SIR—Circulating endothelial progenitor cells (EPCs) are widely recognised to contribute to the reparative process of the vascular endothelium and participate in angiogenesis [1]. Although declines in the circulating population of EPCs are associated with poor cardiovascular disease prognosis and are predictive of future adverse cardiovascular events [2], transplantation of ex vivo expanded EPCs into the coronary artery can rescue ischaemic tissue and significantly improve coronary function in patients with myocardial infarction [3]. The angiogenic potential of these cells can be explained, in part, through their ability to home to local sites of ischaemia and vascular damage and secrete potent proangiogenic factors, such as cytokines, chemokines and growth factors, which are integral in promoting new blood vessel formation and repair. For example, both vascular endothelial growth factor (VEGF) [4] and granulocyte-colony stimulating factor (G-CSF) [5] stimulate recruitment and migration of EPCs from the bone marrow, inhibit apoptosis and support the angiogenic capacity of mature endothelial cells [6, 7]. In addition, interleukin (IL)-8, a proangiogenic cytokine, has been shown to attract EPCs to infarcted tissue and enhance the effect of G-CSF to mobilise progenitor cells from the bone marrow [8–10]. In older adults, endothelial injury and compromised EPC-mediated vascular repair are thought to contribute to atherosclerosis [11]. We have previously reported that EPC colony-forming capacity, migration and telomere length decline with ageing [12, 13]. In the present study, we tested the hypothesis that the capacity of circulating EPCs to release proangiogenic cytokines declines with age in healthy adults.
Methods
Peripheral blood samples were collected from 37 healthy, sedentary adults: 17 young (age range: 21–34 years) and 20 older (age range: 56–70 years) men. All subjects were non-obese (body mass index, BMI ≤ 30 kg/m2), normotensive, non-smokers, non-medicated and free of overt cardiovascular, metabolic and haematologic disease, as assessed by medical history, resting and exercise electrocardiograms, and fasting blood chemistries. Prior to participation, all of the subjects provided written informed consent according to the guidelines of the University of Colorado at Boulder.
Putative EPCs were isolated and identified from peripheral blood as previously described [12]. Briefly, peripheral-blood mononuclear cells were isolated by Ficoll density-gradient centrifugation (Histopaque 1077, Sigma) and plated on six-well plates coated with human fibronectin (BD Biosciences) for 48 h. Thereafter, non-adherent cells were collected and 5 × 105 cells were seeded onto 24-well fibronectin-coated plates (BD Biosciences). Endothelial phenotype of these cells was confirmed by immunofluorescent staining for the uptake of DiL-ac-LDL and expression of VE-cadherin, von Willebrand factor, CD31 and VEGFR-2 [14]. In addition, fluorescent-activated cell sorting analysis utilising endothelial-specific antibodies was performed in selected samples. To determine cytokine release, cells were incubated in growth medium in the absence and presence of the stimulant phytohemagglutinin (PHA; 10 μg/ml) for 72 h. Concentrations of the proangiogenic cytokines VEGF, G-CSF, IL-8 and IL-17 in the medium were determined by enzyme immunoassay (R&D Systems, Minneapolis, MN). The growth medium, without EPCs, did not contain measurable amounts of angiogenic growth factors.
Group differences for all variables were determined by between-group analysis of variance. Relations between variables of interest were assessed by means of linear and stepwise regression analyses. Analysis of covariance (ANCOVA) was performed with the variable of interest serving as the covariate. Data are reported as mean ± SEM. Statistical significance was set at P < 0.05.
Results
Selected subject characteristics are presented in Table 1. Although within clinically normal levels, most haemodynamic and metabolic indices tend to be higher in older compared with young men.
Table 1.
