Abstract
Generation of reactive oxygen species (ROS) by plasma membrane–localized NADPH oxidase (Nox 2) is a major mechanism of cell signaling associated with activation of the enzyme by a variety of agonists. With activation, the integral membrane flavocytochrome of Nox 2 transfers an electron from intracellular NADPH to extracellular O2, generating superoxide anion (O2•−). The latter dismutes to H2O2 which can diffuse through aquaporin channels in the plasma membrane to elicit an intracellular signaling response. O2•− also can initiate intracellular signaling by penetration of the cell membrane through anion channels (Cl- channel-3, ClC-3). Endosomes containing Nox2 and ClC-3 (called signaling endosomes) are composed of internalized plasma membrane and generate O2•− in the endosomal lumen to initiate signaling at intracellular sites. Thus, cellular signaling by Nox2 is dependent on the transmembrane flux of ROS. The role of this pathway has only recently been described and will require additional investigation to appreciate its physiological significance fully. Antioxid. Redox Signal. 11, 1349–1356.
Introduction
The generation of reactive oxygen species (ROS) in tissues was first proposed >50 years ago by Gerschman, Gilbert, and co-workers (20, 21) as a possible mechanism for the toxic effects of oxygen at increased partial pressure. Approximately 10 years later, McCord and Fridovich (42) isolated an enzyme (superoxide dismutase) from cells that degrades superoxide (O2•−) and postulated that it functions to protect against oxidant stress. Subsequently, cells were shown to produce O2•− and other ROS when exposed to redox active agents, but also as a by-product of normal metabolism. For example, O2•− is produced by “leakage” of electrons from the mitochondrial respiratory chain or from microsomal redox-active enzymes (6, 19). Early on, a “physiologic” role for ROS was discovered related to the killing of bacteria by phagocytic cells through a pathway involving superoxide anion plus hypochlorous acid generated by the myeloperoxidase reaction (3). Although it was appreciated that this reaction is beneficial to the host organism, it represents a toxic effect of ROS on the bacteria. These concepts resulted in emphasis on the injurious effects of ROS and their role in various pathologies. More recently, it has become clear that cells generate ROS at lower levels to serve a signaling function that is crucial for cellular homeostasis. These oxygen-derived species function in signal transduction by reversible oxidative modification of proteins, leading to phosphorylation cascades and altered gene transcription (18). ROS-mediated signaling can promote cell proliferation and survival, but also can regulate programmed cell death or even necrosis, thereby playing a key role in the modulation of cell turnover (23, 40, 41, 49).
ROS are produced in the intact cell by both enzymatic and nonenzymatic reactions. The latter include the autooxidation of xenobiotics such as paraquat or of endogenous metabolites such as components of the mitochondrial respiratory chain. These autooxidation reactions are not regulated, and, although the ROS that are generated may interact (or interfere) with signaling pathways, this is unlikely to constitute a physiologically important system. Conversely, the enzymatic generation of ROS can be more finely controlled and can constitute a physiologic signaling pathway. These enzyme systems have specific subcellular localization and thus give rise to the concept of compartmentalization of both ROS production and the signaling response.
Compartmentalization was noted relatively early in the evolution of our knowledge of ROS biology, because the production of superoxide by phagocytic cells was found to occur by a plasma membrane–localized enzyme (NADPH oxidase) into the well-circumscribed space of a phagolysosome. Compartmentalization is a likely explanation for many of the diverse effects of ROS. Antioxidant defenses are likewise compartmentalized, with specific enzymes for mitochondria (MnSOD), cytosol (CuZnSOD, GSH peroxidase), peroxisomes (catalase), and the extracellular space (ECSOD). By accepting the compartmentalization of ROS production, the question arises as to mechanisms for the possible transfer of ROS across membranes. In that regard, it is helpful to start with a discussion of the plasma membrane–associated NADPH oxidases.
Generation of ROS by NADPH Oxidase in Phagocytes
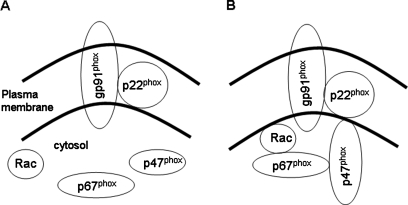
NADPH oxidase has long been recognized as an important generator of ROS in phagocytic cells such as polymorphonuclear leukocytes (PMNs) or macrophages in which it is localized to the plasma membrane (3, 4). This enzyme represents a multiprotein complex that is assembled after appropriate stimulation (16, 59). The assembled complex consists of both plasma membrane and cytosolic proteins (Fig. 1). The intrinsic membrane component is a heterodimeric flavocytochrome with gp91phox and p22phox subunits. These designations were chosen to indicate a protein (p) or glycoprotein (gp) of the phagocyte oxidase (phox), whereas the numeric designation indicates the nominal molecular mass on gel electrophoresis. The heterodimeric flavocytochrome is also called cytochrome b558, based on its spectroscopic properties. It is the catalytic subunit responsible for transferring an electron from NADPH to molecular oxygen, thereby forming O2•−. Activity of cytochrome b558 requires its interaction with cytosolic regulatory subunits. The primary regulatory proteins are p47phox and p67phox, which are phosphorylated during the activation process and translocate to the plasma membrane, where they associate with the flavocytochrome. The p47phox is important for organization of the complex, and p67phox activates the electron-transfer process. A small G protein, rac2 in PMNs, is crucial for initiation of the activation process resulting in phosphorylation and translocation of cytosolic components to the plasma membrane. Several additional proteins (p40phox, p29) also appear to play a role in activation of the complex, although their function is incompletely understood (16, 33, 59). It has been suggested that binding of p67phox to endosomal membranes through its interaction with p40phox is required for its delivery to the plasma membrane (43). The NADPH oxidase complex can be activated after exposure to stimuli such as opsonized bacteria, various cytokines, or bacterial lipopolysaccharides.
