SUMMARY
Background
Depression is common in Alzheimer’s disease [AD], and antidepressants are commonly used for its treatment, yet evidence for antidepressant efficacy in this population is lacking. We conducted a multi-center, randomized, placebo-controlled trial titled “Depression in Alzheimer’s Disease-2” (DIADS-2) to assess the efficacy and tolerability of sertraline for depression in AD.
Methods
One hundred thiry-one participants from 5 U.S. medical centers with mild-to-moderate AD (Mini-Mental State Examination [MMSE] scores 10–26) and depression of AD were randomized to double-blinded treatment with sertraline (N=67) or placebo (N=64), with a target dosage of 100 mg daily. Efficacy was assessed using logistic regressions and mixed effects models in an intention to treat (ITT) analysis with imputation of missing data. Principal outcome measures were modified Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change (mADCS-CGIC), change in Cornell Scale for Depression in Dementia (CSDD) scores, and remission defined by both mADCS-CGIC score ≤2 and CSDD score ≤ 6.
Findings
mADCS-CGIC ratings (OR = 1.01 (95% CI: 0.52, 1.97, p=0.98), CSDD scores (median difference at 12 weeks 1.2,[95% CI -1.65, 4.05], p=0.41), and remission at 12 weeks of followup (OR = 2.06, [95% CI - 0.84, 5.04], p=0.11) did not differ between sertraline (N=67) and placebo (N=64). Sertraline-treated patients experienced more adverse events, most notably gastrointestinal and respiratory, than placebo-treated patients.
Interpretation
Sertraline did not demonstrate efficacy for the treatment depression symptoms in patients with Alzheimer's disease. In addition, its use was associated with an increased incidence of adverse events. Thus, selective serotonin reuptake inhibitors may be of limited value for treating depression in AD patients
BACKGROUND
Alzheimer’s disease (AD) is a neurodegenerative disease associated with substantial financial and emotional burdens for patients, caregivers and society (1). Neuropsychiatric symptoms (NPS) are very common in AD and a significant contributor to morbidity (2) due to their adverse effects on patients and caregivers(3). One NPS cluster is “depression of AD”, an affective syndrome affecting up to 50% of persons with AD and characterized by an atypical presentation with prominent anhedonia, irritability, agitation, and anxiety, but less evidence of guilt, and suicidality than major depressive episode (MDE) (4–7). Its symptoms are relatively persistent with 50–60% of untreated depressed AD patients remaining depressed at 1-year followup (8, 9). Antidepressant use is common in dementia, with a prevalence of 43.2% reported in a recent Danish registry study (10). Yet despite this widespread antidepressant use, there is limited evidence for antidepressant efficacy in this population.
Several antidepressants have been studied in AD with equivocal results (11–18) . One selective serotonin reuptake inhibitor (SSRI) trial with sertraline was positive, but three others with sertraline, fluoxetine, and citalopram were not. Behavioral interventions (19) and exercise (20) appear to improve depression. Because depression is a source of great distress to patients, and associated with worse quality of life as well as significant caregiver burden (21), it is important to develop effective treatments. Additionally, most trials have enrolled participants with MDE. It is possible that enrolling participants with a broader definition of depression in AD would be more generalizable to clinical work.
Based on the aforementioned positive sertraline pilot study and in order to more definitively assess efficacy, we conducted a larger multicenter 12-week double-blind placebo-controlled antidepressant trial of sertraline for depression in AD titled "Depression of Alzheimer’s Disease-2" (DIADS-2), followed by an additional 12 weeks of either randomized treatment for responders and the option for open-label treatment for non-responders. The results of the primary 12-week randomized trial are presented here. We hypothesized that sertraline treatment would be associated with better mood outcomes when compared with placebo.
METHODS
Patients
Participants were recruited from memory clinics at five academic centers in the United States. Participants met criteria for dementia of AD according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria and had Mini-Mental State Examination (MMSE) scores (22) from 10–26, inclusive. They also met criteria for depression of AD (4, 5), which compared with DSM-IV criteria for MDE requires the presence of 3 or more symptoms within a 2-week period, one of which must be depressed mood or anhedonia, with the addition of irritability as a possible symptom. Cholinesterase inhibitors and memantine were allowed, as long as treatment had not been initiated within 3 months of screening; dose adjustments within predetermined therapeutic dose ranges were allowed so long as they occurred ≥1 month prior to screening. Participants were ineligible if they were taking antipsychotics, antidepressants, or benzodiazepines. Anticonvulsants were allowed only for treatment of a pre-existing seizure disorder.
Consent was obtained from participants and their legally authorized representatives using procedures established by individual sites and their Institutional Review Boards (23). Informed consent also was obtained from caregivers for the collection caregiver measures. The study was conducted under the oversight of a Data Safety Monitoring Board (DSMB) operated by the National Institute of Mental Health (NIMH).
Procedures
Participants were randomized by the study’s Coordinating Center (PI: BKM), in a 1:1 ratio, to receive sertraline or placebo as previously described (24). Since the five clinical sites have demographically different patient populations which could affect the distribution of the outcomes, the randomization schedule was stratified by clinical site and it was designed with blocks of permuted length.
