Abstract
Reactive oxygen species (ROS) are generated in response to growth factors, cytokines, G protein–coupled receptor agonists, or shear stress, and function as signaling molecules in nonphagocytes. However, it is poorly understood how freely diffusible ROS can activate specific signaling, so-called “redox signaling.” NADPH oxidases are a major source of ROS and now recognized to have specific subcellular localizations, and this targeting to specific compartments is required for localized ROS production. One important mechanism may involve the interaction of oxidase subunits with various targeting proteins localized in lamellipodial leading edge and focal adhesions/complexes. ROS are believed to inactivate protein tyrosine phosphatases, thereby establishing a positive-feedback system that promotes activation of specific redox signaling pathways involved in various functions. Additionally, ROS production may be localized through interactions of NADPH oxidase with signaling platforms associated with caveolae/lipid rafts, endosomes, and nucleus. These indicate that the specificity of ROS-mediated signal transduction may be modulated by the localization of Nox isoforms and their regulatory subunits within specific subcellular compartments. This review summarizes the recent progress on compartmentalization of redox signaling via activation of NADPH oxidase, which is implicated in cell biology and pathophysiologies. Antioxid. Redox Signal. 11, 1289–1299.
Introduction
Reactive oxygen species (ROS), including superoxide (O2•-) and hydrogen peroxide (H2O2), play a central role in host defense by killing microbes in phagocytic cells. Accumulating evidence suggests that nonphagocytic cells also produce ROS. Although excess amounts of ROS are toxic, physiologic concentrations of ROS function as signaling molecules to mediate various responses, including cell proliferation, migration, differentiation, and gene expression (24, 32). ROS are produced in response to growth factors, cytokines, G protein–coupled receptor agonists, or shear stress. Given that ROS are diffusible and short-lived, localizing the ROS signal at the precise subcellular compartment after receptor activation is essential for specific redox signaling events. Several enzymes, including the mitochondrial electron transport system, xanthine oxidase, cytochrome p450, NADPH oxidase, uncoupled NO synthase (NOS), and myeloperoxidase, have been reported to produce ROS; however, the major source of ROS appears to be the NADPH oxidase. In phagocytic cells, NADPH oxidases consist of membrane-associated cytochrome b558, comprising the catalytic gp91phox and regulatory p22phox subunits, and cytosolic components including p47phox, p67phox, p40phox, and the small GTPase Rac1 (7). In nonphagocytic cells, several homologues of gp91phox (also termed as Nox2) including Nox1, Nox3, Nox4, and Nox5, as well as the Dual oxidases (Duox; Duox1 and Duox2), have been identified (30, 49).
Although the evidence is strong that ROS generated by NADPH oxidases participate in signal transduction, so-called “redox signaling,” the mechanisms by which receptors activate NADPH oxidase and regulate ROS production are poorly understood. NADPH oxidases are now recognized to have specific subcellular localizations, and this targeting is required for localized ROS production and activation of specific redox signaling pathways that mediate various cell functions. One important mechanism may involve the interaction of oxidase subunits with targeting proteins. This review summarizes the recent progress and the information on compartmentalization of redox signaling via activation of NADPH oxidase in various subcellular compartments that are implicated in biology and pathophysiologies.
Redox Signaling in Caveolae/Lipid Rafts
Lipid rafts are cholesterol- and sphingolipid-rich, low-density plasma membrane domains. Specialized microdomains termed caveolae constitute a distinct subset of lipid rafts with cell-surface flask-shaped invaginations that contain caveolin as a major structural protein (36). Caveolae/lipid rafts concentrate multiple signaling molecules including G protein–coupled receptors (GPCRs), receptor tyrosine kinase (RTK), protein kinase C, Src family kinases, and G proteins to form signaling platforms. Compartmentalization of signaling molecules is required to provide the appropriate molecular proximity necessary for rapid, efficient, and specific activation of downstream signaling events (72, 78, 85). The assembly of functionally active NADPH oxidase and subsequent ROS production also is dependent on caveolae/lipid rafts. Thus, these specialized plasma membrane microdomains play an important role in activation of specific redox signaling events (12, 102, 115, 118).
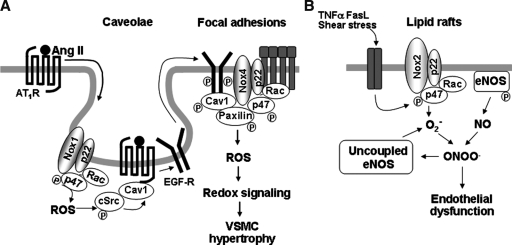
In cultured vascular smooth muscle cells (VSMCs), Nox1 is found in caveolin-1–containing membranes under unstimulated conditions (38). Stimulation of VSMCs with angiotensin II (Ang II), a GPCR agonist, promotes AT1 receptor (AT1R) binding to caveolin-1 as well as trafficking of AT1R from high-density noncaveolar membrane fractions into caveolin-1–containing caveolae/lipid rafts (118). This in turn promotes Rac1 translocation into caveolae/lipid rafts to increase localized ROS production (117), which is required for transactivation of epidermal growth factor receptor (EGFR), whose mechanism is dependent on ROS-mediated activation of cSrc (94). Tyrosine-phosphorylated EGFR and caveolin-1 through cSrc subsequently appear at focal adhesions where Nox4 and paxillin localize, thereby forming redox signaling platforms. Inhibition of either NADPH oxidase or caveolin1 siRNA substantially blocks Ang II–induced increase in [3H]leucine incorporation (97, 114, 118). These results suggest that caveolae/lipid rafts link AT1R signal with NADPH oxidase to provide localized ROS production, thereby activating specific redox signaling pathways involved in vascular hypertrophy, which may contribute to hypertension (12) (Fig. 1A).
FIG. 1.
Redox signaling in caveolae and lipid rafts. (A) In VSMCs, Ang II stimulation promotes AT1-receptor trafficking into caveolin-1–enriched membrane fractions where Nox1 is found. This causes localized ROS production and ROS-cSrc–dependent transactivation of the EGFR and its egress from caveolae. Tyrosine-phosphorylated EGFR and caveolin-1 subsequently appear at focal adhesions where Nox4 and paxillin localize, thereby forming redox signaling platforms. These events are essential for activation of specific redox signaling pathways involved in VSMC hypertrophy. (B) In ECs, stimulation with TNF-α, Fas ligand, and shear stress induces recruitments of Nox2, p47phox, and Rac1 into lipid rafts, thereby promoting raft-localized NADPH oxidase activation and ROS production and eNOS-derived NO within raft domains. This formation of redox signaling platforms in lipid rafts contributes to decrease in NO bioavailability and production of peroxynitrite, which uncouples NOS to produce more O2•-, which contributes to endothelial dysfunction.
Caveolae/lipid rafts interact with actin-cytoskeleton and microtubules via caveolin-1 (86). Ang II stimulation promotes p47phox binding to cortactin, a c-Src–regulated actin-binding protein, which is required for p47phox translocation to the membrane to form active NADPH oxidase complex (91). Studies using GFP-tagged caveolin-1 reveal that reorganization of the actin cytoskeleton causes caveolae redistribution (86, 89). Depolymerization of microtubules reduces the mobility of GFP/caveolin-1 and increases the presence of invaginated caveolae at the cell surface. Disruption of microtubules blocks Ang II–induced Rac1 and AT1R movement into caveolae/lipid rafts, which consequently attenuates ROS production (117). Additionally, small caveolin-containing vesicles “cavicles” are transported, possibly as microtubule cargo, between the plasma membrane and pericentrosomal compartment, “caveosomes” in an actin-cytoskeleton–dependent manner (66). It remains unknown whether Nox complexes are transported within this intracellular compartment.
In endothelial cells (ECs), NADPH oxidase subunits are preassembled in caveolae/lipid rafts in the resting state. Stimulation with TNF-α induces additional recruitment of the p47phox to raft-localized NADPH oxidase and promotes ROS production and eNOS-derived nitric oxide (NO) production within raft domains (113). Thus, caveolae/lipid rafts are sites of spatial regulation of NADPH oxidase and eNOS to promote subsequent generation of peroxynitrite (ONOO-), which induces protein tyrosine nitration (113). Furthermore, death receptors such as Fas and TNF receptor 1 (TNFR1) have been shown to be localized in lipid rafts. They stimulate lipid raft clustering and formation of redox signaling platforms in lipid rafts to increase NADPH oxidase activity, which contributes to impaired endothelium-dependent vasorelaxation (115) (Fig. 1B).
