Abstract
This report demonstrates that even brief inhibition of degradation by the 26S proteasome also inhibits global protein synthesis, mediated through increased phosphorylation of eIF2α by the heme-regulated inhibitor kinase. Exposure of COS-7 cells to the proteasome inhibitor MG132 for 4 h resulted in a 55–60% decrease in protein synthesis rate compared to control cells. This repression of protein synthesis after treatment with MG132 is not due to induction of apoptosis, which is known to occur after longer periods of 26S inhibition. Instead, we observed a significantly increased phosphorylation of eIF2α, which is known to repress global protein synthesis. In three mouse embryonic fibroblast knockout cell lines lacking one of the four kinases known to phosphorylate eIF2α, increased phosphorylation of eIF2α still occurred following inhibition of the 26S proteasome. These three cell lines included a deletion of the double-stranded RNA-activated protein kinase (PKR); a deletion of the PKR-like endoplasmic reticulum resident kinase (PERK); or a deletion of the GCN2 kinase, indicating that none of these kinases were primarily responsible for the observed phosphorylation of eIF2α. In contrast, in a fourth mouse embryonic fibroblast knockout cell line, HRI−/− cells lacking the heme-regulated inhibitor kinase (HRI) fail to increase eIF2α phosphorylation upon proteasome inhibitor treatment (MG132 or various doses of bortezomib), indicating that the HRI kinase is the primary kinase activated by brief treatment of mouse embryonic fibroblasts with 26S proteasome inhibitors.
Keywords: 26S proteasome, apoptosis, eIF2α, protein degradation, protein kinase, protein synthesis
INTRODUCTION
The dominant mechanism of control of global protein synthesis is phosphorylation/dephosphorylation of translational components, although other mechanisms, such as cleavage of initiation factors, can also affect protein synthesis rates, for example during apoptosis or following viral infection. Phosphorylation of eukaryotic translational initiation factor 2 (eIF2) appears to be a general mechanism for inhibiting initiation of protein synthesis [1]. eIF2 is composed of three subunits termed α, β and γ in order of increasing molecular mass. The primary role of eIF2 in translational initiation is to transfer methionyl-tRNA(Met-tRNAi) to the 40S ribosomal subunits [2]. First, eIF2 forms a ternary complex with Met-tRNAi and GTP; this ternary complex then binds to the 40S ribosomal subunit to generate the 43S preinitiation complex [3]. Upon joining of the 60S ribosomal subunit to the 43S preinitiation complex, the GTP moiety is hydrolyzed and an eIF2·GDP complex is released from the ribosome [4]. In order to promote another round of initiation, the GDP bound to eIF2 must be exchanged for GTP, a reaction carried out by guanine nucleotide exchange factor (eIF2B) [2].
The global rate of protein synthesis is mainly regulated by the specific phosphorylation of serine 51 of the eIF2α subunit [5]. Phosphorylated eIF2α (eIF2α (P)) cannot undergo GDP/GTP exchange and forms a non-dissociable eIF2α (P)·eIF2B complex [5, 6]. Since intracellular levels of eIF2B are approximately 10–20% that of eIF2 in the cytoplasm, phosphorylation of as little as 10% of eIF2 can be sufficient to sequester virtually all available eIF-2B, thereby blocking the eIF2B exchange activity and therefore inhibiting protein synthesis completely [4, 6]. eIF2α is known to be specifically phosphorylated at Ser 51 by at least four different kinases including the interferon-inducible double-stranded RNA-activated PKR (in response to viral infection and stress conditions), the heme-regulated inhibitor (HRI) kinase, the nutrient-regulated protein kinase GCN2 (in response to uncharged tRNA in nutrient deprived cells), and PKR-like ER transmembrane protein kinase (PERK, in response to accumulation of unfolded protein in the ER) [4, 5, 7].
The 26S proteasome is an ATP-dependent proteolytic system which is engaged in the selective degradation of short-lived proteins under normal metabolic conditions, bulk degradation of long-lived proteins, partial digestion/processing of some proteins (e.g., NF-κB), and antigen presentation. Cyclin-dependent kinase inhibitors, M-, S-, and G1-phase specific cyclins, p53, ornithine decarboxylase (ODC), the transcription factors c-Jun and c-Fos are a few examples of the multitude of proteins degraded by the 26S proteasome [8]. Previously, several contradictory studies on the effect of the 26S proteasome inhibition on protein synthesis rate have been published. For instance, Schubert et al. indicated that treating HeLa cells with a cocktail of proteasome inhibitors (zLLL/lactacystin) for 2 or 4 h profoundly decreased protein synthesis [9]. Similarly, Mimnaugh et al. also showed that the proteasome inhibitor, lactacystin, decreased the synthesis of most cellular proteins, while it specifically induced the synthesis of stress proteins hsp72 and hsp90 in human SKBr3 breast tumor cells [10]. Jiang et al. later indicated that the reduced levels of translation in response to proteasome inhibition were caused by increased phosphorylation of eIF2α, which was mediated through the activation of GCN2 [11]. In contrast, Bush et al reported that the proteasome inhibitor, MG132, did not affect total protein synthesis even after 18 h treatment of canine kidney cells [12].
During our recent studies on the mechanism of degradation of S-adenosylmethionine decarboxylase (a short-lived, polyamine biosynthetic enzyme), we found that inhibition of the 26S proteasome causes a significant increase in cellular level of the enzyme’s substrate, S-adenosylmethionine (AdoMet) (>2-fold) [13]. The present studies trace this increase in AdoMet levels to an increase in the level of its precursor, methionine. Methionine levels in turn were increased due to a general increase in amino acid levels resulting from decreased protein synthesis and therefore decreased utilization of amino acids. We therefore decided to investigate the molecular mechanisms responsible for the decreased protein synthesis rate after inhibition of the 26S proteasome. Although decreased protein synthesis rates occur during apoptosis, and inhibition of 26S proteasome have been reported to induce apoptosis in several different experimental systems [14–17], our results indicate that loss of protein synthesis activity after short periods of inhibition of the 26S proteasome is not accompanied by any signs of induction of apoptosis. Instead, we show that inhibition of the 26S proteasome significantly increases eIF2α phosphorylation, which is thus the primary cause of loss of protein synthesis activity, in agreement with other published work. By testing 4 knockout cell lines with individual deletions for each of the four kinases known to phosphorylate eIF-2α, we also demonstrate for the first time that HRI is the primary kinase responsible for the increased eIF-2α phosphorylation caused by proteasome inhibitor in mouse embryonic fibroblasts.
EXPERIMENTAL
Materials
Enhanced chemifluorescent (ECF) detection reagent for Western blotting was purchased from Amersham (now GE, Arlington Heights, IL). COS-7 cells were obtained from American Type Culture Collection (Manassas, VA). Wild type (PERK+/+) and PERK-knockout cells were kindly provided by Dr. David Ron (New York University of School of Medicine). Wild type (PKR+/+) and PKR−/− mouse embryonic fibroblast (MEF) cells were a generous gift of Dr. Charles Weissmann (University of Zurich). Wild type (GCN2+/+) and GCN2−/− MEF cells were a generous gift from Dr. Douglas Cavener (The Pennsylvania State University). Velcade bortezomib) was a gift from Dr. Vincent Chau The Pennsylvania State University College of Medicine). MG132 (carbobenzoxy-L-leucyl-L-leucyl-L-leucinal) and caspase inhibitor I (Z-VAD-FMK) were purchased from Calbiochem (San Diego, CA). Dulbecco’s Modified Eagle Medium (DMEM) was from Gibco BRL (Gaithersburg, MD). Fetal Calf Serum (FCS) was from HyClone (Logan, UT). Polyvinylidene difluoride (PVDF) membrane was obtained from Osmonics (Westborough, MA). Anti-caspase 3 and anti-caspase 8 antibodies were kindly provided by Dr. Shao-Cong Sun (Penn State University College of Medicine, currently at the M.D. Anderson Cancer Center, Houston, TX). Alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG) was from Bio-Rad (Hercules, CA). [methyl-3H]methionine (84 Ci/mmol), [5-3H]proline (15–40 Ci/mmol) and biodegradable counting solution (BCS) were from Amersham (now GE). Phenylisothiocyanate (PITC) was from Pierce (Rocford, IL). Cycloheximide, trifluoroacetic acid (TCA), and Protein Standard (BSA) were from Sigma (St. Louis, MO). All other chemicals were obtained from Fisher (Pittsburgh, PA).