Participants began treatment with 50 mg sertraline or identically-appearing placebo tablets daily for one week, and then increased to the target dose of 100 mg sertraline or matching placebo daily. Clinicians had the option of increasing or decreasing the daily dose in the first four weeks after randomization depending on response and tolerability. In addition, caregivers received a standardized psychosocial intervention described below.
Visits occurred at baseline, and at 2, 4, 8, and 12 weeks after randomization. During the treatment phase, clinicians assessed mood at each visit through participant examination and caregiver interview using the modified Alzheimer's Disease Cooperative Study Clinical Global Impression of Change index (mADCS-CGIC), which, in addition to the original scale (25) incorporates a global rating of mood and associated symptoms of depression (e.g., sleep and appetite). Mood was assessed with the Cornell Scale for Depression in Dementia (CSDD) (26), a 19-item scale measuring the severity of depression in dementia, utilizing input from both the caregiver and the participant. All raters underwent a training process consisting of review of operationalized depression of AD criteria (7) as well as discussion of the application of these criteria at annual investigator’s meetings. Interrater reliability for CSDD score was determined by each rater’s observation of three videotaped interviews of persons with AD not enrolled in the trial. For 25 raters, the intraclass coefficient was 0.93 (27).
The mADCS-CGIC requires a clinical assessment of the participant, with input from the caregiver, by the investigator resulting in a rating on a seven-point scale ranging from 7 = “much worse” to 4 = “no change”, to 1 = “much better”. At week 12, those who were rated as improved (scores of 3, 2, or 1) continued on masked study treatment for another 12 weeks. For those patients not rated as improved, the study physician had the option of discontinuing study drug and starting any open-label treatment. In that event, the patients were encouraged to continue study participation and complete all study assessments.
A symptom checklist was derived from the Food and Drug Administration (FDA) approved prescribing information for sertraline (listed in Table 4). Starting at baseline and at all follow-up visits, participants and their caregivers were asked about whether any of these symptoms, or other self-reported adverse effects (AEs), occurred within one month of the baseline visit or since the last study visit once enrolled in the study. Serious adverse events (SAEs) were documented as defined by the FDA (28) (i.e., any adverse drug experience occurring at any dose that resulted in any of the following: death, a life-threatening adverse experience, inpatient hospitalization or prolongation of existing hospitalization, or persistent or significant disability/incapacity).
Table 4.
Occurrence of side effects derived from a checklist during follow-up
| Sertraline | Placebo | ||||
|---|---|---|---|---|---|
| (N=66) | (N=63) | Sertraline vs Placebo | |||
| Unadjusted OR |
95% CI | p- value (Exact) |
|||
| Agitation | 38 | 46 | 0.50 | (0.22, 1,12) | 0.10 |
| Anxiety | 54 | 53 | 0.85 | (0.30, 2.36) | 0.82 |
| Confusion | 38 | 36 | 1.02 | (0.48, 2.17) | 1.00 |
| Constipation | 22 | 18 | 1.25 | (0.55, 2.84) | 0.57 |
| Decreased Libido |
10 | 7 | 1.42 | (0.45, 4.75) | 0.61 |
| Diarrhea | 34 | 19 | 2.44 | (1.13, 5.42) | 0.02 |
| Dizziness | 39 | 19 | 3.31 | (1.52, 7.41) | 0.001 |
| Drug allergy | 4 | 0 | -- | -- | 0.12 |
| Dry mouth | 30 | 17 | 2.24 | (1.02, 5.07) | 0.04 |
| Ejaculatory dysfunction |
7 | 6 | 1.13 | (0.30, 4.32) | 1.00 |
| Falls | 16 | 11 | 1.51 | (0.59, 3.97) | 0.39 |
| Fatigue | 53 | 50 | 1.06 | (0.41, 2.75) | 1.00 |
| Gait instability | 35 | 31 | 1.16 | (0.55, 2.46) | 0.73 |
| Headache | 29 | 22 | 1.46 | (0.68, 3.16) | 0.37 |
| Indigestion | 23 | 11 | 2.51 | (1.04, 6,39) | 0.03 |
| Insomnia | 32 | 32 | 0.91 | (0.43, 1.93) | 0.87 |
| Nausea | 15 | 8 | 2.01 | (0.73, 5.97) | 0.17 |
| Nervousness | 37 | 35 | 1.02 | (0.48, 2.17) | 1.00 |
| Poor Appetite | 29 | 28 | 0.98 | (0.46, 2.08) | 1.00 |
| Somnolence | 35 | 32 | 1.09 | (0.52, 2.31) | 0.91 |
| Tremor | 23 | 15 | 1.70 | (0.74, 4.00) | 0.18 |
| Visual Disturbance |
14 | 13 | 1.04 | (0.41, 2.66) | 1.00 |
| Vomiting | 11 | 4 | 2.93 | (0.81, 13.35) | 0.10 |
| Other Symptoms |
32 | 35 | 0.75 | (0.36, 1.59) | 0.48 |
Fisher’s exact test used to calculate the p-values.