Selected subject characteristics
| Variable | Young | Older |
|---|---|---|
| (n = 17) | (n = 20) | |
| Age (years) | 25 ± 1 | 61 ± 1* |
| Body mass (kg) | 79.8 ± 2.5 | 84.9 ± 2.0* |
| BMI (kg/m2) | 24.1 ± 0.6 | 26.8 ± 0.5* |
| Body fat (%) | 15.5 ± 1.1 | 27.0 ± 1.1* |
| Waist circumference (cm) | 82.8 ± 1.4 | 95.7 ± 1.6* |
| Systolic BP (mmHg) | 113 ± 3.0 | 127 ± 1.7* |
| Diastolic BP (mmHg) | 68 ± 1.7 | 77 ± 1.4* |
| Total cholesterol (mmol/l) | 4.1 ± 0.3 | 5.1 ± 0.2* |
| LDL cholesterol (mmol/l) | 2.5 ± 0.1 | 3.4 ± 0.1* |
| HDL cholesterol (mmol/l) | 1.2 ± 0.1 | 1.3 ± 0.1 |
| Triglycerides (mmol/l) | 1.1 ± 0.1 | 1.3 ± 0.1 |
| Glucose (mmol/l) | 4.7 ± 0.1 | 5.3 ± 0.1* |
| Insulin (pmol/l) | 32.7 ± 3.6 | 36.5 ± 4.0 |
| HOMA-IR | 1.1 ± 0.2 | 1.4 ± 0.2 |
BMI, body mass index; BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance. Values are mean ± SEM. *P < 0.05.
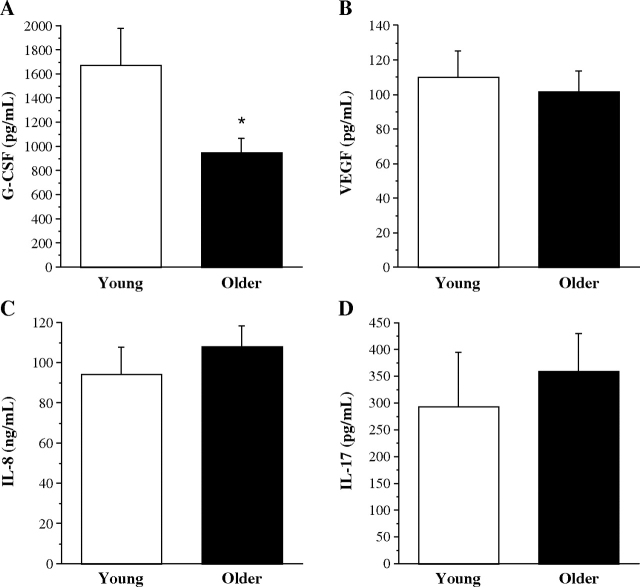
There were no significant differences in basal release of VEGF (22.7 ± 2.1 vs 28.0 ± 4.9 pg/ml), G-CSF (43.1 ± 4.9 vs 38.9 ± 5.7 pg/ml), IL-8 (7.3 ± 1.4 vs 5.9 ± 1.0 ng/ml) and IL-17 (10.6 ± 0.7 vs 12.4 ± 1.0 pg/ml) by EPCs from young and older men. PHA stimulation resulted in increased concentrations of proangiogenic cytokines in the medium from EPCs isolated from both groups. Stimulated EPC release of VEGF (110.4 ± 15.1 vs 101.8 ± 11.9 pg/ml), IL-8 (94.6 ± 13.2 vs 108.4 ± 10.2 ng/ml) and IL-17 (293.6 ± 102.4 vs 360 ± 70.6 pg/ml) were not different between the young and older subjects (Figure 1). However, the amount of G-CSF released was ~45% lower (P < 0.05) in older (954.3 ± 115.3 pg/ml) compared with young (1676.8 ± 304.4 pg/ml) men (Figure 1). In the overall study population, significant (all P < 0.05) univariate correlations were observed with percent body fat (r = −0.46) and low-density lipoprotein (LDL) cholesterol (r = −0.34) and PHA-stimulated G-CSF release. Stepwise regression analysis identified percent body fat as the primary determinant of G-CSF release (R2 = 0.21). However, the main effect of age persisted after statistically (ANCOVA) controlling for percent body fat.
Figure 1.
PHA-stimulated EPC release of proangiogenic cytokines G-CSF (A), VEGF (B), IL-8 (C) and IL-17 (D) from young and older men. Values are mean ± SEM. *P < 0.05.
Discussion
The main finding of the present study is that the capacity of EPCs to release the proangiogenic cytokine G-CSF is significantly reduced in healthy older men. EPC release of VEGF, IL-8 and IL-17, however, is not depressed with advancing age. Reduced EPC-mediated secretion of G-CSF may contribute to deficient vascular repair and regeneration that is characteristic of advancing age.