FIG. 1.
Activation of NADPH oxidase. (A) The unassembled NADPH oxidase complex consists of cytoplasmic and intrinsic membrane components. (B) After an activating stimulus, the enzyme is assembled by translocation of the cytoplasmic components to the cell membrane.
The gp91phox is a membrane-spanning protein oriented so that electron transfer occurs from cytoplasmic NADPH to an acceptor oxygen on the extracellular side of the plasma membrane (Fig. 2). In the phagocyte, this results in the generation of O2•− into the phagolysosome. Activation of the complex and O2•− production by PMNs and other phagocytic cells is a major bactericidal mechanism, and absence of one or more proteins of the enzyme complex is manifested as chronic granulomatous disease, a syndrome characterized by increased susceptibility to chronic infections. In addition to bactericidal activity, the respiratory burst in phagocytes can result in activation of protein tyrosine kinases or inactivation of protein tyrosine phosphatases (14, 54). These observations provided support for the concept of NADPH oxidase involvement in ROS-mediated signaling.
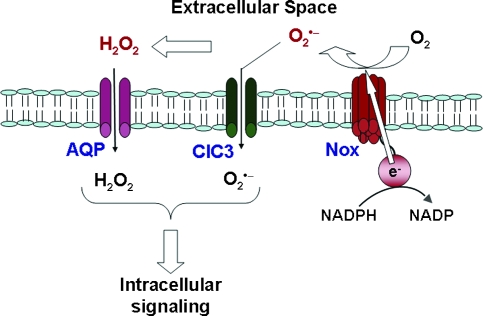
FIG. 2.
Generation of reactive oxygen species by activated NADPH oxidase (Nox). NOX2 transfers an electron from intracellular NADPH across the cell membrane to molecular oxygen to generate O2•− in the extracellular space. Extracellular O2•− can dismute to H2O2, which traverses the cell membrane through aquaporin channels (AQP). Extracellular O2•− also can penetrate cell membranes through a chloride channel-3 (ClC3). H2O2 and O2•− may interact with specific ligands to initiate intracellular signaling. Modified with permission from (14). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
The NADPH Oxidase Family
It is now recognized that the phagocytic NADPH oxidase is the prototype for a family of enzymes that are widely distributed and that differ primarily in the flavocytochrome component (16, 31, 59). These enzyme complexes have been designated the Nox proteins (for NADPH oxidase), and the classic phagocyte oxidase is now called Nox2. Five Nox proteins have been described, along with two additional enzymes called Duox (for dual oxidase). All of the Nox proteins appear to have the membrane-associated p22phox component (except perhaps Nox5) and are activated by proteins analogous to those described for Nox2. Some of these proteins may be expressed on internal (organellar) membranes and may show constitutive activity, but those details are still under investigation. Membrane oxidases identical to the phagocytic NADPH oxidase complex (Nox2) have been demonstrated in endothelial cells, fibroblasts, mesangial cells, smooth muscle cells, neurons, and probably are expressed in nearly all cell types (31, 59). The rate of superoxide generation by Nox2 in nonphagocytic cells is lower than the levels required for microbicidal function, but is sufficient for cell signaling.
ROS-Mediated Signaling in Endothelium
The Nox pathways have been well studied in endothelial cells (1, 2, 22, 28, 58). These cells express Nox2 and also Nox4 and perhaps Nox1; as in the phagocytic cells, endothelial Nox2 is localized to the plasma membrane, although it may also be expressed on endocytic vessels derived from plasma membrane. The location of Nox4 is less certain and may be expressed primarily by the nuclear and other organellar membranes. Endothelial Noxs have been shown to participate in cell signaling and the generation of O2•− results in the activation of tyrosine kinases and protein phosphorylation. Analogous to its localization in PMN, activated Nox2 generates superoxide anion on the external side of the plasma membrane, as indicated by the cytochrome c reduction assay (8, 39). (Cytochrome c added to the medium would be expected to remain in the extracellular space, whereas most superoxide generated intracellularly would dismute before it could cross the plasma membrane; therefore, the reduction of cytochrome c added to the medium, and its inhibition by extracellular SOD, corroborates an extracellular site of O2•− production). O2•− production by activated Nox2 in bovine pulmonary artery cells was ∼50% of the corresponding value for the respiratory burst in PMN, although superoxide production by rat pulmonary microvascular endothelium was only about one tenth as great (8, 39). Nox2 in endothelial cells can be activated by a variety of agents including hormones and agonists such as angiotensin II and thrombin, and inflammatory mediators such as TNF-α and IL-1 (22).
