Abstract
Clinical data and models of human disease indicate that androgen receptor (AR) activity is essential for prostate cancer development, growth and progression. The dependence of prostatic adenocarcinoma on AR signaling at all stages of disease has thereby been exploited in the treatment of disseminated tumors, for which ablation of AR function is the goal of first line therapy. Although these strategies are initially effective, recurrent tumors arise with restored AR activity, and no durable treatment has yet been identified to combat this stage of disease. New insights into AR regulation and the mechanisms underlying resurgent AR activity have provided fertile ground for the development of novel strategies to more effectively inhibit receptor activity and prolong the transition to therapeutic failure.
Keywords: Prostatic adenocarcinoma, androgen, endocrine therapy, hormone action, nuclear receptor
Background
Prostate cancer remains the second leading cause of cancer death in the United States and the most frequently diagnosed non-cutaneous malignancy. Over 186,000 patients are diagnosed each year and greater than 27,000 will succumb to death by prostate cancer(1). The goal of treatment for clinically localized disease is cure, typically by surgery or radiation therapy (2). For patients who recur systemically after definitive treatment, or who present with locoregional or metastatic disease, long-term disease control is the primary objective. Typically, this entails a series of hormonal therapies that suppress androgen receptor (AR) signaling, as prostate cancers are exquisitely dependent on AR function for survival and progression. While AR-directed therapies inhibit tumor growth, disease is rarely eliminated, and resistance to therapy is acquired through restored AR function. Once progression is documented, the course is inevitably fatal. Docetaxel-based chemotherapy can prolong life but is likewise not curative, highlighting the need for more effective treatments (3, 4).
Androgen exerts its biological effects through AR, a ligand dependent transcription factor and member of the nuclear receptor superfamily (5). In prostatic adenocarcinoma cells, the most abundant serum androgen, testosterone, is converted into a higher affinity ligand for AR, dihydrotestosterone (DHT) via the action of 5-alpha reductase (6). Upon ligand binding, the AR sheds inhibitory chaperones such as heat shock proteins, undergoes rapid homodimerization and nuclear translocation, and binds to DNA at specific sequences termed androgen responsive elements (AREs)(7). Once bound, the receptor recruits cooperative transcriptional cofactors (coactivators) that assist in inducing gene expression (8, 9). This AR-dependent gene expression program results in varied biological outcomes dependent on cell context, including the induction of genes encoding secretory products of the prostate (e.g. prostate specific antigen, PSA), cell survival proteins, and genes that promote cell cycle initiation (10). The striking requirement of prostate cancer cells for AR activity is exemplified in the clinic, wherein in therapeutic suppression of AR signaling, as typically achieved through ligand depletion and/or the use of direct AR antagonists, results in decreased PSA production, objective tumor regressions, and palliation of symptoms when present (11). The durability of the effect can range from months to years, but unfortunately, are not permanent, and after a variable period of time tumor regrowth occurs. This is heralded first by rising PSA values (“biochemical failure”), followed by increased tumor size, new metastatic spread, and disease related symptoms (12).
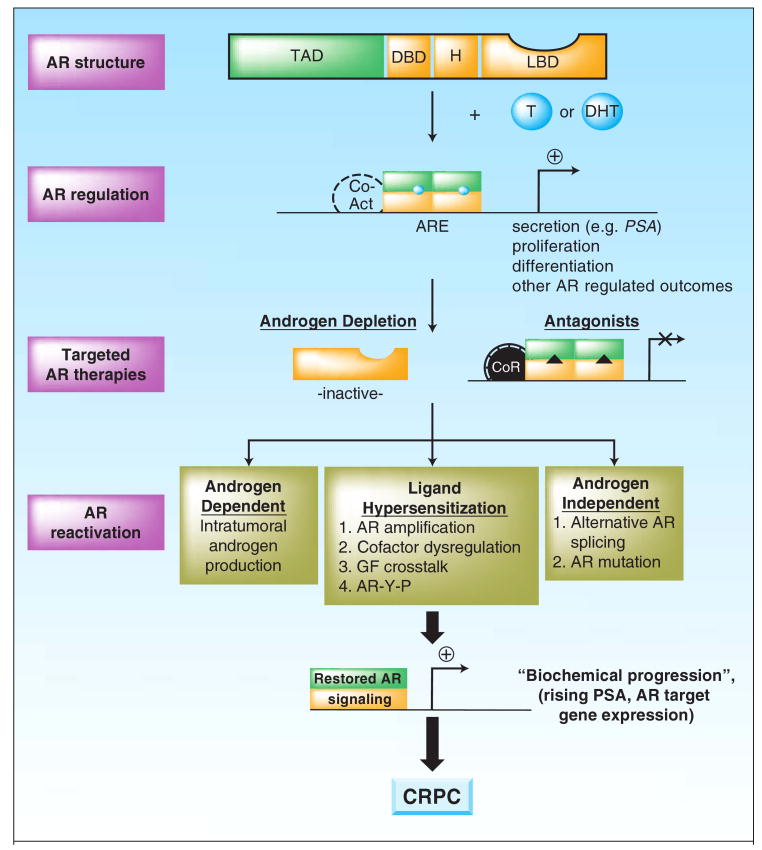