The caregivers of participants in both the sertraline and placebo groups, received a standardized, psychosocial intervention. This intervention consisted of 20- to 30-minute counseling sessions every at every study visit (and occasionally on the phone between visits), provision of educational materials, and 24-hour availability for crisis management assistance. At baseline, checklists guiding supportive care and developed for this trial were reviewed, and supportive care plans were developed with the caregiver. The content of counseling sessions included:
Review and adjustment of the patient and caregiver supportive care plans;
Emotional support and opportunity to ventilate feelings;
Counseling regarding specific caregiving skills (e.g., bathing) as needed;
Assistance with problem-solving of specific issues that the caregiver brought to the sessions; and
Discussion of educational materials: The 36-Hour Day (29) and Practical Dementia Care (30)
Data Analysis
All analyses were performed according to the patients’ original treatment assignment (intention-to-treat). Missing mood outcome data were imputed using the method of multiple imputation(31). Prediction models of the missing outcomes were estimated based on the patients’ other available baseline and follow-up data, and these models were used to impute the missing outcomes five times. The results of the five imputations were synthesized using simple combination rules (32) to yield estimates of the treatment comparisons.
The primary efficacy outcome was the comparison for the two groups at week 12 of ratings on the mood domain of the mADCS-CGIC, assessed by proportional odds logistic regression. Two other mood outcomes were compared by treatment group assignment: (1) the repeated measures of CSDD scores at baseline, and weeks 2, 4, 8, and 12; and (2) the proportion of patients in remission at week 12, defined as a patient having a CSDD score ≤ 6 and mADCS-CGIC ≤ 2 (i.e., ”better” or “much better”)(24). The proportion of patients in each treatment group whose depression remitted was compared using logistic regression.
CSDD scores over the 12 weeks were compared using mixed effects models, allowing a random intercept and slope for each patient. The CSDD scores were skewed to the right so a square-root transformation of the scores was used as the outcome in the regression models. Polynomial terms were used to model the trajectory of CSDD scores over time. To test for different rates of change in CSDD over time, a likelihood ratio test was used to compare a model allowing the changes over time to differ by treatment group to a model that did not allow the changes over time to differ by treatment group. The medians of the CSDD scores were compared at each visit. The standard errors of medians were calculated by bootstrapping. The measures were evaluated for outliers and the distributional assumptions of the models were confirmed.
Since years of education differed by treatment group, the results for the primary and secondary outcomes include adjustment for years of formal education. Models with and without adjustment for site were analyzed; the treatment effect estimates were virtually identical, so the results reported here are from the models without controlling for site. Treatment by site interactions were also considered and found not to be significant for any of the three outcomes.
Exact tests and logistic regressions were used to compare the number of patients experiencing all-cause and specific-cause AEs and SAEs in the treatment groups. Models of AEs were analyzed with and without adjustment for baseline differences in the report of adverse effects in the 28 days prior to randomization.
The planned sample size of 130 was based upon 80% power, 0.05 significance level and 20% attrition, to detect a difference of the following magnitude between the two treatment groups in the distribution of the seven categories of the mADCS-CGIC (from worst to best) (24): 8%, 18%, 22%, 25%, 20%, 5%, 2% in the group assigned to placebo 3%, 8%, 17%, 17%, 36%, 13%, 6% in the group assigned to sertraline
Statistical analyses were performed using R version 2.7.1(33). All p-values are two-sided. p<.05 was used as the threshold for statistical significance. No adjustments were made for multiple comparisons.
Role of the funding source
NIMH scientific collaborators participated on the trial’s Steering Committee. Sertraline and matching placebo were provided by Pfizer, Inc., which did not otherwise participate in the design or conduct of the trial. After database lock and study unblinding, the corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. All co-investigators had access to the raw data.
RESULTS
Demographics and Clinical Variables
A CONSORT flow chart of study recruitment and retention is in Figure 1. 131 patients met eligibility criteria and were randomized: 67 to sertraline and 64 to placebo. Participants had a median age of 79 years, and 54% female; 67% were white, 21% African-American, and 11% Hispanic/Latino (Table 1). The majority were married, living in their own home, and had at least a high school education. Participants randomized to sertraline had more years of formal education than those in the placebo group. The CSDD and MMSE scores reflect a moderately depressed group with mild to moderate dementia severity, and approximately 40% of subjects met criteria for MDE.
Figure 1.
