Abstract
Subcellular compartmentalization of reactive oxygen species (ROS) plays a critical role in transmitting cell signals in response to environmental stimuli. In this regard, signals at the plasma membrane have been shown to trigger NADPH oxidase-dependent ROS production within the endosomal compartment and this step can be required for redox-dependent signal transduction. Unique features of redox-active signaling endosomes can include NADPH oxidase complex components (Nox1, Noxo1, Noxa1, Nox2, p47phox, p67phox, and/or Rac1), ROS processing enzymes (SOD1 and/or peroxiredoxins), chloride channels capable of mediating superoxide transport and/or membrane gradients required for Nox activity, and novel redox-dependent sensors that control Nox activity. This review will discuss the cytokine and growth factor receptors that likely mediate signaling through redox-active endosomes, and the common mechanisms whereby they act. Additionally, the review will cover ligand-independent environmental injuries, such as hypoxia/reoxygenation injury, that also appear to facilitate cell signaling through NADPH oxidase at the level of the endosome. We suggest that redox-active endosomes encompass a subset of signaling endosomes that we have termed redoxosomes. Redoxosomes are uniquely equipped with redox-processing proteins capable of transmitting ROS signals from the endosome interior to redox-sensitive effectors on the endosomal surface. In this manner, redoxosomes can control redox-dependent effector functions through the spatial and temporal regulation of ROS as second messengers. Antioxid. Redox Signal. 11, 1313–1333.
Introduction
Although the concept of a free radical originally emerged in the mid 19th century, its popularity quickly faded when no one was able to isolate free radical compounds (41). Indeed, it was only a chance discovery by Moses Gomberg that led to the first publication detailing radical chemistry in 1900 (37). Gomberg had endeavored to generate hexaphenylethane from triphenylmethyl chloride, but instead produced an unexpected organic yellow compound that was more reactive than expected for hexaphenylethane. This finding led Gomberg to propose the presence of a triphenylmethyl radical as an intermediate in the reaction. Despite this discovery and significant interest in Gomberg's papers, it took many decades for the general scientific community to accept the idea of an organic radical. The field again took significant steps forward in the 1920s and 1930s, with the development of quantum mechanics. In particular, the newly emerging idea of activation energy provided an explanation for the difficulties in isolating highly reactive, short-lived intermediates such as radicals as pure substances. A solution to the difficulty in studying these short-lived radicals came about in the 1950s, with the release of commercial electron paramagnetic resonance (EPR) machines. This allowed for the detection of radicals based on their paramagnetic properties.
Biological free radicals were first identified in 1968, when Joe McCord and Irwin Fridovich examined the production of superoxide by the xanthine oxidase found in milk (76). The report of this observation was soon followed by a second paper discussing the enzymatic function of erythrocuprein (also known as hemocuprein) as a superoxide dismutase that produces hydrogen peroxide and oxygen from two superoxide molecules (77). Superoxide production was subsequently linked to white blood cells (WBCs), and a failure of WBCs to produce superoxide was linked to chronic granulomatous disease. This provided superoxide with a biological function—as a bactericidal agent that is produced within the phagosome of a WBC following phagocytosis of a pathogen (4). Another biological role for superoxide was discovered in the early 1980s, when it was recognized that superoxide levels increase in vascular tissue during reperfusion following an ischemic event (38, 75). The production of superoxide in both cases—in the vasculature and in WBCs—was eventually linked to members of a group of membrane proteins that is known as the NADPH oxidase (Nox) family (60, 96, 103).
Seven known NADPH oxidase catalytic subunits exist (Nox1, Nox2gp91phox, Nox3, Nox4, Nox5, Duox1, and Duox2) (48, 60). NADPH oxidases generate superoxide by transferring an electron from NADPH to molecular oxygen. The most widely characterized NADPH oxidase is phagocytic gp91phox (Nox2). The phagocytic NADPH oxidase is a multi-subunit enzyme complex with both membrane and cytosolic components (60). The membrane subunits gp91phox and p22phox make up the flavocytochrome b558 component of phagocytic Nox. The cytosolic subunits include p47phox, p67phox, p40phox, and the small GTPase Rac1/2. In the resting state the cytosolic components remain quiescent in the cytoplasm, and the membrane-bound cytochrome b558 complex is inactive. Upon stimulation, the cytosolic subunits are translocated to the membrane to bind the cytochrome b558 components, leading to activation of the NADPH oxidase complex. Included in this activation process is the phosphorylation of p47phox and p67phox (28), and the conversion of GDP-bound Rac1/2 into GTP-bound forms through the activation of a Rac guanine nucleotide exchange factor (2, 80). Other NADPH oxidases share several of the co-activator subunits with Nox2, but can also use unique regulators (Noxo1 and Noxa1) (48, 60).
Over the last two decades, superoxide and hydrogen peroxide produced by NADPH oxidases have been linked to a number of highly spatially- and temporally-regulated signaling mechanisms. This review will focus on the biology of one of these signaling mechanisms—those defined by redox-active endosomes. This group of signaling pathways generates superoxide in the endosome lumen as a signaling intermediate, in response to stimuli such as interleukin-1-beta (IL-1β) (68, 78), tumor necrosis factor alpha (TNFα) (67, 78, 119), and hypoxia/reoxygenation (H/R) (69). The available evidence suggests that each of these pathways produces superoxide through the family of NADPH oxidases, and that each of these signaling pathways requires an endocytic event to facilitate redox signaling at the endosomal level. The similarity of these events to those of phagosome formation in macrophages and neutrophils suggests conservation of certain phagosomal mechanisms in the signaling pathways of nonphagocytic cells. As within the phagosome, confinement of membrane-impermeable superoxide within the boundaries of an endosome allows for regulated localization and concentration of ROS at sites of cellular signaling, while preventing more widespread nonspecific redox-dependent damage to proteins, lipids, and DNA within cells.
The current literature strongly supports the idea of luminal production of Nox-derived superoxide in TNFα-, IL-1β-, and H/R-mediated signaling endosomes. To a degree, recent evidence also suggests that platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and angiotensin II (AngII)— and to a lesser degree insulin and fas ligand (FasL)— regulate superoxide production and stimulate specific downstream redox-signaling through Nox-positive superoxide-producing endosomes. This review focuses on emerging knowledge in the fledgling area of cellular signaling through superoxide-producing endosomes, structures that we propose to name redoxosomes (redox-active endosomes) based on conserved features that appear to control redox signaling events. In this review, emphasis will be placed on studies of interleukin -1β (IL-1β), tumor necrosis factor alpha (TNFα), and hypoxia/reoxygenation (H/R) signaling, and to a lesser degree on studies of platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and angiotensin II (AngII), with the goal of outlining a model whereby redoxosomes act as a common redox-signaling compartment for a diverse set of stimuli with stimulus-specific outcomes. We will examine in detail the events of the selected systems—from signal initiation to Nox activation, cellular handling of ROS, signal transduction, and the termination of Nox activity and superoxide production. By dissecting the mechanisms whereby these systems achieve diverse outcomes using the same redox signaling intermediates, we hope to build a basic foundation for redoxosomal signaling that may be expanded upon in the future.
General Overview of Types of Receptor Signaling from the Plasma Membrane: Redoxosomes in Context
Ligands can initiate cellular responses through their binding to receptors at the cell surface. Common to all types of cell surface receptor signaling is the conversion of an extracellular message to an intracellular signal. To promote an appreciation of aspects of receptor signaling that are unique to redoxosomes, we will give a broad overview of major classes of receptor-mediated signaling from the cell surface in this section.
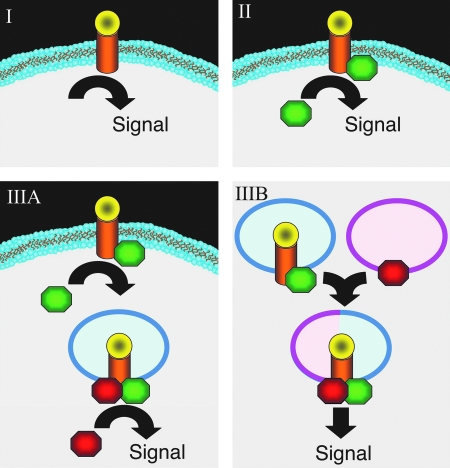
Mechanisms of receptor signaling from the plasma membrane can be generally grouped into three categories (Fig. 1). In the most basic of these, a surface membrane protein/receptor directly gives rise to a functional signal following either exposure to an environmental stimulus or ligand binding (Type I, Fig. 1). In this context, the protein/receptor transmits its intracellular signal while remaining at the plasma membrane. Notable examples of such proteins are ion channels, which can respond to changes in membrane potential or to ligand binding by inhibiting or promoting the passage of ions (98). For instance, the GABA family of receptors includes inhibitory and excitatory ion channels that, in the dendrite of a neuron, may either potentiate or excite a synapse (73). A second mechanism (Type II, Fig. 1), exemplified by G-protein signaling, involves the recruitment of a second messenger to the signaling pathway (61). In this context, structural changes in the intracellular portion of the receptor are induced following ligand binding, and allow for the docking of G-protein components. Downstream signals are then transmitted not directly by the receptors but through receptor-specific effectors. This kind of mechanism inherently allows for a higher level of regulation and signal amplification, through the production of second messengers that directly impart intracellular signals.
FIG. 1.
Mechanisms of receptor signaling following ligand binding at the plasma membrane. (I) Ligand binding directly activates a receptor-mediated cell signaling event at the plasma membrane in the absence of additional cytosolic effectors. (II) Ligand binding leads to the recruitment of a cytosolic effector to the receptor at the plasma membrane, which in turn initiates signaling. (IIIA) Ligand binding and cytosolic effector recruitment initiates endocytosis of the receptor. Following endocytosis and the formation of a specific microenvironment, downstream effectors are recruited to drive formation of an active signaling complex. (IIIB) Ligand binding and cytosolic effector recruitment initiate endocytosis of the receptor, as shown in IIIA. However, the formation of a signaling complex requires endosomal fusion with another cellular compartment to generate the microenvironment needed for receptor complex activation. Such microenvironmental changes may include the docking of secondary membrane associated effectors (as shown) and/or changes in endosomal membrane structure/function (i.e., the composition of phospholipid or ion channels or endosomal pH). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
A third mechanism of cell surface receptor signaling is one whereby the ligand and receptor are endocytosed into a signaling endosome prior to transmission of the intracellular signal (Type III, Fig. 1). The formation of isolated signaling endosomes allows for an advanced level of regulation that is simply not possible in most cases of signaling at the plasma membrane. For example, within an endosome it becomes feasible for localized changes in membrane phospholipids to be confined to areas of active signaling. As discussed later in the review, such changes in phospholipids can control the docking of effectors to the surface of signaling endosome. Furthermore, endosomal regulation of luminal pH, voltage, and osmotic pressure can also be used to influence signaling events in ways that are not possible at the plasma membrane. Endosomal signaling also offers the potential to concentrate signaling agents in a confined region, allowing for rapid receptor processing and termination of signaling activities. Two types of signaling by endosomes are theoretically possible, although research aimed at differentiating these two forms is far from well established. The first type (Type IIIA, Fig. 1) involves direct signal transmission from newly formed endosomes containing the ligand/receptor complex. In this context, the docking of an effector(s) on the ligand-bound receptor at the plasma membrane is required to initiate endocytosis. Following endocytosis, a second effector(s) is recruited to the endosome to facilitate signal transmission. In the second type of endosomal signaling, receptor endocytosis occurs in the same manner as in Type IIIA, but in this case, endocytosis is followed by vesicle fusion, during which a membrane-associated second effector(s) docks with the receptor complex to activate signaling (Type IIIB, Fig. 1). Notably, fusion events allow for new membrane proteins and lipid types to be introduced into the signaling system. Redoxosomal signaling mechanisms appear to utilize Type IIIA and/or Type IIIB signaling pathways, and differentiating between these two mechanisms has been challenging.
Examples of ligand/receptor systems that utilize endosomes for signal transduction include EGF, PDGF, IL-1β, and TNFα. In the case of the EGF receptor (EGFR), it has long been known that receptor endocytosis takes place following ligand binding. What has been significantly more difficult to elucidate is the temporal placement of EGFR signaling with respect to the endocytic event. To date, only a few downstream endpoints have been rigorously demonstrated to be dependent on the endocytosis of EGFR (74, 91, 114, 116). A notable example is the observation that inhibition of EGFR endocytosis following a ligand stimulus also leads to reduced extracellular signal-regulated kinase (ERK) activation by EGFR (74). Other studies of both PDGF and EGF signaling used a series of specific inhibitors to prevent receptor recycling and tyrosine kinase activation of PDGFR and EGFR. In the presence of these inhibitors, ligand/receptor binding and endocytosis proceeded in the absence of cell signaling. When these inhibitors were washed out of the cell, signaling resumed in the absence of plasma membrane stimulation of the receptors. Dissection of this system demonstrated not only that endosomal signaling by PDGFR and EGFR is possible, but also that it is sufficient to generate a normal signaling cascade from these pathways (116, 117). Although these and other studies (14, 108) suggest that PDGFR and EGFR can signal at the endosomal level, they do not rule out that both receptors also signal from the plasma membrane. In fact, internalized EGFR cannot activate PLC-γ because the lipid composition of the endosomal compartment is inappropriate (99). Furthermore, studies evaluating the proliferative effects of EGF stimulation demonstrate that EGFR signaling at the plasma membrane is enhanced in the absence of dynamin-dependent endocytosis (114), suggesting that the endocytosis of EGFR downregulates this pathway. Ligand-bound EGFR also appears to associate with different effectors at the plasma membrane and in endosomal compartments (14). Hence, it appears that EGFR falls into both categories II and III (Fig. 1) and is capable of generating different signals at the plasma membrane and in endosomal compartments.
Further evidence for the importance of endosomal cell signaling comes from a collection of studies involving the IL-1β receptor (IL-1R1) and the TNFα receptor (TNFR1). In the cases of both pathways, inhibiting receptor endocytosis reduces receptor-mediated signaling that is required for activation of the proinflammatory transcription factor NFκB (67, 68). Of interest to this review, both IL-1β and TNFα stimulation promote the production of endosomal reactive oxygen species through NADPH oxidases and this process also requires endocytosis (67, 68, 79, 119). Further discussion of these models with respect to redoxosomal signaling will be highlighted later in the review.
Biogenesis of Receptor-Activated Redoxosomes from the Plasma Membrane
Studies to date suggest that redoxosome formation requires the endocytosis of key plasma membrane components following an environmental trigger such as the binding of cytokine to its receptor. It has become increasingly recognized that the organization of key redoxosomal proteins at the plasma membrane is likely mediated through the association of ligand-activated receptors with lipid rafts that harbor NADPH oxidases in an inactive state. This section will review the current thinking on mechanisms whereby ligand activation of certain receptors triggers a cascade of events leading to Nox activation in the endosomal compartment, as well as methods that are used for the detection of endosomal Nox activation.