Recurrent, “castration resistant” cancers, or CRPC, represent the lethal phenotype of the illness. Considerable effort has been expended to better understand the targets and mechanisms contributing to progression, with the hope that innovative new approaches can be brought forward. Rising PSA levels, however, serve as an indication that AR activity is inappropriately restored in CRPC (13), a hypothesis that has been solidified by a litany of studies investigating mechanisms of therapeutic failure. These mechanisms have been extensively reviewed elsewhere, and include: i) AR amplification and/or overexpression; ii) gain-of-function AR mutations (largely occurring in the ligand binding domain and conferring ligand promiscuity); iii) intracrine androgen production (thus providing tumor-produced ligand to AR); iv) overexpression of AR coactivators (thus sensitizing cells to low level ligand); and v) indirect AR activation via growth factors, cytokines or aberrant AR phosphorylation (Figure 1) (14-21). Strikingly, analyses of circulating tumor cells in patients with metastatic disease revealed up to 50% with AR amplification, further supporting AR as a major effector of CRPC (22). Inflammation has also been proposed to indirectly negate the inhibitory effects of AR antagonists through molecular cascades that convert AR antagonists into agonists (23). Most recently, it was shown that AR mRNA can undergo alternative splicing events that delete the LBD, thus producing a constitutively active receptor which does not require ligand and is refractory to current AR antagonists (24, 25). These observations strongly suggest that androgen deprivation initiates a selective process for AR re-activation and resultant CRPC development. Recent clinical trials with novel AR antagonists further credentialed the AR pathway as one of therapeutic relevance. This premise applies to both chemotherapy naïve and the post-chemotherapy setting, a point when many tumors are considered to be “hormone refractory” and not amenable to further hormonal manipulations. Novel means to durably inhibit AR therefore are urgently needed, and current advances toward this goal are the focus of the present review.
Figure 1. Androgen receptor re-activation in prostate cancer progression.
Structure: The androgen receptor (AR) consists of a 3 domains that are highly conserved among the nuclear receptors (the DNA binding domain, DBD; a hinge region; and a C-terminal ligand binding domain, LBD), and unique N-terminal domain, which contains the principle transactivation domain (TAD). Regulation: Ligand (represented by circles, testosterone, T or dihydrotestosterone, DHT) binding induces homodimerization, intramolecular interaction of the N- and C-termini, and rapid nuclear translocation. Active homodimers bind DNA at AREs within the regulatory regions of target genes, recruits coactivators, and induces a gene expression program that includes activation of Prostate Specific Antigen (PSA). Current therapies: The AR C-terminal domain is targeted clinically by the use of GnRH agonists to deplete androgen systhesis (Androgen Depletion) and direct AR antagonists (represented by triangles) that actively inhibit AR activity and foster corepressor recruitment. Re-activation: AR is reactivated during disease progression by the mechanisms indicated, thus leading to restored androgen synthesis, sensitization to low level or alternate ligands, and/or androgen independent AR activation. These events result in restored AR signaling (“Biochemical Progression”) and promote recurrent, castration resistant tumor formation (CRPC).
Clinical-Translational Advances
Major breakthroughs in the development of novel androgen ablative and AR antagonist strategies have been recently described, which have the potential to improve the efficacy of AR targeting and subsequent therapeutic outcome. As will be discussed, these advances were developed based on substantive evidence that the current standard of practice fails to achieve complete androgen ablation and/or sufficient suppression of AR signaling in the prostate. Paralleling these findings, advances in understanding of AR biology revealed an unexpected need to develop new classes of AR targeting agents directed against the N-terminal domain (Figure 2). The potential utility these new strategies and the likely impact of combination therapy with AR-directed therapies will be discussed below:
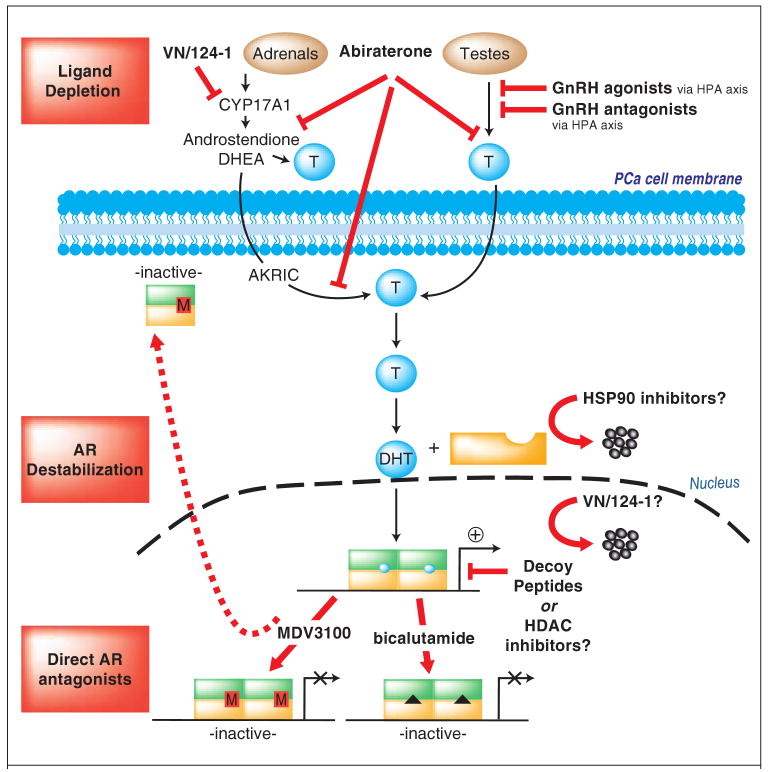
Figure 2. New opportunities for durable AR regulation.
