Abstract
The past decade has seen an exponential increase in the number of cancer therapies with defined molecular targets. Interestingly, many of these new agents are also documented to raise levels of intracellular reactive oxygen species (ROS) in addition to inhibiting a biochemical target. In most cases, the exact link between the primary target of the drug and effects on cellular redox status is unknown. However, it is important to understand the role of oxidative stress in promoting cytotoxicity by these agents, because the design of multiregimen strategies could conceivably build on these redox alterations. Also, drug resistance mediated by antioxidant defenses could potentially be anticipated and circumvented with improved knowledge of the redox-related effects of these targeted agents. Given the large number of targeted chemotherapies, in this review, we focus on selected agents that have shown promise in hematologic malignancies: proteasome inhibitors, histone deacetylase inhibitors, Bcl-2–targeted agents, and a kinase inhibitor called adaphostin. Despite structural differences within classes of these compounds, a commonality of causing increased oxidative stress exists, which contributes to induction of cell death. Antioxid. Redox Signal. 11, 1123–1137.
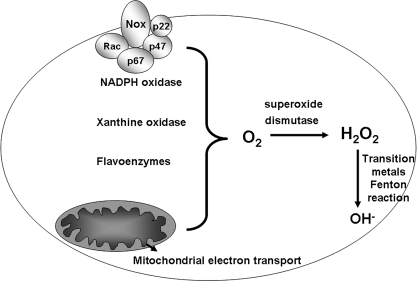
The term oxidative stress refers to an imbalance in the antioxidant-to-prooxidant ratio within a cell. This ratio is constantly negotiated in cells because homeostatic cellular function generates oxidative species that are continuously inactivated by antioxidant systems. Endogenous sources of oxidant stress, such as mitochondrial electron transport and activation of oxidases, generate free radicals as byproducts of their function (Fig. 1). It has been estimated that ∼1–2% of the total oxygen consumption of mitochondria generates reactive oxygen species (ROS); therefore, oxidative phosphorylation is the major endogenous source of oxidative stress (41). Cellular oxidases are another source of ROS. A prototypical example of such an oxidase is the NADPH oxidase complex, which functions to deliver a superoxide burst as a defense against bacteria. Similarly, oxidases like the xanthine oxidases, monoamine oxidases, and other flavoenzymes are also endogenous sources of oxidants (33).
FIG. 1.
Endogenous sources of oxidative stress. Four sources of oxidative stress that represent normal metabolic pathways are represented. The NADPH oxidase is a membrane-bound enzyme complex that generates superoxide. Xanthine oxidase is an enzyme important for uric acid formation, which also generates superoxide as a byproduct of its function. Flavoenzymes are a diverse group of enzymes that are involved in numerous biologic processes and include many monooxidases. Mitochondrial electron transport generates superoxide primarily through complex I and III. Superoxide dismutases inactivate superoxide but generate hydrogen peroxide, which can give rise to hydroxyl radical in the presence of transition metals.
Superoxide is the specific byproduct of both mitochondrial respiration and of the aforementioned oxidases and is one example of a ROS. ROS refers to oxygen-containing breakdown products of molecular oxygen that are highly reactive and are able to damage lipid membranes, proteins, and DNA when present in high amounts. This damage is not necessarily perpetuated by superoxide itself but by further breakdown products of molecular oxygen. For example, superoxide is inactivated primarily by the superoxide dismutase (SOD) enzymes. The reaction by which superoxide is broken down actually generates hydrogen peroxide, another ROS entity. Unlike superoxide, hydrogen peroxide can traverse biologic membranes, thereby expanding its range of reactivity because it can travel from outside the cell to inside the cell and from one subcellular organelle to another. Hydrogen peroxide can be further inactivated by an array of antioxidants. However, in the presence of transition metals such as Fe and Cu, the Fenton reaction catalyzes the generation of hydroxyl radical, the most highly reactive and damaging ROS species. Therefore, overt damage to macromolecules is most often promoted by the hydroxyl radical. Again, endogenous oxidative stress rarely leads to damage, because a healthy cell generally possesses an armory of antioxidants to inactivate and dispel ROS, thereby obviating any harm to the cell. However, when cellular antioxidants are overwhelmed to a great degree, which occurs in the context of external environmental challenges like toxic insults or radiation, cell death is the expected outcome.
The type of cell death triggered by oxidative stress is dependent on the dose and duration of the exposure. Necrotic cell death is thought to result from a higher amount and exposure to oxidant stress than the amount necessary to elicit apoptotic cell death. The examples of cancer therapies to be discussed in this article focus on oxidative stress–induced apoptosis, although we cannot rule out the possibility that some of these agents cause other types of cell death, such as autophagy and necrosis.
A defining feature of apoptotic cell death is activation of cysteine proteases called caspases that function to activate one another and ultimately dismantle the cell (65). A multimember family of proteins, caspases that initiate the cell-death cascade are caspase-8, caspase-9, caspase-2, and caspase-4. Caspase-8 is the initiator for death signals stemming from outside the cell, whereas caspase-9 is triggered by signals within the cell. Mechanisms of caspase-2 and -4 activation are less well understood, but are also internal to the cell. Caspase-4 is thought to be activated by stress initiated in the endoplasmic reticulum (ER), also referred to as ER stress (45).
Mitochondria represent a convergence point for oxidative stress–induced apoptosis. This organelle can serve as a starting point of oxidative stress or a transducer of oxidative stress–induced apoptotic signaling (116). A large percentage of endogenously produced oxidants are generated continuously during mitochondrial oxidative phosphorylation, with superoxide being the predominant entity. A higher rate of oxidative phosphorylation could initiate an oxidative stress if mitochondrial antioxidants are not adequately protective (42). In line with this concept, a loss of mitochondrial antioxidant capacity could also initiate an oxidative stress from within mitochondria, because the normal defenses would be reduced.
A buildup of oxidants outside mitochondria can also promote apoptosis. The mitochondrial permeability transition, a pore complex that allows the exchange of solutes across mitochondrial membranes, is triggered by oxidants (59). A major player in promoting activation of caspases is the mitochondrial resident, cytochrome c. On exposure to an apoptotic trigger perturbing mitochondria, cytochrome c leaves the intermembrane space of mitochondria and enters the cytosol. Once in the cytosol, it forms a complex with dATP, an adaptor molecule called Apaf-1, and caspase-9. This conglomerate, called the apoptosome, functions to activate caspase-9, which then activates caspase-3 (26, 58). Other proapoptotic molecules are known to be released from mitochondria to promote caspase activation, but of these, only the exit of cytochrome c from mitochondria has been linked to redox (43). In the intermembrane space of mitochondria, cardiolipin, a mitochondrial phospholipids, tethers cytochrome c in place. Oxidation of cardiolipin, under conditions of mitochondrial oxidative stress, loosens cytochrome c and promotes its release. Release of cytochrome c, in addition to the other various proteins released when mitochondria are disrupted, is thought to require permeabilization of the mitochondrial outer membrane. This permeabilization exposes the rest of the cell to various oxidant species normally housed (and neutralized) within mitochondria.