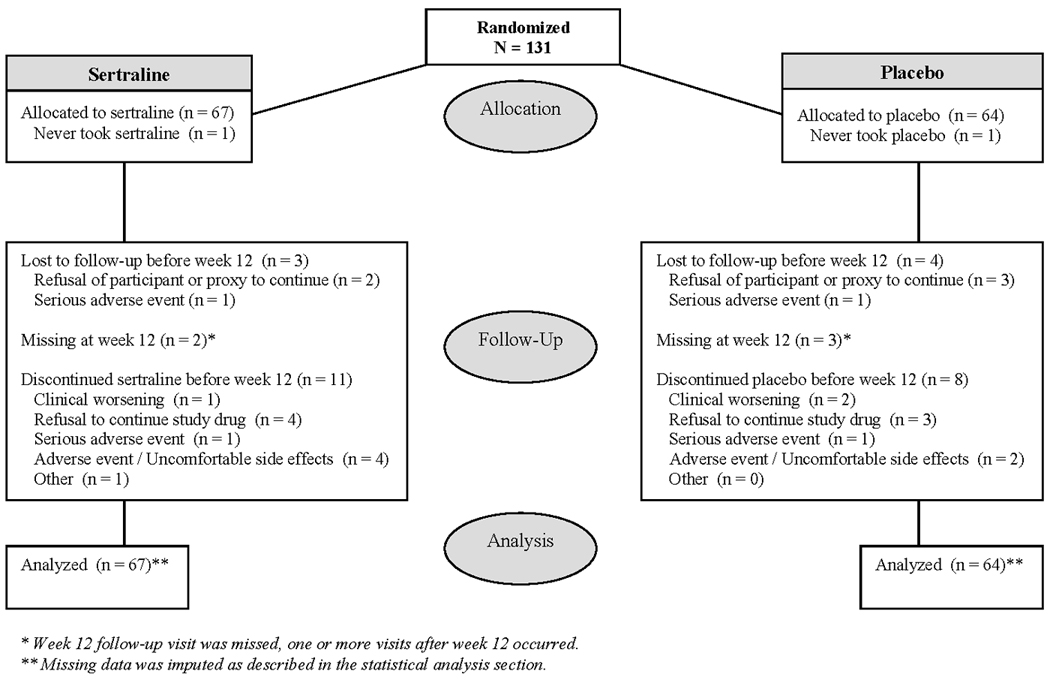
CONSORT chart of patient flow in DIADS-2
Table 1.
Demographics and clinical variables at baseline
| Total | Sertraline | Placebo | |
|---|---|---|---|
| No. randomized | 131 | 67 | 64 |
| Age, mean (sd), years | 77.3 (8.0) | 76.5 (8.0) | 78.2 (8.0) |
| Gender, % | |||
| Female | 54.2 | 59.7 | 48.4 |
| Male | 45.8 | 40.3 | 51.6 |
| Ethnic Group, % | |||
| White, non-Hispanic | 67.2 | 73.1 | 60.9 |
| African-American | 21.4 | 17.9 | 25.0 |
| Hispanic / Latino | 10.7 | 7.5 | 14.1 |
| Asian | 0.8 | 1.5 | 0 |
| Marital status, % | |||
| Married | 64.1 | 64.2 | 64.1 |
| Widowed | 25.2 | 26.9 | 23.4 |
| Divorced / separated | 6.1 | 4.5 | 7.8 |
| Never married | 4.6 | 4.5 | 4.7 |
| Education, mean (sd), years | 12.5 (3.7) | 13.2 (3.6) | 11.8 (3.8) |
| Duration of dementia, mean (sd), years | 2.8 (2.2) | 2.6 (2.1) | 3.1 (2.3) |
| Depression episodes before cognitive symptoms, % | |||
| No episodes | 74.0 | 77.6 | 70.3 |
| One episode | 16.8 | 13.4 | 20.3 |
| Two or more episodes | 7.6 | 6.0 | 9.4 |
| Missing | 1.5 | 3.0 | 0 |
| Depression episodes since cognitive symptoms, % | |||
| One episode | 86.3 | 86.6 | 85.9 |
| Two or more episodes | 12.9 | 12.0 | 14.1 |
| Missing | 0.8 | 1.5 | 0 |
| History of mood disorder in first degree relative, % | 16.0 | 13.4 | 18.8 |
| Personal history of mood disorder, % | 27.5 | 25.4 | 29.7 |
| Cornell Scale for Depression in Dementia, median, (1st quartile, 3rd quartile) |
13 (9, 18) | 13 (9,19) | 13 (9.5, 17) |
| Mini-mental State Examination, mean (sd) | 20.0 (4.6) | 20.6 (4.5) | 19.3 (4.8) |
| Taking only cholinesterase inhibitors (%) | 38 | 40 | 36 |
| Taking only memantine (%) | 6.1 | 6.0 | 6.3 |
| Taking both memantine and cholinesterase inhibitors (%) | 26 | 25 | 26 |
| Diagnosis of Major Depressive Episode (MDE), % | 39.7 | 38.8 | 40.6 |
There were demographic and clinical differences between the enrolled populations at different sites (data not shown) and randomization was stratified by treatment site as described in the Methods..
Retention and adherence to treatment
Patients randomized to sertraline who had not terminated treatment earlier were taking a mean dose of 91.1 mg daily (SD = 20.1) at 4 weeks and 93.1 mg (SD = 19.4) at 12 weeks; those randomized to placebo were taking a mean dose of 97.4 mg daily (SD = 14.6) at 4 weeks and 96.6 mg daily (SD = 14.2) at 12 weeks. Treatment adherence was assessed by pill counts from returned medication bottles: the participants in the sertraline group returned 93.5% (95% CI: 89.0, 97.9) of their study bottles and those in placebo groups similarly returned 92.9% (95% CI: 88.1, 97.7) of their study bottles. The sertraline-treated participants who returned their study medication bottles took 83.1%, 95% CI: (78.1%, 88.1%) of the target study drug dose compared with 90.1%, 95% CI: (86.3%, 93.8%) in the placebo-treated group (t-test for equal means, t =−2.17 with 121 df, p = 0.03).