Endothelial cells in situ are exposed to a variety of mechanical forces, including intraluminal pressure and tangential shear stress. In vitro studies have demonstrated that endothelial cells undergo changes with shear stress, leading to what has been described as a flow-adapted state (10). It is assumed that endothelial cells in situ have been flow adapted by the physiologic exposure to shear. Endothelial cells in situ respond to abruptly decreased shear with ROS production (1, 15, 63), and endothelial cells in vitro respond similarly (8, 47). Increased shear stress also activates signaling through ROS generation associated with the activation of NOX2 (10, 26). Thus, the concept has evolved that endothelial cells respond to an alteration of shear either in a positive or negative direction from the “set” point.
Our laboratory has described the signaling pathways associated with acute loss of shear, an event that models “ischemia” due to vascular obstruction. The loss of shear is sensed by caveoli, either indirectly through cytoskeletal elements or directly through shear sensitivity of these organelles, resulting in a cascade manifested initially by closure (decreased open probability) of cell membrane–associated KATP channels (8, 46). Channel “closure” results in partial depolarization of the endothelial cell plasma membrane, estimated in our studies with pulmonary microvascular endothelium as a change of approximately +20 mv (assuming a resting endothelial cell membrane potential of −60 mv) (8, 63). This signal is transduced by membrane-associated phosphatidylinositol-3 kinase (PI3K) and protein kinase B (Akt) to activate the small G protein (rac1 in endothelium) that orchestrates the assembly of the NADPH oxidase complex and the generation of O2•− (63). Downstream signaling events include the activation of MAP kinases (Erk, Jnk) and transcription factors (NF-κB, AP1), eventuating in endothelial cell proliferation (46, 60). Our preliminary (unpublished) studies indicate increased angiogenic potential of flow-adapted endothelial cells subjected to acute loss of shear, which presumably could result in the formation of new vessels as compensation for the loss of blood flow. The physiologic “purpose” for the signaling response to increased shear has not been determined.
Mediators of ROS-Dependent Transmembrane Signaling
In both the PMN and endothelial cell models, extracellularly generated O2•− results in an intracellular signaling response. How is this response initiated, because the plasma membrane represents an obvious barrier to the free diffusion of O2•−? Superoxide in solution dismutes to H2O2 with a rate constant of ∼105 M−1s−1 at pH 7; extracellular superoxide dismutase (if present) can increase the rate of O2•− dismutation by ∼4 orders of magnitude. Thus, the lifetime and potential diffusion distance for O2•− will be very short, estimated at 0.5 μm (44), and it seems likely that a reaction product of superoxide (i.e., H2O2) rather than the anion itself is responsible for initiating the signaling response (Fig. 2). H2O2 is relatively stable in solution and readily crosses cell membranes, where it could initiate the signaling cascade through a variety of redox-mediated reactions such as the oxidation of protein sulfhydryl moieties (18). Although the plasma membrane has been assumed to be freely permeable to H2O2, current evidence suggests that, similar to H2O, the pathway for H2O2 diffusion is through aquaporin channels (5). The selectivity of these channels for H2O2 is not known but could possibly influence the signaling response. The intracellular concentration of H2O2 is regulated through enzymatic scavenging by peroxiredoxins, glutathione peroxidases, and catalase.
Support for H2O2 as the mediator of signal transduction has been gained from experiments in which scavengers of ROS were added to the extracellular milieu. Thus, the signaling cascade is relatively unaffected by the presence of superoxide dismutase, which converts O2•− to H2O2, but is abrogated by the presence of catalase or other H2O2 scavengers (46). Additional possibilities for the signal transducer include other products formed from O2•−, such as •OH from its interaction with H2O2, peroxynitrite (ONOO-) from its interaction with •NO, or oxidized membrane components including lipid hydroperoxides or protein carbonyls. However, all of these more likely represent toxic by-products of superoxide generation rather than physiologic signaling molecules (27).
The hypothesis that H2O2 represents the signal transducer for extracellularly generated O2•− is compatible with results after the addition of exogenous H2O2 to cells (56). The pitfalls (and strengths) of this approach have been reviewed, and the properties of H2O2 that coincide with the theoretic requirements for a second-messenger function have been described (17, 27): (a) H2O2 is produced enzymatically; (b) its concentration is effectively regulated through rapid removal by various enzymes with specificity for H2O; (c) a steep gradient of H2O2 emanates from its site of production; and (d) H2O2 is relatively specific in its biochemical sites of action. A major caveat is that past studies frequently have relied on supraphysiologic concentrations of exogenous H2O2 for studies of cellular activation, but more recent studies using concentrations of H2O2 within the physiologic range have confirmed many of the previous observations (17, 27, 56).