Ligand depletion strategies: In addition to the currently used GnRH agonists, GnRH antagonists have been developed, which eliminate testosterone surges. Abiraterone provides an advantage through an ability to suppress both testicular and adrenal androgen synthesis, whereas VN/124-01 can both suppress CYP17 activity and also reduce AR levels. AR destabilization: Mechanisms to destabilize AR could be potentially achieved through HSP90 inhibitors or inhibitors of HDAC-HSP90 association. Alternatively, peptides have been used to disrupt AR N-C terminal interaction that could reduce AR association with chromatin, in addition to peptides derived from AR corepressors. Direct AR antagonists: The next generation AR antagonist MDV3100 is in clinical trial, and precludes both AR-DNA association and AR nuclear accumulation.
Improving androgen depletion
The current standard of care for patients with disseminated disease is treatment with gonadotropin releasing hormone (GnRH) agonists (e.g. leuprolide) (3, 26). After an initial “flare” of testosterone production, sustained GnRH agonists desensitize gonadotropin release and suppress testicular androgen synthesis (27), which accounts for as much as 95% of serum testosterone. These regimens are initially effective at suppressing AR activity (as judged by PSA declines) and initiating tumor regression. Upon progression, ketoconazole, which blocks adrenal androgens can been used (28). However, the recent discovery that serum androgen depletion selects for intracrine androgen production (e.g. as achieved by induction of enzymes that convert weak adrenal-derived androgens to testosterone) highlights the transient and/or incomplete efficacy of GnRH agonists to achieve “true” androgen depletion (17-19, 29). Exemplifying this, studies evaluating androgen levels in tissue showed that androgen-depletion therapies reduced intratumoral androgens by only 75% at a time when androgen levels in the sera remained at the castrate range; in these tissues, persistent expression of AR and AR target genes (e.g. PSA and TMPRSS2) validated the concept that the residual androgen is sufficient to sustain AR activity. AR gene amplification can further enhance the ability of the receptor to “adapt” to the environment of low testosterone, in part by using low affinity ligands (30). These observations underscore the emerging view that current androgen depletion strategies are incomplete, and that residual androgen contributes to sustained AR activity and disease progression.
A new means to further deplete androgens is provided by a selective CYP17 inhibitor that blocks 17a-hydroxylase and C17,20-lysase enzymes in the adrenal steroid synthetic pathway, abiraterone, which has entered clinical trial (31). Abiraterone inhibits both testicular-derived androgen production and tumor-derived androgen synthesis, and preclinical studies showed that abiraterone principally reduces weight of androgen dependent/AR-dependent organs (32). Recent clinical data showed that abiraterone can suppress testosterone in noncastrate patients (33). Whether abiraterone can formally suppress intratumoral androgen production remains to be fully tested (34), but recent phase I trials in patients with CRPC showed significant PSA declines, tumor regression, and palliation of symptoms in patients who have not received chemotherapy, as well as in the post-chemotherapy setting, wherein “hormonal” agents are typically not considered. (35). A phase III trial of abiraterone vs. placebo plus prednisone with a primary endpoint of survival is ongoing in postchemotherapy-treated patients (J. DeBono and H. Scher, personal communication). Thus, abiraterone could provide a significant advantage toward the goal of durable androgen depletion and suppression of AR activity. Alternatively, VN/124-1 is a CYP17 inhibitor that both reduces androgen production and among other effects, can also reduce AR expression levels (36). VN/124-1 has entered clinical trial, and it is hoped that the multiple functions of the drug will provide an advance toward the goal of blocking AR activity. Lastly, estrogens (including diethylstilbestrol, DES) can be used to suppress both testicular and adrenal androgens (37), but formal trials of adequate size and power to address a survival impact have not been conducted.