Cell Culture Maintenance
COS-7, wild type PERK+/+ and PERK−/−-knockout cells were maintained in DMEM (plus 4.5 g/l glucose) supplemented with 10% FCS, 2mM glutamine, 100μg/ml streptomycin, and 100 U/ml penicillin in a humidified atmosphere at 37°C with 5% CO2. Wild type (PKR+/+) and PKR−/− cells were maintained in DMEM with 10% FBS, 0.1 mM 2-mercaptoethanol, 1mM sodium pyruvate, 100 μg/ml streptomycin, and 100 U/ml penicillin in a humidified atmosphere at 37°C with 5% CO2. Stock cultures were maintained in 75 cm2 Corning flasks, and experimental cultures were grown in 10 cm2 or 6 cm2 Falcon plates. The cells were subcultured when they were 70–80% confluent.
Western Blotting
The ECF detection system was utilized for Western blotting according to manufacturer’s instructions. Proteins were electrophoretically separated on 12.5% polyacrylamide gels under denaturing conditions in 0.1% sodium dodecyl sulfate (SDS) [18]. The proteins were then transferred to PVDF membrane at 45V for 50 min at room temperature or overnight at 30V, 4°C in the transfer buffer (25 mM Tris, 192 mM glycine, and 20% methanol). When proteins were transferred overnight, the next morning the voltage was raised to 90V for an additional hour to complete the transfer. Membranes were incubated with a rabbit polyclonal antibody (1:4,000 dilution) that specifically recognizes eIF2α, which is phosphorylated at Ser 51 followed by alkaline phosphatase-conjugated anti-rabbit secondary antibody (1:4,000 dilution) in Tris-buffered saline-Tween 20 (TBS-T). The relative amount of total eIF2α in each sample was determined by probing the membranes with a monoclonal antibody (1:500 dilution) that recognizes both the phosphorylated and unphosphorylated forms of eIF2α, followed by alkaline phosphatase-conjugated anti-mouse secondary antibody (1:4,000 dilution). eIF-4G was detected with a rabbit polyclonal antibody (1:3,000 dilution). Proenzymes and the cleaved products indicating activation of caspase 3 or caspase 8 were detected with rabbit polyclonal antibodies used at 1:2,000 or 1:800 dilution, respectively. In each case, the membranes were then extensively washed with TBS-T and developed with ECF substrate for 3–5 min. The fluorescence developed was scanned with a Molecular Dynamics’ FluorImager using a 570 nm filter, and bands were quantified using ImageQuant software.
Determination of Protein Synthesis Rate and Amino Acid Uptake
Cells were seeded in 10 cm2 dishes and treated with MG132 for 4 h (at about 80% confluence). After 4 h of treatment, cells were incubated with 0.4 μCi/ml [5-3H]proline (15–40 Ci/mmol) or with 0.4 μCi/ml [methyl-3H]methionine (84 Ci/mmol) for 20 min. The medium was then removed and cells were washed twice with PBS. Cells were lysed and unincorporated radioactive amino acid was removed by washing cells once for 10 min and twice for 5 min each with 10% TCA on ice. After washing once with methanol, cells lysates were neutralized with 1 ml of 0.3 N NaOH, 1% SDS for 30 min at room temperature. The lysate (800 μl) was added to 10 ml BCS and counted. To measure amino acids uptake, cells were treated similarly labeled with 0.4 μCi/ml [3H]proline or 1 μCi/ml [3H]methionine. After labeling, cells were washed twice with PBS to remove extracellular (unabsorbed) amino acids, and then lysed in 1 ml of 0.3 N NaOH, 1% SDS for 30 min at room temperature. The intracellular radioactivity released into the lysate (800 μl) was counted in 10 ml BCS [19].
Analysis of Apoptotic Cells by Flow Cytometry
Apoptosis was analyzed based on the translocation of phosphatidylserine from the inner side of the plasma membrane to the outer layer during the early stages of apoptosis [20]. Equal numbers of cells were seeded in 10 cm2 dishes, and grown until they were 70% confluent. Then cells were treated with MG132 (50 μM) or DMSO (0.1%) for 4 h. After treatment, cells were washed gently with 10 ml PBS twice, and incubated with trypsin for 3 min. After removal of trypsin, cells were incubated at 37°C just until they appeared to be detached. Cells were then washed with PBS and centrifuged at 200× g for 5 min. Cells were resuspended in 100 μl of annexin V binding buffer (10 mM Hepes/NaOH, pH 7.4, 5 mM CaCl2, 140 mM NaCl) containing FITC-labeled annexin V (annexin V-FITC) and propidium iodide (PI; 50 μg/ml). Cells were incubated in this solution by shaking at room temperature for 15 min. Samples were then diluted in Hepes buffer and subjected immediately to flow cytometric analysis using a FACS Scan II flow cytometer. Early-stage apoptotic cells stain with annexin V only, while late-stage apoptotic cells as well as necrotic cells stain with both annexin V and PI.
Amino Acid Analysis by HPLC
Cells were lysed in 0.1 N HCl by three quick freeze-thaw cycles. After centrifugation at 16,000 × g, 4°C for 10 min, the pellet was resuspended in 0.3 N NaOH for protein assay. The supernatant containing free amino acids was then dried down by centrifugal evaporation (SpeedVac), resuspended in redry solution (20% triethylamine, 40% ethanol), and dried down again. Amino acids were then resuspended in derivatization solution [70% ethanol, 10% triethylamine, 10% phenylisothiocyanate (PITC)] for 10 min. After drying again by centrifugal evaporation, the samples were resuspended in sample diluent buffer [95% 2.6 mM Na2HPO4 pH 7.4 (pH was adjusted with 10% phosphoric acid) and 5% acetonitrile], and were directly injected for HPLC analysis. The HPLC system consisted of a Waters 600E multisolvent delivery system equipped with a Waters 484 tunable absorbance detector and a 30 cm × 3.9 mm C18 column. Amino acids were detected at 254 nm. The mobile phase A (solution A) consisted of 94% 140 mM sodium acetate trihydrate, 0.05% triethylamine (pH was adjusted to pH 6.4 with glacial acetic acid) and 6% acetonitrile. The mobile phase B (solution B) was 60% acetonitrile, which was degassed prior to use by sonication (using a Bronson 2200 ultrasonic bath) under vacuum for 20 sec. The gradient conditions were initially 100% solution A for 13.5 min, followed by an immediate change to 3% solution B and then a linear change to 23% solution B over 16.5 min; then to 71% solution A and 29% solution B over 13 min; then to 34% solution B over 7 min; maintained isocratically at 34% solution B for 10 min; then to 100% solution B over 0.5 min, maintained isocratically at 100% solution B for 2.5 min; then to 100% solution A over 2 min; and maintained at 100% solution A for 3 min. The flow rate was 1 ml/min, but was raised to 1.5 ml/min during the final 3 min washing of the column with 100% solution A. To calculate methionine concentration, a standard curve was obtained as a function of known concentrations of methionine and the corresponding peak areas using linear regression with Prism 3.0 software (GraphPad Software, San Diego, CA). The methionine concentration in each sample was then calculated from the sample absorbance and the standard curve.