Although H2O2 is now generally accepted as a signaling molecule and specifically as the second messenger related to NADPH oxidase activity, several studies have suggested that O2•− also may operate in this context. Further, O2•− and H2O2 may have different and discrete signaling roles. For example, O2•− added to intact HEPG2 cells results in cytochrome c release from mitochondria, an index of programmed cell death or apoptosis, an effect that was not seen with equivalent concentrations of H2O2 (37). In these experiments, pretreatment with a SOD-mimetic abolished the effect of O2•−, indicating that H2O2 produced by dismutation was not responsible for the observed results. As another example, T-cell receptor–stimulated activation of the FAS ligand promoter was dependent on O2•− but independent of H2O2; the activation was blocked by an SOD-mimetic but was unaffected by enzymes that scavenge H2O2 (13).
Finally, extracellular O2•− (generated by activated alveolar macrophages) has been shown to evoke a large intracellular Ca2+ transient in endothelial cells associated with activation of inositol 3-phosphate (IP3) receptors (38). This latter response was inhibited by SOD and was not reproduced by the addition of H2O2. The differential signaling function of O2•− and H2O2 in higher organisms recaps similar observations in bacteria in which the SoxR transcription factor appears to be an O2•−-specific sensor, whereas the Oxy-R transcription factor is sensitive to H2O2 (9). This has been explained as a result of differential chemistry in which H2O2 modification of proteins is primarily through oxidation of sulfhydryl groups, whereas O2•− specifically reacts with iron-sulfur clusters related to high electrostatic attraction (9).
How can extracellular O2•− result in intracellular signaling events? A possible mechanism was indicated by studies of O2•− flux across cell membranes. It was shown many years ago by using extracellular cytochrome c as a trap that O2•− generated inside erythrocyte ghosts could traverse the cell membrane through anion channels (36). More recent studies evaluated transmembrane flux of extracellular O2•− generated by xanthine/xanthine oxidase or by the addition of KO2 (potassium superoxide) to endothelial cells (24). Cells that were loaded with the superoxide-sensitive dye hydroethidine showed a burst of fluorescence after the addition of O2•−, suggesting transmembrane flux of this radical. The burst of fluorescence was followed by a transient increase of cytosolic Ca2+ (24) that, based on previous observations, results from activation of IP3 receptors (38). Both oxidation of hydroethidine and release of intracellular Ca2+ induced by extracellular O2•− were blocked by the presence of an anion channel blocker (DIDS) or the selective silencing of the chloride channel-3 (ClC-3) by treatment with siRNA (24, 38). The increase in intracellular Ca2+ induced by extracellular superoxide in endothelial cells resulted in the activation of mitochondrial superoxide anion production, thereby amplifying the initial signal, as indicated by increasing fluorescence of the hydroethidine indicator (24). The mechanism for Ca2+-induced mitochondrial ROS production appears to be alteration (depolarization) of the mitochondrial membrane. This amplified signal (i.e., ROS release by mitochondria) induced cellular apoptosis, which was inhibited when cell penetration by extracellular O2•− was blocked (24). Thus, these results indicate that extracellular superoxide can penetrate the cell membrane through a chloride channel and thereby activate an intracellular signaling cascade (Fig. 2). Because of its short lifetime and limited diffusivity, the effects of extracellular O2•− must be confined to the area immediately surrounding the channel, although the manifestations of the secondary signaling events can be more widespread. Previous publications indicating inhibition of physiologic effects after receptor activation by Cl− channel blockers raise the possibility that an O2•−-mediated increase of intracellular Ca2+ regulates granule exocytosis by PMNs (29) and possibly other secretory cells (7, 51, 52).
An alternative but unexplored explanation for the observed role of O2•− in signaling is that the anion channel itself serves a second-messenger function, and its oxidation specifically by superoxide (but not H2O2) leads to intracellular signaling, but this would not explain the initial oxidation of hydroethidine on addition of exogenous O2•−. Further complicating the issue is the demonstration that ClC-3 is present in the PMN secretory granules and, through Cl- flux, may play a role in activation of Nox2 (48). Clearly the relation between Nox2 activation, O2•− generation, and ClC-3 will require further study.
Although H2O2 permeation through aquaporin channels and O2•− penetration through anion channels are potential agents for transmitting NOX-generated chemical signals across the cellular plasma membrane, another possibility for signal transduction associated with activation of NADPH oxidase is related to proton (H+) movements. Transfer of electrons from NADPH across the cell membrane to extracellular O2 to generate O2•− results in a relative excess of H+ in the cell cytosol, and extrusion of these protons is necessary to maintain the balance of electrical charge in the cell (12). Recent studies have demonstrated the presence of cellular proton channels that show outward rectification, thereby permitting proton efflux (11). This field of investigation is still controversial, and an alternate explanation is that charge neutrality is maintained through the NADPH oxidase itself (25). The recent identification of a “proton channel” gene indicates that these channels do exist separate from the NADPH oxidase (53, 55). Thus, a so-far-undiscovered effect of the proton efflux pathway could be responsible for cell signaling by NADPH oxidase activity unrelated to a role for either H2O2 or O2•−. However, proton efflux could not explain the specificity of individual ROS or the specific effects of ROS added to the extracellular milieu (in the absence of NOX activity).
In summary, the published data support the conclusion that transmembrane diffusion of H2O2 and probably O2•− is the mechanism for NOX-mediated signaling.