Zhang et al. (115) showed that Fas ligand stimulation promotes recruitment of Nox2, p47phox, and Rac1 into lipid rafts, where an increase in NADPH oxidase activity and ROS production occurs (115). Moreover, death factors bind to their receptors in individual lipid rafts and subsequently stimulate acid sphingomyelinase to produce ceramide from sphingomyelin in ECs. Ceramide-enriched membrane platform formation results in aggregation of NADPH oxidase subunits such as Nox2 and p47phox and other proteins, which in turn promotes O2•- production. O2•- reacts with eNOS-derived NO to decrease NO bioavailability and to produce ONOO-, which uncouples NOS to produce more O2•- but less NO. This mechanism contributes to endothelial dysfunction (55) (Fig. 1B). These findings indicate that caveolae/lipid rafts are signaling domains in which death receptors couple to NADPH oxidase to promote local production of ROS, thereby forming active redox signaling platforms involved in endothelial dysfunction.
Redox Signaling at Cell–Matrix Adhesions (Focal Adhesions)
Integrin-mediated cell adhesion is required for anchorage-dependent cell growth. Focal adhesions, cell–matrix adhesion sites, serve as organizing centers for regulatory and structural proteins to facilitate rapid and precise control of cell function (106). Activation of integrins leads to phosphorylation of focal complex proteins such as paxillin and focal adhesion kinase (FAK), which facilitates the linkage of the actin cytoskeleton and integrin receptors (75) These pathways are involved in the formation of focal contacts cross-talk with tyrosine kinases and small G proteins to coordinate downstream signaling for gene transcription of cell proliferation, survival, motility, and cytoskeletal remodeling (15, 41, 42, 84, 92). Integrin-mediated adhesion also governs the presence of lipid rafts on the plasma membrane by preventing its internalization via retaining tyrosine phosphorylated caveolin-1 in focal adhesions (22), which is required for targeting of active Rac1 and its coupling of p21-activated protein kinase (PAK) to the NADPH oxidase activation. A significant content of tyrosine phosphorylated caveolin-1 localizes near focal adhesion sites in association with cytoskeleton elements (22, 51), where caveolin-1 interacts with focal adhesion protein scaffolds and recruits several signaling proteins involved in cell growth, survival, and transformation (43, 79, 82, 87). As mentioned earlier, AT1R migration into lipid rafts is associated with egress of EGFRs from these microdomains. Ultimately, transactivated EGFRs are found and colocalize with phospho-caveolin-1 at focal adhesions where Nox4 and paxillin colocalize, thereby forming redox signaling complexes at focal adhesions (38, 95) (Fig. 1A).
The signaling properties of ROS are largely due to the reversible oxidation of redox-sensitive target proteins, and especially of protein tyrosine phosphatases (PTPs) (17, 25, 52, 62). The PTP activity is dependent on the reactive cysteine residues (Cys-SH) with a low pKa at their active site (59, 116) that are readily susceptible to reversible oxidation by H2O2 (110). Integrin-induced ROS are required to oxidize/inhibit the low-molecular-weight PTP, thereby preventing the enzyme from dephosphorylating and inactivating FAK. Accordingly, FAK phosphorylation and downstream events, including MAPK and Src phosphorylation, focal adhesion formation, and cell spreading, are all significantly attenuated by inhibition of redox signaling. On cell adhesion, oxidative inhibition of PTPs promotes the phosphorylation/activation and the downstream signaling of FAK and, as a final event, cell adhesion and spreading onto fibronectin (18) (Fig. 2).
FIG. 2.
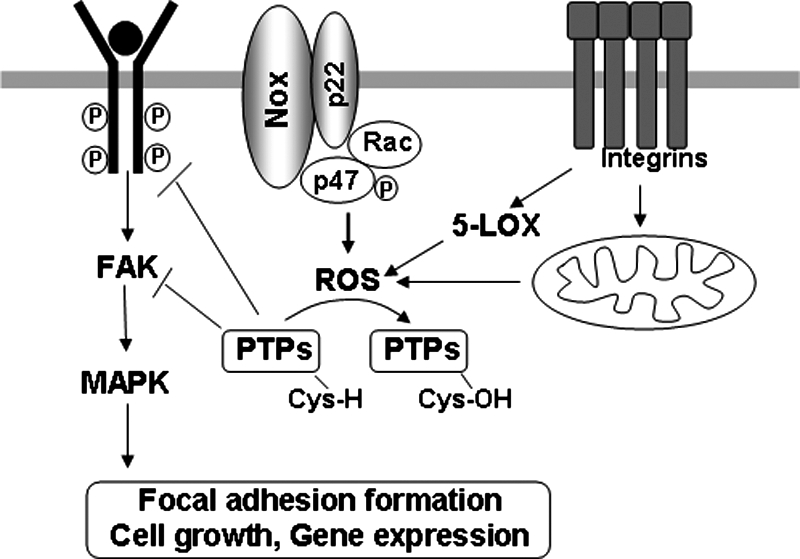
Redox signaling at cell–matrix adhesions (focal adhesions). Growth factor activates NADPH oxidase, whereas integrin activation stimulates mitochondria and 5-lipoxygenase to generate localized ROS production at cell–matrix adhesions. ROS produced by synergistic action of integrins and growth factors induce oxidative inactivation of protein tyrosine phosphatases (PTPs), which negatively regulate RTK and FAK, thereby promoting downstream redox signaling events such as MAPK, leading to cell proliferation, survival, and gene expression.
A synergistic action of integrins and RTKs occurs for redox signaling, which plays an essential role in anchorage-dependent cell growth. Both growth factor and integrin activation generate oxidants independently, specifically, both mitochondria and 5-lipoxygenease for integrins, and NADPH oxidase for growth factors. Thus, their simultaneous stimulation has a cooperative effect in enhancing ROS production (16). Different compartmentalization and kinetics of ROS production can likely account for distinct subsets of molecular targets of ROS during cell adhesion. Thus, synergistic action of integrins and growth-factor receptors exists for activation of redox signaling leading to cell proliferation and survival through the reversible oxidation of proteins such as PTPs, including PTP1B, LMW-PTP, PTEN, and SHP2, as well as RTKs, including insulin receptor, EGFRs, and PDGF-Rs (16) (Fig. 2).
Redox Signaling at Cell–Cell Contacts
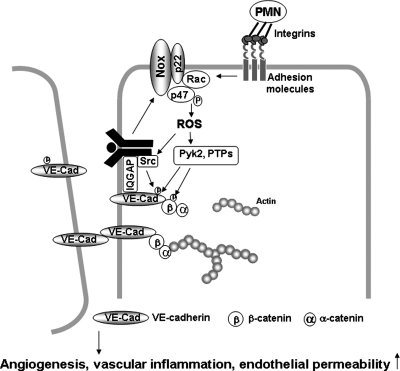
Loss of stable cell–cell contacts between ECs in the parent vessel is an important mechanism for initiating the endothelial migration and proliferation involved in angiogenesis as well as promoting endothelial permeability. The molecule primarily responsible for cell–cell contacts of ECs is the transmembrane homophilic adhesion molecule, vascular endothelial (VE)-cadherin (21). The cytoplasmic domain of VE-cadherin binds to ß-catenin, which is linked to the actin cytoskeleton via α-catenin. Tyrosine phosphorylation of the VE-cadherin complex is required for disruption of the cell–cell junction (23, 68, 107), and it is mediated through cSrc, which is dependent on ROS (58, 100). Although localization of NADPH oxidase at the cell–cell junction has not been clearly demonstrated, NADPH oxidase–derived ROS are involved in VE-cadherin/β-catenin phosphorylation and disruption of cell–cell contacts (71). Activation of Rac1 stimulates generation of ROS, which are capable of activating Pyk2 (28, 101) and Src (4). Rac1-induced ROS disrupt VE-cadherin–mediated cell–cell adhesion through an increase in tyrosine phosphorylation of α-catenin in ECs (100). Inhibition of VE-cadherin function induced by VE-cadherin–blocking antibodies activates Rac1, thereby increasing localized ROS production, which in turn promotes EC barrier dysfunction through ROS-mediated tyrosine phosphorylation of Pyk2 (98), which regulates cell–cell adhesion (47). Thus, a positive feed-forward mechanism exists whereby localized ROS promote endothelial permeability (Fig. 3).