Role of lipid rafts in the organization of Nox signaling
Lipid rafts are specialized microdomains of the cell membrane that are ∼50–150 nm in size and are characterized as regions enriched for cholesterol and glycosphingolipids (90). Early studies of neutrophils demonstrated that Nox2 is constitutively present in lipid rafts, but that the other NADPH oxidase subunits—p47phox, p40phox, and p67phox—are recruited in response to an activating stimulus (97). This view is supported by the demonstration that depleting membranes of lipid rafts by exposing cells to the cholesterol-sequestering agent methyl-B-cyclodextrin inhibits Nox2 activation (115). Nox2 is also found in the lipid rafts of nonphagocytic cells, as shown in coronary arterial endothelial cells, where Nox2-positive lipid rafts cluster following stimulation of the Fas ligand signaling pathway (120).
Nox1 has also been shown to be present in the lipid rafts of nonphagocytic cells. In one study linking Nox1 to the caveolin1 (Cav1) subset of lipid rafts, it was observed that Cav1 knockout (KO) mice have abnormally regulated vasodilation and vasoconstriction, pulmonary hypertension, and dilated cardiomyopathy. These vascular phenomena were eventually linked in part to the dysregulation of AngII signaling via Nox1 in vascular smooth muscle cells (VSMCs) (111). Other direct evidence for the presence of Nox1 in lipid rafts comes from the observation that the TNFα receptor (TNFR1) and the other components of the TNFα signaling pathway are recruited to lipid rafts following ligand stimulation (55, 64). Furthermore, immunofluorescence and immunoisolation experiments in VSMCs revealed that Nox1, Noxo1, Noxa1, and the lipid raft marker caveolin1 also colocalize following TNF stimulation (47, 55).
The TNFα, PDGF, EGF, and AngII pathways have all been associated with lipid rafts (23, 47, 72, 89, 101, 121). Each of these receptors also appears to utilize Nox1 and/or Nox2 during signal transduction (59, 62, 67, 78, 88). Taken together with the apparent constitutive presence of Nox1 and Nox2 in lipid rafts, these findings suggest a common starting point for redoxosomal signaling. In some cases, such as EGF signaling, it appears that the receptor is already present in lipid rafts (89, 91), whereas in cases like TNFα signaling the receptor must be recruited into lipid rafts (64). How each receptor is brought together with Nox is likely to be receptor specific. It is also important to point out that lipid rafts from the plasma membrane and those from the endosomal compartment often cannot be distinguished when using detergent or biophysical methods of characterization, and thus many studies fail to differentiate between the two. Furthermore, the cellular site of Nox activation (at the plasma membrane or within the endosome) is often unclear, depending on the techniques used to evaluate Nox-dependent ROS production. For this reason, the biochemical and imaging methods that are used to evaluate signaling by Nox-dependent ROS will have a significant impact on the mechanistic interpretation of redox signaling events.
Isolation of receptor-activated redoxosomes and analysis of Nox activity
Two of the better-studied receptor pathways that activate Nox in the endosomal compartment include IL-1R1 and TNFR1. Studies on these receptors have utilized both imaging and biochemical techniques to verify that Nox-active endosomes form following ligand stimulation. This section will review the techniques that have been used to study Nox activation in endosomes, as this background is required to understand redoxosomal signaling mechanisms. For further information on several of the ROS quantification techniques discussed below, see the following review (27).
Analysis of NADPH-dependent superoxide production in isolated endosomes
An approach that has commonly been used to isolate and identify redoxosomes is subcellular fractionation by density gradient isolation, followed by analysis of the resulting fractions for NADPH-dependent superoxide production using the chemiluminescent probes or electron spin resonance (ESR) spin-trap probes (67, 68, 83) (Table 1). Of critical importance to these assays is the biophysical separation of endosomes from the plasma membrane and from other organelles known to produce superoxide. Iodixanol gradients have been successfully used to separate Nox-active endosomes from the plasma membrane, mitochondria, and peroxisomes (68). However, the use of iodixanol gradients as a single method of purification fails to separate endosomes from the Golgi or the endoplasmic reticulum (68). Furthermore, many of the assays used to study redoxosomal function rely on the isolation of intact endosomes following a particular stimulus. Thus, the methods used to generate the post-nuclear supernatants (PNS) that are loaded onto iodixanol gradients are critical to the success of this technique. Dounce homogenization (68) and nitrogen cavitation (83) are two methods that have been successfully applied to generate PNS for the isolation of intact redox-active endosomes.
Table 1.
Probes for Detecting NADPH-Dependent Superoxide in Isolated Endosomes
| Probe | Detection method | Probe features | Specific uses | References |
|---|---|---|---|---|
| Lucigenin | Luminescence | Membrane permeable; highly sensitive | Total endosomal superoxide | 67–69, 83 |
| Luminol | Luminescence | Membrane permeable; moderately sensitive | Topology of endosomal superoxide production | 83 |
| Isoluminol | Luminescence | Membrane impermeable; moderately sensitive | Topology of endosomal superoxide production | 83 |
| DMPO | Electron spin resonance (ESR) | Membrane permeable; very quantitative | Total endosomal superoxide | 68 |
DMPO, 5.5-dimethylpyrroline-N-oxide.
Iodixanol gradient subcellular fractionation can be combined with a number of redox-selective probes to quantify changes in NADPH-dependent superoxide production as an index of endosomal Nox activity (Table 1). In this context, lucigenin has been used most commonly as a luminescent probe for superoxide because of it sensitivity. Lucigenin is a membrane-permeable compound that is commonly used for the detection of superoxide in aqueous solutions (33, 84). The basis of detection with lucigenin is its ability to emit photons upon contact with superoxide. The lucigenin reaction is a three-step process. One superoxide molecule reduces lucigenin to form a cation radical, after which a second superoxide reacts with this cation to form a dioxetane molecule. The energetic dioxetane molecule then spontaneously breaks down to methylacridone, and in the process releases a photon with a wavelength of ∼470 nm. This release can be detected using a luminometer (84). Although lucigenin has been challenged as a quantitative assay for superoxide because of its ability to redox-cycle, it is generally accepted as being a reasonably quantitative assay when used at a concentration of 5 μM lucigenin or lower (71, 100). Under these conditions, the rate of superoxide production in isolated endosomes can be measured in the absence or presence of NADPH to assess Nox activities. In this context, relative rates of NADPH-dependent superoxide production increase approximately fourfold after TNFα or IL-1β stimulation (67, 68, 83). Similarly, assays of superoxide production in isolated endosomes have also used less sensitive luminescent probes, including luminol and isoluminol (83). Since luminol and isoluminol have different membrane permeabilities (Table I), a comparison of the results obtained using these two probes has proven useful in addressing the topology of superoxide production by endosomes (as discussed later in this review).
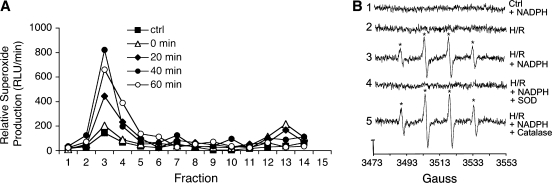
NADPH-dependant superoxide production in isolated endosomes from ligand stimulated cells has been unequivocally demonstrated using electron spin resonance (ESR) (68). The underlying concept of ESR is that all radicals are paramagnetic (i.e., one or more unpaired electrons found in all radicals are attracted to magnetic fields). Thus, when a strong magnetic field is applied to radicals, a small percentage of unpaired electrons will become aligned in the magnetic field. Application of electromagnetic energy (generally in the microwave range) can induce transitions of the unpaired electrons into different spin states. Absorption signatures from the aligned electrons can then be used to identify unique molecules. In the case of radicals such as superoxide, the radical in question is so short-lived at physiologic pH that it is difficult to take a direct reading. Indicators known as spin traps are used to get around this problem. Spin traps are specially designed molecules that interact with radicals to generate a new radical adduct with a longer half-life than the original radical. When using spin traps with specific paramagnetic signatures, it becomes possible to identify the precursor radical (58). Hence, ESR has the advantage of being very specific for various types of free radicals. One common spin trap that has been used in the detection of superoxide is 5,5-dimethylpyrroline-N-oxide (DMPO).
Using each of the above approaches to detect and quantify superoxide in iodixanol fractions, it has been determined that both TNFα- and IL-1β-stimulated redoxosomes have a density of 1.09–1.11 g/ml. While it is not possible to completely isolate redoxosomes from all other cellular compartments by density gradient separation, this procedure is effective for separating the redoxosome from the less dense plasma membrane, as well as from the more dense peroxisomes and mitochondria (68). Furthermore, combining biophysical endosomal separation methods with scavengers of superoxide such as superoxide dismutase (SOD1), or flavoenzyme inhibitors such as diphenyleneiodium (DPI), has helped to demonstrate that endosomal superoxide originates from Nox (68, 78).
Affinity isolation of receptor activated redoxosomes
Enrichment of intact redoxosomes from crude endosomal preparations has been further improved through affinity isolation techniques. This approach has used Rac1 (a co-activator of Nox1 and Nox2) or an endosome-specific marker such as Rab5 (for early endosomes) to further purify redoxosomes with an antibody-bound matrix. In the context of Rab5, two approaches have been used to demonstrate that redoxosomes form in the early endosomal compartment following IL-1β stimulation. The first approach utilized an ectopically expressed HA-tagged Rab5 marker and anti-HA antibodies bound to magnetic beads to enrich for the Rab5 compartment (68). These enriched vesicle were then evaluated biochemically for enrichment of redoxosomal specific proteins and their ability to produce NADPH-dependent superoxides using the chemiluminescent lucigenin assay. A similar approach has used anti-Rab5 antibodies to successfully isolate redoxosomes following IL-1β stimulation (78). Results from both of these studies suggest that redoxosomes form in the early endosomal compartment following receptor internalization.
An alternative, more selective approach for enrichment of redoxosomes utilized an expressed HA-tagged Rac1 protein to mark and purify Nox-active redoxosomes following IL-1β stimulation (67). This approach has the advantage of enriching for endosomes harboring Rac1, which is a requisite activator of the Nox that produces endosomal ROS in response to IL-1β stimulation. Indeed, Rac1 is recruited to endosomes following IL-1β stimulation, and this population of endosomes accounts for the majority of ligand-induced NADPH-dependent superoxide production by the endosomal compartment (67, 68). Findings from studies using this approach suggest that Rac1 can be used as a marker of redoxosomes.
Localization of redoxosomes within intact cells
Two cellular localization techniques have been used to indirectly visualize superoxide production in endosomes within intact cells. These have included fluorescent-based analysis of endosomes loaded with a redox-sensitive fluorochrome, and redox-sensitive cytochemistry (which creates an electron-dense precipitate within endosomes) coupled with analysis by transmission electron microscopy (TEM). Both of these methods are discussed below and have complementary advantages for assessing superoxide within the lumen of endosomes in situ following cellular stimulation.
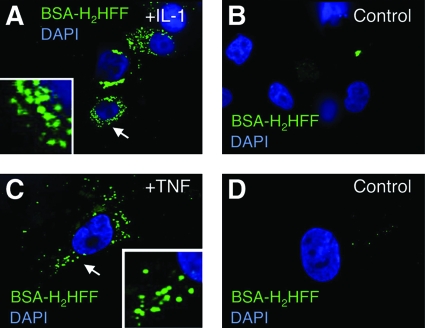
Immunofluorescence approaches to visualize redoxosomes following TNFα or IL-1β stimulation have used a redox-sensitive fluorochrome called dihydro-2′,4,5,6,7,7′-hexafluorofluorescein (H2HFF). Oxidized H2HFF (also called Oxyburst) has an excitation/emission spectrum similar to that of GFP, making it relatively straightforward to use on most fluorescent microscopes. Oxidized H2HFF is ∼2–3 orders of magnitude more fluorescent than its reduced form (16). To limit accumulation of this fluorochrome to the lumen of endosomes, researchers have utilized BSA-conjugated H2HFF to load endosomes at the time of cellular stimulation (67, 68, 78, 83). As depicted in Fig. 2, endosomal loading of BSA-H2HFF at the time of stimulation with IL-1β or TNFα results in increased fluorescence within newly formed endosomes. This method of localizing redoxosomes has also been used in conjunction with co-localized endosomal markers such as EEA1 (to mark early endosomes) (68). Such studies have demonstrated that redoxosomal ROS originates in the early endosomal compartment following IL-1β stimulation, and support the findings from biochemical studies assessing NADPH-dependent superoxide in Rab5 enriched redoxosomes. Studies assessing the specific ROS that give rise to BSA-H2HFF oxidation in live cells have utilized endosomal loading of purified ROS scavenger enzymes such as bovine SOD1 and/or catalase (68). These studies have demonstrated that SOD1, but not catalase, can quench IL-1β-stimulated BSA-H2HFF fluorescence within the endosome, suggesting that oxidation is caused by superoxide production in the endosome. Moreover, treatment of cells with the general Nox inhibitor diphenyleneiodium (DPI) significantly reduces IL-1β-stimulated endosomal BSA-H2HFF fluorescence, suggesting that endosomal superoxide originates from Nox (68, 78).
FIG. 2.
Application of BSA-H2HFF fluorescence to visualize redoxosomal ROS following stimulation by TNFα or IL-1β. (A, B) MCF-7 cells were stimulated for 20 min in the presence or absence of 5 ng/ml IL-1β and BSA-H2HFF, as marked. (C, D) A vascular smooth muscle cell line was stimulated for 20 min in the presence or absence of 10 ng/ml TNFα and BSA-H2HFF, as marked. Nuclei are marked by DAPI (blue) and oxidized H2HFF fluorescence is in green. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
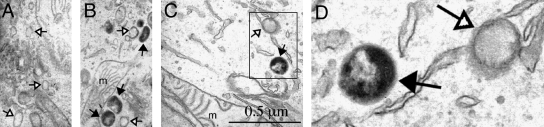
A second method capable of visualizing redoxosomal ROS in intact cells involves cytochemical staining, followed by transmission electron microscopy. Such applications have utilized cerium cytochemistry to localize hydrogen peroxide to IL-1β-stimulated endosomes of VSMCs (78). Cerium chloride is oxidized by hydrogen peroxide and results in an electron-dense cerium perhydroxide precipitate that can be visualized by transmission electron microscopy (TEM) (13). Cerium precipitation does not occur in the presence of superoxide, and hence serves as a marker for hydrogen peroxide (56). However, the detection of superoxides at the ultrastructural EM level has been accomplished by using a 3,3′-diaminobenzidine (DAB)-Mn2+ reaction that detects superoxides (102). In this reaction, superoxide causes the conversion of Mn2+ → Mn3+, which subsequently oxidizes DAB to form an electron-dense polymer. This method has been previously applied to localize superoxide production to vesicular compartments of stimulated neutrophils (57). Ultrastructural TEM confirmation of superoxide in the lumen of IL-1β-stimulated endosomes can also be demonstrated using DAB/Mn2+ cytochemical staining (Fig. 3).