ROS-Mediated Signaling in Endosomes
Recently, a subset of endosomes was identified that contain the Nox1 or Nox2 membrane-spanning proteins and is called “signaling endosomes” (34, 45). These organelles represent a novel pathway for NADPH oxidase–mediated intracellular signaling. The significance of the signaling endosome is that generation of ROS as second messengers is not limited to the cytoplasmic area near the plasma membrane, but rather can occur at intracellular sites, depending on endosomal localization (34, 35). Endosomes are formed by internalization of a patch of plasma membrane. Within endosomes, the cytoplasmic face of the membrane corresponds to the cytoplasmic face of the plasma membrane, whereas the internal (luminal) face and the endosomal contents correspond to the extracellular face of the plasma membrane and the extracellular space, respectively. This configuration reflects that for phagolysosomes of PMNs and other phagocytic cells. Signaling endosomes have been shown to generate O2•− within the luminal space, transferring the electron from cytoplasmic NADPH to “extracellular” O2 (34, 45). Thus, the spatial relation between the membrane protein and electron transfer to O2 are maintained in the endosome as for the cell membrane (Fig. 3A). As in prior studies with the plasma membrane (5, 24), the endosomal membrane is permeable to H2O2 and also to permeation by O2•−, possibly through Cl− channels (50). In a recent study, the efflux of ROS from endosomes of MCF-7 cells stimulated with interleukin-1β showed that 60% was O2•−, and this efflux was abolished by Cl− channel inhibitors (50). Further, these endosomes recruited SOD to their cytoplasmic surface, which would result in the rapid and local generation of H2O2 from O2•− traversing membrane channels. SOD-deficient cells showed defective signaling in response to interleukin 1, suggesting that rapid dismutation of O2•− to H2O2 is necessary for this signaling pathway. A possible role for O2•− itself in endosome-mediated signaling has not yet been described.
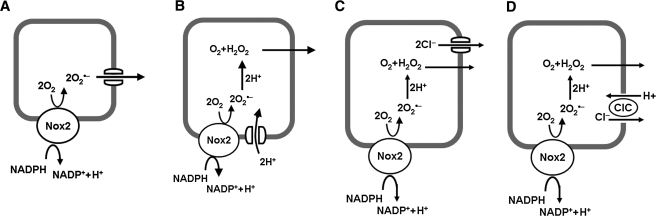
FIG. 3.
Superoxide generation in a signaling endosome. Activated NADPH oxidase (Nox 2) transfers an electron from cytoplasmic NADPH to O2 in the lumen of the endosome to generate O2•−. The O2•− can exit the endosome through a chloride channel (ClC) (A) or dismute to H2O2 (B–D). Charge compensation theoretically can be provided by efflux of O2•− (A), influx of H+ through a proton channel (B), efflux of Cl− through a Cl− channel (C), or exchange of luminal Cl− for cytoplasmic H+ through an H+/Cl− antiporter (D). Modified with permission from (32).
As described earlier for the cell membrane, transfer of electrons from cytosol to the endosomal lumen to generate O2•− requires charge compensation. This can be provided by diffusion of O2•− through endosomal membrane channels (50), which transfers the electrons back to the cytosol (Fig. 3A); as mentioned earlier, this pathway may account for much but not all of the electron flux (50). For the remaining O2•−, dismutation to H2O2 occurs within the endosome, and transmembrane movement of H+ from the cytosol to lumen or of anion from lumen to cytosol is necessary to equilibrate the charges. One possibility is that proton channels on the endosomal membrane allow proton flux, as proposed for the plasma membrane (Fig. 3B). Another possibility is that Cl− efflux from the endosomal lumen to the cytoplasm provides charges compensation; this could occur either through a Cl− channel (Fig. 3C) or through an H+/Cl− exchanger (Fig. 3D). It has been proposed that ClC-3 functions as an H+/Cl− antiporter similar to that described for ClC-4 and ClC-5 (32, 45). Charge compensation through Cl− efflux alone would result in alkalinization of the endosome, because of utilization of H+ in O2•− dismutation. The precise effects of H+/Cl− exchange on endosomal pH would depend on the stoichiometry of the process, which has been reported as 1 H+ for 2 Cl− in bacteria but is not known for mammalian endosomes (30). Inhibition of Cl− translocation in endosomes could explain the effect of Cl− channel inhibitors on Nox activity (because a failure of charge compensation would quickly inhibit activity of the oxidase) but would leave unexplained the mechanism for superoxide permeation through the membrane.
Site-Specific Generation of Signaling Molecules
It has recently become clear that site-specific localization of Nox2-mediated ROS production can play an important role in physiologic function. Localization of Nox2 to the leading edge of migrating endothelial cells has been shown to be important for angiogenesis and wound healing (57, 61). The generation of ROS on the extracellular side of lamellipodia could provide a signaling gradient for directed cell migration. Localization of ROS production to focal complexes of the leading edge may be regulated through binding of the p47 subunit of Nox2 to the orphan adaptor TRAF4 and subsequent binding to the focal contact scaffold Hic-5 (57, 61), whereas caveolae or lipid rafts may be the intramembranous sites for NADPH localization (62). These results suggest that the discrete localization of ROS production in the membrane can provide spatial discrimination to the signaling process.