FIG. 3.
Redox signaling at cell–cell contacts. PMN binding to ECs, inflammatory stimuli, or growth factors simulate localized ROS production via activation of NADPH oxidase at or near the cell–cell contacts in confluent ECs. ROS are involved in activation of Src, Pyk2, FAK, and PKC, or in oxidative inactivation of PTPs, thereby promoting tyrosine phosphorylation of VE-cadherin and β-catenin, which in turn promotes disruption of cell–cell contacts, and thus increasing endothelial permeability, migration, and proliferation, which are involved in angiogenesis.
VEGF stimulation promotes association of cSrc with VEGF receptor type2, which causes Src-dependent tyrosine phosphorylation of VE-cadherin. This in turn stimulates loss of cell–cell contacts, thereby promoting EC migration and proliferation involved in angiogenesis as well as endothelial permeability (19, 48, 103). Of note, cSrc is a downstream target of ROS. IQGAP1, an effector of active Rac1 (37, 46), which colocalizes with VE-cadherin at cell–cell junctions to stabilize cell–cell contact in confluent monolayers of ECs. VEGF stimulation promotes IQGAP1 association with activated VEGFR2 to link VEGFR2 to VE-cadherin to stimulate Rac1/ROS-dependent tyrosine phosphorylation of VE-cadherin, thereby promoting disruption of cell–cell contacts to initiate EC migration (111, 112). Polymorphonuclear (PMN) leukocyte adhesion to ECs via adhesion molecules ICAM-1 stimulates ROS production, which also promotes loss of cell–cell adhesions through activating Src and Pyk2, which phosphorylate VE-cadherin on Tyr658 and Tyr731, respectively (4). These responses are involved in increasing transendothelial migration of PMNs and endothelial permeability, which contributes to inflammatory diseases such as atherosclerosis and diabetes. Nwariaku et al. (71) reported that TNF-induced loss of endothelial junctional integrity is mediated through the PAK1-NADPH oxidase-JNK-VE-cadherin phosphorylation pathway. Thus, NADPH oxidase–derived ROS produced near the adherens junction may activate redox signaling that disrupts the cell–cell contacts, and thus promote angiogenesis, vascular inflammation, and permeability (58, 88) (Fig. 3).
Several PTPs, including PTP1B (8, 67, 83), density-enhanced phosphatase-1 (DEP1)/CD148 (31), vascular endothelial PTP (VE-PTP) (68), and SHP-2 (93), localize at cell–cell adhesion sites to maintain low levels of tyrosine phosphorylation, and thus stabilize junctional integrity (80). Thus, localized production of ROS at cell–cell contacts may induce oxidative inactivation of VE-cadherin or β-catenin–associated PTPs, thereby promoting tyrosine phosphorylation of VE-cadherin complex proteins (Fig. 3).
Redox Signaling in Lamellipodial Leading Edge and Focal Complexes
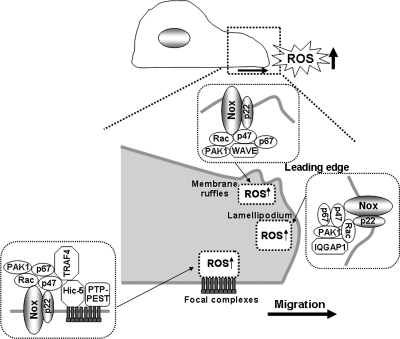
Migrating cells create transitory integrin-containing structures with tyrosine-phosphorylated proteins termed focal complexes (69). Leading-edge focal complexes bear the strongest tensile forces in migrating cells, and focal complex turnover is necessary for the remodeling of lamellipodia to promote membrane ruffling. RhoGTPases Rac1 and Cdc42 are involved in focal complex formation within lamellipodia and filopodia, respectively, whereas RhoA facilitates the maturation of focal complexes into stable focal adhesions (69). Rac1 directs the tyrosine kinase Src to lamellipodia to phosphorylate focal complex proteins, including Pyk2, thus promoting the turnover of focal complexes (50, 90). Directed cell migration is a highly localized process involving the generation of spatially and temporally restricted signaling molecules, including Rac1 (44) and phosphatidylinositol-3,4,5-trisphosphate [PI(3,4,5)P3] (63), a product of phosphatidylinositol 3-kinase (PI3K), at the site of the new leading edge. EC migration is a key event for tissue repair in response to injury, angiogenesis, and wound healing. In ECs, Rac1- and Nox2-dependent NADPH oxidase plays an important role in cell migration (2, 20, 39, 96). The PI3K-Rac pathway is involved in the production of ROS that accumulate at the membrane ruffles (73), which is required for cytoskeletal reorganization and directed cell migration (40, 65). Nox2 and its regulatory subunits, p47phox and p67phox also are targeted to the focal complexes or membrane ruffles in lamellipodia (40, 108, 109). TNF-α–stimulated translocation of these subunits to the cell membrane is required for ROS production, which stimulates cytoskeletal reorganization required for migratory response. Thus, oxidants derived from NADPH oxidase activation and lamellipodial dynamics are likely to be spatially and functionally coupled at the leading edge to promote directional cell migration (40, 65) (Fig. 4).
FIG. 4.
Redox signaling in lamellipodial leading edge and focal complexes. Localized ROS signal at lamellipodial focal complexes is mediated through formation of p47phox-TRAF4-Hic5 complexes and oxidative inactivation of PTP-PEST by ROS, which is required for activation of Rac1 and its effector PAK1, which phosphorylate p47phox through formation of TRAF4-Rac1-PAK1 complexes. These p47phox–containing complexes create a positive-feedback loop to facilitate localized Nox-dependent ROS production, which contributes to directional cell migration. Localized ROS at membrane ruffles and lamellipodial leading edges are mediated through p47phox-WAVE1-Rac1-PAK1 complexes, which phosphorylate p47phox as well as Rac1-IQGAP1-Nox2 targeting to the leading edge through the scaffolding function of IQGAP1, respectively. These NADPH oxidase–targeting mechanisms are required for ROS-dependent directional cell migration.
Wu et al. (109) identified targeting molecules that may specify the site of ROS production at lamellipodial focal complexes during cell migration. In ECs, the p47phox subunit of NADPH oxidase binds to the orphan adaptor TRAF4, which in turn binds to the focal contact scaffold Hic-5, thereby targeting p47phox to the focal complexes. Thus, local activation of NADPH oxidase and ROS production is found. Knockdown of TRAF4 or Hic-5 by using siRNA or disruption of the TRAF4-Hic-5 complex, or inhibition of ROS with antioxidants or of NADPH oxidase with mutant form of p67phox, all block cell migration, indicating that localized ROS signal at lamellipodial focal complexes through formation of the p47phox-TRAF4-Hic-5 complex is important for directional cell migration (Fig. 4).
The mechanism for targeting NADPH oxidase to the lamellipodial leading edge and membrane ruffles is through the interaction of p47phox with moesin and WAVE1, which are enriched within this specific structural compartment (104, 108). WAVE1 catalyzes the actin nucleation responsible for lamellar structure in a Rac1-dependent manner, and thus p47phox-WAVE1 complexes contain Rac1 and Rac1 effectors PAK1, which phosphorylates p47phox (Fig. 4). Antioxidants and inhibition of p47phox-WAVE1 interaction block ROS production and ruffle formation (108). Another important targeting protein is IQGAP1, which is an actin-binding scaffold protein and Rac1 effector that links Rac1 to the cytoskeleton and is required for Rac-mediated polarized cell migration (11, 61). Linkage between the microtubule plus-ends and cortical regions is essential for the establishment of cell polarity and directional migration. IQGAP1 captures and stabilizes microtubules by interacting with the microtubule tip–binding protein CLIP-170 near the cell cortical regions (70). In addition, activated Rac1 promotes capture of CLIP-170–capped microtubules in lamellipodia (61). At the leading edge of cells, Rac1 also links the adenomatous polyposis coli (APC) protein to actin filaments through IQGAP1, thereby regulating polarization and directional migration by forming a complex with APC and CLIP-170. The Nox2 also binds to and colocalizes with IQGAP1 at the leading edge in actively migrating ECs (40). IQGAP1 functions as a scaffold protein to target Nox2 and Rac1 to the specific membrane compartments to localize ROS production, thereby achieving specificity of redox signaling, which may contribute to EC migration (Fig. 4).
