sizes of subjective intoxication between alcohol and placebo
| Effect sizes | ||
|---|---|---|
| Study | Male | Female |
| Marczinski et al. (2008) | 2.768 | 2.617 |
| Marczinski and Fillmore (in press) | 1.682 | 3.306 |
| Weafer and Fillmore (2008) | 0.624 | 2.110 |
| Fillmore and Van Selst (2002) | 0.617 | 5.917 |
| Fillmore et al. (2009) | 0.981 | 1.309 |
| Mean | 1.334 | 3.052 |
| Range | (0.617–2.768) | (1.309–5.917) |
| CI (95%) | (0.203–2.466) | (0.867–5.237) |
DISCUSSION
This research investigated gender differences in the effects of a moderate dose of alcohol on a range of tasks measuring driving performance from data collected in seven previous experiments. The results indicated that alcohol significantly impaired the performance of men and women on all tasks. Moreover, this study found that women displayed greater impairment in response to alcohol than men on all behavioral tests. Women also tended to report higher ratings of subjective intoxication in response to alcohol than men.
Despite the long-standing interest in the possibility that women might be more sensitive to the behaviorally disruptive effects of alcohol, few studies have provided compelling evidence to support such a notion. One reason for the lack of evidence is insufficient statistical power owing to small sample sizes of individual studies. This problem points to the need to aggregate findings across studies that employ a common methodology, such as standard dose administration and testing procedures.
The present study adopted such an approach. First, the testing methodology was standardized across studies and involved a placebo-controlled test of the impairing effects of the same dose of alcohol (0.65 g/kg). In particular, our dosing and testing procedures are designed to produce comparable BACs for men and women during the assessment period under the dose. Indeed, no gender differences were observed in any study, and all BACs were close to the target BAC of 80 mg/100 mL. A reason commonly cited for heightened sensitivity to alcohol among female drinkers is elevated BACs compared with male drinkers. However, given the similar BACs achieved by men and women in each of the current studies, it is unlikely that BACs could explain the gender differences observed in the behavioral and subjective responses to alcohol.
Second, the men and women tested in these experiments were fairly homogenous in terms of their drinking patterns. All studies recruited young adult, non-dependent ‘social’ drinkers. In fact, the men and women in the studies displayed remarkably similar patterns of alcohol consumption in terms of typical quantity consumed and frequency of consumption. Drinking patterns could influence behavioral sensitivity to alcohol in cases where prolonged heavy consumption might result in the development of tolerance to the impairing effects of the drug. However, it is unlikely that the gender differences in this study could be due to differences in tolerance because of the similar drinking patterns observed among the gender groups.
Finally, it is important to note that the sober performance levels on the behavioral tasks were similar for men and women and this was likely due to the task familiarization and training before testing. Some research suggests that the impairing effects of alcohol differ as a function of prior skill level, with more pronounced impairments observed in individuals with low levels of skill (Harrison and Fillmore, 2005). However, given the similar skill among men and women in the current studies, such an explanation for the gender differences is unlikely.
In recent years some biological explanations have been offered to account for observations of increased sensitivity to alcohol in women. In animal models, gonadal steroids, neuroactive steroids and stress hormones are thought to influence the development of gender differences in responses to alcohol (Witt, 2007). There is some evidence that androgens in males inhibit glucocorticoid responses to alcohol while estrogen in females enhances this response, which is thought to contribute to the reinforcing effects of alcohol (Fahlke et al., 1994). Such studies have led some researchers to suggest that females may require smaller doses of alcohol to experience its rewarding effects than males, as evidenced by higher blood levels of corticosterone in response to ethanol (Silveri and Spear, 2004; Witt 2007). It is interesting to note that women in our studies displayed heightened levels of subjective intoxication compared with men in response to the same dose of alcohol. Although our studies did not directly assess the reinforcing effects of alcohol, evidence for heightened sensitivity to the subjective effects in women could suggest that they might also experience more intense rewarding effects from the drug.
