Abstract
Fetal loss in patients with antiphospholipid antibodies (aPL) has been ascribed to thrombosis of placental vessels. However, we have shown that inflammation, specifically complement activation with generation of the anaphylotoxin C5a, is an essential mediator of fetal injury. We have analysed the role of tissue factor (TF) in a mouse model of aPL-induced pregnancy loss. TF is the major cellular activator of the coagulation cascade but also has cell signaling activity. Mice that received aPL-IgG showed strong TF staining throughout the decidua and on embryonic debris. This TF staining was not associated with either fibrin staining or thrombi in deciduas. The absence of fibrin deposition and thrombi suggests that TF-dependent activation of coagulation does not mediate aPL-induced pregnancy loss. We found that either blockade of TF with a monoclonal antibody in wild type mice or a genetic reduction of TF prevented aPL-induced inflammation and pregnancy loss indicated a pathogenic role for TF in aPL-induced pregnancy complications. In response to aPL-generated C5a, neutrophils express TF potentiating inflammation in the deciduas and leading to miscarriages. Importantly, we showed that TF in myeloid cells, but not fetal-derived cells (trophoblasts), was associated with fetal injury, suggesting that the site for pathologic TF expression is neutrophils. We found that TF expression in neutrophils contributes to respiratory burst and subsequent trophoblast injury and pregnancy loss induced by aPL. The identification of TF, acting as an important pro-inflammatory mediator in aPL-induced fetal injury, provides a new target for therapy to prevent pregnancy loss in the aPL syndrome.
Keywords: aPL, pregnancy, tissue factor
Introduction
Thrombosis and inflammation occur simultaneously in many clinical conditions.1 Tissue factor (TF), the major cellular initiator of the coagulation protease cascade, plays important roles in both thrombosis and inflammation.2 Coagulation reactions are initiated by the complex of TF and the serine protease factor VIIa (FVIIa). TF is an integral cell surface protein and obligatory cofactor for FVIIa activity. The TF: FVIIa complex activates its substrates, factor X and factor IX, by limited proteolysis. Activated FX (FXa) then converts prothrombin to thrombin, which cleaves fibrinogen and activates platelets leading to the formation of a hemostatic plug.
The role of TF in blood coagulation is well established. However, non-coagulant effects have also been identified.3–5 For instance, TF-dependent activation of the coagulation cascade generates various proteases that can regulate target cells by cleaving and activating a family of G-protein-coupled protease-activated receptors (PARs), resulting in increased inflammation.
Monocytes from patients with antiphospholipid antibodies (aPL) express TF and in-vitro experiments showed that monocytes and neutrophils incubated with aPL express TF.6,7 Otherstimuli,such asmitogens, bacterial cell products, components of the complement system and cytokines, are known to promote the expression of TF on the surface of endothelial cells, monocytes and neutrophils.8,9 TF expression on these cells is a hallmark of inflammatory conditions, such as sepsis, atherosclerosis, inflammatory bowel disease and systemic lupus erythematosus (SLE).10–13 TF on monocytes and synovial cells promote leukocyte adhesion and transendothelial migration, potentiating inflammation in joints.14 Other studies have shown that decreased TF activity abrogates systemic inflammatory changes in several animal models.15,16
The antiphospholipid syndrome (APS) is considered a thrombophilic disorder, and thrombosis in the placental vessels is thought to be the cause of fetal death. Importantly, complement C3 and C5 have been shown to play a role in aPL-induced thrombosis.17,18 However, animal studies from our laboratory have shown the importance of inflammation in the pathogenesis of aPL-induced pregnancy loss.19,20 Using a mouse model of APS, we showed that complement activation, through the action of anaphylotoxin C5a, promotes neutrophil infiltration into the decidua leading to fetal death.20 Recently, human studies showed that inflammatory mechanisms in the placental bed may contribute to APS pregnancy complications, reinforcing this new concept of the APS as an inflammatory disorder.21 Studies of other coagulation-related disorders suggest the presence of an amplification network in which inflammatory mediators activate the coagulation system, and in turn, coagulation factors induce inflammatory reactions.1 Although activation of complement and recruitment of inflammatory cells within decidual tissue are necessary intermediary steps of aPL-induced pregnancy loss,20 downstream pathogenic mediators of placental and fetal damage have not been defined. In this article, we present evidence that TF, acting as a proinflammatory molecule, enhances neutrophil activity causing trophoblast injury, placental dysfunction and damage to the developing placenta and embryo.