GnRH antagonists are also available and could be used as an alternative means for androgen suppression. Like GnRH agonists, these agents prevent testicular androgen synthesis, but fail to induce the transient testosterone flare associated with GnRH agonists (38, 39). Administration or use of first generation GnRH antagonists such as abarelix was limited by untoward side effects (27, 40), but recent modifications to the structure of the product resulted in reduced immunostimulatory activity and safer administration (41). One such second generation product, degarelix, can suppress circulating androgen levels without inducing testosterone flares or allergic reactions (42, 43). To date, however, GnRH antagonists have not been shown to provide superior antitumor effects relative to a combination of GnRH agonist in combination with an anti-androgen. Past clinical trials attempted to further suppress androgen by combining, ketoconazole, hydrocortisone, and a 5-alpha reductase inhibitor, but advantages relative to conventional treatment remains uncertain (44). Whether a combination of a GnRH antagonist and abiraterone, shown to further reduce serum androgen levels below that achieved with GnRH agonist therapy alone, and separately intratumoral androgens, will prove superior requires prospective testing.
Collectively, these observations indicate that contemporary understanding of androgen regulation in prostate cancer has allowed for the development of new means to inhibit AR through ligand depletion. Using this knowledge, ongoing studies and clinical trials could provide a firm foundation upon which to formally test the prevailing hypothesis that more complete androgen deprivation (especially in combination with AR antagonists, discussed below) can enhance response rates and cure.
AR antagonists
Direct AR antagonists are frequently utilized in combination with orchiectomy or GnRH agonists/antagonists, in an effort to further inhibit AR signaling (45). Although the precise mechanisms of action remain a subject of controversy, substantive evidence supports the contention that docking of AR antagonists such as bicalutamide into the AR C-terminal LBD results in both passive AR inhibition (via competition for agonists) and an active mechanism of AR inhibition (e.g. prevention of coactivator binding and/or induced corepressor recruitment) (46, 47). Thus, AR antagonists would be expected to act in concert with androgen deprivation to further suppress the AR activity. Clinically, the validity of this supposition remains uncertain. Analyses of long-term outcomes in patients receiving “combined androgen blockade” (androgen deprivation plus an AR antagonist) showed varying results with regard to both overall and progression-free survival (11). Indeed, the question of whether AR antagonists (e.g. bicalutamide) or mixed agonists/antagonists (e.g. flutamide) improve outcome has been one of most extensively studied questions in the field. Recognizing the methodologic differences in trials (including variances in dose, differences in scheduling, and distinctions in the timing of androgen blockade), the beneficial effects on long-term outcomes proved to be modest at best.
Uneven results obtained using current AR antagonists are attributed, at least partially, to the observation that these agents show relatively low affinity for AR as compared to DHT, and therefore are required in vast excess for molecular efficacy (48). Thus, recent studies have endeavored to improve the underlying basis by which AR antagonists suppress receptor activity, and several new agents are in clinical trial (4). The BMS-641988 compound was shown in pre-clinical studies to have activity in bicalutamide-resistant cells; however, the drug has been discontinued because clinical findings on safety and efficacy do not support benefit/risk sufficient for further development. A full publication of study results is planned. Alternative strategies emerged through development of MDV3100, a new AR antagonist identified as active in bicalutamide-resistant cancer models that overexpress AR. Preliminary studies suggest that MDV3100 has no agonist effects, and prevents both AR nuclear translocation and DNA binding (49). Given these properties, it may be expected for MDV3100 to provide a mechanistic advantage over bicalutamide, and therefore enforce sustained suppression of AR activity. Preliminary phase I/II trial data are highly suggestive (presented at the 2008 AACR annual meeting), in that MDV3100 treatment correlated with declining serum PSA, reduced circulating tumor cells, and radiographic disease stabilization. Importantly, MDV3100 was active in both pre- and post-chemotherapy treated patients, and a phase III trial is planned. Based on these examples, it is clear that basic knowledge of AR function can foster development of new approaches for targeting the AR LBD. Whether these compounds will result in meaningful clinical outcomes for CRPC should be shortly revealed, as will the impact of these agents on long-term AR and prostate cancer management.
The AR N-terminal domain: a cause for concern?