In addition to pre-clinical studies, a few studies of humans have sought to identify a biological basis for gender differences in behavioral responses to alcohol. Neuro-imaging studies show that alcohol administration decreases regional brain glucose metabolism in humans, and such reductions in neural activity have been suggested to underlie the acute behavioral impairments associated with alcohol consumption (Wang et al., 2000). A more recent study by this same group found that alcohol produced a greater decrement in glucose metabolism in men compared with women (Wang et al., 2003); however, women exhibited greater behavioral impairment in response to alcohol. The neural imagining findings appear to contradict the behavioral observations and there might be several explanations to account for this discrepancy. One possibility is that women are simply more sensitive than men to alcohol-induced reductions of glucose metabolism in terms of its adverse effect on behavioral functioning (Wang et al., 2003). However, the application of neuro-imaging in the search for a neural basis underlying gender differences in response to alcohol is only in its infancy and there is much to be learned from future studies using these promising techniques.
Social and cultural learning also cannot be ruled out as a factor underlying gender differences in behavioral responses to alcohol. It has been known that alcohol affects social behaviors differently in men and women (Wells et al., 2007). For example, studies have shown that men become more aggressive in response to alcohol than do women (Giancola and Zeichner, 1995; Giancola et al., 2002). Such gender differences could be mediated by social norms such that, for men, physical aggression in the drinking situation might be expected, or even encouraged. Indeed there is a wealth of evidence that shows individuals hold expectations about alcohol effects on a variety of social behaviors, affective states and cognitive/motor functions (e.g. Goldman, 1999). With regard to cognitive/motor functions, studies show that individual differences in the degree of impairment that drinkers expect from alcohol can predict their actual level of impairment under the drug and these expectancies actually play a causal role by mediating the observed levels of impairment (e.g. Fillmore et al., 1998, 2002). Evidence that drinkers’ expectations contribute to their impairment has important implications for understanding gender differences in response to alcohol. Studies have reported that men and women differ in their expectancies about alcohol effects on many aspects of behavior (Read et al., 2004). Differences between men and women in their expectancies about the effect of alcohol on cognitive and behavioral functions could contribute to gender differences in the degree of impairment they display under the drug. A challenge for future research on gender differences will be to distinguish between such socially learned influences and those that have a purely biological basis.
Finally, evidence that women are more sensitive to alcohol impairment suggests that women, as a group, are more vulnerable to the risks associated with impaired driving than men. However, reports indicate that actually the converse is true. In fact, women constitute a substantially smaller proportion of drivers involved in alcohol-related fatal crashes than men (Cavaiola and Wuth, 2002). One possible explanation for this paradox might involve the finding that women self-report greater intoxication from a dose of alcohol than men, as was demonstrated in the present study, as well as in previous work (Wang et al., 2003). Perceived intoxication may serve as a cue to help women form judgments that they are unable to drive. As such, greater sensitivity to the perceived intoxicating effects of alcohol might serve an adaptive function that allows the individual to make sound decisions not to drive after drinking. Indeed, the idea that greater subjective ratings of intoxication can influence decisions about one's capability of driving has been demonstrated in previous research (Marczinski et al., 2008; Marczinski and Fillmore, in press). Compared with light drinkers, heavy drinkers are at a greater risk for driving while intoxicated and studies show that these individuals tend to report less intoxication from a dose of alcohol and report being more able to drive after drinking (Marczinski et al., 2008). Thus, although the current study demonstrated that women are more impaired by the behavioral effects of alcohol than men, the fact that such heightened impairment results in an increased risk to women remains uncertain.
Acknowledgments
Funding for this study was provided by grant R01 AA12895 from the National Institute on Alcohol Abuse and Alcoholism and by grant R21 DA021027 from the National Institute on Drug Abuse. These agencies had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report or in the decision to submit the paper for publication. All authors contributed to and have approved of the final manuscript.