aPL and TF
APS is characterized by thrombosis and/or pregnancy morbidity in the presence of aPL. aPL is a term that encompasses a group of heterogeneous, often coexisting antibodies including lupus anticoagulant, anticardiolipin antibodies and antibodies against β2-glycoprotein I (β2GPI) alone. Most pathogenic aPL, detected either as prolongation of the activated partial thromboplastin time (lupus anticoagulant) or by their ability to bind to cardiolipin-coated wells (anticardiolipins), are directed against β2GPI.22–24
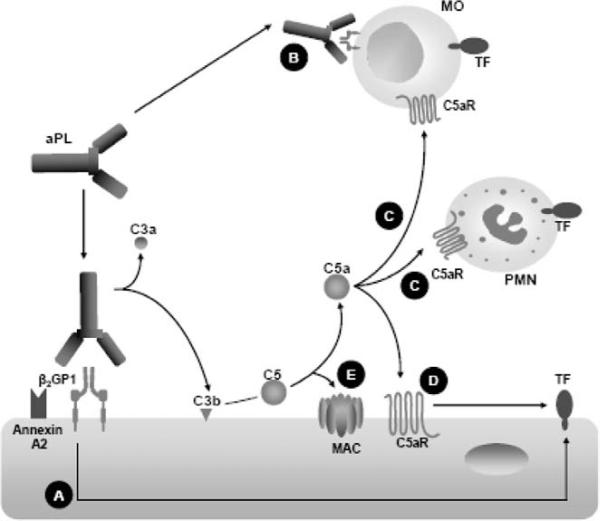
Different mechanisms have been described to explain aPL-induced TF expression and they are summarised in Figure 1. A number of in-vitro studies have shown that certain aPL, specifically those directed against β2GPI, induce the expression of TF25,26 (Figure 1A). It has been shown that the p38 mitogen-activated protein kinase is required for aPL induction of TF expression in endothelial cells.27 In addition, it has been shown that these anti-β2GPI aPL dysregulate the fibrinolytic system by cross-linking with annexin 2 (profibrinolytic endothelial cell surface receptor) on the endothelial surface inducing increased expression of TF (Figure 1A).28 Growing evidence suggests that aPL-dependent induction of TF activity on circulating blood monocytes is an important mechanism of hypercoagulability in APS (Figure 1B). de Prost, et al.29 reported that monocyte procoagulant activity was increased in patients with aPL. Several studies have shown that monocytes isolated from patients with APS exhibit increased expression of TF mRNA and antigen.30–32 Enhanced TF expression and procoagulant activity were observed in monocytes isolated from healthy individuals incubated with serum, plasma and purified total IgG from patients with APS, showing the causative role of autoantibodies of patients with APS in monocyte TF expression.26,33–35 In particular, anti-β2GPI human monoclonal antibodies derived from patients with APS enhance monocyte TF mRNA and activity in a β2GPI-dependent fashion.36
Figure 1.
Different mechanisms explaining aPL-induced TF expression. Antibodies against β2GPI induce TF expression on endothelial cells (A) and on circulating blood monocytes (B). The activation of complement can cause an increase in the expression of functionally active TF. Complement split product C5a induces TF expression in leukocytes (monocytes and neutrophils) and on endothelial cells (D). The terminal or membrane attack complex (MAC) activates endothelial cells resulting in expression of TF (E).