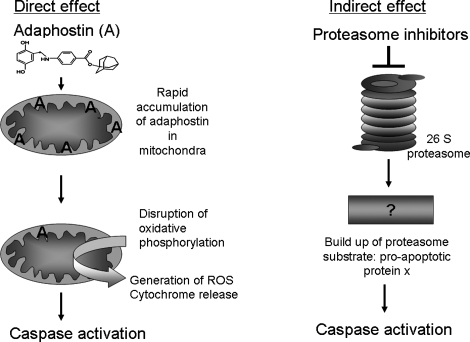
The way in which oxidative stress triggers apoptosis can loosely be characterized as a direct effect on apoptotic machinery or via an indirect effect on apoptosis (Fig. 2). Adaphostin, a kinase inhibitor, which is discussed in greater detail later, is an agent that acts in a direct manner because the active drug accumulates in mitochondria, thereby compromising oxidative phosphorylation (58). Because oxidative phosphorylation is a major generator of ATP, the cellular energy currency, this causes an ATP-depletion/energy crisis and initiates apoptosis. Another example of chemotherapeutic agents that directly modulate oxidative stress and cell death are the Bcl-2 inhibitors (88, 114). In contrast, an indirect effect on apoptosis induction would be a proteasome inhibitor blocking proteasome function and then causing accumulation of an ubiquitinated proapoptotic protein. Similarly, epigenetically targeted agents also induce cell death indirectly because the proximal targets for those agents are gene expression. All four of these classes of agents are discussed in greater detail later.
FIG. 2.
Direct versus indirect effects of new agents on oxidative stress–induced apoptosis. The tyrphostin inhibitor, adaphostin, induces cell death by a more direct route than do proteasome inhibitors. Adaphostin (chemical structure is shown) accumulates in mitochondria and disrupts oxidative phosphorylation, causing mitochondrial membrane depolarization. This perturbation of mitochondria causes an increase in intracellular superoxide, intracellular peroxides, and causes the release of cytochrome c. Cytochrome c is a cofactor in caspase activation, which results in the dismantling of the cell.
Several leukemia and lymphoma model systems have been associated with heightened levels of oxidative stress. Instead of triggering death pathways, this increased oxidative stress in transformed cells, as compared with nontransformed cells, appears to be part of the milieu in which the cancer cell thrives. Consistent with this notion, numerous reports document the ability of a low level of oxidative stress to stimulate signaling, via activation of kinases or inactivation of phosphatases or both, that ultimately transduces a mitogenic signal (27, 44, 52).
The source of the increased oxidative stress seen in hematologic malignancies is likely not attributable to a single entity. Hyperactivation of endogenous sources of ROS, such as electron transport or the NADPH oxidase, has been documented in leukemia cells, potentially accounting for the observed increased levels of ROS. Furthermore, specific oncogenes are known to cause an increase in oxidative stress in addition to exerting effects on downstream signaling molecules. Relevant to leukemia, the BCR/ABL oncogene, which is expressed in chronic myeloid leukemia (CML), increases levels of intracellular peroxides and superoxides (100, 108). Electron transport and PI3-kinase signaling have been fingered as responsible for ROS in BCR/ABL-expressing cells; however, inhibitors of these processes yielded only a partial reduction in ROS levels (48). In AML, mutation of the Flt3 receptor is seen in a subset of poor-prognosis patients and was recently shown to increase ROS levels via a NADPH oxidase–dependent pathway (99). Yet another explanation for heightened ROS in leukemia cells invokes mitochondrial DNA mutations that may alter electron transport (84). As observed in other cancer cells, leukemia cells appear to use glycolytic pathways more than do normal lymphocytes, harkening back to the 1920s when Otto Warburg (14) proposed that fundamental differences in metabolism exist between normal cells and cancer cells.
Proteasome Inhibitors
In 2003, bortezomib became the first-in-class proteasome inhibitor approved by the United States Food and Drug Administration (FDA) for use in refractory multiple myeloma (81, 93). A second FDA approval for mantle cell lymphoma followed in 2006 (107). Bortezomib (the generic designation of the drug) or Velcade was initially called PS-341 while in preclinical development. These clinical indications were the result of a decade of work exploring the role of the proteasome in cancer.
The proteasome represents the terminal step of protein degradation and therefore affects every cellular process imaginable (34). Before proteasomal degradation, proteins are tagged by ubiquitin; thus, this process of protein turnover is called the ubiquitin proteasome pathway (79). Ubiquitination of a protein substrate requires three distinct steps: activation of the ubiquitin molecule by an E1 or ubiquitin-activating enzyme; stringing together of a polyubiquitin chain by an E2 or ubiquitin-conjugating protein; and finally, the labeling of the substrate protein with the polyubiquitin chain by an E3 or ubiquitin ligase (39). Once labeled, the ubiquitinated substrate is recognizable to the proteasome.
The 26S proteasome is a multi-subunit complex, composed of two 19S regulatory caps that flank a 20S core (37). The 19S caps recognize the ubiquitinated substrate protein in an ATP-dependent manner, whereas the 20S core is responsible for carrying out protein degradation. This 20S core contains nonidentical α and β subunits (35). The enzymatic activities that degrade proteins are housed within these β subunits. Two sets of seven distinct β subunits are present in the eukaryotic proteasome (54). Of these, specific enzymatic activities have been attributed to the β5, β1, and β2 subunits (95). The β5 subunits contain the rate-limiting step in proteasomal degradation—the chymotrypsin-like activity. This activity represents the “target” for bortezomib and is the enzymatic activity that bortezomib most effectively inhibits (12). The other two activities, housed within the β2 and β1 subunits, are responsible for the trypsin-like and peptidyl glutamyl– or caspase-like activities. The term caspase-like activity is not to be confused with apoptotic caspases, but instead refers generically to the aspartic acid–directed activity contained within the β1 subunit. All three of the enzymatic activities (chymotrypsin-like, trypsin-like, and caspase-like activity) regulate one another to coordinate their actions on a substrate protein (49). When the chymotrypsin-like activity is turned on, the caspase-like and trypsin-like activities are off. Completion of a cycle of chymotrypsin-like activity then activates the other two activities to process the protein substrate further. This model for concerted and cooperative interactions between the proteasome activities is termed the “bite/chew” model, because the chymotrypsin-like activity bites a chunk out of the substrate protein, and the other two activities chew it into smaller pieces. Follow-up studies of this model for proteasome action have confirmed that all three active sites contribute significantly to protein breakdown and that inhibition of multiple sites is required to decrease proteolysis markedly (50). Furthermore, the relative importance of the active site varies widely with the substrate, so inhibition of one site may be relevant to some proteasome substrates but not all.
The rationale to target the proteasome as a cancer therapy arose from the concept that transformed cells possess more damaged or misfolded proteins than do untransformed cells. These damaged or misfolded cells are substrates for the proteasome; therefore, it is thought that cancer cells rely more heavily on proteasome activity. This concept has been validated by reports showing that transformed cells have a higher number of ubiquitinated proteins than do untransformed cells, specifically by comparing lymphocytes from chronic leukemia patients with lymphocytes from healthy donors (69). Notable selectivity of proteasome inhibitors in transformed cells as compared with noncancerous cells is also seen in multiple model systems (1). Given the clinical success of proteasome inhibitors in two hematologic malignancies (myeloma and mantle cell lymphoma), considerable interest exists in expanding the use of proteasome inhibitors to leukemias and other lymphomas. Unfortunately, bortezomib did not exert substantial clinical activity in these other hematologic malignancies (15), prompting studies to examine other proteasome inhibitors and to understand better the role of the proteasome in leukemia biology.
Although bortezomib is currently the only FDA-approved proteasome inhibitor, two other compounds, carfilzomib and NPI-0052, are in clinical trials (105). These two newer proteasome inhibitors are structurally distinct from bortezomib and from each other, and also exert effects that distinguish them functionally from bortezomib. Proteasome inhibitors can be loosely grouped by their chemical structures as either peptide aldehydes, peptide boronates, β-lactones, or vinyl sulfones (51). Of these, the peptide aldehydes and boronates are reversible, whereas the other two classes are not. Bortezomib is a reversible peptide boronate and has been shown to bind to the β5 subunit, thereby inhibiting the chymotrypsin-like activity of the proteasome.