References
- Avant LL. Alcohol impairs visual presence/absence detection more for males than for females. Percept Psychophys. 1990;48:285–90. doi: 10.3758/bf03211531. [DOI] [PubMed] [Google Scholar]
- Brick J, Nathan PE, Westrick E, et al. The effect of menstrual cycle on blood alcohol levels and behavior. J Stud Alcohol. 1986;47:472–7. doi: 10.15288/jsa.1986.47.472. [DOI] [PubMed] [Google Scholar]
- Burns M, Moskowitz H. Gender-related differences in impairment of performance by alcohol. In: Seixas F, editor. Currents in Alcoholism, Vol. 3: Biological, Biochemical and Clinical Studies. New York: Grune and Stratton; 1978. pp. 479–92. [Google Scholar]
- Cavaiola A, Wuth C. Assessment and Treatment of the DWI Offender. Binghamton: The Hawthorne Press; 2002. [Google Scholar]
- Chutuape MAD, Mitchell SH, de Wit H. Ethanol preloads increase ethanol preference under concurrent random-ratio schedules in social drinkers. Exp Clin Psychopharmacol. 1994;2:310–8. [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edn. Hillsdale, NJ: Lawrence Earlbaum; 1988. [Google Scholar]
- Cole-Harding S, Wilson JR. Ethanol metabolism in men and women. J Stud Alcohol. 1987;48:380–7. doi: 10.15288/jsa.1987.48.380. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Bennett RH. Effects of alcohol on rotary pursuit performance: a gender comparison. Psychol Rec. 1998;48:393–405. [Google Scholar]
- Evans L. Traffic Safety. Bloomfield Hills, MI: Science Serving Society; 2004. [Google Scholar]
- Fahlke C, Engel JA, Eriksson CJ, et al. Involvement of coritcosterone in the modulation of ethanol consumption in the rat. Alcohol. 1994;11:195–202. doi: 10.1016/0741-8329(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Cognitive preoccupation with alcohol and binge drinking in college students: alcohol-induced priming of the motivation to drink. Psychol Addict Behav. 2001;15:325–32. [PubMed] [Google Scholar]
- Fillmore MT. Drug abuse as a problem of impaired control: current approaches and findings. Behav Cognit Neurosci Rev. 2003;2:179–97. doi: 10.1177/1534582303257007. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Blackburn JS, Harrison ELR. Acute disinhibiting effects of alcohol as a factor in risky driving behavior. Drug Alcohol Depend. 2002;95:97–106. doi: 10.1016/j.drugalcdep.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Carscadden J, Vogel-Sprott M. Alcohol, cognitive impairment, and expectancies. J Stud Alcohol. 1998;59:174–9. doi: 10.15288/jsa.1998.59.174. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Ostling EW, Martin CA, et al. Acute effects of alcohol on inhibitory control and information processing in high and low sensation-seekers. Drug Alcohol Depend. 2009;100:91–9. doi: 10.1016/j.drugalcdep.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Van Selst M. Constraints on information processing under alcohol in the context of response execution and response suppression. Exp Clin Psychopharmacol. 2002;10:417–24. doi: 10.1037//1064-1297.10.4.417. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. An alcohol model of impaired inhibitory control and its treatment in humans. Exp Clin Psychopharmacol. 1999;7:49–55. doi: 10.1037//1064-1297.7.1.49. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Response inhibition under alcohol: effects of cognitive and motivational conflict. J Stud Alcohol. 2000;61:239–46. doi: 10.15288/jsa.2000.61.239. [DOI] [PubMed] [Google Scholar]
- Frezza M, Di Padova C, Pozzato G, et al. High blood alcohol levels in women.The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. New Engl J Med. 1990;322:95–9. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Helton EL, Osborne AB, et al. The effects of alcohol and provocation on aggressive behavior in men and women. J Stud Alcohol. 2002;63:64–73. [PubMed] [Google Scholar]
- Giancola PR, Zeichner A. An investigation of gender differences in alcohol related aggression. J Stud Alcohol. 1995;56:573–9. doi: 10.15288/jsa.1995.56.573. [DOI] [PubMed] [Google Scholar]