Foreman et al. (26) reported that localized ROS production by NADPH oxidase in the growing tips of cells is required for polarized root hair growth. As in animal cells, ROS production appears to be regulated by Rho GTPases. Carol et al. (13) provided the evidence that inactivation of Rho GTPase guanine nucleotide dissociation inhibitor (RhoGDI) encoded by the supercentipede (SCN1) gene causes more broad distribution of ROS and mislocalization of root hair cells, leading to ectopic hair-formation sites. These results suggest that the spatial organization of growth in plant cells requires the local RhoGDI-regulated activation of NADPH oxidase and ROS production. Thus, it will be intriguing to investigate whether similar negative regulatory mechanisms that restrict ROS production are observed in polarized cell growth of mammalian cells. Additionally, Rac1 localization is regulated by targeting of Rho GTPase guanine nucleotide exchange factors (GEFs) with Rac1 activity. Nox1 associates with the RacGEF βPIX (74), which activates Rac1, stimulating EGF-dependent oxidant production. Rap1a also binds to Nox2 complex, thus targeting them to membrane protrusions where it locally activates the Rac GEFs Vav2 and Tiam1, and Rac1 itself, which in turn contributes to localized ROS production at the lamellipodial leading edge (6).
Many PTPs, which are subject to oxidative inactivation by ROS, concentrate in specific subcellular compartments, thereby establishing a positive-feedback mechanism that activates redox signaling pathways. PTP-PEST is a cytosolic PTP with a PEST domain, localized to focal complexes through direct binding to paxillin and Hic-5. PTP-PEST inhibits Rac1 activity and phosphorylation of Pyk2 and Src, thereby preventing membrane ruffling, focal complex turnover, and polarized cell movement (81). Myristoylated TRAF4 and p47phox target to nascent focal complex–like structures, which induces local oxidative inactivation of PTP-PEST (109). Inhibition of PTP-PEST in turn activates Rac1 and its effector kinase PAK1, thereby promoting p47phox phosphorylation, creating a positive-feedback loop that facilitates NADPH oxidase activation and local ROS production (109). Furthermore, targeted inactivation of PTP-PEST by localized ROS production through formation of TRAF4-Rac1-PAK1 complexes facilitates activation of redox-sensitive focal complex signaling of Src and Pyk2 (Fig. 4). These responses, in turn, promote focal complex turnover and membrane ruffle formation, as well as cell migration. During chemotaxis, PTEN is specifically localized to the back of the cell membrane, whereas its substrate PI(3,4,5)P3 is concentrated at the front, leading edge (53). These results suggest that PTEN regulates directed cell migration by targeting the opposite site of cells from the leading edge where NADPH oxidase-derived ROS and PI(3,4,5)P3 are accumulated, thereby sensing and amplifying the PI(3,4,5)P3 gradient at the leading edge. Of note, other oxidant-sensitive MAP kinase phosphatase MKP1 and SHP-2, which are predominantly cytosolic, are not the targets of TRAF4-linked oxidants (109). Thus, TRAF4-p47phox-dependent ROS seem to be specific to PTP-PEST because of its specific localization at focal contacts/complexes (Fig. 4).
Redox Signaling in Endosomes
Localization of subunits of NADPH oxidase on internal membranes has been demonstrated. In unstimulated ECs, endogenous Nox2 and its regulatory proteins exist as a preassembled complex in perinuclear compartments (54). Other studies revealed perinuclear localization of GFP-Nox4 in primary human ECs (99), and endogenous Nox1, Nox2, and Nox4 in endothelial EaHy926 and human microvascular ECs (77). With confocal microscopy or fluorescence resonance energy transfer, colocalization of p22phox with Nox1 or Nox4 at the endoplasmic reticulum (ER) was observed in transiently transfected VSMCs or HEK293 cells or both (5, 60). These results suggest that active NADPH oxidase complex is formed in intracellular compartments. Chen et al. (14) recently reported that ER localization of V5-taggeed Nox4 is critical for oxidative inactivation of protein tyrosine phosphatase 1B (PTP1B), which is localized mainly to the cytosolic face of the ER (27, 105). The ER-resident PTP1B specifically dephosphorylates endocytosed RTKs, thereby terminating RTK signal (34). Epidermal growth factor stimulates H2O2 generation, resulting in the reversible oxidation of reactive Cys215 in PTP1B to inactivate the enzyme (52), which in turn increases protein tyrosine phosphorylation and mitogenic response. Thus, PTP1B may be oxidatively inactivated by Nox4-derived ROS at the ER, thereby enhancing RTK activation at this intracellular compartment.
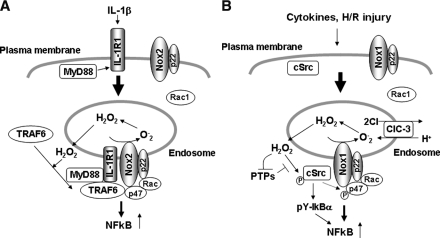
Several reports suggest the mechanism of targeting of NADPH oxidase and the consequence of NADPH oxidase–dependent ROS production in endosomes. Li et al. (56) demonstrated that interleukin-1β (IL-1β) stimulation promotes endocytosis of the IL-1β receptor (IL-1R1), which is required for Nox2-dependent ROS production at early endosomes and subsequent redox-dependent activation of the transcription factor NF-κB. By using lucigenin assay and electron spin-resonance spectroscopy that measures NADPH oxidase activities in isolated vesicular fractions, as well as fluorescence microscopy, they showed that Nox2-dependent O2- production is increased in endosomes. IL-1β binding promotes MyD88 association with IL-1R1, which triggers endocytosis of the IL-1R1/MyD88 complex and subsequent recruitment of Rac1 and Nox2 into the endosomal compartment. Although the mechanism is unknown, Rac1 recruitment into the IL-1R1–containing endosomes is required for translocation of Nox2 from the plasma membrane to this intracellular compartment. Through these processes, endosomes become a source for Nox2-mediated production of H2O2, which facilitates the redox-dependent recruitment of TRAF6 to the ligand-activated IL-1R1/MyD88 complex in endosomes. This establishes the formation of redox-active signaling platforms, thereby leading to activation of downstream IKK kinases, IKK, and, ultimately, NF-κB (Fig. 5A).
FIG. 5.
Redox signaling in endosomes. (A) Binding of IL-1 to IL-1R1 on the plasma membrane promotes MyD88 association with IL-1R1, which triggers endocytosis of the IL-1R1-MyD88 complex and subsequent recruitment of Rac1 and Nox2 into the endosomal compartment. Localized ROS signal in endosomes facilitates the redox-dependent association of TRAF6 with the receptor complex, which contributes to activation of NF-κB. (B) Hypoxia/reoxygenation (H/R) injury induces endosomal recruitment of cSrc and Rac1, thereby activating Nox1-dependent ROS production and its downstream cSrc, which phosphorylates Iκ-Bα, and thus leading to NF-κB activation. In VSMCs, Nox1 colocalizes with ClC-3 in endosomes, which is required for cytokine-induced ROS production within endosomes and its downstream NF-κB activation.
Most recently, the same group demonstrated that both Nox1 and Nox2 are involved in endosomal ROS production after hypoxia/reoxygenation (H/R) injury, and that this is required for c-Src activation and c-Src–mediated, inhibitory IκBα tyrosine phosphorylation (57). This process requires endosomal recruitment of both Rac1 and c-Src. These suggest that Rac1-dependent activation of Nox1 and Nox2 in endosomes plays a critical role in activating c-Src and its downstream NF-κB after H/R injury (Fig. 5B). Miller et al. (64) reported that Nox1 colocalizes with ClC-3, a chloride/proton exchanger, in endosomes of VSMCs, and that ClC-3 is required for TNF-α– and IL-1β–induced Nox1-dependent ROS production within early endosomes and its downstream NF-κB activation (Fig. 5B). ClC-3 seems to be required for charge neutralization of the electron flow generated by Nox1 across the membranes of signaling endosomes. Taken together, these studies suggest that NADPH oxidase–dependent ROS production in endosomes is involved in proinflammatory immune responses.