Complement activation has emerged as a common event in the pathogenesis of many diseases; many of them associated with endothelial activation due to the presence of complement receptors on endothelial cells.37,38 We have shown that complement activation is a critical early mediator linking aPL to fetal injury.19,20 Complement can contribute significantly to thrombosis by increasing TF expression in various cell types.1 The activation of complement, specifically of C5 as part of an inflammatory response, can cause an increase in the expression of functionally active TF in leukocytes39 (Figure 1C). In addition, the complement split product C5a can directly induce expression of TF on endothelial cells40 (Figure 1D). The induction of TF by C5a represents an important interrelationship between the inflammatory and coagulation systems. Recent work has shown that complement activation induced by aPL and downstream signaling through C5a receptors lead to the induction of TF in human neutrophils.6 The terminal or membrane attack complex (MAC) represents the final common pathway of the complement cascade. MAC is a cytolytic pore-forming complex that can destroy cells by permeabilising the plasma membrane. Although MAC may also cause tissue necrosis by lysing cells, non-lethal effects of the MAC that trigger cell activation are likely to be more important to human pathology.41,42 It has been reported that MAC activates endothelial cells resulting in expression of TF, chemokines and adhesion molecules (Figure 1E).43,44
Does TF contribute to aPL-induced fetal loss in mice?
Complement activation has emerged as a common event in pregnancy loss, but the downstream mediators and effectors of placental and fetal damage induced by aPL remains unknown. Knowing that TF expression is a characteristic feature associated with aPL, we sought to investigate whether TF contributes to aPL-induced fetal loss in mice.
To test this hypothesis we used a mouse model of aPL-induced pregnancy loss.19,20 In this model, passive transfer of human and murine aPL induces fetal loss and growth restriction, thereby showing a direct pathogenic role for aPL.19,20
Mice that received aPL-IgG showed strong TF staining throughout the decidua and on embryonic debris. In contrast, mice treated with control IgG (NH-IgG) displayed weak TF staining, which was restricted to the ectoplacental cone region and intact embryo. Surprisingly, there was no increase in fibrin staining associated with increased TF staining in deciduas from aPL-treated mice.45 In addition, no thrombi were observed in deciduas from aPL-treated mice. The absence of fibrin deposition and thrombi suggests that TF-dependent activation of coagulation does not mediate aPL-induced pregnancy loss.
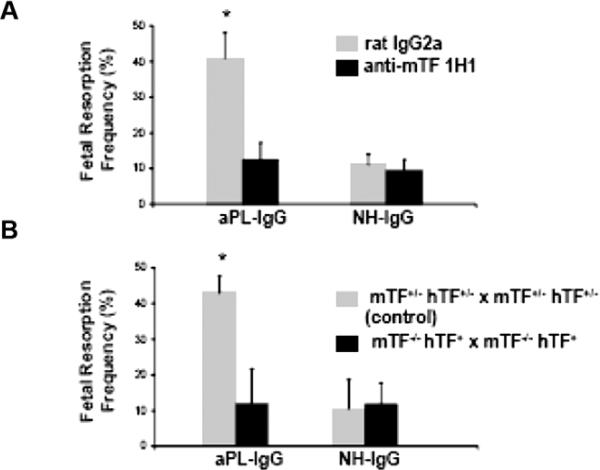
To assess the importance of TF in aPL-induced fetal injury, we inhibited TF with a monoclonal anti-mTF antibody 1H1.46 TF blockade prevented fetal death in aPL-treated mice (Figure 2A). The pregnancy outcomes in mice treated with both aPL-IgG and 1H1 mAb were comparable to that observed in mice receiving NHIgG alone. In deciduas from aPL-treated mice there was increased C3b deposition and neutrophil infiltration as we previously described.19,20 Blockade of TF with anti-TF mAb 1H1 diminished aPL-induced C3 deposition and neutrophil infiltration. The reduction in inflammation and fetal injury by anti-mTF antibody 1H1 shows that TF is a crucial effector molecule in aPL-induced pregnancy loss.
Figure 2.
Blockade of TF with a monoclonal antibody in wild type mice or a genetic reduction of TF prevented aPL-induced pregnancy loss. (A) Mice that received aPL-IgG had a high frequency of fetal resorption than those that received normal human IgG (P < 0.001). Treatment with anti-TF mAb 1H1 led to a significant reduction in the frequency of fetal resorption compared with those mice receiving aPL-IgG (P < 0.01). Rat IgG2a, used as control antibody, did not affect pregnancy outcomes. (B) Approximately 40% of the embryos in control mice (mTF+/-, hTF+) treated with aPL-IgG were resorbed. Mice expressing low activity of TF showed a reduction in aPL-induced fetal resorption frequency than control mice (P < 0.001, originally published by Redecha, et al.45).