Summary
In summary, NADPH oxidases, in addition to their role in microbial killing, are now known to be involved in intracellular signaling. The best studied of this family of enzymes is Nox2 (gp91phox), which is present in phagocytes, endothelial cells, and probably most other cell types. This enzyme transfers an electron to molecular oxygen to generate superoxide on the extracellular face of the plasma membrane or into the lumen of a phagolysosome or signaling endosome. Nox localization in specialized membrane domains may provide spatial resolution to the signaling process. Signaling is accomplished by dismutation of O2•− to H2O2 extracellularly with subsequent plasma-membrane permeation through aquaporin channels. O2•− also can permeate the plasma and endosomal membranes through anion (Cl-) channels, where it can interact with nearby proteins to initiate intracellular signaling. Still unresolved is the precise mechanism for localizing the extracellularly generated oxidants and for directing the signaling molecule to its downstream target. And finally, the major question: what is the biologic advantage for extracellularly generated signaling molecules, and how did the primitive cell co-opt this pathway for its survival advantage? Future study will undoubtedly shed additional light on the roles and mechanisms for cell membrane–associated signaling pathways.
Acknowledgments
I thank Dr. Shampa Chatterjee for providing Fig. 1, Dr. Mortimer Civan for helpful discussions, Dr. Madesh Muniswamy for reviewing the manuscript, and Susan Turbitt for secretarial support. Original research from my laboratory was supported by grants from the NHLBI.
Abbreviations
Akt, protein kinase B; ClC, chloride channel; IL-1, interleukin-1; IP3, inositol 3-phosphate; NOX, NADPH oxidase; PMN, polymorphonuclear leukocyte; PI3K, phosphatidylinositol-3 kinase; ROS, reactive oxygen species; SOD, superoxide dismutase.
References
- 1.Al-Mehdi AB. Zhao G. Dodia C. Tozawa K. Costa K. Muzykantov V. Ross C. Blecha F. Dinauer M. Fisher AB. Endothelial NADPH oxidase as the source of oxidants in lungs exposed to ischemia or high K+ Circ Res. 1998;83:730–737. doi: 10.1161/01.res.83.7.730. [DOI] [PubMed] [Google Scholar]
- 2.Alom-Ruiz SP. Anilkumar N. Shah AM. Reactive oxygen species and endothelial activation. Antioxid Redox Signal. 2008;10:1089–1100. doi: 10.1089/ars.2007.2007. [DOI] [PubMed] [Google Scholar]
- 3.Babior BM. Kipnes RS. Curnutte JT. Biological defense mechanisms: the production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babior BM. Lambeth JD. Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 5.Bienert GP. Moller AL. Kristiansen KA. Schulz A. Moller IM. Schjoerring JK. Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 6.Boveris A. Cadenas E. Stoppani AO. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem J. 1976;156:435–444. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown EM. Pazoles CJ. Creutz CE. Aurbach GD. Pollard HB. Role of anions in parathyroid hormone release from dispersed bovine parathyroid cells. Proc Natl Acad Sci U S A. 1978;75:876–880. doi: 10.1073/pnas.75.2.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee S. Levitan I. Wei Z. Fisher AB. KATP channels are an important component of the shear-sensing mechanism in the pulmonary microvasculature. Microcirculation. 2006;13:633–644. doi: 10.1080/10739680600930255. [DOI] [PubMed] [Google Scholar]
- 9.D'Autreaux B. Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nature Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 10.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeCoursey TE. Voltage-gated proton channels. Cell Mol Life Sci published. 2008:on–line. doi: 10.1007/s00018-008-8056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demaurex N. Petheo GL. Electron and proton transport by NADPH oxidases. Phil Trans R Soc London Series B: Biol Sci. 2005;360:2315–2325. doi: 10.1098/rstb.2005.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devadas S. Zaritskaya L. Rhee SG. Oberley L. Williams MS. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J Exp Med. 2002;195:59–70. doi: 10.1084/jem.20010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 15.Fisher AB. Al-Mehdi AB. Manevich Y. Shear stress and endothelial cell activation. Crit Care Med. 2002;30:S192–S197. doi: 10.1097/00003246-200205001-00004. [DOI] [PubMed] [Google Scholar]
- 16.Fisher AB. Zhang G. NADPH and NADPH Oxidase. In: Laurent GJ, editor; Shapiro SD, editor. Encyclopedia of Respiratory Medicine. New York: Elsevier; 2006. pp. 77–83. [Google Scholar]
- 17.Forman HJ. Use and abuse of exogenous H2O2 in studies of signal transduction. Free Radic Biol Med. 2007;42:926–932. doi: 10.1016/j.freeradbiomed.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forman HJ. Fukuto JM. Torres M. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol. 2004;287:C246–C256. doi: 10.1152/ajpcell.00516.2003. [DOI] [PubMed] [Google Scholar]
- 19.Freeman BA. Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981;256:10986–10992. [PubMed] [Google Scholar]