Protein Determination
The protein concentration was determined using the Bio-Rad dye-binding assay. Bovine serum albumin was used as a standard [21].
Statistical Analysis
Statistical analyses were carried out with Prism 3.0 software (GraphPad Software, San Diego, CA). Student’s two-tailed t-test was used to determine whether observed differences were statistically significant (p<0.05).
RESULTS
During our studies of the mechanism of S-adenosylmethionine decarboxylase degradation, we found that a significant increase in S-adenosylmethionine (AdoMet) level (>2-fold) occurred shortly after inhibition of the 26S proteasome [13]. One potential mechanism to account for this observed increase in [AdoMet] could be that inhibition of the 26S proteasome stabilized AdoMet synthase, the enzyme which catalyzes the production of AdoMet from methionine and ATP, thereby increasing the amount of AdoMet synthase activity. Using Western blot analysis, we determined that the amount of one of the Sam synthase subunits increased only 30% after 4 h inhibition of the 26S proteasome, while other subunits were not increased at all (data not shown), suggesting that there must be additional mechanisms involved in producing the increase in AdoMet level observed after inhibition of the 26S proteasome. A second potential mechanism for the increase [AdoMet] would be an increase in the availability of the methionine substrate for the AdoMet synthase-catalyzed reaction after 26S proteasome inhibition. Analysis of amino acid profiles after 4 h MG132 (50 μM) treatment of COS-7 cells indicated that inhibition of the 26S proteasome resulted in a 1.7- to 2.1-fold increase in the level of all detectable amino acids including methionine (Table 1), with methionine concentrations in MG132 treated cells rising to 12.8 pmol/mg protein as compared to 7.2 pmol/mg protein in control cells. This strongly suggested that the increase in AdoMet level observed was likely due to an increase in the availability of methionine as substrate. This increase in intracellular methionine (or other amino acids) concentration could occur either 1) through an increase in uptake of methionine from the medium; 2) through down-regulation of protein synthesis (less usage of intracellular amino acids for protein synthesis); or 3) through an increase in protein degradation activity, thus supplying more methionine through release from degraded proteins. This 3rd possibility was highly unlikely under these experimental conditions, since the major intracellular protein degradation apparatus (the 26S proteasome) was inhibited. Using [3H]methionine or [3H]proline in separate experiments, it was determined that inhibition of the 26S proteasome did not affect amino acid uptake (data not shown). Therefore, we next decided to investigate possible changes in the rate of protein synthesis following cell exposure to MG132. As seen in Fig. 1, the determination of protein synthesis rate by [3H]proline incorporation into TCA precipitable proteins indicated that treatment of COS-7 cells with MG132 (50 μM) for 4 h reduced the rate of protein synthesis by ~55% as compared to the control cells (treated with 0.1% DMSO). Likewise, a ~60% decrease in protein synthesis rate was observed in MG132 treated cells when [3H]methionine was used as a radiolabel (Fig. 1, plotted on the right side of the graph).
Table 1. Effect of 26S proteasome inhibition on amino acid levels in COS-7 cells.
Cells were treated with MG132 (50 μM) or DMSO (0.1%) for 4 h. The results are presented as fold increase (MG132/control). The values are means±SD (n=2).
| Amino Acid | Fold Increase |
|---|---|
| Thr | 2.07±0.31 |
| Tyr | 2.12±0.20 |
| Val | 2.09±0.18 |
| Met | 1.70±0.12 |
| Leu | 2.14±0.14 |
| Phe | 2.18±0.11 |
| Lys | 1.82±0.22 |
Figure 1. Effect of the 26S proteasome inhibitor on protein synthesis in COS-7 cells.

To determine the protein synthesis rate after MG132 treatment (50 μM) or 0.1% DSMO for 4 h, cells were labeled by addition of either 0.5 μCi/ml [3H]proline or 0.4 μCi/ml [3H]methionine to the complete media for 20 min. Cells were washed extensively with PBS, and with 10% TCA for 10 min to remove unincorporated radioactive precursor. Cells were washed with 10% TCA twice more for 5 min each. After a final wash with absolute MeOH, cells were lysed in 0.3N NaOH, 1% SDS for 30 min at room temperature. Aliquots were counted by liquid scintillation counting in 10 ml BCS. Values are means±SEM (n = 4 in [3H]proline experiment; n = 3 in [3H]methionine experiment). The p values significantly different in comparison with the control are indicated (Student’s t-test). The SEM is small and not seen in the MG132-treated group in [3H]methionine incorporation experiment (plotted on the right Y-axis).
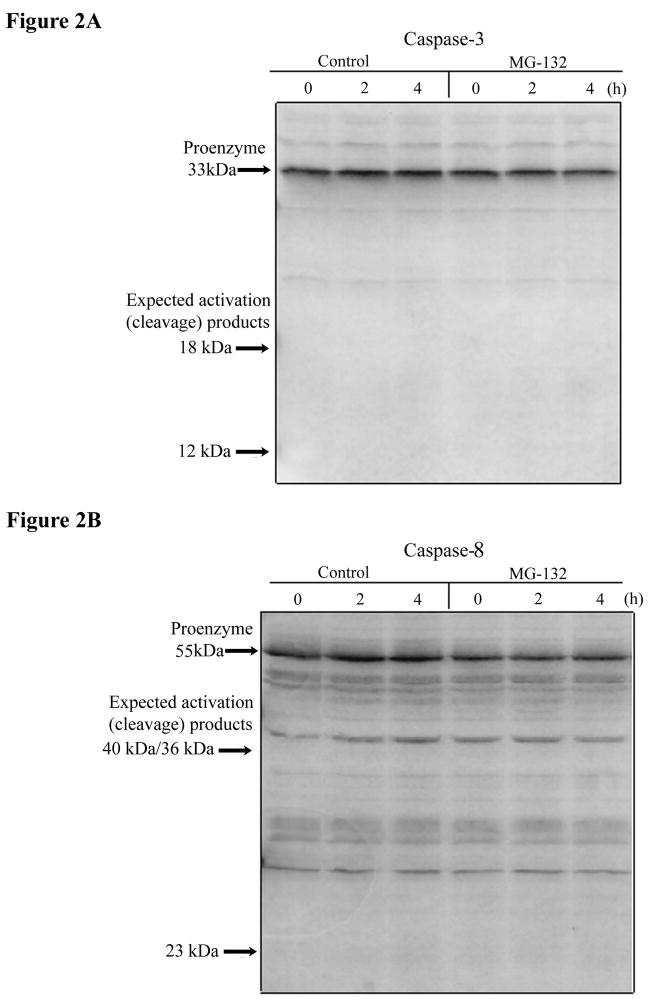
As noted in the Introduction, it has previously been shown that apoptosis can be induced by different proteasome inhibitors, at least at longer exposure times than used in the current experiments, probably as a consequence of the accumulation of some crucial proteins that would normally be degraded by the ubiquitin-proteasome pathway [14–16, 22, 23]. Therefore, we decided to investigate whether apoptosis was induced in COS-7 cells after short-term inhibition of the 26S proteasome, thereby possibly accounting for the observed decrease in protein synthesis. First, we investigated whether the apoptosis executioner caspase 3, which is an enzyme activated in almost every apoptotic process [14, 16], or caspase 8, another early caspase, were cleaved to their active forms after inhibition of the 26S proteasome. As can been seen in the Western blot analyses in Fig. 2A and B, no disappearance of caspase 3 or caspase 8 precursor protein was detected after addition of the proteasome inhibitor MG132 to COS-7 cells for up to 4 h. Secondly, the percentage of cells undergoing apoptosis after 4 h treatment of cells with MG132 was the same as in untreated control cells, as measured by flow cytometry analysis of annexin V and propidium iodide staining (data not shown), again indicating no apoptosis was induced at early time points after MG132 addition. Pre-incubation of COS-7 cells with the broad-spectrum caspase inhibitor I (Z-VAD-FMK, 35 μM) for 1 h before addition of fresh medium containing MG132 and caspase inhibitor I also failed to prevent the loss of protein synthesis activity 4 h later (data not shown). This time-dependent experiment also indicated that the loss of protein synthesis activity occurs as early as 2 h after addition of MG132. Altogether, these results indicate that loss of protein synthesis activity after short-term (2–4 h) inhibition of the 26S proteasome is independent of and precedes any induction of apoptosis.