Similar levels of aPL were detected in plasma and deciduas from aPL+1H1 and aPL+rat IgG-treated mice showing that anti-TF 1H1 antibodies do not interfere with binding of aPL. As an alternative strategy to show that TF is required for aPL-induced fetal loss, we studied pregnancy outcomes in mice expressing low levels of TF.47 Low TF mice express very low levels of human TF (hTF) from a transgene in the absence of murine TF(mTF-/-,hTF+).47 Low TF females were mated with low TF males (mTF-/-, hTF+ × mTF-/-,hTF+). We found that low TF mice treated with aPL-IgG were protected from fetal loss compared with control mice (Figure 2B). Serum t1/2 and peak levels of aPL were comparable in low TF mice and control mice. The reduction of TF expression completely rescued embryos and diminished C3 deposition and neutrophil infiltration in deciduas from aPL-treated mice to the level of mice treated with aPL-IgG and anti-TF mAb. In addition, low TF females mated with wild type males were protected from aPL-induced pregnancy loss, showing that maternal TF is crucial for pathology in this model.
Some studies suggest that aPL induce a procoagulant response in endothelial cells and monocytes through interaction with toll-like receptor 4 (TLR4).48,49 Given these studies and the capacity of TLR4 signaling to induce TF expression on monocytes and endothelial cells, we examined the role of TLR4 in aPL-induced pregnancy loss and increased decidual TF expression. TLR4-/-mice were not protected from aPL-induced pregnancy loss, and maternal-embryo units showed TF staining of deciduas and embryo destruction comparable to aPL-treated wild type mice.
We then studied whether aPL binding to other surface receptors may be sufficient to cause miscarriage. To examine the possibility that direct binding of aPL induce TF expression on trophoblasts, we used two different mouse monoclonal aPL that recognise phospholipids on trophoblast cells50 but differ in their Fc domain and thus differ in their complement activation capacity. FB1 is an IgG2b that can activate complement via the classical pathway, and FD1 is an IgG1 that can not activate complement. Pregnant mice treated with FB1 had a four-fold increase in fetal resorption frequency, which could be prevented by blocking complement activation with the C3 convertase inhibitor Crry-IgG. In contrast, FD1, which shows similar binding to deciduas, did not induce miscarriages. Immunohistochemical analysis of decidual tissue showed an increase in TF staining only in mice treated with FB1, which was markedly decreased by inhibiting complement. Minimal TF staining was present in mice treated with FD1 indicating that aPL binding to trophoblasts is not sufficient to induce TF expression, and that complement activation is required for aPL-induced TF increase in deciduas.
Knowing that TF expression is dependent on complement activation and neutrophils, we were able to show that TF expression in neutrophils is a result of C5a-C5aR interaction.
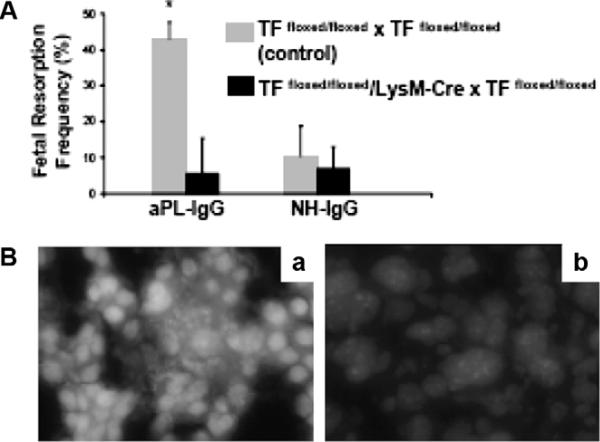
Experiments performed in TFfloxed/floxed/LysMCre mice that do not express TF on myeloid cells allowed us to distinguish the role of trophoblasts TF from that of myeloid cells TF. The protection from aPL-induced pregnancy loss observed in these mice emphasises the crucial role of TF in maternal myeloid cells.45 Moreover, knowing that monocytes are not required for aPL-induced pregnancy loss and that neutrophils from aPL-treated TFfloxed/floxed/LysM-Cre mice do not express TF allow us to conclude that TF expression on maternal neutrophils plays a causative and crucial role in aPL-induced fetal injury. TFfloxed/floxed/LysMCre mice treated with aPL showed normal pregnancies and diminished decidual inflammation compared with wild type mice, suggesting that TF expression on neutrophils modulates the ability of the neutrophil to induce tissue injury. Indeed, neutrophils from TFfloxed/floxed/LysMCre mice treated with aPL showed a lower generation of oxidants and less free radical-mediated lipid peroxidation in deciduas (Figure 3Bb) than TFfloxed/floxed control mice (Figure 3Ba) that express TF, suggesting that TF modulates oxidative burst in neutrophils.45
Figure 3.