- 20.Gerschman R. Gilbert DL. Nye SW. Dwyer P. Fenn WO. Oxygen poisoning and x-irradiation: a mechanism in common. Science. 1954;119:623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert DL. Gerschman R. Ruhm KB. Price WE. The production of hydrogen peroxide by high oxygen pressures. J Gen Physiol. 1958;41:989–1003. doi: 10.1085/jgp.41.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griendling KK. Sorescu D. Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 23.Haddad JJ. Hypoxia and the regulation of mitogen-activated protein kinases: gene transcription and the assessment of potential pharmacologic therapeutic interventions. Int Immunopharmacol. 2004;4:1249–1285. doi: 10.1016/j.intimp.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Hawkins BJ. Madesh M. Kirkpatrick CJ. Fisher AB. Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol Biol Cell. 2007;18:2002–2012. doi: 10.1091/mbc.E06-09-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henderson LM. Meech RW. Evidence that the product of the human X-linked CGD gene, gp91-phox, is a voltage-gated H(+) pathway. J Gen Physiol. 1999;114:771–786. doi: 10.1085/jgp.114.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh HJ. Cheng CC. Wu ST. Chiu JJ. Wung BS. Wang DL. Increase of reactive oxygen species (ROS) in endothelial cells by shear flow and involvement of ROS in shear-induced c-fos expression. J Cell Physiol. 1998;175:156–162. doi: 10.1002/(SICI)1097-4652(199805)175:2<156::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 27.Janssen-Heininger YM. Mossman BT. Heintz NH. Forman HJ. Kalyanaraman B. Finkel T. Stamler JS. Rhee SG. van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones SA. O'Donnell VB. Wood JD. Broughton JP. Hughes EJ. Jones OT. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol. 1996;271:H1626–H1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- 29.Korchak HM. Eisenstat BA. Hoffstein ST. Dunham PB. Weissmann G. Anion channel blockers inhibit lysosomal enzyme secretion from human neutrophils without affecting generation of superoxide anion. Proc Natl Acad Sci U S A. 1980;77:2721–2725. doi: 10.1073/pnas.77.5.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuang Z. Mahankali U. Beck TL. Proton pathways and H+/Cl- stoichiometry in bacterial chloride transporters. Proteins. 2007;68:26–33. doi: 10.1002/prot.21441. [DOI] [PubMed] [Google Scholar]
- 31.Lambeth JD. Kawahara T. Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lassegue B. How does the chloride/proton antiporter ClC-3 control NADPH oxidase? [Comment] Circ Res. 2007;101:648–650. doi: 10.1161/CIRCRESAHA.107.161869. [DOI] [PubMed] [Google Scholar]
- 33.Leavey PJ. Gonzalez-Aller C. Thurman G. Kleinberg M. Rinckel L. Ambruso DW. Freeman S. Kuypers FA. Ambruso DR. A 29-kDa protein associated with p67phox expresses both peroxiredoxin and phospholipase A2 activity and enhances superoxide anion production by a cell-free system of NADPH oxidase activity. J Biol Chem. 2002;277:45181–45187. doi: 10.1074/jbc.M202869200. [DOI] [PubMed] [Google Scholar]
- 34.Li Q. Harraz MM. Zhou W. Zhang LN. Ding W. Zhang Y. Eggleston T. Yeaman C. Banfi B. Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26:140–154. doi: 10.1128/MCB.26.1.140-154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Q. Zhang Y. Marden JJ. Banfi B. Engelhardt JF. Endosomal NADPH oxidase regulates c-Src activation following hypoxia/reoxygenation injury. Biochem J. 2008;411:531–541. doi: 10.1042/BJ20071534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lynch RE. Fridovich I. Effects of superoxide on the erythrocyte membrane. J Biol Chem. 1978;253:1838–1845. [PubMed] [Google Scholar]
- 37.Madesh M. Hajnoczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol. 2001;155:1003–1015. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madesh M. Hawkins BJ. Milovanova T. Bhanumathy CD. Joseph SK. Ramachandrarao SP. Sharma K. Kurosaki T. Fisher AB. Selective role for superoxide in InsP3 receptor-mediated mitochondrial dysfunction and endothelial apoptosis. J Cell Biol. 2005;170:1079–1090. doi: 10.1083/jcb.200505022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manevich Y. Al-Mehdi A. Muzykantov V. Fisher AB. Oxidative burst and NO generation as initial response to ischemia in flow-adapted endothelial cells. Am J Physiol Heart Circ Physiol. 2001;280:H2126–H2135. doi: 10.1152/ajpheart.2001.280.5.H2126. [DOI] [PubMed] [Google Scholar]
- 40.Mates JM. Segura JA. Alonso FJ. Marquez J. Intracellular redox status and oxidative stress: implications for cell proliferation, apoptosis, and carcinogenesis. Arch Toxicol. 2008;82:273–299. doi: 10.1007/s00204-008-0304-z. [DOI] [PubMed] [Google Scholar]