Seven participants (5%) were lost to followup over the first 12 weeks of the study (3 sertraline-treated and 4 placebo-treated). An additional 2 participants in the sertraline group and 3 participants in the placebo group missed their week 12 study visit. The proportion of patients discontinuing study medications prior to week 12 did not differ significantly between treatment groups (Fisher’s test, exact p = 0.64); 12 (18%) participants in the sertraline-treated group and 9 (14%) in the placebo-treated group discontinued study medications prior to week 12.
At week 12, the study physician was asked to guess treatment assignment. Although the proportion of correct guesses was not different than chance overall (proportion = 0.57, 95% C.I. (0.47–0.66, exact binomial test, p = 0.16), the distribution of correct guesses differed by treatment group (Pearson χ2 = 7.15 with 1 df, p = 0.008) with the proportion of correct guesses in the sertraline group being greater than 0.5 (proportion = 0.70, 95% CI 0.56–0.81, exact binomial test, p = 0.005), while the proportion of correct guesses in the placebo group was not different than 0.5 (proportion = 0.45, 95% CI 0.32–0.58, exact binomial test, p = 0.50).
Primary outcome
mADCS-CGIC scores were imputed for the 12 participants that did not have a week 12 visit. Education was imputed for one participant. There were no differences on the mADCS-CGIC at week 12 by treatment assignment (Table 2). The odds ratio of being at or better than a given CGIC category for sertraline versus placebo, was 1.01 (95% CI: 0.52, 1.97, Wald χ2 = 0.001 with 1 df, p = 0.98). There was no significant site × treatment interaction for this primary nor either of the secondary outcomes.
Table 2.
CGI-C by treatment group and visit
| mADCS-CGIC1 rating |
Sertraline (n=67)2 |
Placebo (n=64) |
|---|---|---|
| 7 “much worse” | 1 (1.5%)3 | 0 (0%) |
| 6 “worse” | 5 (7.5%) | 2 (3.1%) |
| 5 “a bit worse” | 6 (9.0%) | 9 (14.1%) |
| 4 “no change” | 10 (14.9%) | 11 (17.2%) |
| 3 “a bit better” | 18 (26.9%) | 18 (28.1%) |
| 2 “better” | 18 (26.9%) | 21 (32.8%) |
| 1 “much better” | 9 (13.4%) | 3 (4.7%) |
Modified ADCS-CGIC was assessed at week 12. ADCS-CGIC, a clinician-rated global impression of change from baseline through week 12, was modified so that the clinician was rating global impression of mood change only.
These numbers include imputed values from the first imputation cycle. The analysis combined the results across five imputation cycles.
Percentages may not add to 100% due to rounding.
Secondary outcomes
CSDD scores were imputed for 2 participants for week 2, 4 participants for week 4, 7 participants for week 8, and 12 participants for week 12. CSDD median scores did not differ by treatment assignment over the 12 weeks (Table 3). The rate of change in the transformed CSDD scores over time did not differ between treatment groups (Likelihood ratio test, χ2 = 0.26, 3 df, p = 0.97). The median trajectories for each treatment group are displayed in Figure 2.
Table 3.
CSDD difference (Placebo – Sertraline) in medians and confidence intervals* by visit.
| Week 2 | Week 4 | Week 8 | Week 12 |
|---|---|---|---|
| 0.80 (−1.63, 3.23) | 0.80 (−1.75, 3.35) | 1.20 (−1.18, 3.58) | 1.20 (−1.65, 4.05) |
Standard errors for medians calculated by bootstrapping. The results from all five imputations were combined.
Figure 2.

CSDD medians* at each visit by treatment group. Error bars represent the range between the first and third quartiles.
There was no statistically significant increase in the estimated odds of remission on sertraline treatment compared with placebo (OR = 2.06, 95% CI: 0.84, 5.04, Wald χ2 = 2.55 with 1 df, p = 0.11), with 33% of sertraline-treated participants achieving remission at week 12 compared with 19% of placebo-treated patients.
Adverse Events
66 participants on sertraline and 63 patients on placebo had at least one follow-up visit and provided AE data using the symptom checklist. Of these, diarrhea, indigestion, dry mouth, and dizziness were more common in the sertraline group, while agitation was more common in the placebo group (Table 4). After controlling for an imbalance in the distribution of history of tremor at baseline, tremor was also more common in the sertraline group during follow-up (OR = 2.94, 95% CI: 1.15, 7.54, Wald χ2 = 5.1 with 1 df, p = 0.02). Report of tremor during follow-up was associated with treatment termination before week 12 only in the sertraline group (Fisher’s test, exact p=0.04), but not associated with being on a dose lower than 100 mg at week 4 (Fisher’s test, exact p=0.68) or week 12 (Fisher’s test, exact p=0.46) among those still on treatment, experiencing an SAE before week 12 (Fisher’s test, exact p=0.60) or being lost-to-follow-up before week 12 (Fisher’s test, exact p=0.67.) There was no difference in the proportion of participants experiencing SAEs in the two groups; 13 sertraline-treated and 7 placebo-treated participants experienced SAEs (Fisher’s test, exact p = 0.23). Four sertraline-treated but no placebo-treated participants experienced SAEs relating to the respiratory system (pneumonias or other respiratory infections). There were no significant differences in SAEs by treatment assignment in other organ systems (neurologic: sertraline 4, placebo 3; musculoskeletal: sertraline 3, placebo 1; cardiac: sertraline 3, placebo 5). There was one death in the placebo group and none in the sertraline group. All four of these participants were women, and three were on study drug at the time of the event.