One of the newer, clinically relevant proteasome inhibitors, NPI-0052, is structurally reminiscent of lactacystin, the first described chemical inhibitor of the 20S proteasome (11). Lactacystin is a microbial metabolite isolated from Streptomyces and, despite significant potency, is not suitable for clinical use. NPI-0052, also known as salinosporamide A, is a marine product and also contains a β-lactone ring and is irreversible. Notably, it targets all three enzymatic activities of the proteasome and is the only compound to do so that has been described. Carfilzomib, formerly known as PR-171, is an epoxyketone irreversible inhibitor (56). Carfilzomib is related to epoxomicin, a natural product isolated from an Actinomycetes, which forms an adduct with the N-terminal threonine of the β5 subunit. Other proteasome inhibitors, such as the vinyl sulfones, have not reached clinical development and also have overlapping specificities with other proteases.
The first report that proteasome inhibitors cause oxidative stress came from a study examining bortezomib action in lung cancer cell lines (61). An increase in superoxide levels was noted that preceded caspase activation and appeared to be coupled with mitochondrial dysfunction. Rotenone and antimycin A, inhibitors of mitochondrial electron-transport chain complexes I and III, or cyclosporin A, an inhibitor of the mitochondrial permeability transition pore, inhibited bortezomib-induced ROS generation and mitochondrial perturbations, as did tiron, a chemical antioxidant. A pan-caspase inhibitor did not affect bortezomib-induced ROS generation but did prevent PARP cleavage and apoptotic death. In prostate cancer cells overexpressing Bcl-2, a reduction in bortezomib-induced ROS generation was observed and correlated with cellular resistance to bortezomib.
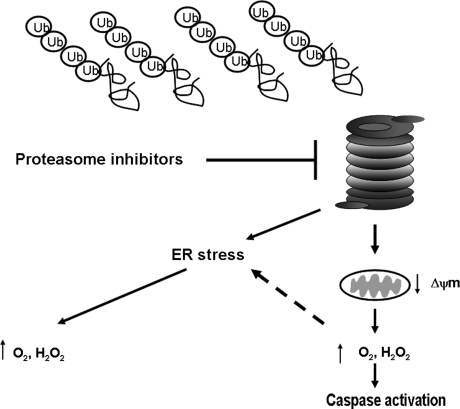
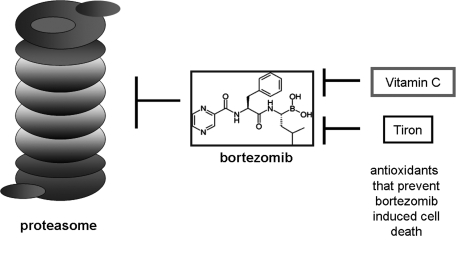
Taken together, these results indicated mitochondria as a source of bortezomib-induced ROS and placed ROS generation upstream of caspase activation in the scheme of bortezomib cytotoxicity (Fig. 3). This conclusion was challenged by another group that used an array of diverse cancer cell lines and found that N-acetylcysteine, butylated hydroxyanisole, and glutathione all failed to protect cells from bortezomib-induced cell death (122). Vitamin C, however, did inhibit bortezomib-induced apoptosis, but this was attributed to interference directly with bortezomib. A vitamin C–bortezomib complex was found to be formed that would render the drug inactive and limit its membrane permeability, thereby preventing the drug from entering into the cell. These same authors concurred with the finding that Tiron protected cells from bortezomib-induced cell death but offered an alternate explanation for the finding—that tiron contains structural features (vicinal diols), which are capable of strongly binding with a boronic acid moiety, as is present in bortezomib. Therefore, they posed the argument that Tiron, instead of working as an antioxidant, is functioning as vitamin C did in their hands, via formation of a biologic inactive complex with bortezomib (Fig. 4).
FIG. 3.
Proteasome inhibitor–induced apoptosis and potential sites of ROS generation. The proteasome inhibitor, bortezomib, and its structurally similar relative, MG132, have been found to increase intracellular ROS levels. At least two explanations have been offered for this observation. An accumulation of polyubiquitinated proteins overwhelms the protein-handling machinery of the cell, causing endoplasmic reticular (ER) stress, which may then cause oxidative stress. Mitochondrial perturbations by proteasome inhibitors can cause release of mitochondrial resident oxidants and cytochrome c, promoting caspase activation. A connection between these two pathways is depicted by the dashed line and includes the ability of ROS generated from mitochondria to cause ER stress.
FIG. 4.
Alternate explanation for antioxidant protection of bortezomib-induced cell death. A role for oxidative stress in the mechanism of action of boretzomib is supported by the ability of antioxidants to protect against cytotoxicity. However, it has been argued that the antioxidants tiron and vitamin C may inactivate bortezomib by binding to the boronate group of the compound.
A more recent study from a separate group proposes that cytochrome p450 activation by bortezomib causes ROS generation, which then deboronates bortezomib, inactivating the drug, because the boronic acid moiety is the portion of the compound thought to bind to the active-site N-terminal threonine residue of the proteasome (57).
Many other studies have probed the role of ROS in bortezomib-induced cell death in model systems, including myeloma (83), mantle cell lymphoma (85), and leukemia (71), as well as solid tumor models like colon cancer (72), endometrial cancer (62), and head and neck squamous cell carcinoma (29). Across the hematologic malignancy models, the ability of bortezomib to increase ROS appears conserved, with a number of different antioxidants, such as NAC, showing cytoprotection. Furthermore, the ability of bortezomib to increase ROS levels in leukemia and myeloma cells has been suggested as the basis for synergy with other agents.
In the solid-tumor model systems, the data are less streamlined. In head and neck squamous cell carcinoma cell lines, ROS and ER stress are implicated as major players in bortezomib cytotoxicity (29). Supporting this model, in myeloma cell lines (U266 and RPMI8226) and in myeloma patient specimens, antioxidants blunt bortezomib toxicity, and depletion of glutathione with buthionine sulfoximine (BSO) sensitizes cells to bortezomib-induced cell death (77). The same study shows that two markers of ER stress, induction of the transcription factors ATF4 and CHOP, occur after bortezomib exposure (77). ER stress as a consequence of proteasome inhibition has been demonstrated in a number of studies (70).
However, distinct cell types may be more susceptible to ER stress or ROS induction or both by bortezomib. An example of a susceptible cell type is the multiple myeloma cell, which is a malignant plasma cell. Plasma cells are terminally differentiated B cells that function to generate antibody and therefore handle high volumes of protein, tipping them toward a higher threshold of ER stress. Proteasome inhibition, which causes a further protein-processing problem, is theorized to be more effective in these cells because of their basal level of high ER stress. In contrast, some cell types do not seem to respond to bortezomib with an oxidative stress or ER stress response. For example, in colon cancer lines, oxidative stress by the preclinical predecessor of bortezomib, MG132, or bortezomib itself, was not observed (22). These varied findings regarding the occurrence of oxidative stress by bortezomib have led to acceptance of the idea that ROS induction by bortezomib may be cell-type specific.
In support of a strong role for redox modulation by proteasome inhibitors are the numerous observations that other classes of proteasome inhibitors also cause an oxidative stress (20). Lipid peroxidation and oxidative DNA damage have been observed in a dose-dependent manner in lactacystin-treated cells. Gene-array analysis of primary neural cells treated with lactacystin shows upregulation of heat-shock proteins, antioxidants, and cell-cycle inhibitors, indicating major gene-expression consequences of the observed oxidative stress (118). As for the newer, clinically relevant proteasome inhibitors, NPI-0052 causes a greater increase of intracellular superoxide than does bortezomib (71). A unique feature of NPI-0052 is its reliance on caspase-8 activation, whereas bortezomib activates both caspase-8 and caspase-9 equivalently. The antioxidant NAC blocks NPI-0052–induced cell death but does not affect proteasome-activity inhibition or caspase-8 processing in leukemia cells; therefore, increased superoxide levels and increased intracellular peroxide levels are likely causing the demise of the cell in a parallel pathway to caspase-8 activation. To date, the effect of carfilzomib on redox has not been explored.