- Goldman MS. Risk for substance abuse: memory as a common etiological pathway. Psychol Sci. 1999;10:196–8. [Google Scholar]
- Harrison ELR, Fillmore MT. Are bad drivers more impaired by alcohol? Sober driving precision predicts impairment from alcohol in a simulated driving task. Accid Anal Prev. 2005;37:882–9. doi: 10.1016/j.aap.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Haut JS, Beckwith BE, Petros TV, et al. Gender differences in retrieval from long-term memory following acute intoxication with ethanol. Physiol Behav. 1989;45:1161–5. doi: 10.1016/0031-9384(89)90103-0. [DOI] [PubMed] [Google Scholar]
- Hindmarch I, Bhatti JZ, Starmer GA, et al. The effects of alcohol on the cognitive function of males and females and on skills related to car driving. Hum Psychopharmacol Clin. 1992;7:105–14. [Google Scholar]
- Hingson RW, Heeren T, Zakocs RC, et al. Magnitude of alcohol-related mortality and morbidity among U.S. college students ages 18 to 24. J Stud Alcohol. 2002;63:136–44. doi: 10.15288/jsa.2002.63.136. [DOI] [PubMed] [Google Scholar]
- Holloway FA. Low-dose alcohol effects on human behavior and performance. Alcohol Drugs Driving. 1995;11:39–56. [Google Scholar]
- Hurst PM, Harte D, Frith WJ. The Grand Rapids dip revisited. Accid Anal Prev. 1994;26:647–54. doi: 10.1016/0001-4575(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Jones BM, Jones MK. Women and alcohol: intoxication, metabolism and the menstrual cycle. In: Greenblatt M, Schuckit MA, editors. Alcoholism Problems in Women and Children. New York: Grune & Stratton; 1976. pp. 103–36. [Google Scholar]
- Jones BM, Jones MK. Alcohol and memory impairment in male and female social drinkers. In: Birnbaum IM, Parker ES, editors. Alcohol and Human Memory. Hillsdale, NJ: Lawrence Erlbaum; 1977. pp. 127–38. [Google Scholar]
- Laberg JC, Loberg T. Expectancy and tolerance: a study of acute alcohol intoxication using the balanced placebo design. J Stud Alcohol. 1989;50:448–55. doi: 10.15288/jsa.1989.50.448. [DOI] [PubMed] [Google Scholar]
- Lammers SMM. Do alcohol pharmacokinetics in women vary due to the menstrual cycle? Addiction. 1995;90:23–30. doi: 10.1046/j.1360-0443.1995.901235.x. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Erwin CW, Cleveland WP, et al. Effects of alcohol on psychomotor performance of men and women. J Stud Alcohol. 1978;39:745–58. doi: 10.15288/jsa.1978.39.745. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Stapleton JM, Lister R, et al. Effects of alcohol on accident risk. Pathologist. 1986;40:36–41. [Google Scholar]
- Marczinski CA, Fillmore MT. Clubgoers and their trendy cocktails: implications of mixing caffeine into alcohol on information processing and subjective reports of intoxication. Exp Clin Psychopharmacol. 2006;14:450–8. doi: 10.1037/1064-1297.14.4.450. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Acute alcohol tolerance on subjective intoxication and simulated driving performance in binge drinkers. Psychol Addict Behav. 2009;23:238–47. doi: 10.1037/a0014633. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Harrison ELR, Fillmore MT. Effects of alcohol on simulated driving and perceived driving impairment in binge drinkers. Alcohol Clin Exp Res. 2008;32:1329–37. doi: 10.1111/j.1530-0277.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- Mills KC, Bisgrove EZ. Body sway and divided attention performance under the influence of alcohol: dose-response differences between males and females. Alcohol Clin Exp Res. 1983;7:393–7. doi: 10.1111/j.1530-0277.1983.tb05492.x. [DOI] [PubMed] [Google Scholar]
- Mitchell MC. Alcohol-induced impairment of central nervous system function: behavioral skills involved in driving. J Stud Alcohol. 1985;10(Suppl):109–16. doi: 10.15288/jsas.1985.s10.109. [DOI] [PubMed] [Google Scholar]
- Moskowitz H, Robinson C. Driving-related skills impairment at low blood alcohol levels. In: Noordzij P, Roszbach R, editors. Proceedings of the Tenth International Conference on Alcohol, Drugs, and Traffic Safety. Amsterdam: Elsevier; 1987. pp. 79–87. [Google Scholar]