DISCUSSION
Over the course of 12 weeks nearly 40% of the study population was judged to be either "better" or "much better" in terms of mood compared with baseline, and nearly 70% were judged at least "a bit better". Moreover, depression severity, as measured by CSDD score, improved by nearly 50%. Yet, there were no significant differences between treatment groups on any of the three primary or secondary mood outcomes. In addition, sertraline treatment was associated with an increased rate of common SSRI adverse effects, and SAEs were common in this group, although the latter finding was not statistically significant. The observations of SAEs, particularly those involving the respiratory system, are of concern in light of case reports of eosinophilic pneumonitis with sertraline (34).
These data may reflect a true “null” finding or that a sertraline effect was confounded by other aspects of study procedures, such as decreased adherence, greater side effects, or higher rate of dropout in the sertraline-treated group. However, there was only a small difference in adherence between sertraline- and placebo-treated participants and a high rate of adherence in both groups, and despite a higher rate of side effects in the sertraline-treated group there was no evidence this led to decreased dose nor to greater rate of dropout. We believe that the achieved dose of 90–100 mg represents an appropriate target dose for this elderly frail population, given the substantial rates of sertraline-related side effects observed in the sertraline-treated group. While treatment adherence was slightly lower in the sertraline-treated group (83%) vs. placebo (90%), this small difference is not likely to account for the results presented. Admittedly, there was likely a beneficial effect from participation in the trial including the psychosocial intervention, given the high rate of observed placebo response which is common in antidepressant trials and would serve to dilute observed drug effect. The observation that investigators were able to guess treatment assignment more accurately than chance in the sertraline group could introduce bias in either direction, because observers who guessed that patients were on sertraline might be more likely to report a beneficial effect or more likely to report adverse events.
Another reason for results differing from prior studies is that most prior studies enrolled patients with MDE, while DIADS-2 used a broader definition of depression in AD with the symptom list tailored toward the NPS clusters common in affective syndromes in AD. It is possible that sertraline is more effective in more narrowly defined major depressive episodes than in the more broadly defined depression of AD syndrome used in this study, or that there are subgroups of responders not identified in the primary analyses.
Strengths of the study include: 1) randomized treatment assignment with inclusion of placebo control; 2) double blind treatment assignments with rigorous adherence to masked rating; 3) high retention rates (>90% over 12 weeks) and a high rate of adherence to study drug, which may have been bolstered by the psychosocial intervention; 4) use of a consensus definition of depression of AD; 5) use of a semi-structured psychosocial intervention in a multi-center trial with centralized training and monitoring of adherence to the protocol; 6) relatively few medical or medication exclusions resulting in a study population that is broadly representative of the AD population.
Limitations of the study include: 1) the psychosocial intervention may have impacted study outcomes as reviewed above; 2) participants comprised a sample of convenience in US academic medical centers, and hence may not generalize to other settings, 3) we lacked sufficient detail on SAE events to identify whether respiratory system SAEs reflected the diagnosis of eosinophilic pneumonitis
In short, 12 weeks of sertraline treatment for depression in AD was not associated with clinical improvement and was associated with a higher risk of adverse events. These results did not reproduce prior results of a smaller trial of sertraline in AD patients with MDE and do not support the widespread use of SSRIs for depression in AD.
Acknowledgements
Grant funding: National Institute of Mental Health, 1U01MH066136, 1U01MH068014, 1U01MH066174, 1U01MH066175, 1U01MH066176, 1U01MH066177.
Footnotes
Trial Registration: www.clinicaltrials.gov NCT00086138
ROLES OF AUTHORS AND CONTRIBUTORS
All authors participated in data collection and revision of the paper. Drafting of the paper was done by PBR. Data analyses were performed by LTD, CF, BKM, CLM.
DISCLOSURES
- Barbara K. Martin is involved in another trial for which Pfizer donated a different drug.
- Paul B. Rosenberg has received research funds from Pfizer, Elan, Lilly and Merck in amounts greater than $10,000.
- Jacobo Mintzer has received research support from Abbot to study donepezil and divalproex sodium, from AstraZeneca to study quetiapine, from BMS to study aripiprazole, from Eli Lilly to study olanzapine, from Forest to study both citalopram and memantine, from Janssen to study galantamine and risperidone, and from Pfizer to study donepezil and memantine; Dr. Mintzer also has been a consultant, paid directly or indirectly, for AstraZeneca, BMS, Eli Lilly, Janssen, Pfizer, Forest, and Aventis. He also has been an unpaid consultant for Targacept and has participated in Speaker's Bureaus for Janssen, Forest, and Pfizer.
- Daniel Weintraub has received research support from Boehringer Ingelheim; Dr. Weintraub also has been a paid consultant for Acadia Pharmaceuticals, Novartis Pharmaceuticals, Boehringer Ingelheim, Osmotica Pharmaceutical, BrainCells Inc., EMD Serono, and Sanofi Aventis, and has participated on a Speaker’s Bureau for Pfizer.