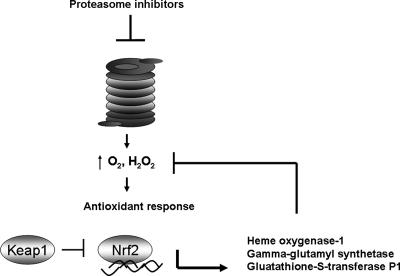
The cellular response to an oxidative stress usually involves the upregulation of antioxidant defenses. In the case of proteasome inhibitor–induced oxidative stress, several antioxidants are noted to increase. In neurons, synthesis of the ubiquitous and plentiful antioxidant, glutathione (GSH), is stimulated by lactacystin, which acts on the Nrf2 transcription factor (117). Nrf2 drives the expression of many antioxidant enzymes, including the enzyme that catalyzes the first and rate-limiting step in GSH synthesis: γ-glutamylcysteine synthetase. Nrf-2 has also been implicated in promoting expression of heme oxygenase-1 (HO-1), a heat-shock protein also referred to as hsp32 and antioxidant. In astrocytes, exposure to MG-132 (a proteasome inhibitor structurally similar to bortezomib) causes an induction of HO-1 (103). Overexpression studies with HO-1 and other antioxidant proteins indicate that these antioxidants also possess antiapoptotic properties, so it is conceivable that HO-1 induction by proteasome inhibitors may ultimately promote drug resistance.
Hsp72, another cytoprotective heat-shock protein, is reported to be upregulated by exposure to MG132 (86). In line with this concept, another small heat-shock protein, hsp27, has been implicated in bortezomib resistance in lymphoma (13). Antisense directed toward hsp27 sensitized bortezomib-resistant lymphoma lines to bortezomib, and overexpression of hsp27 rendered sensitive cells resistant to bortezomib.
The detoxification protein GST P1 is also an Nrf2 target and is upregulated in response to proteasome inhibitors such as MG132 and lactacystin (111). Interestingly, Nrf2 activity is regulated by the proteasome. Nrf2 is sequestered in the cytoplasm by Keap1 (Kelch-like ECH-associated protein-1) (Fig. 5). Ubiquitination of Keap1 and its proteasomal degradation promote the translocation of Nrf2 to the nucleus, enabling it to function as a transcription factor. Therefore, the antioxidant response to proteasome inhibitors is dually regulated by the proteasome: first at the level of ROS generation by proteasome inhibitors, and also by Keap1 degradation by the proteasome.
FIG. 5.
Proteasome inhibitors can promote an antioxidant response. After an oxidative stress is achieved by proteasome inhibitors, a cellular antioxidant response mediated by the transcription factor Nrf2 has been documented. Nrf2 is antagonized by Keap1, which sequesters it in the cytoplasm. Nrf2 drives transcription of several redox-modulating genes that have been implicated in proteasome inhibitor–treated cells including heme oxygenase-1, γ-glutamyl synthetase, and glutathione S-transferase. These antioxidants may in turn soak up ROS and potentially mediate resistance to the drug.
Interestingly, the proteasome itself is amenable to modulation by oxidative stress. With purified proteasomes, electrophile-induced oxidative stress was shown to decrease the ability of the proteasome to degrade substrates, likely through modification of an ATPase on the 19S proteasome (102). Examination of the 20S proteasome showed a similar pattern of regulation—exposure to large amounts of oxidants like hydrogen peroxide or peroxynitrite caused a decrease in the proteasome's ability to break down a particular substrate protein (36). A comparison of the 20S and 26S proteasome revealed that the 20S proteasome is more resistant to oxidative stress than is the 26S proteasome, which was attributed to the ATP dependence of the 26S proteasome (92). Cumulatively, these studies suggest that ROS generated by endogenous sources or by cellular perturbations can modulate proteasome activity, thereby affecting the action of the proteasome inhibitors. Therefore, redox status may be an element to consider in trying to maximize the utility of the proteasome inhibitors.
Epigenetically Targeted Agents
Epigenetics is defined as changes in gene expression that are not due to any alteration in the DNA sequence. The best-known epigenetic markers are DNA methylation and histone acetylation. Both of these processes control gene activity and the architecture of chromatin. DNA methylation and modifications of histone proteins control in tandem the coiling of DNA, thereby enabling or disabling transcription (112). Histones are best known as DNA-packaging proteins, but also participate in the regulation of gene expression. Posttranslational modifications on histones, such as acetylation, methylation, and phosphorylation, affect the binding of histones to DNA, thereby regulating portions of DNA as accessible to transcription factors. Acetylation of histone lysines, for example, is generally associated with transcriptional activation. The term “histone code” refers to distinct histone modifications present in normal tissue; these are maintained by the coordinated activity of histone acetyl transferases (HATs) and histone deacetylases (HDACs). A precise pattern of DNA methylation and histone modification is essential for the physiologic activities of cells and tissues. In cancer, this pattern is thought to be dysregulated, with silencing of tumor suppressors and activation of aberrant genes (96).
The hypomethylating agents have gained momentum in cancer therapeutics with the intense study of epigenetic alterations in cancer. Two such agents, 5-azacytidine (Vidaza) and 5-aza-2'-deoxycytidine (decitabine), have been approved as treatments for myelodysplastic syndrome (MDS) (32). Both of these drugs have been available for >40 years but were always categorized as classic cytostatic agents. More recent work has highlighted their ability to inhibit DNA methylation, prompting their reclassification as epigenetically targeted drugs.
As early as the 1980s, decitabine was found to possess ROS-generating characteristics in AML cell lines (74). At the time, this was attributed to an effect on differentiation, because a myeloid cell line was used, and it was assumed that differentiation of these cells would enable a functional NADPH oxidase. However, this was purely speculation, and no experiments to address this possibility have been conducted. Building on those observations, both azacytidine and 5-aza-2-deoxycytidine are seen to cause increased ROS alone or in combination with other epigenetically targeted agents, such as HDAC inhibitors (31). Antioxidants able to stem this increase in ROS are those that bolster levels of GSH, suggesting that GSH depletion plays a role in the mechanism of action of these agents. These same antioxidants are protective against cell death induced by the hypomethylating agents, indicating that the oxidative stress figures into the induction of cytotoxicity. However, the precise source for the oxidative stress remains unknown for the hypomethylating agents.