Redox Signaling in the Nucleus
Many transcription factors are redox sensitive, including AP-1, NF-κB, Nrf2, p53, glucocorticoid receptor, and others (1, 3, 9, 10, 29, 33). These transcription factors require an oxidative signal in the cytoplasm to initiate signaling for activation (phosphorylation of Jun or dissociation of NF-κB or Nrf2 from inhibitory protein complexes). After activation and translocation into the nucleus, cysteine residues within the DNA-binding domain of each transcription factor are reduced by thioredoxin1 and redox factor-1. Reduction is a prerequisite for transcription-factor binding to DNA and subsequent gene activation. Thus, oxidants in the cytoplasm activate redox signaling, whereas oxidative stress in the nuclear compartment blocks the process (35). Nox4 is localized in the focal adhesions and the nucleus in VSMCs (38, 76). Kuroda et al. (45) demonstrated that the endogenous Nox4 preferentially localizes to the nucleus in human ECs. Nox4 siRNA abrogates nuclear staining of Nox4, as well as basal- and phorbol ester–stimulated NADPH oxidase activity in the nuclear fraction. Nuclear Nox4-dependent ROS production is involved in oxidative stress–responsive gene expression. Thus, local Nox4-dependent ROS production in the nucleus may contribute to regulation of the redox-dependent transcription factor and gene expression involved in cell growth, differentiation, senescence, and apoptosis. Differential localization of the Noxes at distinct intracellular localizations may be due to different cell types or experimental conditions such as antibody specificity or a transfection system using tagged proteins.
Conclusion and Future Directions
NADPH oxidase appears to be activated within discrete subcellular compartments, including caveolae/lipid rafts, focal adhesions, cell–cell contacts, lamellipodial leading edges and membrane ruffles, endosomes, and the nucleus. This facilitates spatially confined ROS production with redox-sensitive targets in proximity, which may allow ROS to activate specific redox signaling events. Future experiments should be directed toward determining the functional importance of localized ROS production and the targeting mechanism of NADPH oxidase. Furthermore, it is important to identify and visualize the novel ROS targets in redox signaling events involved in chemotaxis, proliferation, differentiation, senescence, and apoptosis. These studies will rely on multiple experimental approaches, including highly innovative imaging, redox proteomics, cell biology and biochemistry, and molecular biology, both in vitro and in vivo. A better understanding of compartmentalization of redox signaling will provide further insights into temporally and spatially organized ROS-dependent signaling systems and the relevance of antioxidant therapy with targeting to specific intracellular microdomains for treatment of various oxidant stress–dependent diseases.
Acknowledgments
This work was supported by NIH R01 HL077524, AHA Grant-In-Aid 0555308B, and AHA NCRP Innovative Research Grant 0970336N.
Abbreviations
Ang II, angiotensin II; APC, adenomatous polyposis coli; AT1R, AT1 receptor; DEP1, density-enhanced phosphatase-1; Duox, dual oxidase; ECs, endothelial cells; EGFR, epidermal growth-factor receptor; ER, endoplasmic reticulum; FAK, focal adhesion kinase; GEFs, guanine nucleotide exchange factors; GPCRs, G protein–coupled receptors; H2O2, hydrogen peroxide; H/R, hypoxia/reoxygenation; IL-1β, interleukin-1β; IL-1R1, IL-1β receptor; NO, nitric oxide; NOS, NO synthase; O2•-, superoxide; ONOO-, peroxynitrite; PAK, p21-activated protein kinase; PI3K, phosphatidylinositol 3-kinase; PI(3,4,5)P3, phosphatidylinositol-3,4,5-trisphosphate; PMN, polymorphonuclear leukocyte; PTPs, protein tyrosine phosphatases; PTP1B; protein tyrosine phosphatase 1B; ROS, reactive oxygen species; RhoGDI, Rho GTPase guanine nucleotide dissociation inhibitor; RTK, receptor tyrosine kinase; TNFR1, TNF receptor 1; (VE)-cadherin, vascular endothelial-cadherin; VSMCs, vascular smooth muscle cells.
References
- 1.Abate C. Patel L. Rauscher FJ., 3rd Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science (New York) 1990;249:1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- 2.Abid MR. Kachra Z. Spokes KC. Aird WC. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett. 2000;486:252–256. doi: 10.1016/s0014-5793(00)02305-x. [DOI] [PubMed] [Google Scholar]
- 3.Allen RG. Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 4.Allingham MJ. van Buul JD. Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–4064. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- 5.Ambasta RK. Kumar P. Griendling KK. Schmidt HH. Busse R. Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 6.Arthur WT. Quilliam LA. Cooper JA. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J Cell Biol. 2004;167:111–122. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 8.Balsamo J. Arregui C. Leung T. Lilien J. The nonreceptor protein tyrosine phosphatase PTP1B binds to the cytoplasmic domain of N-cadherin and regulates the cadherin-actin linkage. J Cell Biol. 1998;143:523–532. doi: 10.1083/jcb.143.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloom D. Dhakshinamoorthy S. Jaiswal AK. Site-directed mutagenesis of cysteine to serine in the DNA binding region of Nrf2 decreases its capacity to upregulate antioxidant response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. Oncogene. 2002;21:2191–2200. doi: 10.1038/sj.onc.1205288. [DOI] [PubMed] [Google Scholar]
- 10.Bodwell JE. Holbrook NJ. Munck A. Sulfhydryl-modifying reagents reversibly inhibit binding of glucocorticoid-receptor complexes to DNA-cellulose. Biochemistry. 1984;23:1392–1398. doi: 10.1021/bi00302a009. [DOI] [PubMed] [Google Scholar]
- 11.Briggs MW. Sacks DB. IQGAP proteins are integral components of cytoskeletal regulation. EMBO Rep. 2003;4:571–574. doi: 10.1038/sj.embor.embor867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callera GE. Montezano AC. Yogi A. Tostes RC. Touyz RM. Vascular signaling through cholesterol-rich domains: implications in hypertension. Curr Opin Nephrol Hypertens. 2007;16:90–104. doi: 10.1097/MNH.0b013e328040bfbd. [DOI] [PubMed] [Google Scholar]
- 13.Carol RJ. Takeda S. Linstead P. Durrant MC. Kakesova H. Derbyshire P. Drea S. Zarsky V. Dolan L. A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature. 2005;438:1013–1016. doi: 10.1038/nature04198. [DOI] [PubMed] [Google Scholar]
- 14.Chen K. Kirber MT. Xiao H. Yang Y. Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen KD. Li YS. Kim M. Li S. Yuan S. Chien S. Shyy JY. Mechanotransduction in response to shear stress: roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem. 1999;274:18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- 16.Chiarugi P. From anchorage dependent proliferation to survival: lessons from redox signalling. IUBMB Life. 2008;60:301–307. doi: 10.1002/iub.45. [DOI] [PubMed] [Google Scholar]
- 17.Chiarugi P. Cirri P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem Sci. 2003;28:509–514. doi: 10.1016/S0968-0004(03)00174-9. [DOI] [PubMed] [Google Scholar]