Androgen deprivation therapies and direct AR antagonists have commonality, in that both approaches rely on an intact AR ligand- binding domain, and stand on the premise that agonist binding to the LBD is universally required for AR activation. Recent analyses suggest that this presumption is likely premature. Pioneering work by Dehm, Tindall and colleagues showed first in model systems that AR can be alternatively spliced so that the C-terminal domain is deleted, rendering production of a receptor that is constitutively active (24). This observation is consistent with previous studies which showed that unlike most other nuclear receptors, the predominant transcriptional transactivation function of AR resides in the N-terminus, and that deletion of the LBD confers ligand-independent activity (5, 50). Constitutively active splice variants have recently been observed in tumor tissue, wherein it was shown that variants lacking C-terminal residues are overproduced in CRPC, and that these receptors are constitutively active (25). Together, these unexpected observations highlight yet another mechanism by which tumors bypass androgen deprivation and/or AR antagonists, as it is predicted that the splice variants would be refractory to both. Accordingly, it is evident that a new class of AR inhibitory agents must be developed for successful management of tumors expressing truncated AR, wherein even complete androgen ablation would have no effect on receptor activity.
Several options for potentially suppressing the function of C-terminal deficient ARs may already exist. It has been suggested that either HSP90 inhibitors (e.g. geldanamycin or analogs), or agents that modulate HSP90-HDAC interactions (e.g. genistein) may reduce overall AR levels (51, 52), and could potentially be used to suppress the action of both full-length and truncated AR. Knockdown strategies have also been proposed, as siRNA directed against the AR suppresses prostate cancer growth in model systems (53); however, such strategies are hindered by the uncertain feasibility of using siRNAs for cancer therapy. Alternative means to thwart AR function using expressed peptides have been documented in proof of principle studies, wherein “decoys” of the AR N-terminus were shown to suppress cell growth and survival (54), as has expression of corepressor domains that target AR N-terminal transactivation function (55). Although translating such observations to the clinical setting remains a major challenge, the observation that CRPC tumors can express truncated, androgen deprivation and AR antagonist-resistant receptors is a cause for concern, and underscores the need for intensive development of strategies to target the AR N-terminus.
Combination therapies: what does the future hold?
Since prostate cancers utilize a multitude of genetic alterations to restore AR activity and tumor growth under conditions of androgen deprivation and/or combined androgen blockade, development of successful combinatorial therapies will likely be required to eliminate disease and prevent recurrence. Radiation therapy in combination with androgen depletion can improve response in locally advanced disease (34), and provides benefit for this subset of tumors. Docetaxel can extend survival inpatients with CRPC (56); however, the benefit is modest, with an average extension of only 2-3 months. It has been hypothesized that the scheduling of docetaxel in combination with AR antagonizing strategies may need refinement (57). This posit was initially supported by the observation that cancer cells which survive AR inhibitory strategies accumulate in the G1 phase of the cell cycle (10), whereas docetaxel acts predominantly in later phases (G2/M) to induce cell death. The supposition that concurrent administration of AR antagonizing strategies with docetaxel may impede the cytotoxic effects of the chemotherapeutic was validated in models of androgen dependent cancer (58), and is further supported by clinical data which showed an improved response to docetaxel in the presence of androgen (59). These observations provide the impetus for re-examining how AR ablative therapies might be optimized in combination with anti-mitotics, and emphasize the importance of considering AR biology in the design of combinatorial therapeutic approaches. Furthermore, it should be considered under which conditions AR acts as a survival factor to counteract chemotherapeutic response, and how combination therapy could be optimized to suppress AR-associated survival activity.
Other combinations yet to be rigorously considered include abrogation of growth factor or transcriptional regulatory pathways that contribute to ligand-independent AR activity. Several growth factor and cytokine pathways, including FGF, IGF, EGF, IL-6 and heregulin were shown in model systems to facilitate AR activity in the presence of no or low androgen, and are therefore preliminarily implicated in disease progression (15, 20). Theoretically, antagonists of these pathways could in some cases act in concert with androgen ablation and/of AR antagonists to further suppress AR activity, tumor growth, and development of CRPC. A question to be addressed is whether growth factor pathways are upregulated as a survival mechanism after androgen depletion, so as to determine how potential combinations of AR-directed therapies with growth factor receptor antagonists would be most effective. For all proposed combinations, it will be imperative to also consider the tumor microenvironment, as tumor cells residing near activated stroma and/or neuroendocrine cells that supply growth factors may show a differential response to therapy (60). Lastly, it has been recently shown that AR may require HDACs for transcriptional activation (61), and that HDAC inhibitors may cooperate with AR-directed therapeutics to elicit an enhanced cellular response (62). Combination of HDAC inhibitors with GnRH analogs is the focus of an ongoing inter-prostate cancer SPORE neoadjuvant trial.
Future Directions
The contribution of AR to prostate tumorigenesis and disease progression is incontrovertible. Characterization of CRPC at the molecular level and in model systems has validated the concept that AR activity is regained as part of disease progression. Until recently, development of innovative new strategies for durable suppression of AR activity has been modest. While ligand depletion or the use of C-terminal binding receptor antagonists can induce tumor remission, these strategies do not provide a means to sustain suppression of AR activity and do not completely eliminate the tumor. Under prospective study is whether more complete inhibition of AR signaling (e.g. through combination of a GnRH agonist with an HDAC inhibitor) can completely abrogate AR activity. As the degree of androgen dependence may vary, characterization of the surviving cell population in the neoadjuvant setting may provide important new insights into the mechanisms of resistance and points of therapeutic attack.