Another intriguing question is how redox may affect DNA methylation and vice versa. The DNA methyltransferases (DNMTs) are a group of enzymes that control methylation status. A recent study linked DNMT1 to sensitivity to oxidative stress by generating Rat-1 cells that overexpress DNMT1 and somatic HCT116 knockout cells that do not express either DNMT1 [DNMT1(-/-)] or DNMT3B [DNMT3B(-/-)] (73). The former were relatively resistant to hydrogen peroxide–induced cell death, and the latter were more sensitive to hydrogen peroxide–mediated cytotoxicity as compared with control HCT116 cells. These results highlight a role for DNMT1 as potentially more relevant to cell death and drug sensitivity than other members of the DNMT family in colon cancer. An explanation of this unique effect of DNMT1 may lie in the fact that it is a downstream target of the AP-1 transcription factor complex member, Fos. Increased AP-1 activity has been linked to resistance to oxidative stress induced by chemotherapy, and DNMT1 may be an effector of this response (4). With the success of the hypomethylating agents in MDS, uncovering potential mediators of resistance becomes more urgent. Understanding redox alterations caused by those agents should help this pursuit. Additional basic studies of the enzymes that control epigenetic alterations and the redox dependence of these enzymes also will provide much-needed insight into these processes. These data are likely forthcoming from focused studies of the numerous enzymes that control posttranslational modifications of chromatin-associated proteins. For example, lysine-specific demethylase-1 (LSD1), also known as KDM1, is a flavin-dependent histone demethylase. Activity of LSD1 is limited to mono- and dimethylated lysines; however, it can cooperate with the other classes of histone demethylases that are able to act on mono-, di-, and trimethylated lysine residues. Like other flavin-dependent amine oxidases, LSD1 produces hydrogen peroxide by oxidation of an amino group of its substrates through an FAD-dependent mechanism (28). Therefore, its action can influence the redox environment proximal to chromatin; however, whether LSD1 itself is sensitive to changes in the redox environment as well remains unanswered.
The histone deacetylase (HDAC) inhibitors are a structurally diverse class of agents that induce growth arrest, differentiation, and cell death in vitro (68). Suberoylanilide hydroxamic acid (SAHA or vorinostat) is the first drug of this type to be approved by the FDA and is used for the treatment of cutaneous T-cell lymphoma (23). Many other HDAC inhibitors have emerged and are in various stages of testing. In clinical trials, HDAC inhibitors are associated with a low incidence of adverse events and are generally well tolerated by patients as either monotherapy or in combination with other agents (94). Structurally, the HDAC inhibitors have been grouped into four categories: hydroxamic acids, short fatty chain acids and their derivatives, benzamides, and cyclic peptides (7). The HDAC inhibitors are also grouped based on what HDAC family members they inhibit. Eleven HDAC proteins, grouped into classes I–IV, have been described in mammals and vary with respect to subcellular distribution, tissue-specific expression, and function (66). The majority of the hydroxamic acid HDAC inhibitors (vorinostat, trichostatin A, PXD101, LBH589, and LAQ824) are pan-inhibitors of the HDAC family. However, more specific hydroxamic acid HDAC inhibitors are emerging. CRA-024781/PCI-24781 preferentially inhibits class I and II HDAC family members and displays increased potency to inhibit those enzymes as compared with vorinostat. It is orally bioavailable and is currently in phase I trials (5).
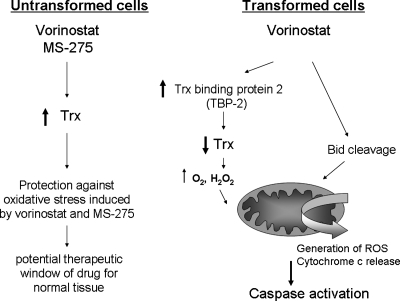
Although several structurally distinct HDAC inhibitors cause oxidative stress, vorinostat, the hydroxamic acid, has been the best characterized with respect to ROS production (Fig. 6). The initial report of vorinostat (SAHA) affecting redox focused on apoptosis induction and concluded that the drug promotes expression of the proapoptotic Bcl-2 family member, Bid. Bid translocation to mitochondria and subsequent disruption of mitochondria was identified as the source of ROS by vorinostat (98). Recent reports highlighted a role for the antioxidant, thioredoxin (Trx), in sensitivity to vorinostat. Trx is a ubiquitous antioxidant and electron donor for ribonucleotide reductase and for several peroxidases (2). The flavoenzyme, thioredoxin reductase, keeps Trx in a reduced state by using NADPH as a cofactor. Vorinostat induces the expression of Trx-binding protein 2 (TBP-2), which binds to and inactivates Trx, providing a model by which an important antioxidant is inactivated by the drug, thereby permitting oxidative stress to proceed (6). A later study attributed Trx levels to vorinostat sensitivity in transformed cells (110). An increase in Trx was observed in normal human fibroblasts cell lines treated with vorinostat, but not in a myeloma cell line or in an SV40-transformed cancer cell line. Similar results were obtained with the benzamide HDAC inhibitor, MS/SNDX-275.
FIG. 6.
Role of oxidative stress in the selective effects of HDACi on transformed cells. The antioxidant thioredoxin may be a key in the observed selectivity of the HDAC inhibitors vorinostat and MS-275 on cancer cells. In normal, untransformed cells, thioredoxin is upregulated and protects cells from oxidative stress. In cancer cells, gene expression of thioredoxin-binding protein-2 (TBP-2) and the cleavage of the proapoptotic protein, Bid, are documented. TBP-2 reduces activity of Trx by binding and inactivating it, thereby promoting oxidant stress. Bid disrupts mitochondria, causing oxidative stress.
MS-275, now being developed by Syndax Pharmaceuticals, hence its renaming as SNDX-275, has been shown to generate ROS by several groups in leukemia cell lines and primary patient specimens (16, 97). Because MS-275 targets class I HDACs, and within this class, exerts specificity for HDAC1, 2, and 3, it is plausible that inhibition of the class I family members accounts for the generation of ROS (Fig. 6).
For both vorinostat and MS-275, the oxidative stress caused by the drug leads to caspase activation, but caspase inhibitors do not prevent ROS generation (64). A similar pattern is seen with other HDAC inhibitors, such as PCI-24781. Like the hypomethylating agents, a source for the oxidative stress has as yet to be identified. However, because chemical antioxidants block cell death by the HDAC inhibitors, it is clear that redox is an important component of the action of these agents.
BCL-2 Inhibitors
The Bcl-2 oncogene, a potent antiapoptotic protein, is a logical candidate for therapeutic targeting. Bcl-2 is found on virtually every cell membrane, including plasma membranes, mitochondrial membranes, ER membranes, and nuclear membranes (78). Localization of Bcl-2 on the mitochondrial membrane has been attributed to its antiapoptotic action, because it can bind proapoptotic Bcl-2 family members such as Bax and Bak, thereby inactivating their ability to exert effects on mitochondria (89). However, several studies also cite other models for Bcl-2 action, including a role in modulating cellular redox status (53). The first tie between apoptosis and ROS came from the observation that, when overexpressed, Bcl-2 protected cells from hydrogen peroxide– and menadione-induced lipid and thiol oxidation. Later work showed that expression of the ubiquitous cellular antioxidant glutathione (GSH) is much higher in cells expressing Bcl-2, suggesting that Bcl-2 promotes GSH retention, stability, or synthesis (113). Other antioxidants, such as MnSOD, also appear to be modulated by Bcl-2 expression; however, this may be through the activation of common transcription factors such as NF-κB that promote both Bcl-2 and MnSOD levels (55).
Overexpression of Bcl-2 protects virtually any cell type from induction of apoptosis, and in CLL, lung cancer, and lymphoma, overexpression of Bcl-2 is an observed feature (90). Consequently, numerous strategies targeting Bcl-2 have evolved in recent years, ranging from antisense to small-molecule inhibitors to natural products (90). Strategies to target Bcl-2 have spanned inhibiting its gene expression with HDACi, reducing mRNA expression with antisense, and preventing the function of the protein with small molecules. The antisense strategies were the first to be explored in a clinical context. Relevant to hematologic malignancies, clinical trials were conducted in relapsed or refractory CLL, acute myeloid leukemia (AML), and myeloma by using antisense oligodeoxynucleotides targeting BCL-2 mRNA. The molecules used clinically (G3139; Genasense; oblimersen sodium) are nuclease-resistant phosphorothioates, which hybridize to the first 18 nucleotides within the open reading frame of BCL-2 mRNAs and induce degradation (91). Despite potentially interesting data in leukemia model systems, concern exists about the specificity of this antisense approach, prompting the development of other modalities to inhibit Bcl-2. A consistent correlation between clinical response and knockdown of BCL-2 mRNA or protein levels in patients undergoing treatment with G3139 has not been shown; however, this could be attributed to the slow degradation of Bcl-2 (67).