Figure 2. Loss of protein synthesis activity after inhibition of the 26S proteasome is not due to activation of caspases.
A) Analysis of caspase 3 and B) caspase 8 activation. After treatment of COS-7 cells with MG132 (50 μM) or 0.1% DMSO, equal amounts of protein (50 μg) from cells lysates were resolved by electrophoresis on a 12.5% polyacrylamide gel, followed by transfer to PVDF membrane at 50V at room temperature for 45 min. The membranes were probed with a caspase 3 polyclonal antibody (1:2,000 diluted) overnight at 4°C. The membrane was then washed extensively with TBS-T and incubated with alkaline phosphatase-conjugated anti-rabbit IgG (1:4,000 diluted). The membrane was developed with ECF detection reagent (Amersham), and visualized with a FluorImager. The same membrane was stripped and reprobed with a caspase 8 polyclonal antibody (1:800 diluted), and developed as described above.
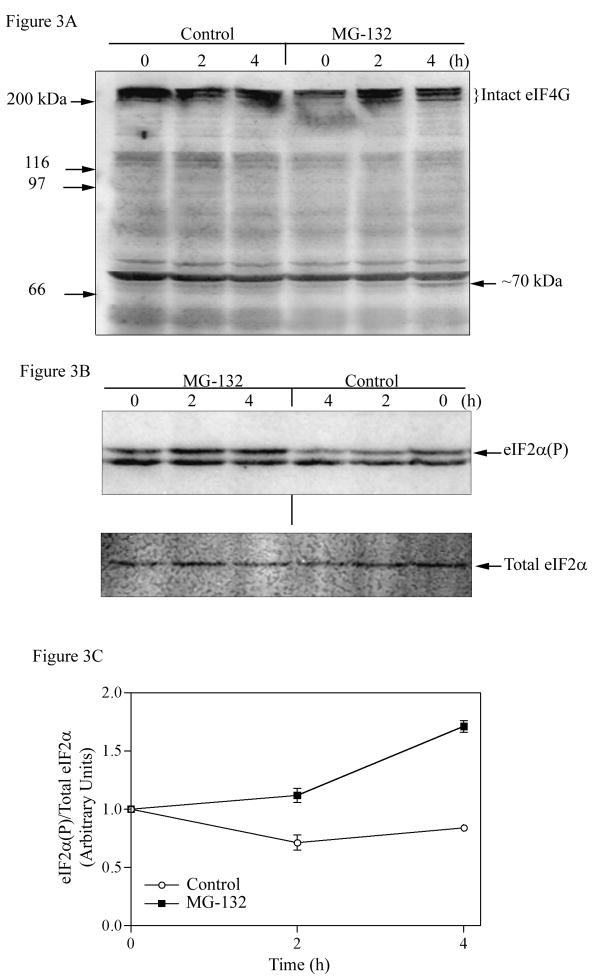
Post-translational processing of some eukaryotic initiation factors, in particular eIF-4G and eIF2, is known to regulate the global rate of protein synthesis in response to physiological insults. For example, picarnovirus infections or antitumor agents cisplatin or etoposide induce the cleavage of eIF-4G, which also occurs during apoptosis and which completely shuts off protein synthesis [24, 25]. Therefore, we next examined the stability of eIF-4G after treatment of cells with MG132. The Western blot in Fig. 3A shows that the intact eIF-4G, which runs as a set of three closely spaced bands about 220 kDa, was not cleaved into any visible smaller fragments after 2 h of MG132 treatment, at which point protein synthesis is already inhibited. Although a faint immunoreactive band of the anticipated size (~70 kDa) was observed in treated cells after 4 h of MG132 treatment, the amount of intact and presumably functional eIF-4G was not significantly affected by MG132 treatment, eliminating eIF-4G cleavage as a viable explanation of the observed 55–60% decrease in protein synthesis rate.
Figure 3. Effect of the proteasome inhibitor on eIF-4G cleavage and phosphorylation of eIF2α in COS-7 cells.
A) COS-7 cells were treated with MG132 (50 μM) for times indicated. Equal amounts of protein (50 μg) were separated on 7.5% SDS-PAGE gel, transferred to PVDF membrane, and detected with an eIF-4G polyclonal antibody as described in Experimental Procedures. B) To detect phosphorylated eIF2α and total eIF2α, equal amounts of protein (50μg) were separated on 12.5% SDS-PAGE gel, followed by Western blot analysis as described in Experimental Procedures. C) The results were quantitated by ImageQuant software, and the data is expressed as the level of phosphorylated eIF2α divided by the total level of eIF2α, with time point 0 set as one. Values are means±SEM (n = 2). The error bars are small and not visible for some data points.
To determine whether 26S proteasome inhibition can cause an increase in eIF2α phosphorylation, COS-7 cells were treated with MG132 (50 μM) in 0.1% DMSO, and the relative phosphorylation state of eIF2α was determined by Western blot analysis. The blots were probed with a polyclonal antibody that only recognizes phosphorylated eIF2α (phosphorylated at Ser 51). The blots were then stripped and reprobed with a monoclonal antibody that equally recognizes both phosphorylated and unphosphorylated forms of the protein, thus measuring total eIF2α levels. As shown in Fig. 3B and quantitated in Fig. 3C, the relative amount of phosphorylated eIF2α increased 20% by 2 h and 67% by 4 h treatment of COS-7 cells with MG132. In contrast, the relative level of eIF2α phosphorylation was slightly decreased in vehicle-control cells treated with 0.1% DMSO for the same time period. The decrease in eIF2α phosphorylation coincides with the increased rate of protein synthesis observed in control cells (data not shown), a well-known effect of addition of fresh serum-containing medium to cells.
There are several possible mechanisms which could explain how inhibition of the 26S proteasome may be inducing eIF2α phosphorylation: 1). Phosphorylated eIF2α might normally be rapidly degraded by the 26S proteasome, and thus accumulate rapidly to higher levels when the 26S proteasome is inhibited; 2). Inhibition of the 26S proteasome could result in an increase in the activity of an eIF-2α kinase; or 3). There could be a decrease in the activity of an eIF2α phosphatase following inhibition of the 26S proteasome. Initially, we examined whether increasing eIF2α phosphorylation might affect the stability of the protein or not. Our own observations and other reported studies indicated that treatment of cells with okadaic acid (an inhibitor of phosphatases 1 and 2A) results in an increase in eIF2α phosphorylation [26, 27]. Therefore, COS-7 cells were pretreated for 1 h with 1 μM okadaic acid to increase the amount of phosphorylated eIF2α and then eIF2α stability was determined by cycloheximide chase using Western blot analysis. Although eIF2α phosphorylation was significantly increased by okadaic acid treatment, the half-life of eIF2α in treated cells was the same as that in the control cells (data not shown). In addition, stoichiometric phosphorylation of purified eIF2α by an in vitro kinase treatment did not change its stability in an in vitro degradation assay (data not shown). These results suggested that phosphorylated eIF2α is not normally subject to rapid degradation by the 26S proteasome, and precludes direct changes in phosphorylated eIF2α stability contributing to its relative increase after 26S proteasome inhibition.