TF expression by myeloids cells but not fetal-derived cells contributes to aPL-induced decidual oxidative stress and fetal loss. (A) Treatment with aPL-IgG caused an increase in fetal resorptions in TFfloxed/floxed mice (*P < 0.001 versus NH-IgG). TFfloxed/floxed/LysM-Cre mice were protected from fetal loss induced by aPL-IgG. Fetal resorption frequency in these mice was comparable to TFfloxed/floxed mice treated with NH-IgG. (B) Superoxide (O2−) generation in decidual tissue was determined using dihydroethydium fluorescence. aPL-induced O2− formation (a) is attenuated in TFfloxed/floxed/LysM-Cre mice (b) to a similar extent to NH-IgG-treated mice. Original magnification ×800 (originally published by Redecha, et al.45).
Anticoagulants hirudin and fondaparinux did not prevent pregnancy loss in this mouse model of APS,51 suggesting that anticoagulation is not sufficient to prevent miscarriages. These anticoagulants inhibit coagulation at the level of thrombin and FXa, respectively, but they do not inhibit the formation of the TF:FVIIa and TF:FVIIa:FXa complexes. These complexes can activate G-protein-coupled PARs and induce inflammation.3 Agonists of PARs, notably of PAR-2, induce inflammation in many diseases associated with neutrophil infiltration and alterations in epithelial permeability.1,3,52 Therefore, TF-mediated signaling through PARs may promote inflammation leading to trophoblast injury and pregnancy loss.
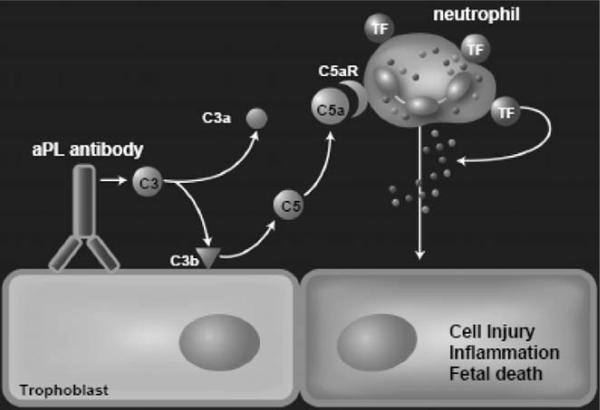
Collectively, our study shows that maternal TF on neutrophils is essential in the pathogenesis of aPL-induced fetal loss and shows a functional linkage between complement components, TF and neutrophils. We propose that after aPL-IgG binding to trophoblasts, complement activation occurs leading to generation of the anaphylatoxin C5a, which attracts and activates neutrophils through C5aR (Figure 4). Trophoblasts do not express complement receptors. C5a-C5aR interaction on neutrophils results in TF expression. Activated neutrophils release reactive oxygen species and proteolytic enzymes leading to decidual damage. TF expression on neutrophils appears to lead to the formation of complexes that amplify inflammation, cell injury and fetal death. The identification of TF, acting as an important pro-inflammatory mediator in aPL-induced fetal injury, provides a new target for therapy to prevent pregnancy loss in the APS.
Figure 4.
Mechanisms of aPL-induced TF increase and fetal death. aPL are preferentially targeted to the placenta where they activate complement leading to the generation of potent anaphylatoxin C5a. C5a attracts and activates neutrophils. As a result of C5a-C5aR interaction, neutrophils express TF. TF on neutrophils contribute to oxidative burst and subsequent trophoblast injury and ultimately fetal death (originally published by Redecha, et al.45).