FIG. 3.
Cytochemical detection of redoxosomal superoxides at the ultrastructural EM level, using a 3,3′-diaminobenzidine (DAB)-Mn2+ reaction. MCF-7 cells were stimulated with 0.5 ng/ml IL-1β for 15 min, followed by staining in Hepes Buffer (10 mM Hepes, pH 7.4, 135 mM NaCl, 5 mM KCl, 1 mM CaCl2) containing 5 mM DAB, 1 mM MnCl2, and 1 mM NaN3 for 5 min at 37°C. After washing with Hepes buffer, cell samples were fixed for electron microscopy using 2% glutaraldehyde at 4°C overnight, and washed with Hepes buffer three times. Post-fixation was performed with 1% osmium tetroxide for 1 h, followed by washing three times in buffer alone. Cells were then dehydrated through a graded series of ethanol solutions (from 50%, 75%, and 95%, to 100%) and embedded in Epon resin. Sections (100 nm) were then evaluated by TEM. (A–D) MCF-7 cells were treated with (A) PBS or (B–D) IL-1β for 15 min and stained in the presence of DAB/Mn2+ for 5 min, followed by fixation and evaluation by TEM. Two endosomal populations were seen, including those with electron-dense precipitates in their interiors (solid arrows) and those lacking a precipitate (open arrows). An enlargement of the boxed region in (C) is shown in (D). Mitochondria are marked by an “m” for reference. Arrows mark regions enlarged in the inset.
Role of receptor endocytosis in redoxosomal Nox activation
A unique feature of ligand/receptor-initiated redoxosomal signaling is a requirement for endocytosis. For example, studies evaluating the formation of redoxosomes following IL-1β or TNFα activation demonstrate that inhibiting endocytosis by expressing dominant negative dynamin significantly reduced NADPH-dependent superoxide production in the endosomal compartment, while also inhibiting downstream NFκB activation (67, 68). The mechanism by which Nox becomes active in the endosomal compartment following IL-1β or TNFα stimulation remains to be fully elucidated. However, Rac1-siRNA knockdown studies in IL-1β-activated epithelial cells have demonstrated that Rac1 is required for the recruitment of Nox2 from the plasma membrane into the endosomal compartment (68). Activation of Nox2 in this model system appears to occur first in EEA1- and Rab5-positive endosomes (both markers of the early endosome), based on H2HHF-BSA staining and endosomal affinity isolation studies (68). These findings suggest that Nox activation occurs very early following redoxosome biogenesis. Interestingly, depending on the cell type that is stimulated, IL-1β or TNFα activation appear to be able to use either Nox1 or Nox2 to facilitate endosomal ROS production (67, 68, 78).
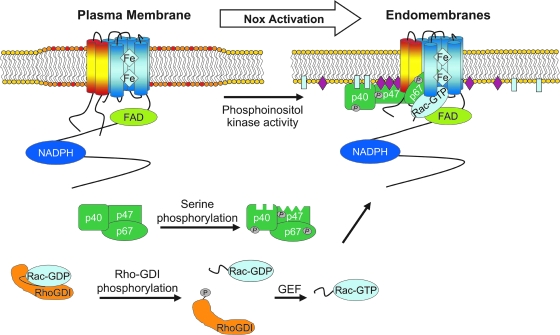
Activation of Nox1 and Nox2 requires the recruitment of cofactors prior to superoxide production. In the case of Nox1, the subunits Noxo1, Noxa1, and Rac bind to the Nox1/p22phox heterodimer to activate superoxide production (5). In the case of Nox2, the Nox2/p22phox complex recruits the cytosolic proteins Rac, p67phox, p47phox, and p40phox (60). The mechanism and sequence of events that lead to activation of the Nox complex has been best defined in the case of Nox2 in phagocytes (Fig. 4). In the cytosol, p40phox, p47phox, and p67phox are found in a complex. Serine phosphorylation of p47phox exposes its SH3 domains, allowing this domain to bind to the proline-rich domain on p22phox. This complex is stabilized by interaction of the p47phox PX domain with PI(4)P and PI(3,4)P2 on the membrane (87). By contrast, the PX domain of p40phox associates with PI(3)P (29). Interestingly from the standpoint of redoxosome formation, both p47phox and p40phox bind to inositol phospholipid products of PI3 kinase in the early endosomal compartment (29, 54). Such studies have clearly demonstrated that a GFP-tagged p40phox PX domain co-localizes with EEA1 positive endosomes. Hence, changes in phosphoinositol composition in the endosome may be a key feature in limiting Nox activation at the plasma membrane and promoting subsequent activation following endocytosis.
FIG. 4.
Model for Nox2 activation during redoxosomal signaling. In the resting state, Nox2 (protein with six blue transmembrane domains) and p22phox (protein with two yellow/red transmembrane domains) make up the flavocytochrome b558 complex, which is located in lipid rafts of the plasma membrane (marked by a widened lipid domain). The Nox subunits p40phox, p47phox, and p67phox are found in a complex in the cytosol, and Rac is in its inactive GDP-bound state associated with RhoGDI. In response to a stimulus, RhoGDI is phosphorylated, causing it to disassociate from Rac-GDP, which exposes its prenylated tail. Prenylated Rac-GDP subsequently interacts with a GEF that exchanges GDP for GTP, generating an activated Rac-GTP complex. Rac1 then moves to the membrane, where it interacts with Nox2. Phosphorylation of p40phox, p47phox, and/or p67phox recruits these subunits to the Nox complex. The serine-phosphorylated p47phox is stabilized on the Nox complex, by interacting with both p22phox (via its N-terminal SH3 domain) and PI(3,4)P2 (via its PX domain). p40phox may further stabilize the complex along the membrane by interacting with PI(3)P via its PX domain. p67phox binds to Nox2 via its activation domain, and also associates with Rac-GTP via its N-terminal tetratricopeptide repeats. Following the recruitment of all members of the Nox complex, superoxide production takes place as NADPH binds to the cytoplasmic tail of Nox2. Electrons are transferred to FAD, which is also bound to the Nox2 cytoplasmic tail. FAD then sequentially transfers the electrons to the Nox complex, where they pass through the two heme centers, and are ultimately transferred to oxygen on the luminal side of the redoxosome. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
p40phox and p67phox are brought to the Nox2 complex as a consequence of the association of p47phox with p22phox (87). Unlike p47phox, which is believed to have a solely regulatory role in the Nox2 complex, p67phox is absolutely required for the production of superoxide, as it interacts directly with the flavocytochrom b558 to induce conformational changes that are believed to directly affect the catalytic activity of b558. The role of p40phox is currently controversial. Like p47phox, p40phox is considered to have a function that is entirely regulatory in nature. However, unlike p47phox, it is debated as to whether p40phox is actually required for the activation of Nox2 in phagocytes (7, 87). The recruitment of phox effectors to redoxosomes has been studied in the context of IL-1β stimulation; both p47phox and p67phox specifically recruit to the endosomal compartment following IL-1β stimulation of MCF-7 cells (68).
Another recruited effector of the Nox1 and Nox2 complexes is Rac (Fig. 4). The two relevant isoforms of the Rac GTPase are Rac1 and Rac2. Rac2 is primarily expressed in phagocytes, while Rac1 appears to be ubiquitously expressed. Prior to activation of the Nox complex, GDP-Rac is bound to RhoGDI in the cytosol, and thereby inhibited. Following exposure of a cell to a Nox-activating stimulus, RhoGDI phosphorylation releases GDP-Rac into the cytosol. Rac is subsequently activated by the exchange of GDP for GTP, and is partially inserted into the membrane via its isoprenylated tail (12). It is speculated that the membrane-inserted portion of Rac directly interacts with, and modulates, the flavocytochrome b558 while its C-terminus associates with p67phox (87).
How ligand stimulation of receptors such as IL-1R1 and TNFR1 initiates the endocytosis of Nox complexes and the recruitment of Nox activators is only beginning to be understood. In the context of IL-1R1, ligand binding recruits MyD88 to the plasma membrane, an event that must take place prior to endocytosis of the receptor and Nox (68). Similarly, it is thought that TRADD recruitment to ligand-activated TNFR1 at the plasma membrane initiates endocytosis of the receptor with Nox (67). Lipid rafts that harbor Nox/p22phox complexes at the plasma membrane likely play a key role in coordinating these events for both IL-1R1 and TNFR1, although this has been formally studied only in the context of TNFR1 (47, 64). Since Rac1 is specifically recruited to IL-1β- and TNFα-stimulated redoxosomes, and is also required for the recruitment of Nox2 into IL-1β stimulated redoxosomes (67, 68), it is currently thought that Rac1 likely recruits Nox into redoxosomes by tethering the receptor to the Nox complex. Indeed Rac1 binds to IL-1R1 (51, 68) and is also a co-factor of the active Nox2 complex. However, since endocytosis of at least IL-1R1 is not dependent on Rac1, Nox recruitment and endocytosis appear to be independently-regulated events (68). Whether similar events control TNFR1-mediated redoxosomal activation remains to be determined. However, Rac1 has been shown to be essential for TNFR1-mediated activation of Nox1 (55). Given the similarities associated with redoxosome activation by IL-1β and TNFα receptors, we speculate that these two receptors share common events in the early biogenesis of redoxosomes (Fig. 4).
Endosomal ROS metabolism: Topology of production and superoxide channels
Nox complexes produce superoxide on the extracellular side of cellular membranes. Although this topology has been well studied in phagocytes, only recently has it been examined in endosomes, using the assays discussed earlier in this review. In the context of both IL-1β and TNFα stimulation, the loading of purified bovine SOD1 into endosomes at the time of stimulation significantly reduced superoxide production by redoxosomes (67, 68, 83). Such SOD1 quenching of lumenal endosomal superoxide has been confirmed by lucigenin and ESR assays on isolated endosomes, as well as by H2HHF-BSA imaging. These findings strongly suggest that superoxide is produced within the lumen of the redoxosome following biogenesis. Although superoxide is formed in the lumen of the redoxosome, it is much less clear how and where superoxide is converted to hydrogen peroxide to facilitate downstream redox signaling. For example, isolated IL-1β-stimulated endosomes have been shown to rapidly transport NADPH-dependent superoxides across the endosomal membrane through a DIDS-sensitive chloride channel (83). Additionally, endogenous cytoplasmic SOD1 is recruited to IL-1β- and TNFα-stimulated endosomes, suggesting that dismutation of superoxide on the endosomal surface could play a role in transmitting redox signals through the generation of local hydrogen peroxide gradients (67, 68). Indeed, SOD1-deficient fibroblasts have significantly blunted IL-1β-mediated activation of NFκB, as compared to wild-type fibroblasts (83). Together, these findings suggest that redoxosomes may have intricate methods of regulating ROS metabolism, and that these mechanisms may be important for the transmission of redox signals. In this context, it is important to understand the redox chemistries of the endosome that can influence ROS production and metabolism.
As a direct consequence of Nox activity, negatively charged superoxide can accumulate in the lumen of phagosomes and redoxosomes, whereas positively charged NADP+ and protons accumulate on the cytosolic side. Hence, NADPH oxidase activity will lead to a voltage potential and an osmotic gradient across the membrane, as well as to acidification of the cytosol and alkalization of the endosomal lumen (see Lamb et al. in this issue for further discussion). This phenomenon has been examined in the context of the phagosome (21, 85). For example, given the rate of superoxide production in the phagocytic system, the positive flux of protons in the cytosol should theoretically cause the intracellular pH to drop by 1 unit per minute (25). This clearly does not occur, in part to the large buffering capacity of the cytoplasm. However, it is also widely accepted that phagosomes contain proton and potassium channels that neutralize the charge, pH, and osmotic difference caused by the electron and proton flux generated by NADPH oxidases. This has led to many hypotheses about the identity of the proton channel, including the suggestion that Nox itself acts as a proton channel (25); however a consensus has yet to be reached.
Like the phagosome, the redoxosome must control the voltage, and also the osmotic and pH gradients that are generated by Nox activity. Additionally, maturation of early endosomes to late endosomes is accompanied by the lowering of luminal pH (39, 112). Regulation of the redoxosome microenvironment becomes particularly relevant to redox-signaling when one considers that changes in lumenal pH will have a significant impact on the rate of spontaneous dismutation of superoxide to hydrogen peroxide, and the fact that these two ROS have different membrane permeabilities. The second-order rate of decay for superoxide decreases by roughly an order of magnitude or 10 M−1s−1 for every increase of 1.0 pH unit in an aqueous environment between pH 6.0 and 14.0; this translates into a 10-fold increase in the half-life of superoxide for every increase of 1.0 pH unit (8). Therefore, even a small change in pH in the redoxosome lumen can dramatically change the steady-state level of superoxide and hydrogen peroxide that is available for redox signaling.
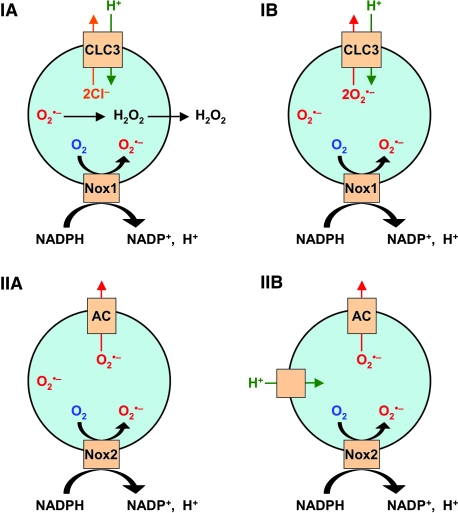
Two studies have attempted to directly identify the channel and/or channels involved in the charge/pH neutralization of redoxosomes. In one set of studies looking at Nox1-positive redoxosomes in VSMCs, it was observed that two chloride channel inhibitors, 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) and niflumic acid (NFA), repress IL-1β-mediated superoxide production and NFκB activation in cell culture (78). Of the many chloride channels in mammals that DIDS or NFA can inhibit, the ClC family is the largest, with ClC-3, ClC-4, and ClC-5 having been identified as chloride-proton antiporters (6, 52). Furthermore, ClC-3 is a voltage-regulated channel that has been associated with the acidification of endosomes, making it a good candidate for a channel involved in the neutralization of the Nox-generated proton gradient. Indeed, in VSMCs that are deficient for ClC-3, TNFα and IL-1β fail to induce NADPH-dependent superoxide production in redoxosomes and show a reduction in cytokine-mediated NFκB activation (78). These studies have implicated ClC-3 as necessary for charge neutralization of redoxosomes. In the absence of ClC-3, Nox1 fails to function due to the build up of an unfavorable membrane potential.