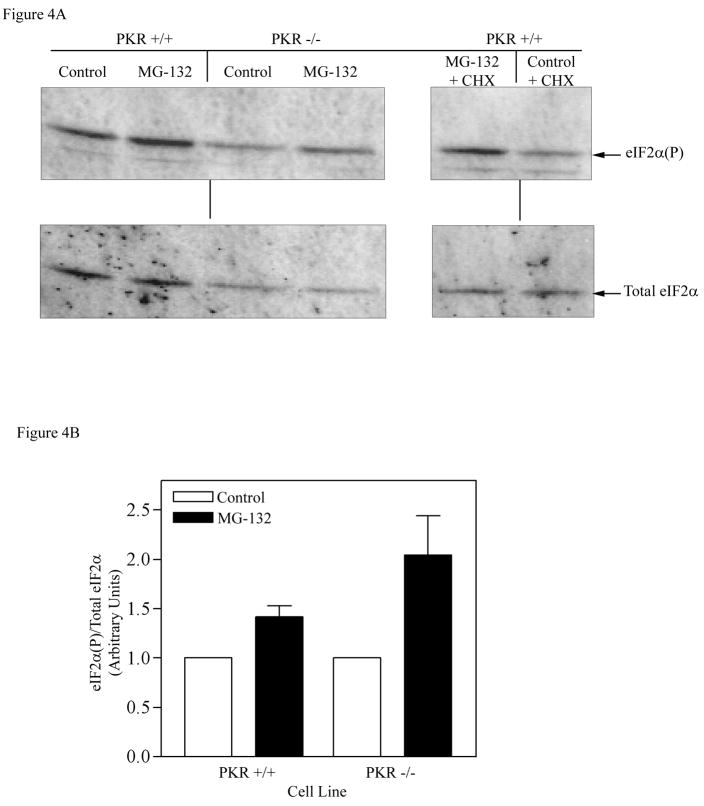
Next, we investigated which of the known eIF2α kinases might be mediating the observed phosphorylation of eIF2α following 26S proteasome inhibition, by testing whether knockout cell lines of each of the known eIF2α kinases no longer showed increased phosphorylation of eIF2α following 26S proteasome inhibition. The first kinase tested was the double-stranded RNA-dependent protein kinase (PKR), which is a ubiquitously expressed serine/threonine protein kinase that has been implicated in mediating phosphorylation of eIF2α in response to dsRNA, cytokine, growth factor and stress signals [28]. Fibroblasts containing a chromosomal deletion in the PKR gene (PKR-KO cells) were utilized to determine whether this kinase is responsible for phosphorylation of eIF2α after inhibition of the 26S proteasome. The results presented in Fig. 4A and B indicated that treatment with the MG132 proteasome inhibitor for 4 h resulted in 1.54-fold increase in eIF2α phosphorylation in parental wild type cells (PKR+/+) as compared to 1.65-fold increase in PKR-KO cells (PKR−/−). This indicates that PKR is not the primary kinase involved in the elevation of eIF2α phosphorylation after inhibition of 26S proteasome function, since a significant rise in the amount of eIF2α phosphorylation following MG132 treatment still occurred in the cells lacking PKR activity. MG132 also stimulated eIF2α phosphorylation even if the protein synthesis was completely blocked by the cycloheximide treatment in these MEF cells, suggesting that de novo protein synthesis is not required for the effect of the proteasome inhibitor on eIF2α phosphorylation (Fig. 4A, right panel).
Figure 4. Effect of the proteasome inhibitor on eIF2α phosphorylation in PKR KO and PERK KO cells.
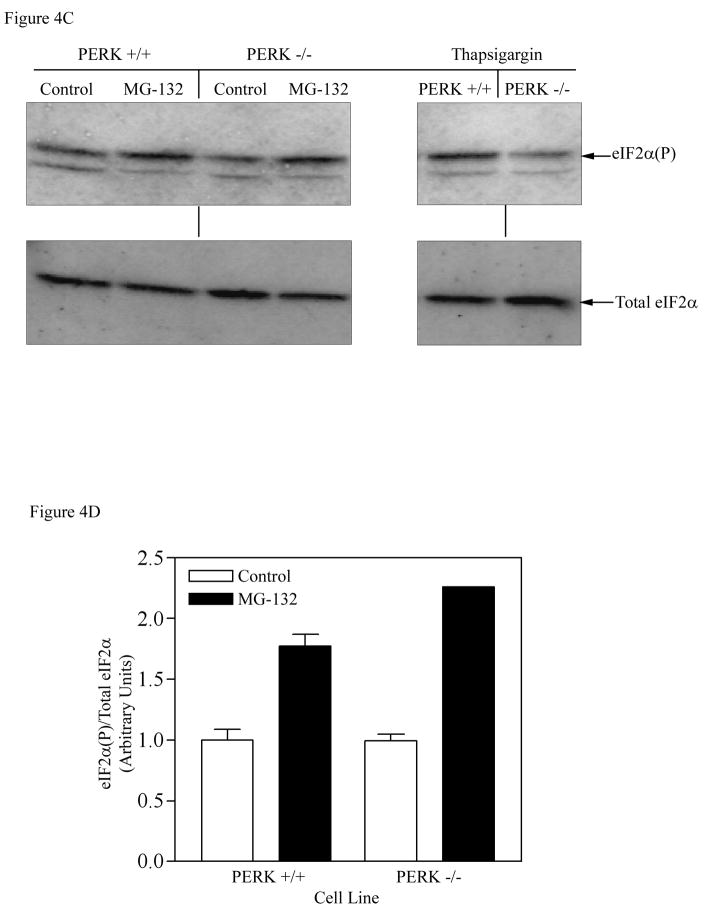
A) Wild type and PKR KO cells were treated with MG132 (50 μM) or 0.1% DMSO for 4 h. Equal amounts of protein (30 μg) were separated on 12.5% SDS-PAGE gel, followed by Western blot analysis as described in Experimental Procedures. B) The results were quantitated by ImageQuant software, and expressed as the amount of phosphorylated eIF2α divided by the total eIF2α level, with control (DMSO treated) set as 1. Cells were also treated with MG132 + CHX to determine whether complete inhibition of protein synthesis lowers the increase in eIF2α phosphorylation (right panel). C) Wild type and PERK KO cells were similarly treated with MG132 (50 μM) or 0.1% DMSO for 4 h and the total and phosphorylated eIF2α was determined by Western blot analysis. Wild type and PERK-KO cells were also treated with thapsigargin (1 μM) for 1 h, an agent known to activate PERK. D) Quantitation of the membrane shown in Fig. 4C (left panel). Each bar in Fig. 4B and D is the mean±SEM of an experiment run in duplicate. The error bars are small and not visible in certain bars.
As stated in the Introduction, in addition to its role in the degradation of essentially all short-lived proteins and the great bulk of long-lived proteins, the 26S proteasome also degrades proteins that are denatured or improperly folded [29]. In addition to leading to rapid degradation, the accumulation of unfolded proteins (e.g., in response to ER stresses) results in increased transcription of the genes for ER-resident chaperones (which would increase the protein-folding activity), and repression of protein synthesis through the increased phosphorylation of eIF2α. This response to ER stresses is known as the unfolded protein response (UPR) [30]. Recently, PKR-like ER resident kinase (PERK) has been shown to be activated in response to accumulation of misfolded proteins in the ER, and to phosphorylate eIF2α, implicating PERK in UPR-mediated repression of protein synthesis [31, 32]. Since inhibition of 26S proteasome activity could lead to the accumulation of unfolded proteins, we utilized a cell line containing a deletion of the transmembrane domain in the PERK gene (PERK-KO cells) to examine whether PERK plays a role in mediating phosphorylation of eIF2α under the conditions of blocking the 26S proteasome function. As a positive control, treatment of cells with thapsigargin, an agent that causes ER stress and subsequently activates PERK, was shown to significantly induce phosphorylation of eIF2α in wild type (Perk+/+) cells, but not in the PERK knockout (PERK−/−) cells, a finding consistent with the deletion of PERK in these cells (Figure 4C). As shown in Fig. 4C and D treatment with MG132 (50 μM) for 4 h increased the relative eIF2α phosphorylation 1.76-fold in the parental wild type (Perk+/+) cells and 2.1-fold in PERK-KO (PERK−/−) cells. Again, since continued induction of eIF2α phosphorylation by MG132 treatment occurred even in the absence of the PERK kinase, this indicates that PERK is not involved in mediating eIF2α phosphorylation following inhibition of 26S proteasome function.