References
- 1.Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131:417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 2.Chu AJ. Tissue factor upregulation drives a thrombosis-inflammation circuit in relation to cardiovascular complications. Cell Biochem Funct. 2006;24:173–192. doi: 10.1002/cbf.1200. [DOI] [PubMed] [Google Scholar]
- 3.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-Activated Receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- 4.Strukova S. Blood coagulation-dependent inflammation. Coagulation-dependent inflammation and inflammation-dependent thrombosis. Front Biosci. 2006;11:59–80. doi: 10.2741/1780. [DOI] [PubMed] [Google Scholar]
- 5.Randolph GJ, Luther T, Albrecht S, Magdolen V, Muller WA. Role of tissue factor in adhesion of mononuclear phagocytes to and trafficking through endothelium in vitro. Blood. 1998;92:4167–4177. [PubMed] [Google Scholar]
- 6.Ritis K, Doumas M, Mastellos D, et al. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177:4794–4802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 7.Roubey RA. Tissue factor pathway and the antiphospholipid syndrome. J Autoimmun. 2000;15:217–220. doi: 10.1006/jaut.2000.0397. [DOI] [PubMed] [Google Scholar]
- 8.Broze GJ., Jr. The tissue factor pathway of coagulation: factor VII, tissue factor,and tissue factor pathway inhibitor. In: Bloom AL, Forbes CD, Thomas DP, Tuddenham EDG, editors. Haemostasis and Thrombosis. 3rd ed. Churchill Livingstone; New York, NY: 1994. pp. 349–377. [Google Scholar]
- 9.Osterud B, Bjorklid E. Sources of tissue factor. Semin Thromb Hemost. 2006;32:11–23. doi: 10.1055/s-2006-933336. [DOI] [PubMed] [Google Scholar]
- 10.Taylor FB, Jr, Chang AC, Esmon CT, Hinshaw LB. Baboon model of Escherichia coli sepsis: description of its four stages and the role of tumor necrosis factor, tissue factors, and the protein C system in septic shock. Curr Stud Hematol Blood Transfus. 1991;58:8–14. doi: 10.1159/000419328. [DOI] [PubMed] [Google Scholar]
- 11.Wakita Y, Wada H, Nakase T, et al. Aberrations of the tissue factor pathway in patients positive for lupus anticoagulant. Clin Appl Thromb Hemost. 1999;5:10–15. doi: 10.1177/107602969900500103. [DOI] [PubMed] [Google Scholar]
- 12.Tremoli E, Camera M, Toschi V, Colli S. Tissue factor in atherosclerosis. Atherosclerosis. 1999;144:273–283. doi: 10.1016/s0021-9150(99)00063-5. [DOI] [PubMed] [Google Scholar]
- 13.More L, Sim R, Hudson M, Dhillon AP, Pounder R, Wakefield AJ. Immunohistochemical study of tissue factor expression in normal intestine and idiopathic inflammatory bowel disease. J Clin Pathol. 1993;46:703–708. doi: 10.1136/jcp.46.8.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bokarewa MI, Morrissey JH, Tarkowsky A. Tissue factor as a proinflammatory agent. Arthritis Res. 2002;4:190–195. doi: 10.1186/ar405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuyama M, Yoshimura R, Akioka K, et al. Tissue factor antisense oligonucleotides prevent renal ischemia-reperfusion injury. Transplantation. 2003;76:786–791. doi: 10.1097/01.TP.0000079630.68668.C2. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham MA, Kitching AR, Tipping PG, Holdsworth SR. Fibrin independent proinflammatory effects of tissue factor in experimental crescentic glomerulonephritis. Kidney Int. 2004;66:647–654. doi: 10.1111/j.1523-1755.2004.00785.x. [DOI] [PubMed] [Google Scholar]
- 17.Pierangeli SS, Girardi G, Vega-Ostertag M, Liu X, Espinola RG, Salmon J. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheum. 2005;52:2120–2124. doi: 10.1002/art.21157. [DOI] [PubMed] [Google Scholar]
- 18.Fischetti F, Durigutto P, Pellis V, et al. Thrombus formation induced by antibodies to beta2-glycoprotein I is complement dependent and requires a priming factor. Blood. 2005;106:2340–2346. doi: 10.1182/blood-2005-03-1319. [DOI] [PubMed] [Google Scholar]