The majority of efforts to inhibit Bcl-2 as a therapeutic modality have focused on small-molecule inhibitors that directly bind Bcl-2 or related antiapoptotic proteins. Of these, gossypol and its analogues, originating from a natural product used as a herbal remedy in China, have been the most extensively studied. Gossypol binds a regulatory site on the surface of antiapoptotic Bcl-2 family proteins, preventing docking with proapoptotic proteins, which usually inactivates the proapoptotic family members (87). The net result is that the proapoptotic proteins are free to promote cell death. Conceptually similar but structurally different small-molecule inhibitors of Bcl-2 and related antiapoptotic family members such as Bcl-XL and Mcl-1 have been developed, and many are on the cusp of entering clinical trials. HA14-1, a chromene derivative (115), and antimycin analogues (109) were identified by computational modeling. BH3I-1 and -2 are a thiazolidin derivative and a benzene sulfonyl derivative, respectively, identified by screening by using a BH3 peptide displacement assay (19). Theaflavins and epigallechatechins (EGCGs) are natural products abundant in black and green tea, respectively (60). ABT737 is a synthetic small-molecule inhibitor produced by Abbott Laboratories by using NMR-guided, structure-based drug design, representing the most deliberate attempt at designing a Bcl-2 inhibitor (80). GX15-070 (also generated by a pharmaceutical firm, in this case, Gemin X), is a synthetic broad-spectrum inhibitor of Bcl-2–family proteins. No side-by-side comparisons of these chemical inhibitors of antiapoptotic Bcl-2 proteins has been reported, but one major point difference between the compounds is their binding affinity for Bcl-2 or Bcl-XL, with ABT737 being the strongest binder and antimycin having the weakest binding properties (90).
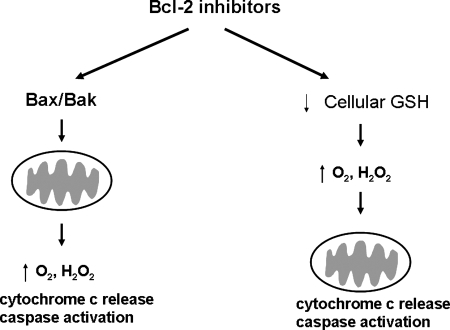
A handful of studies have examined the ability of Bcl-2 inhibitors to modulate redox status. Interestingly, many of these compounds, including 2-methoxy antimycin A, BH3I-2, HA14-1, ABT-737, gossypol (and the gossypol enantiomer, AT101), and the stapled BH3 helical peptide SAHB-BID also inhibit Bcl-xL (120). Bcl-xL overexpression has also been linked to heightened GSH levels; therefore, these compounds may exert redox effects by inhibition of multiple Bcl-2 family members (3) (Fig. 7). Given the antioxidant functions of Bcl-2 mentioned earlier (increased expression of GSH, MnSOD, etc.) it is not surprising that the Bcl-2 inhibitors cause an increase in intracellular peroxide and superoxide levels. This has been documented for HA14-1 (121) and for ABT-737, a small-molecule BH3 mimetic from Abbott Pharmaceuticals (Fig. 7). ABT-737 is the parent compound, whereas ABT-263 is the second-generation drug, currently in clinical trial. For these agents, it has not been carefully determined whether decreased GSH or MnSOD, as a consequence of Bcl-2 inhibition, is responsible for the oxidative stress, or whether mitochondrial dysfunction is the reason for ROS generation.
FIG. 7.
Two models for how Bcl-2 inhibitors can cause an oxidative stress: mitochondrial disruption and depletion of glutathione. Functional studies of Bcl-2 indicate that this antiapoptotic protein can bind and sequester proapoptotic family members like Bax and Bak from localizing to mitochondria. Bcl-2 also can increase levels of cellular glutathione, likely by preventing efflux. Therefore, Bcl-2 inhibitors that antagonize the function of Bcl-2 can antagonize those two pathways, as depicted.
Adaphostin: A Unique Kinase Inhibitor
Many kinase inhibitors elicit changes in cellular redox status, and this topic alone could be covered in a dedicated review article. The majority of kinase inhibitors (the clinically approved BCR/ABL kinase inhibitor, imatinib, being the architypical example) are directed toward the ATP-binding site. Adaphostin is unique in that it is a second-generation tyrphostin kinase inhibitor. The term tyrphostin refers to a class of drugs designed to mimic tyrosine binding to kinases (24). The parent drug of adaphostin, AG957, was studied extensively as a BCR/ABL kinase inhibitor (46). A key feature of AG957 was that its affinity for BCR/ABL was competitive with regard to substrate rather than ATP (like imatinib). However, owing to a serum half-life of <20 min, AG957 was modified. Adaphostin, formerly known as NSC 680410, emerged as a more-promising compound, with a longer serum half-life and greater potency (47). As imatinib gained momentum in treating chronic myeloid leukemia (CML), adaphostin was shown to exert a distinct pattern of activity in BCR/ABL-expressing cells, characterized by induction of ROS and an ability to degrade BCR/ABL protein (9). These two effects are independent of one another, because an antioxidant does not prevent the degradation of BCR/ABL (8). However, the ROS induction is clearly linked to apoptosis, because it precedes caspase activation, and because diverse antioxidants prevent apoptotic DNA fragmentation.
These unique attributes of adaphostin (ROS generation and BCR/ABL degradation) are retained in imatinib-resistant cells (10). Regardless of whether imatinib resistance was conferred by BCR/ABL mutation or mutation-independent alterations, adaphostin induced apoptosis in these lines. Notably, even the most potently imatinib-resistant form of BCR/ABL, the T315I mutant (which is resistant to second-generation BCR/ABL inhibitors such as nilotinib and dasatinib), was susceptible to adaphostin-induced oxidative stress and cell death. Closer inspection of the chemical structure of adaphostin reveals that it is a dihydroquinone derivative, and from studies in primary acute myelogenous leukemia (AML) (119) and chronic lymphocytic leukemia (CLL) cells (101), as well as a host of cell lines that do not express BCR/ABL (63, 75), it is clear that the activity of adaphostin activity does not depend on the presence of the BCR/ABL kinase. Transcriptional and proteomic profiling of adaphostin effects in a panel of cells that included BCR/ABL-positive and BCR/ABL-nonexpressing cells, provides independent confirmation of these findings (40, 106). Upregulation of oxidative stress–related genes such as heat-shock proteins and proteins such as glutathione S-transferase and superoxide dismutase were described in these studies. Data from our laboratory indicate that the heat-shock protein and antioxidant, heme oxygenase-1, is upregulated after adaphostin exposure in glioblastoma cells (63). An explanation for the induction of oxidative stress by adaphostin was recently proposed. HPLC analyses conducted by Scott Kaufmann and colleagues (58) indicated that adaphostin accumulates in mitochondria 3,000-fold over extracellular concentrations. Although the mechanism for this mitochondrial accumulation is not known, it is responsible for disrupting electron transport, promoting ROS generation at complex III. Taken together, these various independent studies indicate that adaphostin is a redox-modulatory agent. The selectivity of adaphostin for AML, CML, and CLL cells may potentially be explained by increased endogenous oxidative stress in these leukemic cells. Therefore, the further oxidative stress generated by adaphostin is more effective at inducing cell death in these leukemia cells than in normal lymphocytes.