Studies in a transformed mammary epithelial cell line (MCF-7 cells) suggest that chloride channels necessary for redoxosomal superoxide production are quite different from those in VSMCs. In MCF-7 cells, neither DIDS nor NFA inhibit superoxide production in IL-1β-induced redoxosomes (83). An in vivo assessment of IL-1β-induced redoxosomal superoxides in MCF-7 cells also demonstrated an induction in lumenal superoxide in the presence of DIDS (as detected by H2HHF-BSA fluorescence) (83). This is in stark contrast to VSMCs in which NFA inhibits H2HHF-BSA-detected redoxosomal superoxide following IL-1β induction (78). The explanation for these cell-type specific differences appears to involve a mechanism for superoxide movement out of redoxosomes in MCF-7 cells. Using membrane-permeable (luminol) and membrane-impermeable (isoluminol) luminescent probes for superoxides, it has been shown that MCF-7 cells have a DIDS/NFA-inhibitable superoxide channel that is capable of rapidly moving superoxide out of redoxosomes and into the cytoplasm (83). Furthermore, using endomembrane-reconstituted proteoliposomes from MCF-7 cells, these studies also demonstrated that X/XO-derived superoxide and Cl36 move across endomembranes in an electrogenic fashion, through a similar DIDS-sensitive channel(s) (83). The ability of X/XO-derived superoxide to move out of endomembrane-reconstituted proteoliposomes in a DIDS-sensitive fashion suggests that the superoxide channel function is independent of NADPH oxidase activity. Cumulatively, these studies in MCF-7 cells suggest that the transport of superoxide anion out of the redoxosome could be another mechanism for the charge neutralization required to maintain NADPH oxidase activity.
Taken together, the reports discussed above provide evidence for the role of chloride channels in redoxosome signaling. However, they also make some contradictory predictions as to the precise function of the chloride channel(s). In the case of the ClC-3 studies on Nox1 signaling in VSMCs, the authors favor a model whereby ClC-3 acts as a proton-chloride antiporter, and redox signaling occurs by the spontaneous dismutation of superoxide to hydrogen peroxide and the subsequent diffusion of this product through the redoxosomal membrane (Fig. 5. IA). However, this study does not rule out the possibility that superoxide leaves the lumen of the endosome intact (Fig. 5. IB). Interestingly, ClC-3 at the plasma membrane has been suggested to act as a superoxide transporter in endothelial cells (43). Moreover, even if ClC-3 does act as a superoxide transporter, this does not reconcile the fact that IL-1β-induced superoxide production by Nox1 is inhibited by NFA/DIDS in VSMCs, yet continues to be produced in the lumen of redoxosomes by Nox2 in MCF-7 epithelial cells in the presence of these inhibitors (Fig. 5. IIA). One potential explanation that could reconcile these two findings would be that MCF-7 cells have an alternative non-ClC-3 channel(s) that is capable of moving protons and chloride across redoxosomal membranes to dissipate Nox-induced changes in membrane potential, whereas ClC-3 may be the sole pathway for charge neutralization and potentially also superoxide transport in VSMCs (Fig. 5. IB). In looking at the Nox2-positive phagosomes for guidance, it was found that superoxide production and charge neutralization are only partially ClC-3 dependent (82). This lends credence to the idea that the charge/pH neutralization by channels in the redoxosome may be cell-type specific. Perhaps in the case of Nox2, the primary mechanism of charge neutralization involves two channels: a DIDS-insensitive channel that transports protons (possibly Nox2 itself) and a DIDS-sensitive anion channel that exports superoxide (Fig. 5. IIB). In this case, even if the superoxide channel were inhibited, the proton channel would neutralize the voltage potential, thus allowing superoxide production to continue in the presence of the inhibitor. Clearly, at this point we can only speculate on the possible reasons for the observed differences between the two systems, and much work needs to be done before we can fully understand the complexities of charge neutralization in the redoxosome and how this may affect redox-signaling.
FIG. 5.
Possible mechanisms of charge neutralization and ROS production by redoxosomes. (IA) The mechanism proposed by Miller et al. (78) for Nox1 redoxosomes in IL-1β-stimulated aortic smooth muscle cells. In this model, the voltage gradient is neutralized by the anion channel ClC-3, which acts as a proton-chloride antiporter. ROS signaling relies on spontaneous dismutation of superoxide to hydrogen peroxide within the redoxosome lumen, followed by diffusion of hydrogen peroxide into the cytosol for redox signaling. (IB) In an open alternative possibility to the Miller model, ClC-3 acts as a proton-superoxide antiporter, and thereby accomplishes charge neutralization and the transport of superoxide into the cytosol for redox signaling. (IIA) A proposed model for IL-1β-stimulated Nox2 redoxosome charge neutralization and ROS production by MCF-7 mammary epithelial cells (83). In this model, an anion channel neutralizes charge by transporting superoxide out of the redoxosome. (IIB) In an extension of model IIA, a NFA/DIDS-insensitive proton channel compensates for changes in lumenal pH produced by Nox2. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Redox-dependent events that spatially control receptor signals in redoxosomes
Redoxosomes provide a framework for thinking about spatially controlled ROS production and the downstream oxidative processes that are involved in transmitting the receptor-mediated signal from the plasma membrane. By definition, redoxosomes are signaling endosomes that harbor ligand-activated receptors and Nox complexes, and produce superoxide and hydrogen peroxide as locally confined second messengers. In most organic systems, superoxide is only mildly reactive with most organic molecules. Lipid peroxidation occurs to only a very minor degree, if at all, in the presence of superoxide. In a few proteins, particularly those of the TCA cycle and those with iron centers, superoxide has an inhibitory effect. (41). Superoxide is nevertheless associated with a short half-life in an aqueous environment because it reacts rapidly with other radicals such as nitric oxide to form peroxinitrite, or with other superoxide molecules to form molecular oxygen and hydrogen peroxide. The nonradical compound hydrogen peroxide results from the addition of two electrons to molecular oxygen, and is produced by the dismutation of two molecules of superoxide. Like superoxide, hydrogen peroxide is poorly reactive at physiologic levels, although it too has been associated with the inhibition of various proteins (41). It appears that much of the oxidative damage caused by hydrogen peroxide and superoxide is the indirect results of conversion to more reactive intermediates. An example of this is the Haber–Weiss reaction. In a pure system, the reaction rate of this system is near 0. However, transition metals (Cu+ or Fe2+) can catalyze this reaction to produce the extremely reactive hydroxyl radical. Another example of cytotoxic species being generated from superoxide is the formation of peroxinitrite through the reaction of superoxide with nitric oxide. In some cases, the cell intentionally generates these highly toxic ROS. For example, phagocytes use myeloperoxidase to generate hypochloric acid from hydrogen peroxide and a chloride anion. The controlled environment of the redoxosome, on the other hand, provides a means for generating favorable ROS gradients at sites of receptor signaling, while minimizing the generation of the more damaging ROS intermediates or affecting the global cellular redox state.
Although the exact identities of the ROS intermediates responsible for the transmission of redoxosomal signals remain largely unexplored, it is generally thought that hydrogen peroxide produced following superoxide dismutation plays an important role. Redox modification of proteins by hydrogen peroxide can include the controlled oxidation of cysteines to form sulfenic (Cys-SOH), sulfinic (Cys-SO2H), or sulfonic (Cys-SO3H) acid (9). One of the best characterized signaling mechanisms involving hydrogen peroxide is the inhibition of cellular phosphatases by cysteine oxidation (17, 92, 93). In addition, inactivation of peroxiredoxins by cysteine sulfinic acid formation has been shown to regulate PDGF signaling (18, 94). Another important mechanism for redox-modulation includes the alteration of protein structure through the hydrogen peroxide-mediated oxidation of reactive thiols, leading to the formation of disulfide bonds (9, 10, 35). Ultimately these small modifications enable structural changes to redox-sensitive proteins, allowing for downstream signaling.
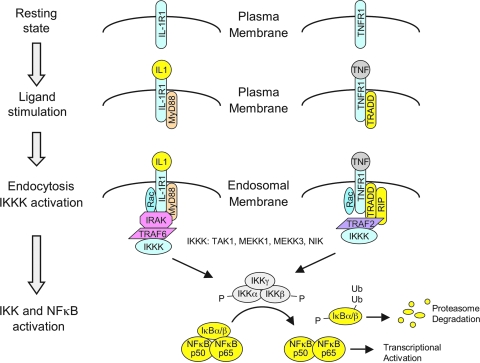
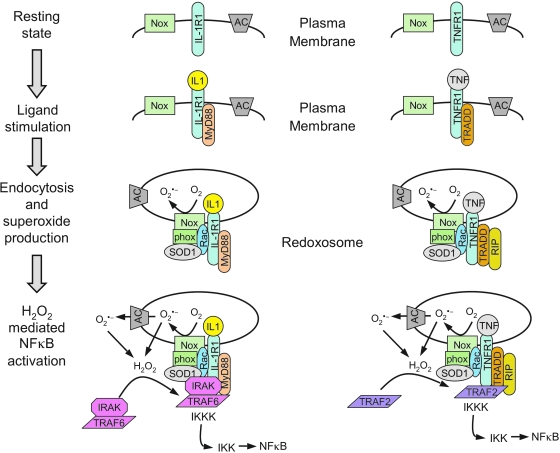
The redox-dependent signaling events that are responsible for redoxosomal receptor activation have been best characterized for IL-1β and TNFα activation of the IκB kinase complex (IKK) and NFκB. Both of these receptor activation pathways appear to share similar mechanisms of redox activation, but maintain unique adaptor/effector partners responsible for IKK complex activation (Fig. 6). In the context of IL-1R1, the binding of IL-1β to its receptor initiates the ordered recruitment of adaptors and effectors (IL-1β→IL-1R1→MyD88→IRAK→TRAF6→IKK kinases–MEKK3, TAK1, and/or NIK) that ultimately leads to the formation of an active IKK kinase (IKKK) receptor complex responsible for phosphorylation of the IKK complex (50, 113). Once the IKK complex is activated, IκB is phosphorylated and NFκB is subsequently mobilized to the nucleus where it can promote transcription of downstream genes. In the context of TNFR1, TNFα binding to its receptor initiates the ordered recruitment of different adaptors and effectors (TNFα→TNFR1→TRADD→TRAF2 / RIP→IKK kinases MEKK1, MEKK3, and TAK1) that ultimately leads to IKK complex activation, IκB phosphorylation, and NFκB activation (65, 113). These described effectors and adaptors for both the IL-1R1 and TNFR1 pathways are abbreviated, but are sufficient for an interpretation of the literature on redoxosomes. For a more comprehensive overview of these signaling pathways, see the following reviews (44, 50, 65, 113).
FIG. 6.
Relevant adaptors and effectors of the IL-1R1 and TNFR1 signaling pathways that lead to NFκB activation. Receptors are drawn as monomers for simplicity of presentation, but actually occur as dimers of IL-1R1 and IL-1 receptor accessory protein (IL-1RAcP), and tetramers of TNFR1, in their ligand-bound states. The molar ratios of adaptor/effectors are also not drawn for accuracy, but for clarity of the types of molecular interactions. Potential IKK kinases (IKKKs) that phosphorylate the IKK complex are listed. Not all potential adaptors of these receptor pathways are listed, but we have attempted to include those most relevant to redoxosomal signaling. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Following ligand binding, adapter recruitment (IL-1R1→MyD88 or TNFR1→TRADD) occurs at the plasma membrane, and this event appears to be required for endocytosis of the receptor and Nox into the endosomal compartment (67, 68). The critical redox-dependent events that subsequently control activation of receptor-associated IKKKs appear to involve H2O2-dependent recruitment of TRAFs to their cognate receptors within redoxosomes (i.e., IL-1R1→TRAF6 or TNFR1→TRAF2). The redox-dependent recruitment of TRAFs at the endosomal level has been studied using several approaches. For example, the loading of IL-1β-stimulated redoxosome with purified catalase and SOD1 proteins inhibits TRAF6 recruitment to the endosomal compartment, but does not affect the recruitment of IL-1R1, MyD88, Nox2, p47phox, p67phox, or Rac1 to the redoxosome (68). This same approach to neutralizing ROS within the redoxosome also prevents IKK and NFκB activation following IL-1β-stimulation. Furthermore, siRNA-mediated Rac1 knockdown, which inhibits Nox recruitment to redoxosomes and hence endosomal ROS production, also reduced TRAF6 recruitment to endosomal IL-1R1, while leaving MyD88 recruitment intact. Presumably Nox (bound to Rac1) is recruited into redoxosomes at the plasma membrane by the known direct interaction between Rac1 and IL-1R1 (68) or IL-1R1/MyD88 (51). These findings suggest that TRAF6 recruitment to IL-1R1/MyD88 requires the formation of Nox-dependent ROS within the redoxosome. Similar approaches have been used to study TNFR1 activation, and the results also implicate H2O2-dependent recruitment of TRAF2 to redoxosomal TNFR1/TRADD complexes in the regulation of IKK activation (67).
Although the composition of the IKKK complex components that recruit to IL-1β and TNFα stimulated redoxosomes has not been investigated, functional studies evaluating the IL-1R1 pathway in epithelial cells suggest that IKKK activity can indeed be associated with purified redoxosome (68). In these studies, isolated endosomes were purified from IL-1β-stimulated cells in the presence or absence of endosome-loaded catalase and SOD1 proteins (to degrade redoxosomal ROS). In an in vitro reconstitution assay, the addition of purified IL-1β-stimulated redoxosomes to immunoprecipitated IKK complexes isolated from unstimulated cells led to IKK activation, as assessed by the ability of these two components to phosphorylate GST-IκBα. However, IL-1β-stimulated redoxosomes loaded with catalase/SOD1 failed to activate a naive immunoprecipitated IKK complex, demonstrating that endosomal ROS were critical for the recruitment of IKKK activity to redoxosomes. Hence, redoxosomes appear to harbor all the IL-1R1 receptor complex components that are necessary for IKK phosphorylation, and formation of this receptor complex is redox-dependent.
The ability of redoxosomes to partition ligand-activated receptors into a Nox-active microenvironment capable of facilitating localized redox-dependent events that are required for receptor activation was highlighted by a study evaluating HA-tagged-Rac1 affinity-isolated redoxosomes (67). Using TNFα or IL-1β stimulated cells, this study demonstrated that Rac1 specifically recruits to the Nox-active endosomal compartment along with several other common redoxosomal factors (SOD1 and Nox-activator phox subunits). However, enrichment of IL-1R1/TRAF6 or TNFR1/TRAF2 complexes in Rac1-containing redoxosomes maintained ligand specificity. These findings demonstrate that redoxosomes share similar redox-modulator proteins, while also retaining ligand-specificity for a given receptor pathway. Hence, redoxosomes for these two independent receptor pathways appear uniquely equipped to carry out superoxide and hydrogen peroxide production at intracellular sites where ligand-activated receptors are concentrated.