- Mumenthaler MS, Taylor JL, O’Hara R, et al. Gender differences in moderate drinking effects. Alcohol Res Health. 1999;23:55–64. [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Drinking and driving. Alcohol Alert. 1996;31:362. [Google Scholar]
- NHTSA (National Highway Traffic Safety Administration) Traffic Safety Facts 2002: A Compilation of Motor Vehicle Crash Data from the Fatality Analysis Reporting System and the General Estimates System. Washington, DC: U.S. Department of Transportation; 2003. [Google Scholar]
- Niaura RS, Nathan PE, Frankenstein W, et al. Gender differences in acute psychomotor, cognitive, and pharmacokinetic response to alcohol. Addict Behav. 1987;12:345–56. doi: 10.1016/0306-4603(87)90048-7. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Gender differences in risk factors and consequences for alcohol use and problems. Clin Psychol Rev. 2004;24:981–1010. doi: 10.1016/j.cpr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Wahlstrom G, Backstrom T, et al. No difference in responsiveness to a low dose of alcohol between healthy women and men. Pharmacol Biochem Behav. 2004;78:603–10. doi: 10.1016/j.pbb.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Price DL, Radwan MA, Terquo DE. Gender, alcohol, pacing and incentive effects on an electronics assembly task. Ergonomics. 1986;29:393–406. doi: 10.1080/00140138608968273. [DOI] [PubMed] [Google Scholar]
- Read JP, Wood MD, Lejuez CW, et al. Gender, alcohol consumption, and differing alcohol expectancy dimensions in college drinkers. Exp Clin Psychopharmacol. 2004;12:298–308. doi: 10.1037/1064-1297.12.4.298. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, Van Rooijen LA. Self-administered short version of the Michigan alcoholism screening test. J Stud Alcohol. 1975;36:117–26. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Characterizing the ontogeny of ethanol-associated increases in coricosterone. Alcohol. 2004;32:145–55. doi: 10.1016/j.alcohol.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Stapleton JM, Guthrie S, Linnoila M. Effects of alcohol and other psychotropic drugs on eye movements: relevance to traffic safety. J Stud Alcohol. 1986;47:426–32. doi: 10.15288/jsa.1986.47.426. [DOI] [PubMed] [Google Scholar]
- Sutker PB, Goist KCJ, King AR. Acute alcohol intoxication in women: relationship to dose and menstrual cycle phase. Alcohol Clin Exp Res. 1987;11:74–9. doi: 10.1111/j.1530-0277.1987.tb01266.x. [DOI] [PubMed] [Google Scholar]
- Vogel-Sprott M. New York: Guilford; 1992. Alcohol Tolerance and Social Drinking: Learning the Consequences. [Google Scholar]
- Wang G-J, Volkow ND, Fowler JS, et al. Alcohol intoxication induces greater reductions in brain metabolism in male than in female subjects. Alcohol Clin Exp Res. 2003;27:909–17. doi: 10.1097/01.ALC.0000071740.56375.BA. [DOI] [PubMed] [Google Scholar]
- Wang G-J, Volkow ND, Franceschi D, et al. Regional brain metabolism during alcohol intoxication. Alcohol Clin Exp Res. 2000;24:822–9. [PubMed] [Google Scholar]
- Weafer J, Fillmore MT. Impairment of alcohol in men and women in a task of psychomotor coordination (unpublished data) 2008.
- Wechsler H, Lee JE, Nelson TF, et al. Drinking and driving among college students: the influence of alcohol-control policies. Am J Prev Med. 2003;25:212–8. doi: 10.1016/s0749-3797(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Wells S, Speechley M, Koval JJ, et al. Gender differences in the relationship between heavy episodic drinking, social roles, and alcohol-related aggression in a U.S. sample of late adolescent and young adult drinkers. Am J Drug Alcohol Abuse. 2007;33:21–9. doi: 10.1080/00952990601082613. [DOI] [PubMed] [Google Scholar]
- Witt ED. Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicol Teratol. 2007;29:81–95. doi: 10.1016/j.ntt.2006.10.013. [DOI] [PubMed] [Google Scholar]