- Anton P. Porsteinsson is involved in research sponsored by Pfizer to study donepezil and PF04494700, Eli Lilly to study atomoxetine, a gamma-secretase inhibitor and a beta amyloid antibody, Wyeth to study a beta amyloid antibody, GSK to study a PPAR inhibitor and Forest to study memantine and neramexane; Dr. Porsteinsson has been a paid consultant and participated on a Speaker's Bureau for Pfizer and Forest.
- Lon S. Schneider is involved in research sponsored by Pfizer; Dr. Schneider has been a paid consultant for Forest, GlaxoSmithKline, Lilly, Merck, and Wyeth.
- Constantine Frangakis has no conflict of interests.
- Lea T. Drye has no conflict of interests.
- Peter V. Rabins has participated on Speaker's Bureaus for Wyeth, Eli Lilly, and Pfizer, and has received reimbursement for legal testimony from Janssen Pharmaceuticals.
- Cynthia A. Munro has no conflict of interests.
- Curtis L. Meinert is involved in another trial for which Pfizer donated a different drug; Dr. Meinert owns shares of GSK stock.
- Constantine G. Lyketsos was involved in another trial for which Pfizer donated a different drug; he also was involved in research sponsored by Forest to study escita.
Contributor Information
Paul B. Rosenberg, Division of Geriatric Psychiatry and Neuropsychiatry, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine.
Lea T. Drye, Johns Hopkins Bloomberg School of Public Health.
Barbara K. Martin, Center for Clinical Trials, Johns Hopkins Bloomberg School of Public Health.
Constantine Frangakis, J Center for Clinical Trials, Johns Hopkins Bloomberg School of Public Health.
Jacobo E. Mintzer, Department of Neurosciences, Medical University of South Carolina.
Daniel Weintraub, Section of Geriatric Psychiatry, University of Pennsylvania and Mental Illness Research, Education and Clinical Center (MIRECC), Philadelphia Veterans Affairs Medical Center.
Anton P. Porsteinsson, AD-CARE Program, Department of Psychiatry, University of Rochester School of Medicine and Dentistry.
Lon S. Schneider, Departments of Psychiatry, Neurology, and Gerontology University of Southern California Keck School of Medicine.
Peter V. Rabins, Johns Hopkins School of Medicine.
Cynthia A. Munro, Division of Medical Psychology, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine.
Curtis L. Meinert, Center for Clinical Trials, Johns Hopkins Bloomberg School of Public Health.
Constantine G. Lyketsos, Division of Geriatric Psychiatry and Neuropsychiatry, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine and Department of Mental Health, Johns Hopkins Bloomberg School of Public Health..
References
- 1.Jonsson L, Eriksdotter Jonhagen M, Kilander L, et al. Determinants of costs of care for patients with alzheimer's disease. Int J Geriatr Psychiatry. 2006;21:449–459. doi: 10.1002/gps.1489. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg M, Shao H, Zandi P, et al. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: The cache county study. Int J Geriatr Psychiatry. 2007 doi: 10.1002/gps.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyketsos CG, Sheppard JM, Steinberg M, et al. Neuropsychiatric disturbance in alzheimer's disease clusters into three groups: The cache county study. Int J Geriatr Psychiatry. 2001;16:1043–1053. doi: 10.1002/gps.448. [DOI] [PubMed] [Google Scholar]
- 4.Olin JT, Schneider LS, Katz IR, et al. Provisional diagnostic criteria for depression of alzheimer disease. Am J Geriatr Psychiatry. 2002;10:125–128. [PubMed] [Google Scholar]
- 5.Olin JT, Katz IR, Meyers BS, Schneider LS, Lebowitz BD. Provisional diagnostic criteria for depression of alzheimer disease: Rationale and background. Am J Geriatr Psychiatry. 2002;10:129–141. [PubMed] [Google Scholar]
- 6.Lyketsos CG, Olin J. Depression in alzheimer's disease: Overview and treatment. Biol Psychiatry. 2002;52:243–252. doi: 10.1016/s0006-3223(02)01348-3. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg PB, Onyike CU, Katz IR, et al. Clinical application of operationalized criteria for 'depression of alzheimer's disease'. Int J Geriatr Psychiatry. 2005;20:119–127. doi: 10.1002/gps.1261. [DOI] [PubMed] [Google Scholar]
- 8.Garre-Olmo J, Lopez-Pousa S, Vilalta-Franch J, et al. Evolution of depressive symptoms in alzheimer disease: One-year follow-up. Alzheimer Dis Assoc Disord. 2003;17:77–85. doi: 10.1097/00002093-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg M, Tschanz JT, Corcoran C, et al. The persistence of neuropsychiatric symptoms in dementia: The cache county study. Int J Geriatr Psychiatry. 2004;19:19–26. doi: 10.1002/gps.1025. [DOI] [PubMed] [Google Scholar]
- 10.Kessing LV, Harhoff M, Andersen PK. Treatment with antidepressants in patients with dementia--a nationwide register-based study. Int Psychogeriatr. 2007;19:902–913. doi: 10.1017/S1041610206004376. [DOI] [PubMed] [Google Scholar]