- 19.Holers VM, Girardi G, Mo L, et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med. 2003;195:211–220. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girardi G, Berman J, Redecha P, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2004;112:1644–1654. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone S, Pijnenborg R, Vercruysse L, et al. The placental bed in pregnancies complicated by primary antiphospholipid syndrome. Placenta. 2006;27:457–467. doi: 10.1016/j.placenta.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Galli M, Comfurius P, Maassen C, et al. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet. 1990;335:1544–1547. doi: 10.1016/0140-6736(90)91374-j. [DOI] [PubMed] [Google Scholar]
- 23.McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Antiphospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H). Proc Natl Acad Sci U S A. 1990;87:4120–4124. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galli M, Luciani D, Bertolini G, Barbui T. Anti-beta 2-glycoprotein I, antiprothrombin antibodies, and the risk of thrombosis in the antiphospholipid syndrome. Blood. 2003;102:2717–2723. doi: 10.1182/blood-2002-11-3334. [DOI] [PubMed] [Google Scholar]
- 25.Amengual O, Atsumi T, Khamashta MA, Hughes GR. The role of the tissue factor pathway in the hypercoagulable state in patients with the antiphospholipid syndrome. Thromb Haemost. 1998;79:276–281. [PubMed] [Google Scholar]
- 26.Zhou H, Wolberg AS, Roubey RA. Characterization of Monocyte Tissue Factor Activity Induced by IgG Antiphospholipid Antibodies and Inhibition by Dilazep. Blood. 2004;104:2353–2358. doi: 10.1182/blood-2004-01-0145. [DOI] [PubMed] [Google Scholar]
- 27.Vega-Ostertag M, Casper K, Swerlick R, Ferrara D, Harris EN, Pierangeli SS. Involvement of p38 MAPK in the up-regulation of tissue factor on endothelial cells by antiphospholipid antibodies. Arthritis Rheum. 2005;52:1545–1554. doi: 10.1002/art.21009. [DOI] [PubMed] [Google Scholar]
- 28.Cesarman-Maus G, Ríos-Luna NP, Deora AB, et al. Autoantibodies against the fibrinolytic receptor, annexin 2, in antiphospholipid syndrome. Blood. 2006;107:4375–4382. doi: 10.1182/blood-2005-07-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Prost D, Ollivier V, Ternisien C, Chollet-Martin S. Increased monocyte procoagulant activity independent of the lupus anticoagulant in patients with systemic lupus erythematosus. Thromb Haemost. 1990;64:216–221. [PubMed] [Google Scholar]
- 30.Cuadrado MJ, López-Pedrera C, Khamashta MA, et al. Thrombosis in primary antiphospholipid syndrome: a pivotal role for monocyte tissue factor expression. Arthritis Rheum. 1997;40:834–841. doi: 10.1002/art.1780400509. [DOI] [PubMed] [Google Scholar]
- 31.Amengual O, Atsumi T, Khamashta MA, Hughes GR. The role of the tissue factor pathway in the hypercoagulable state in patients with the antiphospholipid syndrome. Thromb Haemost. 1998;79:276–281. [PubMed] [Google Scholar]
- 32.Dobado-Berrios PM, López-Pedrera C, Velasco F, Aguirre MA, Torres A, Cuadrado MJ. Increased levels of tissue factor mRNA in mononuclear blood cells of patients with primary antiphospholipid syndrome. Thromb Haemost. 1999;82:1578–1582. [PubMed] [Google Scholar]
- 33.Schved JF, Gris JC, Ollivier V, Wautier JL, Tobelem G, Caen J. Procoagulant activity of endotoxin or tumor necrosis factor activated monocytes is enhanced by IgG from patients with lupus anticoagulant. Am J Hematol. 1992;41:92–96. doi: 10.1002/ajh.2830410205. [DOI] [PubMed] [Google Scholar]
- 34.Kornberg A, Blank M, Kaufman S, Shoenfeld Y. Induction of tissue factor-like activity in monocytes by anti-cardiolipin antibodies. J Immunol. 1994;153:1328–1332. [PubMed] [Google Scholar]