A third eIF2α kinase (GCN2) was similarly tested using GCN2 knockout cell lines, and again the MG132 induced increase in eIF2α phosphorylation was not prevented by the absence of GCN2 upon exposure to 50 μM concentrations of the proteasome inhibitor MG132 for 4 h, indicating that GCN2, usually activated by amino acid starvation, is not the primary kinase mediating proteasome inhibitor-induced eIF2α phosphorylation. As seen in Figure 5A, treatment with MG132 increased eIF2α phosphorylation in both the wild-type and GCN2 knockout cell lines, and the quantitation of normalized band densities in Figure 5B shows a 2.2-fold increase in phosphorylated eIF2α in the parental wild type cells and a 5-fold increase in GCN2-KO cells following MG132 exposure. The greater fold increase in eIF2α phosphorylation in the GCN2-KO cells is presumably due to the significantly lower baseline level of phosphorylation of eIF2α in these GCN2 knockout cells, possibly indicating a GCN2 role in producing the basal levels of eIF2α phosphorylation seen in wild-type cells.
Figure 5. Analysis of eIF2α phosphorylation in GCN2 KO and HRI KO cells.

Cells were treated with MG132 as in Figure 4, and eIF2α phosphorylation was determined by Western blotting. A). Induction of eIF2α phosphorylation in GCN2 KO cells following MG132 treatment. Total and phosphorylated eIF2α were determined by Western blot analysis after treatment of wild type (GCN2+/+) and GCN2 knock out cells (GCN2−/−) with 50 μM MG132 for 4 h. B). Quantitation of the level of eIF2α phosphorylation seen in Panel A. The data are plotted as means±SEM (n = 3), normalized to the amount of total eIF2α. C). Phosphorylation of eIF2α in HRI KO cells. The total and phosphorylated eIF2α was determined by Western blot analysis after treatment of wild type (HRI+/+) and HRI knock out cells (HRI−/−) with 50 μM MG132 for 4 h. D). Quantitation of the level of eIF2α phosphorylation seen in Panel C. The data are plotted as means±SEM (n = 2).
Finally, we examined whether the heme-regulated inhibitor kinase (HRI) plays a role in eIF2α phosphorylation using HRI knockout cells harboring a deletion of the HRI genes (HRI−/− cells) following proteasome inhibition. HRI is the most important eIF2α kinase in erythroid cells where it is expressed abundantly and becomes activated in the absence of heme and inhibits protein synthesis through phosphorylation of eIF2α to prevent accumulation of inactive (misfolded) globin with no heme co-factor [33]. It was therefore initially thought that HRI was unlikely to play a role in the eIF2α phosphorylation observed in non-erythroid fibroblast cells investigated in this study. Contrary to our expectations, MEF cells which lacked HRI showed almost none of the eIF2α phosphorylation seen in parental MEF cells following 50 μM MG132 treatment for 4 h (Fig. 5C and 5D). There was 2.8-fold increase in wild type cells, but only a non-significant 1.2-fold increase in HRI−/− cells, indicating that HRI is the major eIF2α kinase involved in the increased phosphorylation of eIF2α in response to inhibition of the 26S proteasome. While the 20% increase in phosphorylation still observed in HRI−/− cells might be within the range of experimental error, it could also imply that there is another kinase, probably playing a secondary role, which phosphorylates eIF2α during 26S proteasome inhibition.
Although MG132 has been a widely used proteasome inhibitor and considered to be quite specific, there has been some evidence that it may have inhibitory effects on other proteases (e.g., calpains, cathepsins and cysteine proteases) [34, 35]. Therefore, HRI+/+ and HRI−/− cells were also treated with various concentrations of Bortezomib (also known as PS-341 or Velcade), a highly selective and novel dipeptide boronate proteasome inhibitor [36]. Since the effective concentration of Bortezomib varies in different cell lines, we tested a wide range of inhibitor concentrations. As can be seen in Figure 6, Western blot analysis demonstrated that eIF2α is significantly phosphorylated in HRI+/+ cells exposed to various concentrations of Bortezomib (0.1–10 μM) for 4 h; however, no change in the level of eIF2α phosphorylation could be seen in the knockout HRI−/− cells at any Bortezomib concentration. Taken together, the MG132 and Bortezomib results indicate that eIF2α phosphorylation was induced specifically by 26S proteasome inhibition and that HRI is the primary kinase mediating eIF2α phosphorylation in response to 26S proteasome inhibition.
Figure 6. Effect of Bortezomib on eIF2α phosphorylation in HRI wild type and HRI knock out cells.
A) Cells were treated for 4 h with the various concentrations of Bortezomib indicated above the lanes, and eIF2α phosphorylation level was determined by Western blot analysis. B) Quantitation of the level of eIF2α phosphorylation in HRI wild type and HRI knock out cells. The experiment shown in Panel 6A was carried out in triplicate and the data shown in Panel 6B is the mean±SD (n = 3).
DISCUSSION
The 26S proteasome has been shown to be involved in the various biologically important processes, such as the cell cycle, cellular metabolism, apoptosis, signal transduction, immune response, and protein quality control [37], and decreases in protein synthesis rate caused by inhibition of the 26S proteasome have been previously reported [9, 10]. Decreases in protein synthesis following longer periods of 26S proteasome inhibition have been noted to accompany induction of apoptosis [14, 16, 22, 23, 39]; however, no evidence of apoptosis was observed in the current experiments at the very early time points where inhibition of the 26S proteasome clearly caused decreased protein synthesis rates, nor were protein synthesis rates restored upon inhibition of apoptosis-related caspases. These results strongly suggest that the loss of protein synthesis activity after inhibition of the 26S proteasome is independent of the induction of apoptosis. Recently, Brophy et al. also indicated that MG132 treatment does not stimulate apoptosis in COS-7 cells; in fact, they showed that when COS-7 cells were pretreated with the proteasome inhibitors before addition of staurosporine (a known inducer of apoptosis in many cell lines), both caspase 3 activity and percentage of apoptotic cells were reduced as compared to the cells treated with staurosporine alone [40].
As others have observed, we showed that the apoptosis-independent inhibition of protein synthesis that occurs after brief inhibition of 26S proteasome function is likely due to elevated eIF2α phosphorylation [38]. Of the four protein kinases known to phosphorylate eIF2α, the results presented here indicate for the first time that HRI is the primary kinase activated by inhibition of the 26S proteasome. While it is well known that HRI mediates phosphorylation of eIF2α and is expressed at a significant level in erythroid cells, several previous studies have also provided evidence that HRI is a ubiquitous eIF2α kinase of mammalian cells, and is expressed in a wide range of non-erythroid cells [41, 42]. The present study clearly demonstrates that the HRI kinase can mediate translational attenuation in non-erythroid cells, although the mechanism of activation of the kinase is not yet clear.