Several studies have demonstrated the importance of proangiogenic cytokines, particularly G-CSF, for promoting EPC-mediated angiogenesis in response to vascular injury. For example, in animal models of myocardial infarction and limb ischaemia, treatment with G-CSF limits tissue damage, enhances vascular repair, stimulates new vessel formation and restores coronary function [15–17]. Clinically, G-CSF administration after myocardial infarction has been associated with improvements in coronary perfusion, ejection fraction and limits left ventricular remodelling [18, 19]. The angiogenic efficacy of G-CSF treatment is mediated, in part, through recruitment and mobilization of haematopoietic stem cells and EPCs from the bone marrow into the peripheral circulation [5, 20]. Moreover, G-CSF stimulates migration and homing of EPCs to local sites of vascular injury, promotes EPC proliferation and differentiation into a mature endothelial phenotype and enhances cell survival [1, 20]. With respect to human ageing, there is evidence of diminished G-CSF-mediated vascular repair and angiogenesis. Lehrke et al. [21] demonstrated that the favourable effects of G-CSF treatment on cardiac remodelling and regeneration following experimental myocardial infarction are blunted with advancing age. The results of the present study demonstrating significantly blunted (~45%) EPC G-CSF release may also contribute, at least in part, to reduced angiogenic potential and neovascularization capacity in older adults, as well as decreased efficacy of autologous transplantation of ex vivo expanded EPCs from older adults for cytokine- and cell-based therapeutic angiogenesis [22].
Although, in the present study, we investigated a select few proangiogenic cytokines, proteomic analysis reveals that EPCs secrete ~250 different soluble factors with potent paracrine effects in vitro [23]. Indeed, it is now recognised that the release of proangiogenic paracrine factors is a primary means by which EPCs orchestrate their reparative and protective effects on vascular endothelium and cardiac tissue [24]. However, the influence of advancing age on the global release of proangiogenic factors or the ‘secretome’ from EPCs is unclear. The use of proteomics to characterise the secretome may provide further insight into the differential effect of ageing on EPC cytokine release. This area represents a fertile ground for future research as alterations in the secretome profile of EPCs may be a key determinant of their reduced angiogenic capacity with age.
A number of experimental considerations regarding the present study should be mentioned. Firstly, as with all cross-sectional experimental designs, we cannot ignore the possibility that genetic and/or lifestyle behaviours influenced the results of our study. We attempted to minimise potential lifestyle influences by studying healthy men across the adult age range who were non-medicated, non-smokers and not habitually physically active. Secondly, our study involved men only, limiting the generalizability of our results. Oestrogen has been shown to affect both circulating EPC number and function [25] and upregulates VEGF gene expression in EPCs [26]. Thus, the impact of age on EPC release of proangiogenic cytokines may differ in women. Thirdly, there is no agreed-upon method of EPC isolation, identification and cultivation. Some studies have used prolonged ex vivo culture for >14 days which yields a cell population with an endothelial cell-like phenotype [27]. However, extensive culture time under endothelial cell differentiation-specific culture conditions may yield cells with little in vivo or clinical relevance. We chose to avoid this approach to limit phenotypic drift away from the in vivo state. In addition, we used well-characterised immunofluorescent markers to identify putative EPCs with proven functional and clinical utility [28, 29].
In conclusion, advancing age is associated with a marked reduction in the capacity of EPCs to release the potent angiogenic cytokine G-CSF. The ability of EPCs to release other proangiogenic cytokines such as VEGF, IL-8 and IL-17 is, however, preserved in older adults. Impaired release of G-CSF may contribute to limited vascular regenerative capacity of EPCs with increasing age. Further studies of the EPC secretome are required to better understand, and more comprehensively address, the influence of ageing on EPC biology.
Key points
Diminished endothelial progenitor cell (EPC)-mediated vascular repair is thought to contribute to the increased risk of atherosclerosis with age.
EPCs originate from the bone marrow and home to sites of vascular damage/injury and contribute to vascular repair, initiating reendothelialization and neovascularization.
Currently, it is unknown whether the ability of EPCs to release proangiogenic factors is also impaired with age. If so, this may contribute to reduced EPC-mediated vascular repair associated with ageing.
Our results demonstrate that EPCs from older, healthy sedentary men have a diminished capacity to release G-CSF compared with young controls.
Interestingly, the ability of EPCs harvested from older men to secrete VEGF, IL-8 and IL-17 was preserved; this suggests that ageing, per se, may differentially affect the release of proangiogenic factors.
Acknowledgments
We would like to thank all of the subjects who participated in the study as well as Yoli Casas for her technical assistance. This study was supported by the National Institutes of Health awards HL077450, HL076434 and MO1 #RR00051 as well as the American Heart Association award 0555678Z.