- 18.Chiarugi P. Pani G. Giannoni E. Taddei L. Colavitti R. Raugei G. Symons M. Borrello S. Galeotti T. Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol. 2003;161:933–944. doi: 10.1083/jcb.200211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou MT. Wang J. Fujita DJ. Src kinase becomes preferentially associated with the VEGFR, KDR/Flk-1, following VEGF stimulation of vascular endothelial cells. BMC Biochem. 2002;3:32. doi: 10.1186/1471-2091-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colavitti R. Pani G. Bedogni B. Anzevino R. Borrello S. Waltenberger J. Galeotti T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J Biol Chem. 2002;277:3101–3108. doi: 10.1074/jbc.M107711200. [DOI] [PubMed] [Google Scholar]
- 21.Dejana E. Corada M. Lampugnani MG. Endothelial cell-to-cell junctions. FASEB J. 1995;9:910–918. [PubMed] [Google Scholar]
- 22.del Pozo MA. Balasubramanian N. Alderson NB. Kiosses WB. Grande-Garcia A. Anderson RG. Schwartz MA. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esser S. Wolburg K. Wolburg H. Breier G. Kurzchalia T. Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol. 1998;140:947–959. doi: 10.1083/jcb.140.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finkel T. Reactive oxygen species and signal transduction. IUBMB Life. 2001;52:3–6. doi: 10.1080/15216540252774694. [DOI] [PubMed] [Google Scholar]
- 25.Finkel T. Signal transduction by reactive oxygen species in non-phagocytic cells. J Leukoc Biol. 1999;65:337–340. doi: 10.1002/jlb.65.3.337. [DOI] [PubMed] [Google Scholar]
- 26.Foreman J. Demidchik V. Bothwell JH. Mylona P. Miedema H. Torres MA. Linstead P. Costa S. Brownlee C. Jones JD. Davies JM. Dolan L. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 27.Frangioni JV. Beahm PH. Shifrin V. Jost CA. Neel BG. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell. 1992;68:545–560. doi: 10.1016/0092-8674(92)90190-n. [DOI] [PubMed] [Google Scholar]
- 28.Frank GD. Eguchi S. Yamakawa T. Tanaka S. Inagami T. Motley ED. Involvement of reactive oxygen species in the activation of tyrosine kinase and extracellular signal-regulated kinase by angiotensin II. Endocrinology. 2000;141:3120–3126. doi: 10.1210/endo.141.9.7630. [DOI] [PubMed] [Google Scholar]
- 29.Galter D. Mihm S. Droge W. Distinct effects of glutathione disulphide on the nuclear transcription factor kappa B and the activator protein-1. Eur J Biochem. 1994;221:639–648. doi: 10.1111/j.1432-1033.1994.tb18776.x. [DOI] [PubMed] [Google Scholar]
- 30.Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res. 2006;71:289–299. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Grazia Lampugnani M. Zanetti A. Corada M. Takahashi T. Balconi G. Breviario F. Orsenigo F. Cattelino A. Kemler R. Daniel TO. Dejana E. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol. 2003;161:793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griendling KK. Sorescu D. Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 33.Hainaut P. Milner J. Redox modulation of p53 conformation and sequence-specific DNA binding in vitro. Cancer Res. 1993;53:4469–4473. [PubMed] [Google Scholar]
- 34.Haj FG. Verveer PJ. Squire A. Neel BG. Bastiaens PI. Imaging sites of receptor dephosphorylation by PTP1B on the surface of the endoplasmic reticulum. Science (New York) 2002;295:1708–1711. doi: 10.1126/science.1067566. [DOI] [PubMed] [Google Scholar]
- 35.Hansen JM. Go YM. Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol. 2006;46:215–234. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- 36.Harder T. Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- 37.Hart MJ. Callow MG. Souza B. Polakis P. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J. 1996;15:2997–3005. [PMC free article] [PubMed] [Google Scholar]
- 38.Hilenski LL. Clempus RE. Quinn MT. Lambeth JD. Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda S. Ushio-Fukai M. Zuo L. Tojo T. Dikalov S. Patrushev NA. Alexander RW. Novel role of ARF6 in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2005;96:467–475. doi: 10.1161/01.RES.0000158286.51045.16. [DOI] [PubMed] [Google Scholar]
- 40.Ikeda S. Yamaoka-Tojo M. Hilenski L. Patrushev NA. Anwar GM. Quinn MT. Ushio-Fukai M. IQGAP1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with Nox2. Arterioscler Thromb Vasc Biol. 2005;25:2295–2300. doi: 10.1161/01.ATV.0000187472.55437.af. [DOI] [PubMed] [Google Scholar]
- 41.Ishida T. Peterson TE. Kovach NL. Berk BC. MAP kinase activation by flow in endothelial cells: role of beta 1 integrins and tyrosine kinases. Circ Res. 1996;79:310–316. doi: 10.1161/01.res.79.2.310. [DOI] [PubMed] [Google Scholar]
- 42.Jalali S. Li YS. Sotoudeh M. Yuan S. Li S. Chien S. Shyy JY. Shear stress activates p60src-Ras-MAPK signaling pathways in vascular endothelial cells. Arterioscler, Thromb Vasc Biol. 1998;18:227–234. doi: 10.1161/01.atv.18.2.227. [DOI] [PubMed] [Google Scholar]
- 43.Kawabe J. Okumura S. Nathanson MA. Hasebe N. Ishikawa Y. Caveolin regulates microtubule polymerization in the vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;342:164–169. doi: 10.1016/j.bbrc.2006.01.125. [DOI] [PubMed] [Google Scholar]
- 44.Kraynov VS. Chamberlain C. Bokoch GM. Schwartz MA. Slabaugh S. Hahn KM. Localized Rac activation dynamics visualized in living cells. Science (New York) 2000;290:333–337. doi: 10.1126/science.290.5490.333. [DOI] [PubMed] [Google Scholar]
- 45.Kuroda J. Nakagawa K. Yamasaki T. Nakamura K. Takeya R. Kuribayashi F. Imajoh-Ohmi S. Igarashi K. Shibata Y. Sueishi K. Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells. 2005;10:1139–1151. doi: 10.1111/j.1365-2443.2005.00907.x. [DOI] [PubMed] [Google Scholar]
- 46.Kuroda S. Fukata M. Kobayashi K. Nakafuku M. Nomura N. Iwamatsu A. Kaibuchi K. Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J Biol C. 1996;271:23363–23367. doi: 10.1074/jbc.271.38.23363. [DOI] [PubMed] [Google Scholar]
- 47.Lakkakorpi PT. Nakamura I. Nagy RM. Parsons JT. Rodan GA. Duong LT. Stable association of PYK2 and p130(Cas) in osteoclasts and their co-localization in the sealing zone. J Bioll Chem. 1999;274:4900–4907. doi: 10.1074/jbc.274.8.4900. [DOI] [PubMed] [Google Scholar]
- 48.Lambeng N. Wallez Y. Rampon C. Cand F. Christe G. Gulino-Debrac D. Vilgrain I. Huber P. Vascular endothelial-cadherin tyrosine phosphorylation in angiogenic and quiescent adult tissues. Circ Res. 2005;96:384–391. doi: 10.1161/01.RES.0000156652.99586.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 50.Laukaitis CM. Webb DJ. Donais K. Horwitz AF. Differential dynamics of alpha 5 integrin, paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol. 2001;153:1427–1440. doi: 10.1083/jcb.153.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee H. Volonte D. Galbiati F. Iyengar P. Lublin DM. Bregman DB. Wilson MT. Campos-Gonzalez R. Bouzahzah B. Pestell RG. Scherer PE. Lisanti MP. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol Endocrinol. 2000;14:1750–1775. doi: 10.1210/mend.14.11.0553. [DOI] [PubMed] [Google Scholar]
- 52.Lee SR. Kwon KS. Kim SR. Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 53.Leslie NR. Yang X. Downes CP. Weijer CJ. The regulation of cell migration by PTEN. Biochem Soc Trans. 2005;33:1507–1508. doi: 10.1042/BST0331507. [DOI] [PubMed] [Google Scholar]
- 54.Li J-M. Shah AM. Intracellular of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem. 2002;277:19952–19960. doi: 10.1074/jbc.M110073200. [DOI] [PubMed] [Google Scholar]