Although the exact redox-mediated events responsible for the recruitment of TRAFs to redoxosomal IL-1R1 and TNFR1 remain unclear, it is apparent that hydrogen peroxide is necessary for this process. It is possible that the inhibition of phosphatases by hydrogen peroxide production at the redoxosome surface may influence these events. However, the addition of exogenous hydrogen peroxide to ligand-activated cells at 4°C (a temperature at which endocytosis is blocked) appears to be able to drive the recruitment of TRAFs to cell surface IL-1R1 or TNFR1 (67, 68), suggesting that hydrogen peroxide alone, in the absence of functioning phosphatases, is capable of mediating TRAF effector docking events. However, it remains unclear if the redox-dependent changes in protein structure required for TRAF docking occur on the receptor, adaptors, and/or TRAF itself.
Novel ROS Sensor for Redoxosomal Nox Regulation
The Rac1 GTPase is a critical activator of Nox1 and Nox2. Additionally, both Nox1 and Nox2 have been associated with the redox activity of redoxosomes. Overexpression of Rac1 in certain systems is sufficient to activate Nox2. Conversely, the absence of Rac1 prevents the Nox complex from producing superoxide. For Rac1 to activate Nox, it must be in a GTP-bound state. A GXXXXGK(S/T)C domain has been identified as a superoxide-sensitive domain on Rho GTPases (including Rac1) (45, 46), and it appears to control the redox-dependent association and disassociation of the guanine nucleotides GDP and GTP. In the case of GDP-bound Rac1, low millimolar to high micromolar levels of XO-generated superoxide increase GDP dissociation from Rac1 by ∼600 fold (45). Mutational disruption of the GXXXXGK(S/T)C domain in Rac1 (C18S) inhibits this redox-sensitive GDP dissociation. Additionally, mutation of the phenylalanine (F28) that resides 3.6 A from the cysteine of the domain (C18) disrupts redox-mediated dissociation of GDP from Rac1. This finding suggests the need for a phenylalanine in close proximity to the redox-sensing domain. One hypothesis that has been put forward to explain this redox-sensing mechanism is that the cysteine and phenylalanine play a critical role in the controlled withdrawal of an electron from superoxide.
Superoxide may also play a role in the GTP loading of Rac1 (45). Rac1-GDP treated with superoxide in the low millimolar to high micromolar range demonstrates an ∼10- fold increase in GTP loading compared to untreated Rac1-GDP. This increase is enhanced another ∼200- fold when the Rac1-GDP is treated with superoxide followed by treatment with a radical scavenger such as ascorbate or NO. Given the high levels of superoxide that are needed to facilitate changes in GDP/GTP on Rac1, it is difficult to envision how this mechanism may function under physiologic conditions to regulate Rac1 on redoxosomes. However, this pathway may be relevant to levels of superoxide encountered during pathologic conditions such as severe inflammation, or in the context of phagocytes where high μM to low mM concentrations of superoxide are generated by the phagosome.
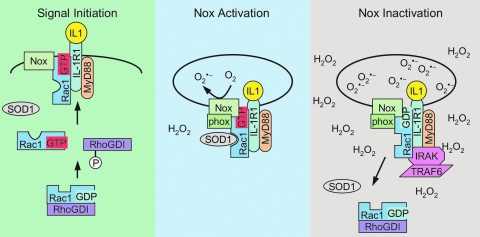
Rac1 can also be redox regulated through its interaction with SOD1 (42), a cytoplasmic protein that rapidly dismutates superoxide into hydrogen peroxide at a rate of 4 × 109 M−1s−1. Immunolocalization experiments have shown that SOD1 is actively recruited to IL-1β stimulated redoxosomes harboring IL-1R1 (83). Interestingly, SOD1 is recruited to redoxosomes following IL-1β stimulation at a molar ratio roughly equal to that of IL-1R1, a finding that is consistent with the fact that Rac1 binds to both IL-1R1 (51, 68) and SOD1 (42). Immunoprecipitation studies have also demonstrated that Rac1 is associated with SOD1 in multiple organs including the kidney, liver, and brain (42). SOD1 most efficiently binds a region of Rac1 contained within amino acids 35 to 70. This region of Rac1 spans several domains that are important for nucleotide binding (i.e., switch I, G2, switch II, and G3 domains). Interestingly, mutations within this region of Rac have been shown to influence Nox2 activation. For example, the Rac2 mutations Asp38Asn and Met45Thr lead to inhibition of Nox2 activity (34). Additionally, several Rac2 mutations including Thr35Ala, Asp38Ala, and Try40Lys lead to lack of sustained Nox2 activity (26). These findings suggest that this SOD1 binding region of Rac may regulate Nox2 activation. Reconstitution assays using His-tagged Rac1 have demonstrated that Rac1 and SOD1 binding depend on the redox state of Rac1, as well as on the nucleotide-bound state of Rac1 (42). When Rac1 is in a reduced state and bound to GTP, it most efficiently binds to SOD1. Oxidation of Rac1 with as little as 50–100 pM of hydrogen peroxide prevents SOD1 association with Rac1. This process is reversible when Rac1 is reduced with 100–300 μM DTT. The association of SOD1 with Rac1 leads to an inhibition in the intrinsic GTPase activity of Rac1 (42), and hence would be expected to activate Rac1-regulated NADPH oxidases under reducing conditions. Indeed, the addition of SOD1 to isolated fibroblast endosomes in vitro leads to enhanced NADPH-dependent superoxide production by Nox2 (42); however, this enhancement is transient since the accumulation of ROS leads to the dissociation of SOD1 from Rac1 and inactivation of Rac1 through the hydrolysis of GTP to GDP. In this manner, SOD1 appears to act as a redox-sensor to control Rac1 activation of NADPH oxidases in the redoxosomes (Fig. 7). Although X/XO-derived superoxide dissociates SOD1 from Rac1-GTP in vitro, leading to enhanced GTP hydrolysis by Rac1 (42), it is currently unclear if a local build-up of hydrogen peroxide on the redoxosome surface, as a consequence of either spontaneous superoxide dismutation or a SOD1-catalyzed dismutation reaction, is responsible for Rac1/SOD1 dissociation. Although it is clear that the oxidation of Rac1 by very low levels of hydrogen peroxide plays an important role in this process, it remains to be determined if SOD1 locally provides this source of hydrogen peroxide for Rac1 oxidation on the redoxosome surface.
FIG. 7.
Redox-sensor model for controlling Nox activation in redoxosomes. The proposed regulatory model for IL-1β signaling builds on the finding that SOD1 is actively recruited to IL-1β-stimulated redoxosomes, and binds to Rac1 in a redox-dependent fashion to control Rac1 GTPase activity. Left panel: In the resting state, Rac1-GDP remains inactive because it is bound to RhoGDI. In this state the majority of Rac1 is most likely in a reduced state, due to the reducing conditions in the cytoplasm. Following IL-1β stimulation, RhoGDI is phosphorylated, releasing Rac-GDP into the cytosol. A Rac1-GEF then activates Rac1 by exchanging GDP for GTP, allowing for the association of Rac1-GTP with the Nox complex at the plasma membrane. IL-1β binding to IL-1R1 also leads to docking of MyD88 at the plasma membrane, which initiates endocytosis of the receptor/Nox complex. Center panel: Following or during early stages of endocytosis, SOD1 binds to reduced Rac1-GTP associated with the Nox complex, maintaining Rac1 in its active GTP-bound conformation by inhibiting the GTPase activity of Rac1. Superoxide production by redoxosomes is initiated at this stage, and Nox activity is maximal. Right panel: The localized buildup of superoxide and hydrogen peroxide around the redoxosome creates an oxidative microenvironment that promotes IRAK/TRAF6 complex docking on the receptor. Eventually, the increased levels of hydrogen peroxide oxidize Rac1, resulting in the disassociation of SOD1 from Rac-GTP, and hydrolysis of GTP by Rac1. Nox activity is terminated as Rac-GDP is formed on the redoxosome and Rac1 is recycled from the membrane by RhoGDI. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Redoxosomal Signaling Via c-Src in the Absence of a Ligand Following Hypoxia/Reoxygenation
As discussed earlier in this review, lipid rafts have been proposed to be important plasma membrane-localized microdomains that house NADPH oxidases (53). These structures provide a framework for better understanding how redoxosomes may be formed through the organization of signal-dependent factors required for Nox complex internalization and function. According to one proposed model, redoxosome formation may be the consequence of internalized caveolin-associated lipid rafts and raft-membrane constituents. In support of this model, a number of studies have identified key molecules from a wide array of signaling pathways—many of which have been implicated in cellular changes caused by oxidative stress—within these lipid microdomains (66). Importantly, various forms of NADPH oxidases (47, 53, 66, 97, 115) and Src-kinases (3) have been reported to cluster in lipid raft microdomains, as well as in the endomembrane compartment following exposure to environmental stimuli (24, 69). For example, caveolin has been shown to bind Src (63), and in this context to negatively regulate c-Src kinase activity (70, 118). Furthermore, there is compelling evidence that ligand-independent pathways for the activation of protein tyrosine kinases such as c-Src exist within lipid rafts, and that these pathways are influenced by oxidative stress (86).
c-Src is a ubiquitously expressed membrane-associated protein tyrosine kinase that plays important roles in redox-dependent signaling cascades (31, 32, 104, 107, 109). As a member of the Src family of nonreceptor protein tyrosine kinases (SFKs), c-Src functions as an important modulator of an array of pathways that transduce signals from the cell surface to the nucleus, including signals promoting cell growth, differentiation, and migration, as well as cellular responses to oxidative stress. Src localization to cellular membranes is accomplished by myristoylation at its N-terminal domain, which is required for c-Src kinase activity at either the plasma membrane or endomembrane level (24). Src consists of modular domains: a N-terminal unique domain; the Src Homology (SH) domains SH3, SH2, and SH1, which serve as protein interaction motifs, and an autoinhibitory C-terminal tail that contains a conserved phosphorylation site (Tyr530) required for the modulation of Src kinase activity (11, 30). Although the tyrosine kinase activity of c-Src is regulated by an array of mechanisms, the most important is modulation of c-Src phosphorylation at residues Tyr419 and Tyr530. Note that the numbering of these tyrosines in the literature is confusing, since it changes according species (Tyr419/Tyr530 in human, Tyr418/Tyr529 in mouse, and Tyr416/Tyr527 in chicken), and phospho-specific antibodies to these residues have been generated in several species. Phosphorylation of Tyr530 on its C-terminal tail maintains the inactive c-Src conformation, creating an inhibitory intramolecular interaction between Tyr530 and the SH2 domain, thereby preventing Tyr419 autophosphorylation and abrogating tyrosine kinase activity. Dephosphorylation of Tyr530 results in autophosphorylation of Tyr419 within the kinase domain (SH1) activation loop, resulting in tyrosine kinase activation. The status of Tyr530 phosphorylation is also regulated by protein tyrosine phosphatases (PTP) and the tyrosine kinase Csk.
While the manner in which c-Src protein tyrosine kinase modulates NADPH oxidase activity remains poorly understood, it is now clear that c-Src plays both initiating and propagating roles in NADPH oxidase-dependent redox signaling (15, 20, 69, 95). In this context, c-Src has been shown to be involved in both ligand-dependent mechanisms of Nox activation (as seen after AngII and aldosterone stimulation) (15, 81) and ligand-independent mechanisms of Nox activation (as seen after hypoxia/reoxygenation injury) (69). Furthermore, the redox-activation of Src kinases following hypoxia/reoxygenation injury has been well established (22, 31, 32, 40, 105), although the link to Nox activation in this context remains understudied. This section will focus on mechanisms by which c-Src may play a role in the initiation, maintenance, and/or modulation of redoxosome-dependent signaling cascades that utilize NADPH oxidase, with a focus on the mechanisms involved in reoxygenation injury.
ROS have been shown to influence c-Src at several levels, both directly by acting on c-Src to modulate its kinase activity (1, 69, 104) and indirectly by modulating factors that regulate c-Src kinase activity (11, 30, 86, 106). Similarly, c-Src kinase activity appears to influence NADPH oxidase-dependent ROS generation at several levels, by facilitating the activation of NADPH oxidase co-factors (Rac and p47phox) required for complex activation (19, 36, 110). For example, hyperoxia induces c-Src-dependent tyrosine phosphorylation of p47phox, induces recruitment of p47phox to the cell membrane, enhances the association of p47phox with Src, and induces NADPH oxidase-mediated superoxide production in human pulmonary artery endothelial cells (19). Additionally, c-Src has been shown to indirectly activate Rac1 via tyrosine phosphorylation of the guanine nucleotide exchange factor (Vav2) (36). Activation of the Rac1-GEF Vav2 via c-Src protein tyrosine kinase phosphorylation has been observed to generate Nox1-dependent ROS in HT29 cells. These features of redox-dependent functions of c-Src are highlighted in redoxosomal pathways that have been found to be important for NFκB activation following hypoxia/reoxygenation (69).
In contrast to pro-inflammatory cytokine pathways that utilize redoxosomes to signal NFκB activation (described earlier in this review), redoxosomal pathways responsible for NFκB activation following hypoxia/reoxygenation appear to be independent of a ligand signal (69). Other important differences between these two pathways include the mechanisms of IκBα phosphorylation required for NFκB activation. The proinflammatory (i.e., TNFα and IL-1β) canonical pathway of NFκB activation occurs through IKK-dependent phosphorylation of IκBα on Ser32/36, which directs IκBα to be degraded by the proteasome. In contrast, NFκB activation following hypoxia/reoxygenation injury is dependent on c-Src-mediated tyrosine phosphorylation of IκBα on Tyr42 and occurs in a proteasome-independent manner (32, 49).
Of interest to this review on endosomal Nox signaling, a recent study has demonstrated that c-Src is required for the formation of redoxosomes following hypoxia/reoxygenation (H/R) and that this process is required for the redox-dependent activation of NFκB (69). In this study, c-Src recruitment from the plasma membrane into the endosomal compartment was initiated during the reoxygenation phase of H/R injury. This recruitment of c-Src to endosomes correlated with NADPH-dependent superoxide production by the endosomal compartment. This study was performed in HeLa cells, and similar findings have been obtained in the mouse hepatocyte TIB73 cell line (Fig. 8). Studies defining the NADPH oxidase involved in H/R-induced redoxosomal ROS were performed in primary fibroblasts generated from Nox1 and Nox2 knockout mice (69). These studies demonstrated that Nox1 was primarily responsible for the induction of redoxosomal superoxides. As in the case of ligand-activated redoxosomes, H/R induction of redoxosomes required endocytosis; the expression of dominant negative dynamin inhibited the formation of endosomal superoxide, the recruitment of both c-Src and Rac1 to the endosomal fraction, and the activation of NFκB following H/R (69).