A recent study reported that eIF2α was significantly phosphorylated in GCN2 wild type cells treated with 1 μM MG132 but that MG132-induced phosphorylation of this translational initiation factor was greatly decreased in knockout GCN2−/− cells; however, a closer examination of the data in that paper shows increasing phosphorylation of eIF2α even in the knockout GCN2−/− cells at later time points of treatment with MG132, e.g., after 6 hours [11]. This suggests that another kinase might also be involved, albeit more slowly, at those lower MG132 doses. We did not observe any reduction in eIF2α phosphorylation in GCN2−/− cells in response to 26S proteasome inhibition in our experimental conditions; the different results obtained in the current study may be partly due to the different concentrations used (1 μM vs. 50 μM MG132 in our study). The concentration used in our study (a standard dose used in many studies of 26S proteasome inhibition [see for example 43, 44]) may cause more complete or more rapid activation of HRI than the lower dose used in the study of Jiang et al [11], where possible phosphorylation of eIF2α in the absence of GCN2 only occurred at later time points (an HRI kinase knockout was not tested in those experiments); under the conditions tested in the current experiments, GCN2 kinase activity may still have been activated, but contributing a relatively minor portion of the observed eIF2α phosphorylation compared to that arising from HRI Kinase activity. Thus, in the GCN2−/− cells tested herein, no appreciable decrease in MG132-stimulated would be observable because of the still-present and dominant eIF2α phosphorylation occurring through HRI.
The activation of HRI might be attained by several different mechanisms, including possible interactions with Heat shock proteins (Hsps). Previous studies indicated that proteasome inhibitors enhance expression of Hsp70 and Hsp27 without heat shock in MEF [45] as well as Hsp 90 expression in lens cells [46]. In addition, another study showed that Hsp70 or Hsp90 is required for folding and transformation of HRI into an active kinase in rabbit reticulocyte lysates and, in living cells, Hsp70 is essential for the activation of HRI under stress conditions [47]. Further experiments are required to investigate links between the HRI activation and heat shock proteins during proteasome inhibition in living cells.
In summary, the results presented here reveal that inhibition of the 26S proteasome results in repression of global protein synthesis through elevated phosphorylation of eIF2α, mediated primarily through activation of HRI kinase activity. Given that proteasome inhibition induces apoptosis in several experimental models through the accumulation of a number of proapoptotic proteins such as Bax, Bid, cyclin-dependent kinase inhibitors (p21 and p27) and p53, several 26S proteasome inhibitors are currently being tested in clinical trials as anti-cancer agents that will induce apoptosis in rapidly proliferating cells. Velcade (Bortezomib) specifically has recently been approved for the treatment of multiple myeloma [48]. In spite of the obvious efficacy of bortezomib in many cases, a phase II trial of bortezomib treatment in 202 patients with refractory relapsed multiple myeloma demonstrated that as many as 65% of patients did not respond to treatment [49]. Other studies indicate that resistance to proteasome inhibition, which may account for some of the non-responsive patients in the bortezomib study cited above, may be partly due to induction of Hsp70, which is known to be an inhibitor of apoptosis acting by preventing the processing of caspases into active forms [50]. Induction of Hsp70 would also be expected to enable HRI kinase activation as mentioned above, and increased phosphorylation of eIF2α and the resulting decrease in protein synthesis rate in some patients might prevent the accumulation of normally rapidly-degraded proteins, proteins whose increased levels would play an important and necessary role in the apoptotic effects of proteasome inhibition. While inhibitors like bortezomib appear to be highly specific for 26S proteasome inhibition, better understanding of the downstream effects of 26S proteasome inhibition and the extent to which they differ between different patients may be important for understanding the differences between inhibitor-responsive and inhibitor non-responsive patients in multiple myeloma and possibly other cancer patients. Further experiments will greatly increase our understanding of molecular links between the 26S proteasome inhibition and eIF2α phosphorylation, and information obtained from such experiments may help to define novel molecular targets and therapeutic strategies to overcome what appears to be clinical resistance to proteasome inhibition in some patients.
Acknowledgments
We would like to thank Dr. Isis Rivera-Walsh for excellent assistance with the flow cytometry-based apoptosis assays; Dr. An Do and Rick Horetsky for help with eIF2α phosphorylation analysis in GCN2 and HRI knock out cells. This work was supported by NSF grant MCB-9603740 to BAS, and NIH grant DK-13499 to SRK.
Abbreviations used
- AdoMet
S-adenosylmethionine
- CHX
cycloheximide
- DMEM
Dulbecco’s Modified Eagle Medium
- DMSO
dimethyl sulfoxide
- ECF
Enhanced chemifluorescence
- eIF2
eukaryotic translational initiation factor 2
- eIF2α (P)
phosphorylated eIF2α
- eIF2B
guanine nucleotide exchange factor
- HRI
Heme-regulated inhibitor kinase
- Hsp
heat shock protein
- MEF
mouse embryonic fibroblasts
- PERK
PKR-like endoplasmic reticulum resident kinase
- PKR
Double-stranded RNA-dependent protein kinase
- PVDF
polyvinylidene difluoride
- TCA
trifluoroacetic acid
- UPR
unfolded protein response
References
- 1.Hershey JW. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- 2.Kimball SR. Eukaryotic initiation factor eIF2. Int J Biochem Cell Biol. 1999;31:25–29. doi: 10.1016/s1357-2725(98)00128-9. [DOI] [PubMed] [Google Scholar]
- 3.Satoh S, Hijikata M, Handa H, Shimotohno K. Caspase-mediated cleavage of eukaryotic translation initiation factor subunit 2alpha. Biochem J. 1999;342:65–70. [PMC free article] [PubMed] [Google Scholar]
- 4.Pain VM. Initiation of protein synthesis in eukaryotic cells. Eur J Biochem. 1996;236:747–771. doi: 10.1111/j.1432-1033.1996.00747.x. [DOI] [PubMed] [Google Scholar]
- 5.Marissen WE, Guo Y, Thomas AA, Matts RL, Lloyd RE. Identification of caspase 3-mediated cleavage and functional alteration of eukaryotic initiation factor 2alpha in apoptosis. J Biol Chem. 2000;275:9314–9323. doi: 10.1074/jbc.275.13.9314. [DOI] [PubMed] [Google Scholar]
- 6.Kumar KU, Srivastava SP, Kaufman RJ. Double-stranded RNA-activated protein kinase (PKR) is negatively regulated by 60S ribosomal subunit protein L18. Mol Cell Biol. 1999;19:1116–1125. doi: 10.1128/mcb.19.2.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 8.Hilt W, Wolf DH. Proteasomes: destruction as a programme. Trends Biochem Sci. 1996;21:96–102. [PubMed] [Google Scholar]
- 9.Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 10.Mimnaugh EG, Chen HY, Davie JR, Celis JE, Neckers L. Rapid deubiquitination of nucleosomal histones in human tumor cells caused by proteasome inhibitors and stress response inducers: effects on replication, transcription, translation, and the cellular stress response. Biochemistry. 1997;36:14418–14429. doi: 10.1021/bi970998j. [DOI] [PubMed] [Google Scholar]
- 11.Jiang HY, Wek RC. Phosphorylation of the alpha-subunit of the eukaryotic initiation factor-2 (eIF2alpha) reduces protein synthesis and enhances apoptosis in response to proteasome inhibition. J Biol Chem. 2005;280:14189–14202. doi: 10.1074/jbc.M413660200. [DOI] [PubMed] [Google Scholar]