At present, new understandings of AR function during disease progression have already led to potential breakthroughs in the development of novel AR antagonists and ligand depletion strategies. While it is hoped that these agents will be of clinical benefit, several hurdles remain. First, it should be determined how new agents function under disparate conditions of AR reactivation, and whether patient stratification based on these criteria would be of benefit. For example, if recurrence is associated with AR mutations or splice variants that induce resistance to AR antagonists, it is unlikely that use of these agents would be of benefit. A notable advance toward this end is the development of mechanisms to characterize CRPC at the molecular level using circulating tumor cells. This innovation is expected to provide needed new insight into the mechanisms governing castration resistance, and could provide a basis for personalized medicine (49). Second, new strategies to target the AR N-terminal domain are needed, given the recent observations of recurrence-associated, C-terminal deficient AR splice variants. Third, mechanisms to destabilize AR or required cofactors would be expected to produce a marked improvement in the durability of response, and should be prioritized for development. Fourth, given advances in understanding of AR-dependent cell cycle control, it should be considered how use of AR ablative strategies might be more effectively combined with existing anti-mitotics to improve outcome. Finally, it should be considered that even if achieved, “durable” AR ablation may not provide a cure, as tumor cells are likely to adapt to true AR inhibition through development of secondary dependencies or signaling pathways, and it remains possible that putative prostate cancer stem cells would be therapy-resistant. Thus, it is imperative to identify alternative targets that may act in concert with AR antagonists. Through these collective strategies, it is hoped that the goal of sustained AR management, will lead to predicted improvements in clinical care and reduce death from prostate cancer.
Acknowledgments
The authors thank their respective laboratories for ongoing discussions, and are grateful to Dr. D. Tindall, M. Schiewer, and S. Shah for critical commentary.
Literature Cited
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Klein EA, Ciezki J, Kupelian PA, Mahadevan A. Outcomes for intermediate risk prostate cancer: Are there advantages for surgery, external radiation, or brachytherapy? Urol Oncol. 2009;27:67–71. doi: 10.1016/j.urolonc.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 4.Taplin ME. Drug insight: role of the androgen receptor in the development and progression of prostate cancer. Nat Clin Pract Oncol. 2007;4:236–44. doi: 10.1038/ncponc0765. [DOI] [PubMed] [Google Scholar]
- 5.Claessens F, Denayer S, Van Tilborgh N, Kerkhofs S, Helsen C, Haelens A. Diverse roles of androgen receptor (AR) domains in AR-mediated signaling. Nucl Recept Signal. 2008;6:e008. doi: 10.1621/nrs.06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penning TM, Jin Y, Rizner TL, Bauman DR. Pre-receptor regulation of the androgen receptor. Mol Cell Endocrinol. 2008;281:1–8. doi: 10.1016/j.mce.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centenera MM, Harris JM, Tilley WD, Butler LM. The contribution of different androgen receptor domains to receptor dimerization and signaling. Mol Endocrinol. 2008;22:2373–82. doi: 10.1210/me.2008-0017. [DOI] [PubMed] [Google Scholar]
- 8.Agoulnik IU, Weigel NL. Androgen receptor coactivators and prostate cancer. Adv Exp Med Biol. 2008;617:245–55. doi: 10.1007/978-0-387-69080-3_23. [DOI] [PubMed] [Google Scholar]
- 9.Chmelar R, Buchanan G, Need EF, Tilley W, Greenberg NM. Androgen receptor coregulators and their involvement in the development and progression of prostate cancer. Int J Cancer. 2007;120:719–33. doi: 10.1002/ijc.22365. [DOI] [PubMed] [Google Scholar]
- 10.Balk S, Knudsen K. AR, the cell cycle, and prostate cancer. Nuclear Receptor Signaling (NURSA) 2007 doi: 10.1621/nrs.06001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beekman KW, Hussain M. Hormonal approaches in prostate cancer: application in the contemporary prostate cancer patient. Urol Oncol. 2008;26:415–9. doi: 10.1016/j.urolonc.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Pienta KJ, Bradley D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1665–71. doi: 10.1158/1078-0432.CCR-06-0067. [DOI] [PubMed] [Google Scholar]