Combinations of These Agents
The past and future of cancer therapy relies on combining agents for maximal activity. In hematologic malignancies in particular (with the exception of CML), multidrug regimens are a mainstay of treatment. All the agents described in this review article have been tested for synergy with other existing or emerging drugs in cell lines, and clinical trials applying these combinations are being formulated. Interestingly, all of the classes of compounds covered in this review have been combined with each other, and ROS have been implicated in most of the additive or synergistic effects in vitro.
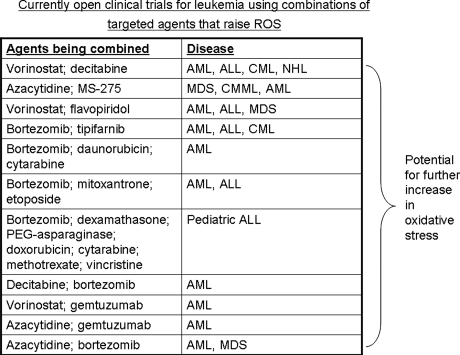
Of these combinations, the proteasome inhibitors and HDAC inhibitors are furthest along in development, with several clinical trials open testing bortezomib with either vorinostat or other HDAC inhibitors (Fig. 8). In multiple myeloma (83), mantle cell lymphoma (38), CML (83), and non–small cell lung cancer (21), oxidative stress is seen with the boretzomib/vorinostat combination. Reports of synergistic cell death extend to additional cell-line models including pancreatic cancer (76), hepatomas (25), and MDS (30). Aside from bortezomib, other proteasome inhibitors such as NPI-0052 also synergize with HDAC inhibitors. Our work shows that oxidative stress is potentiated in leukemia cells treated with NPI-0052 and MS-275 (71).
FIG. 8.
Combinations of targeted agents that increase levels of intracellular ROS that are currently in clinical trials for leukemia. A partial list of currently accruing clinical trials for leukemia with agents discussed in this review. Information was condensed from web postings current as of August 2008 on www.clinical trials.gov. Expansion of abbreviations used in the table are AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia; CML, chronic myeloid leukemia; NHL, non-Hodgkins lymphoma; MDS, myelodysplastic syndrome; CMML, chronic myelomonocytic leukemia. Because at least one agent listed in these combination trials is documented to increase intracellular ROS levels, a potential exists to measure a further increase in oxidative stress in the context of these trials as a putative biomarker for drug action.
Bortezomib has also been combined with adaphostin or with Bcl-2 inhibitors in cell lines. Oxidative stress was cited as the major mechanism of cytotoxicity in AML and CML cells treated with bortezomib and adaphostin. For the bortezomib/Bcl-2 inhibitor combinations, numerous instances of synergistic or additive effects have been reported in liquid and solid cancer cell lines; however, a role for ROS has not been addressed (17, 18, 82, 104).
Conclusions and Future Directions
The fact that the agents discussed in this review cause an oxidative stress that figures prominently in their mechanism of action is of direct clinical value and is an end point that is underused. Monitoring oxidative stress as a biologic marker in clinical trials may provide insight into clinical responses or lack thereof. Methods to quantitate ROS levels by flow cytometry are straightforward and easily applied in mononuclear cells isolated from peripheral blood of cancer patients. Monitoring ROS in cancer patients will enable the answering of many interesting questions. Do ROS levels after drug exposure predict clinical response? Does dietary supplementation with antioxidants such as vitamin C, vitamin E, or selenium, for example, provide protection from targeted chemotherapy? Because of the bias that induction of oxidative stress is a nonspecific cellular event, it is challenging to argue for monitoring it in the context of a clinical trial for a biologically targeted agent. However, oxidative stress can be cancer cell specific, because a different spectrum and activity of endogenous oxidant sources and antioxidant enzymes is present in normal versus malignant cells. Widespread acceptance of the fact that oxidative stress is a relevant and requisite outcome of many new cancer therapies will enable a better understanding of this field and provide insight into using these new agents more intelligently.
Acknowledgments
I regret not being able to cite original articles for some of the work discussed because of space limitations. I thank the current graduate students in my laboratory, Claudia Miller, M.S., Nilsa Rivera, M.S., and Adrienne Howard, for their dedication and efforts in studying many of the agents described in this review and for assistance with formatting references. Grant funding from the National Institutes of Health (RO1 CA115811), University of Texas M.D. Anderson Cancer Center (MDACC), Leukemia SPORE at MDACC, and the Children's Leukemia Research Association is also gratefully acknowledged.
Abbreviations
ALL, acute lymphocytic leukemia; AML, acute myelogenous leukemia; ATP, adenosine triphosphate; Bcl-2, B-cell CLL/lymphoma 2; BCR/ABL, breakpoint cluster region/abelson oncogene; Cu, copper; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; dATP, deoxyadenosine triphosphate; DNA, deoxyribonucleic acid; DNMT, DNA methyl transferase; ER, endoplasmic reticulum; FDA, Food and Drug Administration; Fe, iron; Flt3, FMS-related tyrosine kinase-3; GSH, glutathione; GSTP1, glutathione S-transferase P1; HAT, histone acetyltransferase; HDAC, histone deacetylase; HO-1, heme oxygenase-1; HPLC, high-pressure liquid chromatography; Keap1, Kelch-like ECH-associated protein-1; LSD1, lysine-specific demethylase-1; MDS, myelodysplastic syndrome; MnSOD, manganese superoxide dismutase; NAC, N-acetylcysteine; NADPH, nicotinamide adenine dinucleotide phosphate; NF-κB, nuclear factor-κB; NHL, non-Hodgkins lymphoma; Nrf2, nuclear erythroid 2 p45-related factor 2; PARP, poly-ADP ribose polymerase; ROS, reactive oxygen species; S, Svedberg; SAHA, suberoylanilide hydroxamic acid; SOD, superoxide dismutase; TBP-2, thioredoxin-binding protein-2; Trx, thioredoxin.