- 11.Reifler BV, Teri L, Raskind M, et al. Double-blind trial of imipramine in alzheimer's disease patients with and without depression. Am J Psychiatry. 1989;146:45–49. doi: 10.1176/ajp.146.1.45. [DOI] [PubMed] [Google Scholar]
- 12.Nyth AL, Gottfries CG A nordic multicentre study. The clinical efficacy of citalopram in treatment of emotional disturbances in dementia disorders. Br J Psychiatry. 1990;157:894–901. doi: 10.1192/bjp.157.6.894. [DOI] [PubMed] [Google Scholar]
- 13.Magai C, Kennedy G, Cohen CI, Gomberg D. A controlled clinical trial of sertraline in the treatment of depression in nursing home patients with late-stage alzheimer's disease. Am J Geriatr Psychiatry. 2000;8:66–74. doi: 10.1097/00019442-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Petracca GM, Chemerinski E, Starkstein SE. A double-blind, placebo-controlled study of fluoxetine in depressed patients with alzheimer's disease. Int Psychogeriatr. 2001;13:233–240. doi: 10.1017/s104161020100761x. [DOI] [PubMed] [Google Scholar]
- 15.Petracca G, Teson A, Chemerinski E, Leiguarda R, Starkstein SE. A double-blind placebo-controlled study of clomipramine in depressed patients with alzheimer's disease. J Neuropsychiatry Clin Neurosci. 1996;8:270–275. doi: 10.1176/jnp.8.3.270. [DOI] [PubMed] [Google Scholar]
- 16.Roth M, Mountjoy CQ, Amrein R. Moclobemide in elderly patients with cognitive decline and depression: An international double-blind, placebo-controlled trial. Br J Psychiatry. 1996;168:149–157. doi: 10.1192/bjp.168.2.149. [DOI] [PubMed] [Google Scholar]
- 17.Lyketsos CG, Sheppard JM, Steele CD, et al. Randomized, placebo-controlled, double-blind clinical trial of sertraline in the treatment of depression complicating alzheimer's disease: Initial results from the depression in alzheimer's disease study. Am J Psychiatry. 2000;157:1686–1689. doi: 10.1176/appi.ajp.157.10.1686. [DOI] [PubMed] [Google Scholar]
- 18.Lyketsos CG, DelCampo L, Steinberg M, et al. Treating depression in alzheimer disease: Efficacy and safety of sertraline therapy, and the benefits of depression reduction: The DIADS. Arch Gen Psychiatry. 2003;60:737–746. doi: 10.1001/archpsyc.60.7.737. [DOI] [PubMed] [Google Scholar]
- 19.Teri L. Behavior and caregiver burden: Behavioral problems in patients with alzheimer disease and its association with caregiver distress. Alzheimer Dis Assoc Disord. 1997;11 Suppl 4:S35–S38. [PubMed] [Google Scholar]
- 20.Teri L, Gibbons LE, McCurry SM, et al. Exercise plus behavioral management in patients with alzheimer disease: A randomized controlled trial. JAMA. 2003;290:2015–2022. doi: 10.1001/jama.290.15.2015. [DOI] [PubMed] [Google Scholar]
- 21.Garre-Olmo J, Lopez-Pousa S, Vilalta-Franch J, et al. Carer's burden and depressive symptoms in patients with alzheimer s disease. state after twelve months. Rev Neurol. 2002;34:601–607. [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Alzheimer's Association. Research consent for cognitively impaired adults: Recommendations for institutional review boards and investigators. Alzheimer Dis Assoc Disord. 2004;18:171–175. doi: 10.1097/01.wad.0000137520.23370.56. [DOI] [PubMed] [Google Scholar]
- 24.Martin BK, Frangakis CE, Rosenberg PB, et al. Design of depression in alzheimer's disease study-2. Am J Geriatr Psychiatry. 2006;14:920–930. doi: 10.1097/01.JGP.0000240977.71305.ee. [DOI] [PubMed] [Google Scholar]
- 25.Schneider LS, Olin JT, Doody RS, et al. Validity and reliability of the alzheimer's disease cooperative study-clinical global impression of change. the alzheimer's disease cooperative study. Alzheimer Dis Assoc Disord. 1997;11 Suppl 2:S22–S32. doi: 10.1097/00002093-199700112-00004. [DOI] [PubMed] [Google Scholar]
- 26.Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell scale for depression in dementia. Biol Psychiatry. 1988;23:271–284. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 27.Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 28.Food and Drug Administration. Food and drug administration, code of federal regulations title 21, section 310, part D. [online] Available at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?FR=310.305.
- 29.Mace NL, Rabins PV. The 36-Hour Day: A Family Guide to Caring for Persons with Alzheimer Disease, Related Dementing Illness, and Memory Loss in Later Life. Baltimore: Johns Hopkins University Press; 2000. [Google Scholar]
- 30.Rabins PV, Lyketsos CG, Steele C. Practical Dementia Care. New York: Oxford University Press; 1999. [Google Scholar]
- 31.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley; 1996. [Google Scholar]
- 32.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91:473–489. [Google Scholar]
- 33.R Development Core Team. R: A language and environment for statistical computing. [online]. Available at http://www.R-project.org.
- 34.Haro M, Rubio M, Xifre B, Castro P. Acute eosinophilic pneumonia associated to sertraline. Med Clin (Barc) 2002;119:637–638. doi: 10.1016/s0025-7753(02)73522-7. [DOI] [PubMed] [Google Scholar]