- 13.Ryan CJ, Smith A, Lal P, et al. Persistent prostate-specific antigen expression after neoadjuvant androgen depletion: an early predictor of relapse or incomplete androgen suppression. Urology. 2006;68:834–9. doi: 10.1016/j.urology.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 15.Culig Z, Bartsch G. Androgen axis in prostate cancer. J Cell Biochem. 2006;99(2):373–81. doi: 10.1002/jcb.20898. [DOI] [PubMed] [Google Scholar]
- 16.Yuan X, Balk SP. Mechanisms mediating androgen receptor reactivation after castration. Urol Oncol. 2009;27:36–41. doi: 10.1016/j.urolonc.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–15. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 20.Zhu ML, Kyprianou N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr Relat Cancer. 2008;15:841–9. doi: 10.1677/ERC-08-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Z, Dai B, Jiang T, et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10:309–19. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Leversha M, Han J, Asgari A, et al. Fluorescence in situ hybridization analysis of circulating tumor cells in metastatic prostate cancer. Clin Cancer Res. 2009 doi: 10.1158/1078-0432.CCR-08-2036. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu P, Baek SH, Bourk EM, et al. Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell. 2006;124:615–29. doi: 10.1016/j.cell.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 24.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–77. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu R, Dunn TA, Wei S, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loblaw DA, Mendelson DS, Talcott JA, et al. American Society of Clinical Oncology recommendations for the initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer. J Clin Oncol. 2004;22:2927–41. doi: 10.1200/JCO.2004.04.579. [DOI] [PubMed] [Google Scholar]
- 27.van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology. 2008;71:1001–6. doi: 10.1016/j.urology.2007.12.070. [DOI] [PubMed] [Google Scholar]
- 28.Small EJ, Ryan CJ. The case for secondary hormonal therapies in the chemotherapy age. J Urol. 2006;176:S66–71. doi: 10.1016/j.juro.2006.06.071. [DOI] [PubMed] [Google Scholar]
- 29.Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–27. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 31.Reid AH, Attard G, Barrie E, de Bono JS. CYP17 inhibition as a hormonal strategy for prostate cancer. Nat Clin Pract Urol. 2008;5:610–20. doi: 10.1038/ncpuro1237. [DOI] [PubMed] [Google Scholar]
- 32.Barrie SE, Potter GA, Goddard PM, Haynes BP, Dowsett M, Jarman M. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17-20 lyase) J Steroid Biochem Mol Biol. 1994;50:267–73. doi: 10.1016/0960-0760(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 33.O'Donnell A, Judson I, Dowsett M, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004;90:2317–25. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6:76–85. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–71. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 36.Vasaitis T, Belosay A, Schayowitz A, et al. Androgen receptor inactivation contributes to antitumor efficacy of 17{alpha}-hydroxylase/17,20-lyase inhibitor 3beta-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene in prostate cancer. Mol Cancer Ther. 2008;7:2348–57. doi: 10.1158/1535-7163.MCT-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitahara S, Umeda H, Yano M, et al. Effects of intravenous administration of high dose-diethylstilbestrol diphosphate on serum hormonal levels in patients with hormone-refractory prostate cancer. Endocr J. 1999;46:659–64. doi: 10.1507/endocrj.46.659. [DOI] [PubMed] [Google Scholar]
- 38.Huhtaniemi I, White R, McArdle CA, Persson BE. Will GnRH antagonists improve prostate cancer treatment? Trends Endocrinol Metab. 2009;20:43–50. doi: 10.1016/j.tem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Morote J, Esquena S, Abascal JM, et al. Failure to maintain a suppressed level of serum testosterone during long-acting depot luteinizing hormone-releasing hormone agonist therapy in patients with advanced prostate cancer. Urol Int. 2006;77:135–8. doi: 10.1159/000093907. [DOI] [PubMed] [Google Scholar]
- 40.Sundaram K, Didolkar A, Thau R, Chaudhuri M, Schmidt F. Antagonists of luteinizing hormone releasing hormone bind to rat mast cells and induce histamine release. Agents Actions. 1988;25:307–13. doi: 10.1007/BF01965036. [DOI] [PubMed] [Google Scholar]
- 41.Jiang G, Stalewski J, Galyean R, et al. GnRH antagonists: a new generation of long acting analogues incorporating p-ureido-phenylalanines at positions 5 and 6. J Med Chem. 2001;44:453–67. doi: 10.1021/jm0003900. [DOI] [PubMed] [Google Scholar]
- 42.Van Poppel H, Tombal B, de la Rosette JJ, Persson BE, Jensen JK, Kold Olesen T. Degarelix: a novel gonadotropin-releasing hormone (GnRH) receptor blocker--results from a 1-yr, multicentre, randomised, phase 2 dosage-finding study in the treatment of prostate cancer. Eur Urol. 2008;54:805–13. doi: 10.1016/j.eururo.2008.04.065. [DOI] [PubMed] [Google Scholar]