FIG. 8.
H/R induction of NADPH-dependent superoxide production by the endosomal compartment. (A) TIB73 cells (a hepatocyte cell line) were treated in a hypoxic atmosphere for 5 h, and were then reoxygenated for various periods prior to subcellular fractionation by iodixanol gradient separation of postnuclear supernatants. For each fraction, relative NADPH-dependent superoxide production was detected by lucigenin luminescence, and is shown as Relative Light Units (RLU) per minute. Control (ctrl) cells were not subjected to H/R. H/R experimental samples are shown at times post-reoxygenation (0–60 min). The zero minute post-reoxygenation time point underwent hypoxia for 5 h, but did not undergo reoxygenation. (B) Superoxide production in the peak vesicular fractions (#3-4) of normoxic (ctrl) and 40-min post-reoxygenation TIB73 cell samples was evaluated by ESR, using the DMPO spin-trap in the presence or absence of 100 μM of NADPH to stimulate Nox activity (conditions 1–3). Assays were also performed with isolated vesicular fractions pre-loaded with SOD1 or catalase proteins, by adding 1 mg/ml to the reoxygenation media (conditions 4 and 5). Asterisks mark DMPO/•OH adducts derived from superoxides (as demonstrated by SOD1, but not catalase, quenching following loading of the endosomal lumen). The y-axis represents 5 × 104 arbitrary units of intensity, and the x-axis represents the magnetic field in Gauss.
Studies evaluating the genesis of H/R-induced redoxosomes suggest that both Rac1 and c-Src are necessary for this process (69); siRNAs targeting either Rac1 or c-Src reduces Nox activation in the endosomal compartment and NFκB activation following H/R. siRNA inhibition of Rac1 or c-Src also reduces the recruitment of both proteins (Rac1 and c-Src) to endomembranes following H/R, demonstrating the co-dependence of these two factors in the formation of redoxosomes. Lumenal ROS generated by Nox-active redoxosomes is critical for the redox-activation of c-Src, since loading of endosomes at the time of reoxygenation with SOD1/catalase reduces endosomal ROS and c-Src activation (as evident by Try419 phosphorylation of c-Src in the endosomal fractions). Redox-dependent activation of c-Src on redoxosomes was also necessary for c-Src-mediated Tyr42 phosphorylation of IκBα. In this manner, redoxosomal activation of c-Src following H/R drives activation of NFκB following H/R.
The exact mechanism(s) that underlies c-Src activation on redoxosomes remains to be clearly defined, but may involved both direct redox-activation of c-Src and the inactivation of protein tyrosine phosphatases that keep c-Src in an inactive dephosphorylated state (86, 106). It is hypothesized that ROS can directly activate c-Src by modifying specific cysteine residues that activate the c-Src kinase domain (86). In this context, the crosslinking of Src family kinases in lipid rafts, through oxidant-driven formation of disulfide bonds, is proposed to activate the intrinsic autophosphorylation activity of the kinase. Alternatively, ROS may regulate c-Src function by modifying redox-sensitive cysteine residues in protein tyrosine phosphatases (PTP) that inactivate c-Src (86, 106). Since PTPs are susceptible to oxidative inactivation in their catalytic regions, ROS-induced inhibition of phosphatase activity may keep c-Src in an active conformation by preventing dephosphorylation of Tyr419 at its SH1 kinase domain. However, PTP inhibition may also promote the inactive conformation of c-Src by facilitating Tyr530 phosphorylation—the modification responsible for maintaining the inhibitory intramolecular interaction between phospho-Tyr530 and the SH2 domain—and thereby prevent kinase activation in the SH1 activation loop. Hence, the mechanism of redox-dependent c-Src regulation in redoxosomes remains unclear, but may be directly affected by the types of PTPs and kinases that associate with various endomembrane compartments. However, it is clear that c-Src is important for ligand-independent mechanisms of redoxosomal signaling in the context of pro-oxidant injuries such as H/R.
Concluding Remarks and Future Directions
Studies evaluating the importance of endosomal Nox regulation in cell signaling have led to the discovery of a unique redox-active endosomal compartment we call the redoxosome. Ligand-activated redoxosomes by definition produce NADPH-dependent superoxide in their lumen in a receptor-dependent fashion to control redox-mediated signal transduction. The genesis of redoxosomes appears to be uniquely regulated at the plasma membrane, through the association of ligand-activated receptors with lipid raft-bound NADPH oxidases (Nox1 and Nox2). In the context of the best-studied IL-1β signaling pathway, Rac1 appears to play a critical role in bringing Nox into receptor-bearing endosomes. The localized production of superoxides by ligand-activated redoxosomes appears to spatially control redox-dependent processes that are important for receptor activation. In the context of both IL-1β and TNFα signaling pathways, this redox-dependent process involves TRAF recruitment to the cytoplasmic tail of the receptor. However, the exact redox-dependent events that control TRAF docking on redoxosomal receptor complexes remain to be elucidated. Additionally, chloride channels unique to redoxosomes may regulate both the activity of Nox and the availability of superoxides at the redoxosome surface. It is likely that cell-specific processes dictate whether superoxides pass out of redoxosomes or spontaneously dismutate within the lumen to create the redox-gradients necessary for signal transduction. Given the changing pH of endosomes, this process may be intricately regulated to provide varying degrees of superoxide or hydrogen peroxide at the redoxosome surface. Additionally, certain studies suggest that SOD1 recruitment to the redoxosome surface plays a critical role in ROS production by this compartment by acting as redox-sensor. In this context, the ability of SOD1 to regulate Rac1 GTPase activity in a redox-dependent fashion provides a sensitive mechanism for controlling Rac-dependent NADPH oxidases within redoxosomes. Such a mechanism may be important for controlling ROS at levels required for signal transduction, while preventing a toxic build up of ROS.
The finding that H/R injury also induces Nox-active endosomes that are important for the redox activation of NFκB suggests unexpected diversity in redoxosomal signaling pathways. Although H/R-induced redoxosomes are currently thought to form in a ligand-independent manner, it has not been formally ruled out that unknown ligands contribute to the coordination of H/R responses. For example, oxidative stress induced by reoxygenation may lead to the immediate post-translational secretion of ligands that stimulate, in an autocrine fashion, receptors that coordinate Rac1/c-Src-dependent redoxosome formation. Alternatively, reoxygenation may activate proteases at the cell surface to release ligands capable of activating receptors. Further research into these potential mechanisms is needed to identify the initiating events that control H/R induction of redoxosomes. Despite the potential differences in mechanisms that control the genesis of cytokine- and H/R-induced redoxosomes, downstream events that control redox signaling likely share common features. These appear to include Rac1 control of NADPH oxidases, and the localized production of hydrogen peroxide on the redoxosomal surface to control signaling events (i.e., TRAF recruitment in the case of cytokines and c-Src activation in the case of H/R). Whether superoxide channels also play a role in both redoxosomal pathways remains to be clarified.
The accompanying video (see Supplementary Material; for supplementary video, see www.liebertonline.com/ars)* on redoxosomal activation of NFκB by IL-1β serves as a conceptual framework for how redoxosomes may function in the context of cell signaling; in addition, Fig. 9 summarizes concepts relevant to both TNFα and IL-1β redoxosomal signaling pathways. These models are built on data generated in the following publications (42, 67, 68, 78, 83). Although several aspects of these models have been evaluated only in the case of IL-1β signaling, many aspects of redoxosomal signaling are likely conserved when signaling is triggered by TNFα (67, 78). It is also likely that other proteins unique to redoxosomes have yet to be discovered and/or placed into the context of endosomal regulation. For example, other ROS-metabolizing proteins such as GPx-1 and peroxiredoxins, which degrade hydrogen peroxide, may also control local ROS gradients and/or phosphatases on the redoxosome surface and thereby affect signaling, as has been suggested by work on PDGF activation. Further research into the importance of spatially regulated redoxosomal signaling will likely uncover other unique mechanisms that can explain the astounding specificity with which ROS are able to control diverse redox-dependent signaling events in response to environmental stimuli.
FIG. 9.
Model of redoxosomal signaling for IL-1β and TNFα pathways. In the resting state, the cell expresses Nox, an anion channel(s) (AC), and IL-1R1 or TNFR1 on the plasma membrane. Following receptor binding by IL-1β or TNFα, pathway-specific early effectors (MyD88 or TRADD) are recruited to their cognate receptor at the plasma membrane and initiate endocytosis. Rac1 recruits Nox into the early endosome by tethering (indirectly or directly) the receptor to the Nox complex. Phox subunits (p47phox and p67phox in the case of Nox2) are recruited to the newly formed endosomes to produce superoxide-generating redoxosomes. It is currently unclear at which point SOD1 is recruited to Rac1 on redoxosomes. However, this process appears to be important for maintaining Rac1 in a GTP-bound active state. Membrane-impermeable superoxide generated in the redoxosome lumen may spontaneously dismutate to hydrogen peroxide and pass through the endosomal membrane and out of the redoxosome. Alternatively, a DIDS/NFA-sensitive chloride channel may transport the superoxide out of the redoxosome, where it is subsequently converted to hydrogen peroxide. The localized production of hydrogen peroxide at the redoxosome surface leads to transmission of redox-specific signals to either the receptor or one of the downstream effectors (IRAK/TRAF6 or TRAF2), allowing for docking of these TRAF effectors and subsequent activation of pathway-specific IKKKs. These IKKKs in turn phosphorylate the IKK complex, which leads to NFκB activation. The redoxosomal-signaling pathway is downregulated as the build up of cytosolic hydrogen peroxide oxidizes Rac1 on the surface of the endosome, and this causes disassociation of SOD1 from Rac1 (not shown). In the absence of SOD1 binding to Rac1, Rac1 quickly hydrolyzes GTP and becomes inactive, leading to the termination of Nox-dependent superoxide production. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Supplementary Material
Footnotes
Video of redoxosomal signaling for IL-1β. (Step 1) In the resting state, the cell expresses Nox, an anion channel(s) (AC), and IL-1R1 on the plasma membrane. Following IL-1β binding, early effectors including MyD88 are recruited to the receptor at the plasma membrane and initiate endocytosis. (Step 2) Rac1 recruits Nox into the early endosome by tethering (indirectly or directly) the receptor with the Nox complex. It remains unclear how anion channel(s) are recruited into the endosome. (Step 3) Phox subunits (p47phox and p67phox in the case of Nox2) recruit to the Nox complex in the newly formed endosomes. This may be facilitated by changes in inositol phospholipids in the endosomal membrane (not shown). To maintain Rac1 in a GTP-bound active state, SOD1 is recruited to Rac1. In the movie, the SOD binding event is shown to occur following endocytosis, but it is still unclear at what point SOD1 recruits to Rac1. The activated Nox complex (blue protein complex) transfers an electron from NADPH to molecular oxygen (blue molecules) to produce membrane impermeable superoxide (red molecules) in the lumen of the redoxosome. The superoxide may spontaneously dismutate to hydrogen peroxide (yellow molecules), which can pass through the endosomal membrane and out of the redoxosome (not shown). Alternatively as shown in the movie, a DIDS/NFA-sensitive chloride channel may transport the superoxide outside of the redoxosome, where it is subsequently converted to hydrogen peroxide. (Step 4) The localized production of hydrogen peroxide at the surface of redoxosomes transmits redox-specific signals to either the receptor or one of the downstream effectors IRAK or TRAF6 (as shown in movie), allowing for docking of the IRAK/TRAF6 effector complex and subsequent activation of pathway-specific IKKKs. (Step 5) Activation of NFκB is then initiated through the action of IKKK-mediated phosphorylation of the IKK complex, which leads phosphorylation of the IκB complex and mobilization NFκB to the nucleus (not shown). (Step 6) The redoxosomal-signaling pathway is downregulated as the buildup of cytosolic hydrogen peroxide oxidizes Rac1 on the surface of the endosome, resulting in the disassociation of SOD1 from Rac1. In the absence of SOD1 binding to Rac1, Rac1 quickly hydrolyzes GTP and becomes inactive, leading to the termination of Nox generated superoxide production. This movie is based on findings from refs. 42, 68, 78, and 83.
Acknowledgments
This work was supported by NIDDK (RO1 DK067928 and DK051315 to JFE), NIEHS (F30 ES015950 to FDO), and the Roy J. Carver Chair in Molecular Medicine (JFE). We also gratefully acknowledge Dr. Christine Blaumueller for editorial assistance.
Abbreviations
Ang-II, angiotensin II; BSA, bovine serum albumin; ClC-3, chloride channel-3; DAB, 3,3′-diaminobenzidine; DAPI, 4′, 6′-diamidino-2-phenylindole; DIDS, 4,4-diisothiocyanatostilbene-2,2′-disulfonic acid; DMPO, 5,5-dimethylpyrroline-N-oxide; DNA, deoxyribonucleic acid; DPI; diphenylene iodonium; Duox, dual oxidase; EEA1, early endosomal antigen 1; EGF, epidermal growth factor; EPR, electron paramagnetic resonance; ERK, extracellular signal-regulated kinase; ESR, electron spin resonance; FAD, flavin adenine dinucleotide; FasL, fas ligand; GPx-1, glutathione peroxidase 1; GDP, guanosine diphosphate; GFP, green fluorescent protein; GST, glutathione-s-transferase; GTP, guanosine triphosphate; HA, influenza A viral hemagglutinin (tag); H2HFF-BSA, dihydro-2′,4,5,6,7,7′-hexafluorofluorescein-bovine serum albumin; H2O2, hydrogen peroxide; H/R, hypoxia/reoxygenation; IL-1β, interleukin-1 β; IL-1R1, interleukin-1 receptor 1; IRAK, IL-1R-associated kinase-1; IKK, IκB kinase; IKKK, IκB kinase kinase; MEKK1, mitogen-activated protein kinase kinase kinase; MEKK3, mitogen-activated protein kinase kinase kinase 3; MyD88, myeloid differentiation primary response gene; NADPH, nicotinamide–adenine dinucleotide phosphate (reduced); NIK, nuclear factor kappaB-inducing kinase; NFA, niflumic acid, NFκB, nuclear factor κB; Nox, NADPH oxidase; PBS, phosphate buffered saline; PDGF, platelet-derived growth factor; PLC, phospholipase C; PNS, post-nuclear supernatant; PTP, protein tyrosine phosphatase; PX domain, phox homology domain; Rac1, Ras-related C3 botulinum toxin substrate 1; redoxosome, redox-active endosome; RhoGDI, Rho GDP-dissociation inhibitor; RIP, receptor-interacting protein; ROS, reactive oxygen species; SOD1, superoxide dismutase 1; siRNA, small interfering RNA; TAK1, mitogen-activated protein kinase kinase kinase 7 (MAP3K7); TCA cycle, tricarboxylic acid cycle; TEM, transmission electron microscopy; TNFα, tumor necrosis factor α; TNFR1, tumor necrosis factor receptor 1; TRAF2, tumor necrosis factor receptor-associated factor 2; TRAF6, tumor necrosis factor receptor-associated factor 6; TRADD, tumor necrosis factor receptor-associated death domain; WBC, white blood cell; XO, xanthine oxidase.