- 35.Reverter JC, Tàssies D, Font J, et al. Effects of human monoclonal anticardiolipin antibodies on platelet function and on tissue factor expression on monocytes. Arthritis Rheum. 1998;41:1420–1427. doi: 10.1002/1529-0131(199808)41:8<1420::AID-ART11>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 36.Roubey RA, Pratt CW, Buyon JP, Winfield JB. Lupus anticoagulant activity of autoimmune antiphospholipid antibodies is dependent upon beta 2-glycoprotein I. J Clin Invest. 1992;90:1100–1104. doi: 10.1172/JCI115926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makrides SC. Therapeutic inhibition of the complement system. Pharmacol Rev. 1998;50:59–87. [PubMed] [Google Scholar]
- 38.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171:715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muhlfelder TW, Niemetz J, Kreutzer D, Beebe D, Ward PA, Rosenfeld SI. C5 chemotactic fragment induces leukocyte production of tissue factor activity: a link between complement and coagulation. J Clin Invest. 1979;63:147–150. doi: 10.1172/JCI109269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikeda K, Nagasawa K, Horiuchi T, Tsuru T, Nishizaka H, Niho Y. C5a induces tissue factor activity on endothelial cells. Thromb Haemost. 1997;77:394–398. [PubMed] [Google Scholar]
- 41.Tudesco F, Pausa M, Nardon E, Introna M, Mantovani A, Sobrina A. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J Exp Med. 1997;185:1619–1627. doi: 10.1084/jem.185.9.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morgan BP. Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J. 1989;264:1–14. doi: 10.1042/bj2640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin ML, Rus HG, Nicolescu FI. Membrane attack by complement: assembly and biology of terminal complement complexes. Biomembranes. 1996;4:123–149. doi: 10.1007/s12026-011-8239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saadi S, Holzknecht RA, Patte CP, Platt JL. Endothelial cell activation by pore-forming structures: pivotal role for interleukin-1alpha. Circulation. 2000;101:1867–1873. doi: 10.1161/01.cir.101.15.1867. [DOI] [PubMed] [Google Scholar]
- 45.Redecha P, Tilley R, Tencati M, et al. Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 2007;110:2423–2431. doi: 10.1182/blood-2007-01-070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirchhofer D, Moran P, Bullens S, Peale F, Bunting S. A monoclonal antibody that inhibits mouse tissue factor function. J Thromb Haemost. 2005;3:1098–1099. doi: 10.1111/j.1538-7836.2005.01253.x. [DOI] [PubMed] [Google Scholar]
- 47.Pawlinski R, Pedersen B, Erlich J, Mackman N. Role of tissue factor in haemostasis, thrombosis, angiogenesis and inflammation: lessons from low tissue factor mice. Thromb Haemost. 2004;92:444–450. doi: 10.1160/TH04-05-0309. [DOI] [PubMed] [Google Scholar]
- 48.Pierangeli S, Vega-Ostertag M, Raschi E, et al. Toll like Receptor 4 is involved in antiphospholipid mediated thrombosis: In vivo studies. Ann Rheum Dis. 2007;66:1327–1333. doi: 10.1136/ard.2006.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moroni P, Raschi E, Testoni C, Parisio A, Borghi MO. Innate immunity in the antiphospholipid syndrome: role of toll-like receptors in endothelial cell activation by antiphospholipid antibodies. Autoimmun Rev. 2004;3:510–515. doi: 10.1016/j.autrev.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Monestier M, Kandiah DA, Kouts S, et al. Monoclonal antibodies from NZW × BXSB F1 mice to beta2 glycoprotein I and cardiolipin. Species specificity and charge-dependent binding. J Immunol. 1996;156:2631–2641. [PubMed] [Google Scholar]
- 51.Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med. 2004;10:1222–1226. doi: 10.1038/nm1121. [DOI] [PubMed] [Google Scholar]
- 52.Chu AJ. Role of tissue factor in thrombosis. Coagulation-inflammation-thrombosis circuit. Front Biosci. 2006;11:256–271. doi: 10.2741/1796. [DOI] [PubMed] [Google Scholar]