- 12.Bush KT, Goldberg AL, Nigam SK. Proteasome inhibition leads to a heat-shock response, induction of endoplasmic reticulum chaperones, and thermotolerance. J Biol Chem. 1997;272:9086–9092. doi: 10.1074/jbc.272.14.9086. [DOI] [PubMed] [Google Scholar]
- 13.Yerlikaya A, Stanley BA. S-adenosylmethionine decarboxylase degradation by the 26 S proteasome is accelerated by substrate-mediated transamination. J Biol Chem. 2004;279:12469–12478. doi: 10.1074/jbc.M312625200. [DOI] [PubMed] [Google Scholar]
- 14.Pasquini LA, Besio Moreno M, Adamo AM, Pasquini JM, Soto EF. Lactacystin, a specific inhibitor of the proteasome, induces apoptosis and activates caspase-3 in cultured cerebellar granule cells. J Neurosci Res. 2000;59:601–611. doi: 10.1002/(SICI)1097-4547(20000301)59:5<601::AID-JNR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Wagenknecht B, Hermisson M, Eitel K, Weller M. Proteasome inhibitors induce p53/p21-independent apoptosis in human glioma cells. Cell Physiol Biochem. 1999;9:117–125. doi: 10.1159/000016308. [DOI] [PubMed] [Google Scholar]
- 16.Drexler HC. Activation of the cell death program by inhibition of proteasome function. Proc Natl Acad Sci U S A. 1997;94:855–860. doi: 10.1073/pnas.94.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giuliano M, Lauricella M, Calvaruso G, Carabillo M, Emanuele S, Vento R, Tesoriere G. The apoptotic effects and synergistic interaction of sodium butyrate and MG132 in human retinoblastoma Y79 cells. Cancer Res. 1999;59:5586–5595. [PubMed] [Google Scholar]
- 18.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Freshney RI. Culture of animal cells: A manual of basic techniques. Wiley-Liss Inc; New York: 1994. [Google Scholar]
- 20.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 21.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 22.Naujokat C, Sezer O, Zinke H, Leclere A, Hauptmann S, Possinger K. Proteasome inhibitors induced caspase-dependent apoptosis and accumulation of p21WAF1/Cip1 in human immature leukemic cells. Eur J Haematol. 2000;65:221–236. doi: 10.1034/j.1600-0609.2000.065004221.x. [DOI] [PubMed] [Google Scholar]
- 23.Drexler HC, Risau W, Konerding MA. Inhibition of proteasome function induces programmed cell death in proliferating endothelial cells. Faseb J. 2000;14:65–77. doi: 10.1096/fasebj.14.1.65. [DOI] [PubMed] [Google Scholar]
- 24.Marissen WE, Lloyd RE. Eukaryotic translation initiation factor 4G is targeted for proteolytic cleavage by caspase 3 during inhibition of translation in apoptotic cells. Mol Cell Biol. 1998;18:7565–7574. doi: 10.1128/mcb.18.12.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Etchison D, Milburn SC, Edery I, Sonenberg N, Hershey JW. Inhibition of HeLa cell protein synthesis following poliovirus infection correlates with the proteolysis of a 220,000-dalton polypeptide associated with eucaryotic initiation factor 3 and a cap binding protein complex. J Biol Chem. 1982;257:14806–14810. [PubMed] [Google Scholar]
- 26.Babu SV, Ramaiah KV. Type 1 phosphatase inhibitors reduce the restoration of guanine nucleotide exchange activity of eukaryotic initiation factor 2B inhibited reticulocyte lysates rescued by hemin. Arch Biochem Biophys. 1996;327:201–208. doi: 10.1006/abbi.1996.0110. [DOI] [PubMed] [Google Scholar]
- 27.Redpath NT, Proud CG. Activity of protein phosphatases against initiation factor-2 and elongation factor-2. Biochem J. 1990;272:175–180. doi: 10.1042/bj2720175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams BR. PKR; a sentinel kinase for cellular stress. Oncogene. 1999;18:6112–6120. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- 29.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 31.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 32.Brewer JW, Diehl JA. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc Natl Acad Sci U S A. 2000;97:12625–12630. doi: 10.1073/pnas.220247197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen JJ, London IM. Regulation of protein synthesis by heme-regulated eIF-2 alpha kinase. Trends Biochem Sci. 1995;20:105–108. doi: 10.1016/s0968-0004(00)88975-6. [DOI] [PubMed] [Google Scholar]
- 34.Zhou J, Köhl R, Herr B, Frank R, Brüne B. Calpain mediates a von Hippel-Lindau Protein-independent Destruction of Hypoxia-inducible Factor-1. Mol Biol Cell. 2006;15:1549–1558. doi: 10.1091/mbc.E05-08-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 36.Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliot PJ. Proteasome Inhibitors: A Novel Class of Potent and Effective Antitumor Agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 37.Tanaka K. Molecular biology of the proteasome. Biochem Biophys Res Commun. 1998;247:537–541. doi: 10.1006/bbrc.1998.8617. [DOI] [PubMed] [Google Scholar]
- 38.Cowan JL, Morley SJ. The proteasome inhibitor, MG132, promotes the reprogramming of translation in C2C12 myoblasts and facilitates the association of hsp25 with the eIF4F complex. Eur J Biochem. 2004;271:3596–3611. doi: 10.1111/j.0014-2956.2004.04306.x. [DOI] [PubMed] [Google Scholar]
- 39.Wagenknecht B, Hermisson M, Groscurth P, Liston P, Krammer PH, Weller M. Proteasome inhibitor-induced apoptosis of glioma cells involves the processing of multiple caspases and cytochrome c release. J Neurochem. 2000;75:2288–2297. doi: 10.1046/j.1471-4159.2000.0752288.x. [DOI] [PubMed] [Google Scholar]
- 40.Brophy VA, Tavare JM, Rivett AJ. Treatment of COS-7 cells with proteasome inhibitors or gamma-interferon reduces the increase in caspase 3 activity associated with staurosporine-induced apoptosis. Arch Biochem Biophys. 2002;397:199–205. doi: 10.1006/abbi.2001.2679. [DOI] [PubMed] [Google Scholar]
- 41.Berlanga JJ, Herrero S, de Haro C. Characterization of the hemin-sensitive eukaryotic initiation factor 2alpha kinase from mouse non-erythroid cells. J Biol Chem. 1998;273:32340–32346. doi: 10.1074/jbc.273.48.32340. [DOI] [PubMed] [Google Scholar]
- 42.Mellor H, Flowers KM, Kimball SR, Jefferson LS. Cloning and characterization of cDNA encoding rat hemin-sensitive initiation factor-2 alpha (eIF-2 alpha) kinase. Evidence for multi-tissue expression. J Biol Chem. 1994;269:10201–10204. [PubMed] [Google Scholar]
- 43.Tanaka H, Miake J, Notsu T, Sonyama K, Sasaki N, litsuka K, Kato M, et al. Proteasomal degradation of Kirb.2 channel protein and its inhibition by a Na+ channel blocker aprindine. Biochem Biophys Res Commun. 2005;331:1001–1006. doi: 10.1016/j.bbrc.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Alao JP, Stavropoulou AV, Lam EWF, Coombes RC, Vigushin DM. Histone deacetylase inhibitor, Trichostatin A induces ubiquitin-dependent cyclin D1 degradation in MCF-7 breast cancer cells. Mol Cancer. 2006;5:8. doi: 10.1186/1476-4598-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim D, Kim SH, Li GC. Proteasome inhibitors MG132 and lactacystin hyperphosphorylate HSF1 and induce hsp70 and hsp27 expression. Biochem Biophys Res Commun. 1999;254:264–268. doi: 10.1006/bbrc.1998.9840. [DOI] [PubMed] [Google Scholar]
- 46.Awasthi N, Wagner BJ. Upregulation of heat shock protein expression by proteasome inhibition: an anti-apoptotic mechanism in the lens. Invest Ophthalmol Vis Sci. 2005;46:2082–2091. doi: 10.1167/iovs.05-0002. [DOI] [PubMed] [Google Scholar]
- 47.Lu L, Han AP, Chen JJ. Translation initiation control by heme-regulated eukaryotic initiation factor 2alpha kinase in erythroid cells under cytoplasmic stresses. Mol Cell Biol. 2001;21:7971–7980. doi: 10.1128/MCB.21.23.7971-7980.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5:417–421. doi: 10.1016/s1535-6108(04)00120-5. [DOI] [PubMed] [Google Scholar]
- 49.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 50.Jones EL, Zhao MJ, Stevenson MA, Calderwood SK. The 70 kiloDalton heat shock protein is an inhibitor of apoptosis in prostate cancer. Int J Hyperthermia. 2004;20:835–849. doi: 10.1080/02656730410001721807. [DOI] [PubMed] [Google Scholar]