- 55.Li PL. Gulbins E. Lipid rafts and redox signaling. Antioxid Redox Signal. 2007;9:1411–1415. doi: 10.1089/ars.2007.1736. [DOI] [PubMed] [Google Scholar]
- 56.Li Q. Harraz MM. Zhou W. Zhang LN. Ding W. Zhang Y. Eggleston T. Yeaman C. Banfi B. Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26:140–154. doi: 10.1128/MCB.26.1.140-154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Q. Zhang Y. Marden JJ. Banfi B. Engelhardt JF. Endosomal NADPH oxidase regulates c-Src activation following hypoxia/reoxygenation injury. Biochem J. 2008;411:531–541. doi: 10.1042/BJ20071534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin MT. Yen ML. Lin CY. Kuo ML. Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol Pharmacol. 2003;64:1029–1036. doi: 10.1124/mol.64.5.1029. [DOI] [PubMed] [Google Scholar]
- 59.Lohse DL. Denu JM. Santoro N. Dixon JE. Roles of aspartic acid-181 and serine-222 in intermediate formation and hydrolysis of the mammalian protein-tyrosine-phosphatase PTP1. Biochemistry. 1997;36:4568–4575. doi: 10.1021/bi963094r. [DOI] [PubMed] [Google Scholar]
- 60.Martyn KD. Frederick LM. von Loehneysen K. Dinauer MC. Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 61.Mateer SC. Wang N. Bloom GS. IQGAPs: integrators of the cytoskeleton, cell adhesion machinery, and signaling networks. Cell Motil Cytoskeleton. 2003;55:147–155. doi: 10.1002/cm.10118. [DOI] [PubMed] [Google Scholar]
- 62.Meng TC. Fukada T. Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 63.Merlot S. Firtel RA. Leading the way: directional sensing through phosphatidylinositol 3-kinase and other signaling pathways. J Cell Sci. 2003;116:3471–3478. doi: 10.1242/jcs.00703. [DOI] [PubMed] [Google Scholar]
- 64.Miller FJ Jr. Filali M. Huss GJ. Stanic B. Chamseddine A. Barna TJ. Lamb FS. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 65.Moldovan L. Moldovan NI. Sohn RH. Parikh SA. Goldschmidt-Clermont PJ. Redox changes of cultured endothelial cells and actin dynamics. Circ Res. 2000;86:549–557. doi: 10.1161/01.res.86.5.549. [DOI] [PubMed] [Google Scholar]
- 66.Mundy DI. Machleidt T. Ying YS. Anderson RG. Bloom GS. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J Cell Sci. 2002;115:4327–4339. doi: 10.1242/jcs.00117. [DOI] [PubMed] [Google Scholar]
- 67.Nakamura Y. Patrushev N. Inomata H. Mehta D. Urao N. Kim HW. Razvi M. Kini V. Mahadev K. Goldstein BJ. McKinney R. Fukai T. Ushio-Fukai M. Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells. Circ Res. 2008;102:1182–1191. doi: 10.1161/CIRCRESAHA.107.167080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nawroth R. Poell G. Ranft A. Kloep S. Samulowitz U. Fachinger G. Golding M. Shima DT. Deutsch U. Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 2002;21:4885–4895. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nobes CD. Hall A. Rho, rac and cdc42 GTPases: regulators of actin structures, cell adhesion and motility. Biochem Soc Trans. 1995;23:456–459. doi: 10.1042/bst0230456. [DOI] [PubMed] [Google Scholar]
- 70.Noritake J. Watanabe T. Sato K. Wang S. Kaibuchi K. IQGAP1: a key regulator of adhesion and migration. J Cell Sci. 2005;118:2085–2092. doi: 10.1242/jcs.02379. [DOI] [PubMed] [Google Scholar]
- 71.Nwariaku FE. Liu Z. Zhu X. Nahari D. Ingle C. Wu RF. Gu Y. Sarosi G. Terada LS. NADPH oxidase mediates vascular endothelial cadherin phosphorylation and endothelial dysfunction. Blood. 2004;104:3214–3220. doi: 10.1182/blood-2004-05-1868. [DOI] [PubMed] [Google Scholar]
- 72.Okamoto T. Schlegel A. Scherer PE. Lisanti MP. Caveolins, a family of scaffolding proteins for organizing "preassembled signaling complexes" at the plasma membrane. J Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 73.Park HS. Lee SH. Park D. Lee JS. Ryu SH. Lee WJ. Rhee SG. Bae YS. Sequential activation of phosphatidylinositol 3-kinase, beta Pix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol Cell Biol. 2004;24:4384–4394. doi: 10.1128/MCB.24.10.4384-4394.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park HS. Park D. Bae YS. Molecular interaction of NADPH oxidase 1 with betaPix and Nox Organizer 1. Biochem Biophys Res Commun. 2006;339:985–990. doi: 10.1016/j.bbrc.2005.11.108. [DOI] [PubMed] [Google Scholar]
- 75.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 76.Pedruzzi E. Guichard C. Ollivier V. Driss F. Fay M. Prunet C. Marie JC. Pouzet C. Samadi M. Elbim C. O'Dowd Y. Bens M. Vandewalle A. Gougerot-Pocidalo MA. Lizard G. Ogier-Denis E. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Petry A. Djordjevic T. Weitnauer M. Kietzmann T. Hess J. Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal. 2006;8:1473–1484. doi: 10.1089/ars.2006.8.1473. [DOI] [PubMed] [Google Scholar]
- 78.Quest AF. Leyton L. Parraga M. Caveolins, caveolae, and lipid rafts in cellular transport, signaling, and disease. Biochem Cell Biol. 2004;82:129–144. doi: 10.1139/o03-071. [DOI] [PubMed] [Google Scholar]
- 79.Radel C. Rizzo V. Integrin mechanotransduction stimulates caveolin-1 phosphorylation and recruitment of Csk to mediate actin reorganization. Am J Physiol. 2005;288:H936–H945. doi: 10.1152/ajpheart.00519.2004. [DOI] [PubMed] [Google Scholar]
- 80.Sallee JL. Wittchen ES. Burridge K. Regulation of cell adhesion by protein-tyrosine phosphatases, II: cell-cell adhesion. J Biol Chem. 2006;281:16189–16192. doi: 10.1074/jbc.R600003200. [DOI] [PubMed] [Google Scholar]
- 81.Sastry SK. Lyons PD. Schaller MD. Burridge K. PTP-PEST controls motility through regulation of Rac1. J Cell Sci. 2002;115:4305–4316. doi: 10.1242/jcs.00105. [DOI] [PubMed] [Google Scholar]
- 82.Sedding DG. Hermsen J. Seay U. Eickelberg O. Kummer W. Schwencke C. Strasser RH. Tillmanns H. Braun-Dullaeus RC. Caveolin-1 facilitates mechanosensitive protein kinase B (Akt) signaling in vitro and in vivo. Circ Res. 2005;96:635–642. doi: 10.1161/01.RES.0000160610.61306.0f. [DOI] [PubMed] [Google Scholar]
- 83.Sheth P. Seth A. Atkinson KJ. Gheyi T. Kale G. Giorgianni F. Desiderio DM. Li C. Naren A. Rao R. Acetaldehyde dissociates the PTP1B-E-cadherin-beta-catenin complex in Caco-2 cell monolayers by a phosphorylation-dependent mechanism. Biochem J. 2007;402:291–300. doi: 10.1042/BJ20060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sieg DJ. Hauck CR. Ilic D. Klingbeil CK. Schaefer E. Damsky CH. Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 85.Simons K. Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 86.Stahlhut M. van Deurs B. Identification of filamin as a novel ligand for caveolin-1: evidence for the organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol Biol Cell. 2000;11:325–337. doi: 10.1091/mbc.11.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Swaney JS. Patel HH. Yokoyama U. Head BP. Roth DM. Insel PA. Focal adhesions in (myo)fibroblasts scaffold adenylyl cyclase with phosphorylated caveolin. J Biol Chem. 2006;281:17173–17179. doi: 10.1074/jbc.M513097200. [DOI] [PubMed] [Google Scholar]
- 88.Tang FY. Nguyen N. Meydani M. Green tea catechins inhibit VEGF-induced angiogenesis in vitro through suppression of VE-cadherin phosphorylation and inactivation of Akt molecule. Int J Cancer. 2003;106:871–878. doi: 10.1002/ijc.11325. [DOI] [PubMed] [Google Scholar]