- 43.Gittelman M, Pommerville PJ, Persson BE, Jensen JK, Olesen TK. A 1-year, open label, randomized phase II dose finding study of degarelix for the treatment of prostate cancer in North America. J Urol. 2008;180:1986–92. doi: 10.1016/j.juro.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 44.Taplin M, Ko Y, Regan M, et al. Phase II trial of ketoconazole, hydrocortisone, and dutasteride (KHAD) for castration resistant prostate cancer (CRPC). J Clin Oncol; 2008 ASCO Annual Meeting; 2008. May 20, abstr 5068. [Google Scholar]
- 45.Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol. 2008;8:440–8. doi: 10.1016/j.coph.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9:601–10. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 47.Hodgson MC, Shen HC, Hollenberg AN, Balk SP. Structural basis for nuclear receptor corepressor recruitment by antagonist-liganded androgen receptor. Mol Cancer Ther. 2008;7:3187–94. doi: 10.1158/1535-7163.MCT-08-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh SM, Gauthier S, Labrie F. Androgen receptor antagonists (antiandrogens): structure-activity relationships. Curr Med Chem. 2000;7:211–47. doi: 10.2174/0929867003375371. [DOI] [PubMed] [Google Scholar]
- 49.Scher H, Danila D, Leversha M, Lilja H, Heller G, Fleisher M. Credentialing circulating tumor cells as biomarkers in castration resistant prostate cancer: toward the development of the “liquid biopsy”. AACR Special Topics Meeting; 2009; 2009. [Google Scholar]
- 50.Dehm SM, Tindall DJ. Androgen receptor structural and functional elements: role and regulation in prostate cancer. Mol Endocrinol. 2007;21:2855–63. doi: 10.1210/me.2007-0223. [DOI] [PubMed] [Google Scholar]
- 51.Solit DB, Scher HI, Rosen N. Hsp90 as a therapeutic target in prostate cancer. Semin Oncol. 2003;30:709–16. doi: 10.1016/s0093-7754(03)00346-4. [DOI] [PubMed] [Google Scholar]
- 52.Basak S, Pookot D, Noonan EJ, Dahiya R. Genistein down-regulates androgen receptor by modulating HDAC6-Hsp90 chaperone function. Mol Cancer Ther. 2008;7:3195–202. doi: 10.1158/1535-7163.MCT-08-0617. [DOI] [PubMed] [Google Scholar]
- 53.Snoek R, Cheng H, Margiotti K, et al. In vivo knockdown of the androgen receptor results in growth inhibition and regression of well-established, castration-resistant prostate tumors. Clin Cancer Res. 2009;15:39–47. doi: 10.1158/1078-0432.CCR-08-1726. [DOI] [PubMed] [Google Scholar]
- 54.Quayle SN, Mawji NR, Wang J, Sadar MD. Androgen receptor decoy molecules block the growth of prostate cancer. Proc Natl Acad Sci U S A. 2007;104:1331–6. doi: 10.1073/pnas.0606718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schiewer MJ, Morey LM, Burd CJ, et al. Cyclin D1 repressor domain mediates proliferation and survival in prostate cancer. Oncogene. 2008 doi: 10.1038/onc.2008.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Dosso S, Berthold DR. Docetaxel in the management of prostate cancer: current standard of care and future directions. Expert Opin Pharmacother. 2008;9:1969–79. doi: 10.1517/14656566.9.11.1969. [DOI] [PubMed] [Google Scholar]
- 57.Mazhar D, Waxman J. Early chemotherapy in prostate cancer. Nat Clin Pract Urol. 2008;5:486–93. doi: 10.1038/ncpuro1204. [DOI] [PubMed] [Google Scholar]
- 58.Hess-Wilson JK, Daly HK, Zagorski WA, Montville CP, Knudsen KE. Mitogenic action of the androgen receptor sensitizes prostate cancer cells to taxane-based cytotoxic insult. Cancer Res. 2006;66:11998–2008. doi: 10.1158/0008-5472.CAN-06-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rathkopf D, Carducci MA, Morris MJ, et al. Phase II trial of docetaxel with rapid androgen cycling for progressive noncastrate prostate cancer. J Clin Oncol. 2008;26:2959–65. doi: 10.1200/JCO.2007.15.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taylor RA, Risbridger GP. Prostatic tumor stroma: a key player in cancer progression. Curr Cancer Drug Targets. 2008;8:490–7. doi: 10.2174/156800908785699351. [DOI] [PubMed] [Google Scholar]
- 61.Welsbie DS, Xu J, Chen Y, et al. Histone deacetylases are required for androgen receptor function in hormone-sensitive and castrate-resistant prostate cancer. Cancer Res. 2009;69:958–66. doi: 10.1158/0008-5472.CAN-08-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marrocco DL, Tilley WD, Bianco-Miotto T, et al. Suberoylanilide hydroxamic acid (vorinostat) represses androgen receptor expression and acts synergistically with an androgen receptor antagonist to inhibit prostate cancer cell proliferation. Mol Cancer Ther. 2007;6:51–60. doi: 10.1158/1535-7163.MCT-06-0144. [DOI] [PubMed] [Google Scholar]