References
- 1.Abe J. Takahashi M. Ishida M. Lee JD. Berk BC. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1. J Biol Chem. 1997;272:20389–20394. doi: 10.1074/jbc.272.33.20389. [DOI] [PubMed] [Google Scholar]
- 2.Ando S. Kaibuchi K. Sasaki T. Hiraoka K. Nishiyama T. Mizuno T. Asada M. Nunoi H. Matsuda I. Matsuura Y, et al. Post-translational processing of rac p21s is important both for their interaction with the GDP/GTP exchange proteins and for their activation of NADPH oxidase. J Biol Chem. 1992;267:25709–25713. [PubMed] [Google Scholar]
- 3.Arcaro A. Aubert M. Espinosa del Hierro ME. Khanzada UK. Angelidou S. Tetley TD. Bittermann AG. Frame MC. Seckl MJ. Critical role for lipid raft-associated Src kinases in activation of PI3K-Akt signalling. Cell Signal. 2007;19:1081–1092. doi: 10.1016/j.cellsig.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Babior BM. Kipnes RS. Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banfi B. Clark RA. Steger K. Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 6.Barg S. Huang P. Eliasson L. Nelson DJ. Obermuller S. Rorsman P. Thevenod F. Renstrom E. Priming of insulin granules for exocytosis by granular Cl(-) uptake and acidification. J Cell Sci. 2001;114:2145–2154. doi: 10.1242/jcs.114.11.2145. [DOI] [PubMed] [Google Scholar]
- 7.Bedard K. Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 8.Bielski B. Allen AO. Mechanism of the disproportionation of superoxide radicals. J Phys Chem. 1977;81:1048–1050. [Google Scholar]
- 9.Bindoli A. Fukuto JM. Forman HJ. Thiol Chemistry in Peroxidase Catalysis and Redox Signaling. Antioxid Redox Signal. 2008;10:1549–1564. doi: 10.1089/ars.2008.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biswas S. Chida AS. Rahman I. Redox modifications of protein-thiols: Emerging roles in cell signaling. Biochem Pharmacol. 2006;71:551–564. doi: 10.1016/j.bcp.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 11.Boggon TJ. Eck MJ. Structure and regulation of Src family kinases. Oncogene. 2004;23:7918–7927. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- 12.Bokoch GM. Zhao T. Regulation of the phagocyte NADPH oxidase by Rac GTPase. Antioxid Redox Signal. 2006;8:1533–1548. doi: 10.1089/ars.2006.8.1533. [DOI] [PubMed] [Google Scholar]
- 13.Briggs RT. Drath DB. Karnovsky ML. Karnovsky MJ. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol. 1975;67:566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burke P. Schooler K. Wiley HS. Regulation of epidermal growth factor receptor signaling by endocytosis and intracellular trafficking. Mol Biol Cell. 2001;12:1897–1910. doi: 10.1091/mbc.12.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callera GE. Touyz RM. Tostes RC. Yogi A. He Y. Malkinson S. Schiffrin EL. Aldosterone activates vascular p38MAP kinase and NADPH oxidase via c-Src. Hypertension. 2005;45:773–779. doi: 10.1161/01.HYP.0000154365.30593.d3. [DOI] [PubMed] [Google Scholar]
- 16.Chen CS. Phorbol ester induces elevated oxidative activity and alkalization in a subset of lysosomes. BMC Cell Biol. 2002;3:21. doi: 10.1186/1471-2121-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho SH. Lee CH. Ahn Y. Kim H. Ahn CY. Yang KS. Lee SR. Redox regulation of PTEN and protein tyrosine phosphatases in H(2)O(2) mediated cell signaling. FEBS Lett. 2004;560:7–13. doi: 10.1016/s0014-5793(04)00112-7. [DOI] [PubMed] [Google Scholar]
- 18.Choi MH. Lee IK. Kim GW. Kim BU. Han YH. Yu DY. Park HS. Kim KY. Lee JS. Choi C. Bae YS. Lee BI. Rhee SG. Kang SW. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 19.Chowdhury AK. Watkins T. Parinandi NL. Saatian B. Kleinberg ME. Usatyuk PV. Natarajan V. Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. J Biol Chem. 2005;280:20700–20711. doi: 10.1074/jbc.M411722200. [DOI] [PubMed] [Google Scholar]
- 20.Cifuentes ME. Pagano PJ. c-Src and smooth muscle NAD(P)H oxidase: assembling a path to hypertrophy. Arterioscler Thromb Vasc Biol. 2003;23:919–921. doi: 10.1161/01.ATV.0000077235.97226.82. [DOI] [PubMed] [Google Scholar]
- 21.Cross AR. Segal AW. The NADPH oxidase of professional phagocytes. Prototype of the NOX electron transport chain systems. Biochim Biophys Acta. 2004;1657:1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cursio R. Miele C. Filippa N. Van Obberghen E. Gugenheim J. Alterations in protein tyrosine kinase pathways in rat liver following normothermic ischemia-reperfusion. Transplant Proc. 2006;38:3362–3365. doi: 10.1016/j.transproceed.2006.10.165. [DOI] [PubMed] [Google Scholar]
- 23.Das M. Gherghiceanu M. Lekli I. Mukherjee S. Popescu LM. Das DK. Essential role of lipid raft in ischemic preconditioning. Cell Physiol Biochem. 2008;21:325–334. doi: 10.1159/000129391. [DOI] [PubMed] [Google Scholar]
- 24.de Diesbach P. Medts T. Carpentier S. D'Auria L. Van Der Smissen P. Platek A. Mettlen M. Caplanusi A. van den Hove MF. Tyteca D. Courtoy PJ. Differential subcellular membrane recruitment of Src may specify its downstream signalling. Exp Cell Res. 2008;314:1465–1479. doi: 10.1016/j.yexcr.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Demaurex N. Petheo GL. Electron and proton transport by NADPH oxidases. Philos Trans R Soc Lond B Biol Sci. 2005;360:2315–2325. doi: 10.1098/rstb.2005.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diekmann D. Abo A. Johnston C. Segal AW. Hall A. Interaction of Rac with p67phox and regulation of phagocytic NADPH oxidase activity. Science. 1994;265:531–533. doi: 10.1126/science.8036496. [DOI] [PubMed] [Google Scholar]
- 27.Dikalov S. Griendling KK. Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49:717–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dusi S. Della Bianca V. Grzeskowiak M. Rossi F. Relationship between phosphorylation and translocation to the plasma membrane of p47phox and p67phox and activation of the NADPH oxidase in normal and Ca(2+)-depleted human neutrophils. Biochem J. 1993;290:173–178. doi: 10.1042/bj2900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellson CD. Gobert-Gosse S. Anderson KE. Davidson K. Erdjument-Bromage H. Tempst P. Thuring JW. Cooper MA. Lim ZY. Holmes AB. Gaffney PR. Coadwell J. Chilvers ER. Hawkins PT. Stephens LR. PtdIns(3)P regulates the neutrophil oxidase complex by binding to the PX domain of p40(phox) Nat Cell Biol. 2001;3:679–682. doi: 10.1038/35083076. [DOI] [PubMed] [Google Scholar]
- 30.Engen JR. Wales TE. Hochrein JM. Meyn MA., 3rd Banu Ozkan S. Bahar I. Smithgall TE. Structure and dynamic regulation of Src-family kinases. Cell Mol Life Sci. 2008;65:3058–3073. doi: 10.1007/s00018-008-8122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan C. Li Q. Ross D. Engelhardt JF. Tyrosine phosphorylation of I kappa B alpha activates NF kappa B through a redox-regulated and c-Src-dependent mechanism following hypoxia/reoxygenation. J Biol Chem. 2003;278:2072–2080. doi: 10.1074/jbc.M206718200. [DOI] [PubMed] [Google Scholar]
- 32.Fan C. Li Q. Zhang Y. Liu X. Luo M. Abbott D. Zhou W. Engelhardt JF. IkappaBalpha and IkappaBbeta possess injury context-specific functions that uniquely influence hepatic NF-kappaB induction and inflammation. J Clin Invest. 2004;113:746–755. doi: 10.1172/JCI17337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faulkner K. Fridovich I. Luminol and lucigenin as detectors for O2. Free Radic Biol Med. 1993;15:447–451. doi: 10.1016/0891-5849(93)90044-u. [DOI] [PubMed] [Google Scholar]
- 34.Freeman JL. Kreck ML. Uhlinger DJ. Lambeth JD. Ras effector-homologue region on Rac regulates protein associations in the neutrophil respiratory burst oxidase complex. Biochemistry. 1994;33:13431–13435. doi: 10.1021/bi00249a031. [DOI] [PubMed] [Google Scholar]
- 35.Georgiou G. How to flip the (redox) switch. Cell. 2002;111:607–610. doi: 10.1016/s0092-8674(02)01165-0. [DOI] [PubMed] [Google Scholar]
- 36.Gianni D. Bohl B. Courtneidge SA. Bokoch GM. The involvement of the tyrosine kinase c-Src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Mol Biol Cell. 2008;19:2984–2994. doi: 10.1091/mbc.E08-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomberg M. An instance of trivalent carbon: Triphenylmethyl. J Am Chem Soc. 1900;22:773–771. [Google Scholar]
- 38.Granger DN. Rutili G. McCord JM. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981;81:22–29. [PubMed] [Google Scholar]
- 39.Gruenberg J. Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- 40.Guo J. Meng F. Zhang G. Zhang Q. Free radicals are involved in continuous activation of nonreceptor tyrosine protein kinase c-Src after ischemia/reperfusion in rat hippocampus. Neurosci Lett. 2003;345:101–104. doi: 10.1016/s0304-3940(03)00483-x. [DOI] [PubMed] [Google Scholar]
- 41.Halliwell B. Gutteridge J. Free Radicals in Biology and Medicine. Oxford: Oxford University Press; 2007. [Google Scholar]
- 42.Harraz MM. Marden JJ. Zhou W. Zhang Y. Williams A. Sharov VS. Nelson K. Luo M. Paulson H. Schoneich C. Engelhardt JF. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008;118:659–670. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawkins BJ. Madesh M. Kirkpatrick CJ. Fisher AB. Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol Biol Cell. 2007;18:2002–2012. doi: 10.1091/mbc.E06-09-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayden MS. Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 45.Heo J. Campbell SL. Mechanism of redox-mediated guanine nucleotide exchange on redox-active Rho GTPases. J Biol Chem. 2005;280:31003–31010. doi: 10.1074/jbc.M504768200. [DOI] [PubMed] [Google Scholar]
- 46.Heo J. Raines KW. Mocanu V. Campbell SL. Redox regulation of RhoA. Biochemistry. 2006;45:14481–14489. doi: 10.1021/bi0610101. [DOI] [PubMed] [Google Scholar]
- 47.Hilenski LL. Clempus RE. Quinn MT. Lambeth JD. Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 48.Hordijk PL. Regulation of NADPH oxidases: Tthe role of Rac proteins. Circ Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- 49.Imbert V. Rupec RA. Livolsi A. Pahl HL. Traenckner EB. Mueller–Dieckmann C. Farahifar D. Rossi B. Auberger P. Baeuerle PA. Peyron JF. Tyrosine phosphorylation of I kappa B-alpha activates NF-kappa B without proteolytic degradation of I kappa B-alpha. Cell. 1996;86:787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 50.Janssens S. Beyaert R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell. 2003;11:293–302. doi: 10.1016/s1097-2765(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 51.Jefferies C. Bowie A. Brady G. Cooke EL. Li X. O'Neill LA. Transactivation by the p65 subunit of NF-kappaB in response to interleukin-1 (IL-1) involves MyD88, IL-1 receptor-associated kinase 1, TRAF-6, and Rac1. Mol Cell Biol. 2001;21:4544–4552. doi: 10.1128/MCB.21.14.4544-4552.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jentsch TJ. Chloride and the endosomal-lysosomal pathway: Emerging roles of CLC chloride transporters. J Physiol. 2007;578:633–640. doi: 10.1113/jphysiol.2006.124719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin S. Zhang Y. Yi F. Li PL. Critical role of lipid raft redox signaling platforms in endostatin-induced coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2008;28:485–490. doi: 10.1161/ATVBAHA.107.159772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanai F. Liu H. Field SJ. Akbary H. Matsuo T. Brown GE. Cantley LC. Yaffe MB. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat Cell Biol. 2001;3:675–678. doi: 10.1038/35083070. [DOI] [PubMed] [Google Scholar]
- 55.Kim YS. Morgan MJ. Choksi S. Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi T. Garcia del Saz E. Hendry J. Seguchi H. Detection of oxidant producing-sites in glutaraldehyde-fixed human neutrophils and eosinophils stimulated with phorbol myristate acetate. Histochem J. 1999;31:181–194. doi: 10.1023/a:1003547121574. [DOI] [PubMed] [Google Scholar]
- 57.Kobayashi T. Robinson JM. Seguchi H. Identification of intracellular sites of superoxide production in stimulated neutrophils. J Cell Sci. 1998;111:81–91. doi: 10.1242/jcs.111.1.81. [DOI] [PubMed] [Google Scholar]
- 58.Kopani M. Celec P. Danisovic L. Michalka P. Biro C. Oxidative stress and electron spin resonance. Clin Chim Acta. 2006;364:61–66. doi: 10.1016/j.cca.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 59.Kreuzer J. Viedt C. Brandes RP. Seeger F. Rosenkranz AS. Sauer H. Babich A. Nurnberg B. Kather H. Krieger–Brauer HI. Platelet-derived growth factor activates production of reactive oxygen species by NAD(P)H oxidase in smooth muscle cells through Gi1,2. FASEB J. 2003;17:38–40. doi: 10.1096/fj.01-1036fje. [DOI] [PubMed] [Google Scholar]
- 60.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 61.Lattin J. Zidar DA. Schroder K. Kellie S. Hume DA. Sweet MJ. G-protein-coupled receptor expression, function, and signaling in macrophages. J Leukoc Biol. 2007;82:16–32. doi: 10.1189/jlb.0107051. [DOI] [PubMed] [Google Scholar]
- 62.Lavigne MC. Malech HL. Holland SM. Leto TL. Genetic demonstration of p47phox-dependent superoxide anion production in murine vascular smooth muscle cells. Circulation. 2001;104:79–84. doi: 10.1161/01.cir.104.1.79. [DOI] [PubMed] [Google Scholar]