- 41.Matkar SS. Wrischnik LA. Hellmann-Blumberg U. Production of hydrogen peroxide and redox cycling can explain how sanguinarine and chelerythrine induce rapid apoptosis. Arch Biochem Biophys. 2008;477:43–52. doi: 10.1016/j.abb.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 42.McCord JM. Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 43.McLaughlin NJ. Banerjee A. Khan SY. Lieber JL. Kelher MR. Gamboni-Robertson F. Sheppard FR. Moore EE. Meirau GW. Elzi DJ. Silliman CC. Platelet-activating factor-mediated endosome formation causes membrane translocation of p67phox and p40phox that requires recruitment and activation of p38 MAPK, Rab5a, and phosphatidylinositol 3-kinase in human neutrophils. J Immunol. 2008;180:8192–8203. doi: 10.4049/jimmunol.180.12.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mikkelsen RB. Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22:5734–5754. doi: 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
- 45.Miller FJ Jr. Filali M. Huss GJ. Stanic B. Chamseddine A. Barna TJ. Lamb FS. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. [see Comment] Circ Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 46.Milovanova T. Chatterjee S. Hawkins BJ. Hong NK. Sorokina EM. DeBolt K. Moore JS. Madesh M. Fisher AB. Caveolae are an essential component of the pathway for endothelial cell signaling associated with abrupt reduction of shear stress. Biochim Biophys Acta. 2008;1783:1866–1875. doi: 10.1016/j.bbamcr.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milovanova T. Chatterjee S. Manevich Y. Kotelnikova I. Debolt K. Madesh M. Moore JS. Fisher AB. Lung endothelial cell proliferation with decreased shear stress is mediated by reactive oxygen species. Am J Physiol Cell Physiol. 2006;290:C66–C76. doi: 10.1152/ajpcell.00094.2005. [DOI] [PubMed] [Google Scholar]
- 48.Moreland JG. Davis AP. Bailey G. Nauseef WM. Lamb FS. Anion channels, including ClC-3, are required for normal neutrophil oxidative function, phagocytosis, and transendothelial migration. J Biol Chem. 2006;281:12277–12288. doi: 10.1074/jbc.M511030200. [DOI] [PubMed] [Google Scholar]
- 49.Morgan MJ. Kim YS. Liu ZG. TNFalpha and reactive oxygen species in necrotic cell death. Cell Res. 2008;18:343–349. doi: 10.1038/cr.2008.31. [DOI] [PubMed] [Google Scholar]
- 50.Mumbengegwi DR. Li Q. Li C. Bear CE. Engelhardt JF. Evidence for a superoxide permeability pathway in endosomal membranes. Mol Cell Biol. 2008;28:3700–3712. doi: 10.1128/MCB.02038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pollard HB. Pazoles CJ. Creutz CE. Ramu A. Strott CA. Ray P. Brown EM. Aurbach GD. Tack-Goldman KM. Shulman NR. A role for anion transport in the regulation of release from chromaffin granules and exocytosis from cells. J Supramol Struct. 1977;7:277–285. doi: 10.1002/jss.400070302. [DOI] [PubMed] [Google Scholar]
- 52.Pollard HB. Tack-Goldman K. Pazoles CJ. Creutz CE. Shulman NR. Evidence for control of serotonin secretion from human platelets by hydroxyl ion transport and osmotic lysis. Proc Natl Acad Sci U S A. 1977;74:5295–5299. doi: 10.1073/pnas.74.12.5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramsey IS. Moran MM. Chong JA. Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rinna A. Torres M. Forman HJ. Stimulation of the alveolar macrophage respiratory burst by ADP causes selective glutathionylation of protein tyrosine phosphatase 1B. Free Radic Biol Med. 2006;41:86–91. doi: 10.1016/j.freeradbiomed.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sasaki M. Takagi M. Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. [see Comment] Science. 2006;312:589–592. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- 56.Stone JR. Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- 57.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Science's Stke [electronic resource]: signal transduction knowledge environment. 2006;2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 58.Van Buul JD. Fernandez-Borja M. Anthony EC. Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 59.van der Vliet A. NADPH oxidases in lung biology and pathology: host defense enzymes, and more. Free Radic Biol Med. 2008;44:938–955. doi: 10.1016/j.freeradbiomed.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei Z. Costa K. Al-Mehdi AB. Dodia C. Muzykantov V. Fisher AB. Simulated ischemia in flow-adapted endothelial cells leads to generation of reactive oxygen species and cell signaling. Circ Res. 1999;85:682–689. doi: 10.1161/01.res.85.8.682. [DOI] [PubMed] [Google Scholar]
- 61.Wu RF. Xu YC. Ma Z. Nwariaku FE. Sarosi GA Jr. Terada LS. Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol. 2005;171:893–904. doi: 10.1083/jcb.200507004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang B. Rizzo V. TNF-alpha potentiates protein-tyrosine nitration through activation of NADPH oxidase and eNOS localized in membrane rafts and caveolae of bovine aortic endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:H954–H962. doi: 10.1152/ajpheart.00758.2006. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Q. Chatterjee S. Wei Z. Liu WD. Fisher AB. Rac and PI3 kinase mediate endothelial cell-reactive oxygen species generation during normoxic lung ischemia. Antioxid Redox Signal. 2008;10:679–689. doi: 10.1089/ars.2007.1521. [DOI] [PubMed] [Google Scholar]