- 89.Thomsen P. Roepstorff K. Stahlhut M. van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol Biol Cell. 2002;13:238–250. doi: 10.1091/mbc.01-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Timpson P. Jones GE. Frame MC. Brunton VG. Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr Biol. 2001;11:1836–1846. doi: 10.1016/s0960-9822(01)00583-8. [DOI] [PubMed] [Google Scholar]
- 91.Touyz RM. Yao G. Quinn MT. Pagano PJ. Schiffrin EL. p47phox Associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: role in NAD(P)H oxidase regulation by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:512–518. doi: 10.1161/01.ATV.0000154141.66879.98. [DOI] [PubMed] [Google Scholar]
- 92.Tzima E. del Pozo MA. Shattil SJ. Chien S. Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ukropec JA. Hollinger MK. Salva SM. Woolkalis MJ. SHP2 association with VE-cadherin complexes in human endothelial cells is regulated by thrombin. J Biol Chem. 2000;275:5983–5986. doi: 10.1074/jbc.275.8.5983. [DOI] [PubMed] [Google Scholar]
- 94.Ushio-Fukai M. Griendling KK. Becker PL. Hilenski L. Halleran S. Alexander RW. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:489–495. doi: 10.1161/01.atv.21.4.489. [DOI] [PubMed] [Google Scholar]
- 95.Ushio-Fukai M. Hilenski L. Santanam N. Becker PL. Ma Y. Griendling KK. Alexander RW. Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J Biol Chem. 2001;276:48269–48275. doi: 10.1074/jbc.M105901200. [DOI] [PubMed] [Google Scholar]
- 96.Ushio-Fukai M. Tang Y. Fukai T. Dikalov S. Ma Y. Fujimoto M. Quinn MT. Pagano PJ. Johnson C. Alexander RW. Novel role of gp91phox-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 97.Ushio-Fukai M. Zafari AM. Fukui T. Ishizaka N. Griendling KK. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 98.van Buul JD. Anthony EC. Fernandez-Borja M. Burridge K. Hordijk PL. Proline-rich tyrosine kinase 2 (Pyk2) mediates vascular endothelial-cadherin-based cell-cell adhesion by regulating beta-catenin tyrosine phosphorylation. J Biol Chem. 2005;280:21129–21136. doi: 10.1074/jbc.M500898200. [DOI] [PubMed] [Google Scholar]
- 99.Van Buul JD. Fernandez-Borja M. Anthony EC. Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 100.van Wetering S. van Buul JD. Quik S. Mul FP. Anthony EC. ten Klooster JP. Collard JG. Hordijk PL. Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. J Cell Sci. 2002;115:1837–1846. doi: 10.1242/jcs.115.9.1837. [DOI] [PubMed] [Google Scholar]
- 101.van Wetering S. van den Berk N. van Buul JD. Mul FP. Lommerse I. Mous R. ten Klooster JP. Zwaginga JJ. Hordijk PL. VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration. Am J Physiol Cell Physiol. 2003;285:C343–C352. doi: 10.1152/ajpcell.00048.2003. [DOI] [PubMed] [Google Scholar]
- 102.Vilhardt F. van Deurs B. The phagocyte NADPH oxidase depends on cholesterol-enriched membrane microdomains for assembly. EMBO J. 2004;23:739–748. doi: 10.1038/sj.emboj.7600066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wallez Y. Cand F. Cruzalegui F. Wernstedt C. Souchelnytskyi S. Vilgrain I. Huber P. Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site. Oncogene. 2007;26:1067–1077. doi: 10.1038/sj.onc.1209855. [DOI] [PubMed] [Google Scholar]
- 104.Wientjes FB. Reeves EP. Soskic V. Furthmayr H. Segal AW. The NADPH oxidase components p47(phox) and p40(phox) bind to moesin through their PX domain. Biochem Biophys Res Commun. 2001;289:382–388. doi: 10.1006/bbrc.2001.5982. [DOI] [PubMed] [Google Scholar]
- 105.Woodford-Thomas TA. Rhodes JD. Dixon JE. Expression of a protein tyrosine phosphatase in normal and v-src-transformed mouse 3T3 fibroblasts. J Cell Biol. 1992;117:401–414. doi: 10.1083/jcb.117.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wozniak MA. Modzelewska K. Kwong L. Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 107.Wright TJ. Leach L. Shaw PE. Jones P. Dynamics of vascular endothelial-cadherin and beta-catenin localization by vascular endothelial growth factor-induced angiogenesis in human umbilical vein cells. Exp Cell Res. 2002;280:159–168. doi: 10.1006/excr.2002.5636. [DOI] [PubMed] [Google Scholar]
- 108.Wu RF. Gu Y. Xu YC. Nwariaku FE. Terada LS. Vascular endothelial growth factor causes translocation of p47phox to membrane ruffles through WAVE1. J Biol Chem. 2003;278:36830–36840. doi: 10.1074/jbc.M302251200. [DOI] [PubMed] [Google Scholar]
- 109.Wu RF. Xu YC. Ma Z. Nwariaku FE. Sarosi GA Jr. Terada LS. Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol. 2005;171:893–904. doi: 10.1083/jcb.200507004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu Y. Kwon KS. Rhee SG. Probing cellular protein targets of H2O2 with fluorescein-conjugated iodoacetamide and antibodies to fluorescein. FEBS Lett. 1998;440:111–115. doi: 10.1016/s0014-5793(98)01415-x. [DOI] [PubMed] [Google Scholar]
- 111.Yamaoka-Tojo M. Tojo T. Kim HW. Hilenski L. Patrushev NA. Zhang L. Fukai T. Ushio-Fukai M. IQGAP1 mediates VE-cadherin-based cell-cell contacts and VEGF signaling at adherence junctions linked to angiogenesis. Arterioscler, Thromb Vasc Biol. 2006;26:1991–1997. doi: 10.1161/01.ATV.0000231524.14873.e7. [DOI] [PubMed] [Google Scholar]
- 112.Yamaoka-Tojo M. Ushio-Fukai M. Hilenski L. Dikalov SI. Chen YE. Tojo T. Fukai T. Fujimoto M. Patrushev NA. Wang N. Kontos CD. Bloom GS. Alexander RW. IQGAP1, a novel vascular endothelial growth factor receptor binding protein, is involved in reactive oxygen species-dependent endothelial migration and proliferation. Circ Res. 2004;95:276–283. doi: 10.1161/01.RES.0000136522.58649.60. [DOI] [PubMed] [Google Scholar]
- 113.Yang B. Rizzo V. TNF-alpha potentiates protein-tyrosine nitration through activation of NADPH oxidase and eNOS localized in membrane rafts and caveolae of bovine aortic endothelial cells. Am J Physiol. 2007;292:H954–H962. doi: 10.1152/ajpheart.00758.2006. [DOI] [PubMed] [Google Scholar]
- 114.Zafari AM. Ushio-Fukai M. Akers M. Yin Q. Shah A. Harrison DG. Taylor WR. Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 115.Zhang AY. Yi F. Zhang G. Gulbins E. Li PL. Lipid raft clustering and redox signaling platform formation in coronary arterial endothelial cells. Hypertension. 2006;47:74–80. doi: 10.1161/10.1161/01.HYP.0000196727.53300.62. [DOI] [PubMed] [Google Scholar]
- 116.Zhang ZY. Dixon JE. Active site labeling of the Yersinia protein tyrosine phosphatase: the determination of the pKa of the active site cysteine and the function of the conserved histidine 402. Biochemistry. 1993;32:9340–9345. doi: 10.1021/bi00087a012. [DOI] [PubMed] [Google Scholar]
- 117.Zuo L. Ushio-Fukai M. Hilenski LL. Alexander RW. Microtubules regulate angiotensin II type 1 receptor and Rac1 localization in caveolae/lipid rafts: role in redox signaling. Arterioscler Thromb Vasc Biol. 2004;24:1223–1228. doi: 10.1161/01.ATV.0000132400.25045.2a. [DOI] [PubMed] [Google Scholar]
- 118.Zuo L. Ushio-Fukai M. Ikeda S. Hilenski L. Patrushev N. Alexander RW. Caveolin-1 is essential for activation of Rac1 and NAD(P)H oxidase after angiotensin II type 1 receptor stimulation in vascular smooth muscle cells: role in redox signaling and vascular hypertrophy. Arterioscler Thromb Vasc Biol. 2005;25:1824–1830. doi: 10.1161/01.ATV.0000175295.09607.18. [DOI] [PubMed] [Google Scholar]