- 63.Lee H. Woodman SE. Engelman JA. Volonte D. Galbiati F. Kaufman HL. Lublin DM. Lisanti MP. Palmitoylation of caveolin-1 at a single site (Cys-156) controls its coupling to the c-Src tyrosine kinase: Targeting of dually acylated molecules (GPI-linked, transmembrane, or cytoplasmic) to caveolae effectively uncouples c-Src and caveolin-1 (TYR-14) J Biol Chem. 2001;276:35150–35158. doi: 10.1074/jbc.M104530200. [DOI] [PubMed] [Google Scholar]
- 64.Legler DF. Micheau O. Doucey MA. Tschopp J. Bron C. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFalpha-mediated NF-kappaB activation. Immunity. 2003;18:655–664. doi: 10.1016/s1074-7613(03)00092-x. [DOI] [PubMed] [Google Scholar]
- 65.Li H. Lin X. Positive and negative signaling components involved in TNFalpha-induced NF-kappaB activation. Cytokine. 2008;41:1–8. doi: 10.1016/j.cyto.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 66.Li PL. Zhang Y. Yi F. Lipid raft redox signaling platforms in endothelial dysfunction. Antioxid Redox Signal. 2007;9:1457–1470. doi: 10.1089/ars.2007.1667. [DOI] [PubMed] [Google Scholar]
- 67.Li Q. Spencer NY. Oakley FD. Buettner GR. Engelhardt JF. Antioxid Redox Signal. Vol. 11. Spencer NY, Oakley FD, Buettner GR: 2009. Endosomal Nox2 facilitates redox-dependent induction of NFkappaB by TNFalpha; pp. 1249–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Q. Harraz MM. Zhou W. Zhang LN. Ding W. Zhang Y. Eggleston T. Yeaman C. Banfi B. Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26:140–154. doi: 10.1128/MCB.26.1.140-154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Q. Zhang Y. Marden JJ. Banfi B. Engelhardt JF. Endosomal NADPH oxidase regulates c-Src activation following hypoxia/reoxygenation injury. Biochem J. 2008;411:531–541. doi: 10.1042/BJ20071534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li S. Couet J. Lisanti MP. Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem. 1996;271:29182–29190. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Y. Zhu H. Kuppusamy P. Roubaud V. Zweier JL. Trush MA. Validation of lucigenin (bis-N-methylacridinium) as a chemilumigenic probe for detecting superoxide anion radical production by enzymatic and cellular systems. J Biol Chem. 1998;273:2015–2023. doi: 10.1074/jbc.273.4.2015. [DOI] [PubMed] [Google Scholar]
- 72.Liu J. Oh P. Horner T. Rogers RA. Schnitzer JE. Organized endothelial cell surface signal transduction in caveolae distinct from glycosylphosphatidylinositol-anchored protein microdomains. J Biol Chem. 1997;272:7211–7222. doi: 10.1074/jbc.272.11.7211. [DOI] [PubMed] [Google Scholar]
- 73.Lujan R. Shigemoto R. Lopez–Bendito G. Glutamate and GABA receptor signalling in the developing brain. Neuroscience. 2005;130:567–580. doi: 10.1016/j.neuroscience.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 74.Maudsley S. Pierce KL. Zamah AM. Miller WE. Ahn S. Daaka Y. Lefkowitz RJ. Luttrell LM. The beta(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J Biol Chem. 2000;275:9572–9580. doi: 10.1074/jbc.275.13.9572. [DOI] [PubMed] [Google Scholar]
- 75.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 76.McCord JM. Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968;243:5753–5760. [PubMed] [Google Scholar]
- 77.McCord JM. Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 78.Miller FJ., Jr. Filali M. Huss GJ. Stanic B. Chamseddine A. Barna TJ. Lamb FS. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 79.Miller PJ. Wenzel RP. Etiologic organisms as independent predictors of death and morbidity associated with bloodstream infections. J Infect Dis. 1987;156:471–477. doi: 10.1093/infdis/156.3.471. [DOI] [PubMed] [Google Scholar]
- 80.Mizuno T. Kaibuchi K. Ando S. Musha T. Hiraoka K. Takaishi K. Asada M. Nunoi H. Matsuda I. Takai Y. Regulation of the superoxide-generating NADPH oxidase by a small GTP-binding protein and its stimulatory and inhibitory GDP/GTP exchange proteins. J Biol Chem. 1992;267:10215–10218. [PubMed] [Google Scholar]
- 81.Montezano AC. Callera GE. Yogi A. He Y. Tostes RC. He G. Schiffrin EL. Touyz RM. Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated redox-sensitive RhoA pathways. Arterioscler Thromb Vasc Biol. 2008;28:1511–1518. doi: 10.1161/ATVBAHA.108.168021. [DOI] [PubMed] [Google Scholar]
- 82.Moreland JG. Davis AP. Bailey G. Nauseef WM. Lamb FS. Anion channels, including ClC-3, are required for normal neutrophil oxidative function, phagocytosis, and transendothelial migration. J Biol Chem. 2006;281:12277–12288. doi: 10.1074/jbc.M511030200. [DOI] [PubMed] [Google Scholar]
- 83.Mumbengegwi DR. Li Q. Li C. Bear CE. Engelhardt JF. Evidence for a superoxide permeability pathway in endosomal membranes. Mol Cell Biol. 2008;28:3700–3712. doi: 10.1128/MCB.02038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Munzel T. Afanas'ev IB. Kleschyov AL. Harrison DG. Detection of superoxide in vascular tissue. Arterioscler Thromb Vasc Biol. 2002;22:1761–1768. doi: 10.1161/01.atv.0000034022.11764.ec. [DOI] [PubMed] [Google Scholar]
- 85.Murphy R. DeCoursey TE. Charge compensation during the phagocyte respiratory burst. Biochim Biophys Acta. 2006;1757:996–1011. doi: 10.1016/j.bbabio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 86.Nakashima I. Kato M. Akhand AA. Suzuki H. Takeda K. Hossain K. Kawamoto Y. Redox-linked signal transduction pathways for protein tyrosine kinase activation. Antioxid Redox Signal. 2002;4:517–531. doi: 10.1089/15230860260196326. [DOI] [PubMed] [Google Scholar]
- 87.Nauseef WM. Assembly of the phagocyte NADPH oxidase. Histochem Cell Biol. 2004;122:277–291. doi: 10.1007/s00418-004-0679-8. [DOI] [PubMed] [Google Scholar]
- 88.Park HS. Lee SH. Park D. Lee JS. Ryu SH. Lee WJ. Rhee SG. Bae YS. Sequential activation of phosphatidylinositol 3-kinase, beta Pix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol Cell Biol. 2004;24:4384–4394. doi: 10.1128/MCB.24.10.4384-4394.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patra SK. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim Biophys Acta. 2008;1785:182–206. doi: 10.1016/j.bbcan.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 90.Pike LJ. Lipid rafts: Heterogeneity on the high seas. Biochem J. 2004;378:281–292. doi: 10.1042/BJ20031672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Puri C. Tosoni D. Comai R. Rabellino A. Segat D. Caneva F. Luzzi P. Di Fiore PP. Tacchetti C. Relationships between EGFR signaling-competent and endocytosis-competent membrane microdomains. Mol Biol Cell. 2005;16:2704–2718. doi: 10.1091/mbc.E04-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 93.Rhee SG. Bae YS. Lee SR. Kwon J. Hydrogen peroxide: A key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;2000:PE1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 94.Rhee SG. Kang SW. Jeong W. Chang TS. Yang KS. Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 95.Rosado JA. Redondo PC. Salido GM. Gomez–Arteta E. Sage SO. Pariente JA. Hydrogen peroxide generation induces pp60src activation in human platelets: Evidence for the involvement of this pathway in store-mediated calcium entry. J Biol Chem. 2004;279:1665–1675. doi: 10.1074/jbc.M307963200. [DOI] [PubMed] [Google Scholar]
- 96.Royer–Pokora B. Kunkel LM. Monaco AP. Goff SC. Newburger PE. Baehner RL. Cole FS. Curnutte JT. Orkin SH. Cloning the gene for an inherited human disorder–chronic granulomatous disease–on the basis of its chromosomal location. Nature. 1986;322:32–38. doi: 10.1038/322032a0. [DOI] [PubMed] [Google Scholar]
- 97.Shao D. Segal AW. Dekker LV. Lipid rafts determine efficiency of NADPH oxidase activation in neutrophils. FEBS Lett. 2003;550:101–106. doi: 10.1016/s0014-5793(03)00845-7. [DOI] [PubMed] [Google Scholar]
- 98.Sheng M. Pak DT. Ligand-gated ion channel interactions with cytoskeletal and signaling proteins. Annu Rev Physiol. 2000;62:755–778. doi: 10.1146/annurev.physiol.62.1.755. [DOI] [PubMed] [Google Scholar]
- 99.Singh AB. Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell Signal. 2005;17:1183–1193. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 100.Skatchkov MP. Sperling D. Hink U. Mulsch A. Harrison DG. Sindermann I. Meinertz T. Munzel T. Validation of lucigenin as a chemiluminescent probe to monitor vascular superoxide as well as basal vascular nitric oxide production. Biochem Biophys Res Commun. 1999;254:319–324. doi: 10.1006/bbrc.1998.9942. [DOI] [PubMed] [Google Scholar]
- 101.Stehr M. Adam RM. Khoury J. Zhuang L. Solomon KR. Peters CA. Freeman MR. Platelet derived growth factor-BB is a potent mitogen for rat ureteral and human bladder smooth muscle cells: Dependence on lipid rafts for cell signaling. J Urol. 2003;169:1165–1170. doi: 10.1097/01.ju.0000041501.01323.b9. [DOI] [PubMed] [Google Scholar]
- 102.Steinbeck MJ. Khan AU. Appel WH., Jr. Karnovsky MJ. The DAB-Mn++ cytochemical method revisited: Validation of specificity for superoxide. J Histochem Cytochem. 1993;41:1659–1667. doi: 10.1177/41.11.8292156. [DOI] [PubMed] [Google Scholar]
- 103.Suh YA. Arnold RS. Lassegue B. Shi J. Xu X. Sorescu D. Chung AB. Griendling KK. Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 104.Suzaki Y. Yoshizumi M. Kagami S. Koyama AH. Taketani Y. Houchi H. Tsuchiya K. Takeda E. Tamaki T. Hydrogen peroxide stimulates c-Src-mediated big mitogen-activated protein kinase 1 (BMK1) and the MEF2C signaling pathway in PC12 cells: potential role in cell survival following oxidative insults. J Biol Chem. 2002;277:9614–9621. doi: 10.1074/jbc.M111790200. [DOI] [PubMed] [Google Scholar]
- 105.Takikita–Suzuki M. Haneda M. Sasahara M. Owada MK. Nakagawa T. Isono M. Takikita S. Koya D. Ogasawara K. Kikkawa R. Activation of Src kinase in platelet-derived growth factor-B-dependent tubular regeneration after acute ischemic renal injury. Am J Pathol. 2003;163:277–286. doi: 10.1016/S0002-9440(10)63651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tao Q. Spring SC. Terman BI. Comparison of the signaling mechanisms by which VEGF, H2O2, and phosphatase inhibitors activate endothelial cell ERK1/2 MAP-kinase. Microvasc Res. 2005;69:36–44. doi: 10.1016/j.mvr.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 107.Tatosyan AG. Mizenina OA. Kinases of the Src family: structure and functions. Biochemistry (Mosc) 2000;65:49–58. [PubMed] [Google Scholar]
- 108.Taub N. Teis D. Ebner HL. Hess MW. Huber LA. Late endosomal traffic of the epidermal growth factor receptor ensures spatial and temporal fidelity of mitogen-activated protein kinase signaling. Mol Biol Cell. 2007;18:4698–4710. doi: 10.1091/mbc.E07-02-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thomas SM. Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 110.Touyz RM. Yao G. Schiffrin EL. c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:981–987. doi: 10.1161/01.ATV.0000069236.27911.68. [DOI] [PubMed] [Google Scholar]
- 111.Ushio–Fukai M. Alexander RW. Caveolin-dependent angiotensin II type 1 receptor signaling in vascular smooth muscle. Hypertension. 2006;48:797–803. doi: 10.1161/01.HYP.0000242907.70697.5d. [DOI] [PubMed] [Google Scholar]
- 112.van der Goot FG. Gruenberg J. Intra-endosomal membrane traffic. Trends Cell Biol. 2006;16:514–521. doi: 10.1016/j.tcb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 113.Verstrepen L. Bekaert T. Chau TL. Tavernier J. Chariot A. Beyaert R. TLR-4, IL-1R and TNF-R signaling to NF-kappaB: Variations on a common theme. Cell Mol Life Sci. 2008;65:2964–2978. doi: 10.1007/s00018-008-8064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vieira AV. Lamaze C. Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 115.Vilhardt F. van Deurs B. The phagocyte NADPH oxidase depends on cholesterol-enriched membrane microdomains for assembly. EMBO J. 2004;23:739–748. doi: 10.1038/sj.emboj.7600066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Y. Pennock S. Chen X. Wang Z. Endosomal signaling of epidermal growth factor receptor stimulates signal transduction pathways leading to cell survival. Mol Cell Biol. 2002;22:7279–7290. doi: 10.1128/MCB.22.20.7279-7290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Y. Pennock SD. Chen X. Kazlauskas A. Wang Z. Platelet-derived growth factor receptor-mediated signal transduction from endosomes. J Biol Chem. 2004;279:8038–8046. doi: 10.1074/jbc.M311494200. [DOI] [PubMed] [Google Scholar]
- 118.Williams TM. Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med. 2004;36:584–595. doi: 10.1080/07853890410018899. [DOI] [PubMed] [Google Scholar]
- 119.Woo CH. Kim TH. Choi JA. Ryu HC. Lee JE. You HJ. Bae YS. Kim JH. Inhibition of receptor internalization attenuates the TNFalpha-induced ROS generation in non-phagocytic cells. Biochem Biophys Res Commun. 2006;351:972–978. doi: 10.1016/j.bbrc.2006.10.154. [DOI] [PubMed] [Google Scholar]
- 120.Zhang AY. Yi F. Zhang G. Gulbins E. Li PL. Lipid raft clustering and redox signaling platform formation in coronary arterial endothelial cells. Hypertension. 2006;47:74–80. doi: 10.1161/10.1161/01.HYP.0000196727.53300.62. [DOI] [PubMed] [Google Scholar]
- 121.Zuo L. Ushio–Fukai M. Ikeda S. Hilenski L. Patrushev N. Alexander RW. Caveolin-1 is essential for activation of Rac1 and NAD(P)H oxidase after angiotensin II type 1 receptor stimulation in vascular smooth muscle cells: Role in redox signaling and vascular hypertrophy. Arterioscler Thromb Vasc Biol. 2005;25:1824–1830. doi: 10.1161/01.ATV.0000175295.09607.18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.