References
- 1.Walter DH, Dimmeler S. Endothelial progenitor cells: regulation and contribution to adult neovascularization. Herz. 2002;27:579–88. doi: 10.1007/s00059-002-2427-y. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt-Lucke C, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–7. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 3.Assmus B, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–17. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 4.Asahara T, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–72. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell TM, et al. Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2005;25:296–301. doi: 10.1161/01.ATV.0000151690.43777.e4. [DOI] [PubMed] [Google Scholar]
- 6.Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998;273:13313–6. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 7.Bussolino F, et al. In vitro and in vivo activation of endothelial cells by colony-stimulating factors. J Clin Invest. 1991;87:986–95. doi: 10.1172/JCI115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koch AE, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 9.Kocher AA, et al. Myocardial homing and neovascularization by human bone marrow angioblasts is regulated by IL-8/Gro CXC chemokines. J Mol Cell Cardiol. 2006;40:455–64. doi: 10.1016/j.yjmcc.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe T, et al. Endogenous interleukin-8 (IL-8) surge in granulocyte colony-stimulating factor-induced peripheral blood stem cell mobilization. Blood. 1999;93:1157–63. [PubMed] [Google Scholar]
- 11.Reed M.J., Edelberg J.M. Impaired angiogenesis in the aged. Sci Aging Knowledge Environ. 2004;2004:pe7. doi: 10.1126/sageke.2004.7.pe7. [DOI] [PubMed] [Google Scholar]
- 12.Hoetzer GL, et al. Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men. J Appl Physiol. 2007;102:847–52. doi: 10.1152/japplphysiol.01183.2006. [DOI] [PubMed] [Google Scholar]
- 13.Kushner EJ, et al. Aging and endothelial progenitor cell telomere length in healthy men. Clin Chem Lab Med. 2009;47:47–50. doi: 10.1515/CCLM.2009.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peichev M., et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 15.Minamino K, et al. Macrophage colony-stimulating factor (M-CSF), as well as granulocyte colony-stimulating factor (G-CSF), accelerates neovascularization. Stem Cells. 2005;23:347–54. doi: 10.1634/stemcells.2004-0190. [DOI] [PubMed] [Google Scholar]
- 16.Kanellakis P, et al. Granulocyte colony-stimulating factor and stem cell factor improve endogenous repair after myocardial infarction. Cardiovasc Res. 2006;70:117–25. doi: 10.1016/j.cardiores.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Orlic D, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–9. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang HJ, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004;363:751–6. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 19.Ince H, et al. Preservation from left ventricular remodeling by front-integrated revascularization and stem cell liberation in evolving acute myocardial infarction by use of granulocyte-colony-stimulating factor (FIRSTLINE-AMI) Circulation. 2005;112:3097–106. doi: 10.1161/CIRCULATIONAHA.105.541433. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi T, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 21.Lehrke S, et al. Aging impairs the beneficial effect of granulocyte colony-stimulating factor and stem cell factor on post-myocardial infarction remodeling. Circ Res. 2006;99:553–60. doi: 10.1161/01.RES.0000238375.88582.d8. [DOI] [PubMed] [Google Scholar]
- 22.Dzau VJ, et al. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46:7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 23.Pula G, et al. Proteomics identifies thymidine phosphorylase as a key regulator of the angiogenic potential of colony-forming units and endothelial progenitor cell cultures. Circ Res. 2009;104:32–40. doi: 10.1161/CIRCRESAHA.108.182261. [DOI] [PubMed] [Google Scholar]
- 24.Gnecchi M, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strehlow J, et al. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation. 2003;107:3059–65. doi: 10.1161/01.CIR.0000077911.81151.30. [DOI] [PubMed] [Google Scholar]
- 26.Haas DW, et al. MDR1 gene polymorphisms and phase 1 viral decay during HIV-1 infection: an adult AIDS Clinical Trials Group study. J Acquir Immune Defic Syndr. 2003;34:295–8. doi: 10.1097/00126334-200311010-00006. [DOI] [PubMed] [Google Scholar]
- 27.Fadini G.P., et al. Technical notes on endothelial progenitor cells: ways to escape from the knowledge plateau. Atherosclerosis. 2008;197:496–503. doi: 10.1016/j.atherosclerosis.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 28.Hill J, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. New Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 29.Werner N, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]