References
- 1.Agrawal SG. Liu FT. Wiseman C. Shirali S. Liu H. Lillington D. Du MQ. Syndercombe-Court D. Newland AC. Gribben JG. Jia L. Increased proteasomal degradation of Bax is a common feature of poor prognosis chronic lymphocytic leukemia. Blood. 2008;111:2790–2796. doi: 10.1182/blood-2007-10-110460. [DOI] [PubMed] [Google Scholar]
- 2.Arner ES. Holmgren A. The thioredoxin system in cancer. Semin Cancer Biol. 2006;16:420–426. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Bojes HK. Datta K. Xu J. Chin A. Simonian P. Nunez G. Kehrer JP. Bcl-xL overexpression attenuates glutathione depletion in FL5.12 cells following interleukin-3 withdrawal. Biochem J. 1997;325:315–319. doi: 10.1042/bj3250315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradbury CM. Locke JE. Wei SJ. Rene LM. Karimpour S. Hunt C. Spitz DR. Gius D. Increased activator protein 1 activity as well as resistance to heat-induced radiosensitization, hydrogen peroxide, and cisplatin are inhibited by indomethacin in oxidative stress-resistant cells. Cancer Res. 2001;61:3486–3492. [PubMed] [Google Scholar]
- 5.Buggy JJ. Cao ZA. Bass KE. Verner E. Balasubramanian S. Liu L. Schultz BE. Young PR. Dalrymple SA. CRA-024781: a novel synthetic inhibitor of histone deacetylase enzymes with antitumor activity in vitro and in vivo. Mol Cancer Ther. 2006;5:1309–1317. doi: 10.1158/1535-7163.MCT-05-0442. [DOI] [PubMed] [Google Scholar]
- 6.Butler LM. Zhou X. Xu WS. Scher HI. Rifkind RA. Marks PA. Richon VM. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc Natl Acad Sci U S A. 2002;99:11700–11705. doi: 10.1073/pnas.182372299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carey N. La Thangue NB. Histone deacetylase inhibitors: gathering pace. Curr Opin Pharmacol. 2006;6:369–375. doi: 10.1016/j.coph.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Chandra J. Hackbarth J. Le S. Loegering D. Bone N. Bruzek LM. Narayanan VL. Adjei AA. Kay NE. Tefferi A. Karp JE. Sausville EA. Kaufmann SH. Involvement of reactive oxygen species in adaphostin-induced cytotoxicity in human leukemia cells. Blood. 2003;102:4512–4519. doi: 10.1182/blood-2003-02-0562. [DOI] [PubMed] [Google Scholar]
- 9.Chandra J. Hackbarth J. Le S. Loegering D. Bone N. Bruzek LM. Narayanan VL. Adjei AA. Kay NE. Tefferi A. Karp JE. Sausville EA. Kaufmann SH. Involvement of reactive oxygen species in adaphostin-induced cytotoxicity in human leukemia cells. Blood. 2003;102:4512–4519. doi: 10.1182/blood-2003-02-0562. [DOI] [PubMed] [Google Scholar]
- 10.Chandra J. Tracy J. Loegering D. Flatten K. Verstovsek S. Beran M. Gorre M. Estrov Z. Donato N. Talpaz M. Sawyers C. Bhalla K. Karp J. Sausville E. Kaufmann SH. Adaphostin-induced oxidative stress overcomes bcr/abl mutation-dependent and -independent imatinib resistance. Blood. 2006;107:2501–2506. doi: 10.1182/blood-2005-07-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chauhan D. Hideshima T. Anderson KC. A novel proteasome inhibitor NPI-0052 as an anticancer therapy. Br J Cancer. 2006;95:961–965. doi: 10.1038/sj.bjc.6603406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauhan D. Hideshima T. Anderson KC. Targeting proteasomes as therapy in multiple myeloma. Adv Exp Med Biol. 2008;615:251–260. doi: 10.1007/978-1-4020-6554-5_12. [DOI] [PubMed] [Google Scholar]
- 13.Chauhan D. Li G. Shringarpure R. Podar K. Ohtake Y. Hideshima T. Anderson KC. Blockade of Hsp27 overcomes bortezomib/proteasome inhibitor PS-341 resistance in lymphoma cells. Cancer Res. 2003;63:6174–6177. [PubMed] [Google Scholar]
- 14.Chen Z. Lu W. Garcia-Prieto C. Huang P. The Warburg effect and its cancer therapeutic implications. J Bioenerget Biomembr. 2007;39:267–274. doi: 10.1007/s10863-007-9086-x. [DOI] [PubMed] [Google Scholar]
- 15.Cortes J. Thomas D. Koller C. Giles F. Estey E. Faderl S. Garcia-Manero G. McConkey D. Ruiz SL. Guerciolini R. Wright J. Kantarjian H. Phase I study of bortezomib in refractory or relapsed acute leukemias. Clin Cancer Res. 2004;10:3371–3376. doi: 10.1158/1078-0432.CCR-03-0508. [DOI] [PubMed] [Google Scholar]
- 16.Dai Y. Rahmani M. Dent P. Grant S. Blockade of histone deacetylase inhibitor-induced RelA/p65 acetylation and NF-kappaB activation potentiates apoptosis in leukemia cells through a process mediated by oxidative damage, XIAP downregulation, and c-Jun N-terminal kinase 1 activation. Mol Cell Biol. 2005;25:5429–5444. doi: 10.1128/MCB.25.13.5429-5444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dasmahapatra G. Nguyen TK. Dent P. Grant S. Adaphostin and bortezomib induce oxidative injury and apoptosis in imatinib mesylate-resistant hematopoietic cells expressing mutant forms of Bcr/Abl. Leuk Res. 2006;30:1263–1272. doi: 10.1016/j.leukres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Dasmahapatra G. Rahmani M. Dent P. Grant S. The tyrphostin adaphostin interacts synergistically with proteasome inhibitors to induce apoptosis in human leukemia cells through a reactive oxygen species (ROS)-dependent mechanism. Blood. 2006;107:232–240. doi: 10.1182/blood-2005-06-2302. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Degterev A. Lugovskoy A. Cardone M. Mulley B. Wagner G. Mitchison T. Yuan J. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nat Cell Biol. 2001;3:173–182. doi: 10.1038/35055085. [DOI] [PubMed] [Google Scholar]
- 20.Demasi M. Davies KJ. Proteasome inhibitors induce intracellular protein aggregation and cell death by an oxygen-dependent mechanism. FEBS Lett. 2003;542:89–94. doi: 10.1016/s0014-5793(03)00353-3. [DOI] [PubMed] [Google Scholar]
- 21.Denlinger CE. Rundall BK. Jones DR. Proteasome inhibition sensitizes non-small cell lung cancer to histone deacetylase inhibitor-induced apoptosis through the generation of reactive oxygen species. J Thorac Cardiovasc Surg. 2004;128:740–748. doi: 10.1016/j.jtcvs.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Ding WX. Ni HM. Chen X. Yu J. Zhang L. Yin XM. A coordinated action of Bax, PUMA, and p53 promotes MG132-induced mitochondria activation and apoptosis in colon cancer cells. Mol Cancer Ther. 2007;6:1062–1069. doi: 10.1158/1535-7163.MCT-06-0541. [DOI] [PubMed] [Google Scholar]
- 23.Duvic M. Vu J. Vorinostat: a new oral histone deacetylase inhibitor approved for cutaneous T-cell lymphoma. Expert Opin Invest Drugs. 2007;16:1111–1120. doi: 10.1517/13543784.16.7.1111. [DOI] [PubMed] [Google Scholar]
- 24.Dvir A. Milner Y. Chomsky O. Gilon C. Gazit A. Levitzki A. The inhibition of EGF-dependent proliferation of keratinocytes by tyrphostin tyrosine kinase blockers. J Cell Biol. 1991;113:857–865. doi: 10.1083/jcb.113.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emanuele S. Lauricella M. Carlisi D. Vassallo B. D'Anneo A. Di Fazio P. Vento R. Tesoriere G. SAHA induces apoptosis in hepatoma cells and synergistically interacts with the proteasome inhibitor bortezomib. Apoptosis. 2007;12:1327–1338. doi: 10.1007/s10495-007-0063-y. [DOI] [PubMed] [Google Scholar]
- 26.Fariss MW. Chan CB. Patel M. Van Houten B. Orrenius S. Role of mitochondria in toxic oxidative stress. Mol Interv. 2005;5:94–111. doi: 10.1124/mi.5.2.7. [DOI] [PubMed] [Google Scholar]
- 27.Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 28.Forneris F. Binda C. Battaglioli E. Mattevi A. LSD1: oxidative chemistry for multifaceted functions in chromatin regulation. Trends Biochem Sci. 2008;33:181–189. doi: 10.1016/j.tibs.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Fribley A. Zeng Q. Wang CY. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol Cell Biol. 2004;24:9695–9704. doi: 10.1128/MCB.24.22.9695-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galimberti S. Canestraro M. Khan R. Buda G. Orciuolo E. Guerrini F. Fazzi R. Maffei R. Marasca R. Petrini M. Vorinostat and bortezomib significantly inhibit WT1 gene expression in MO7-e and P39 cell lines. Leukemia. 2008;22:628–631. doi: 10.1038/sj.leu.2404918. [DOI] [PubMed] [Google Scholar]
- 31.Gao S. Mobley A. Miller C. Boklan J. Chandra J. Potentiation of reactive oxygen species is a marker for synergistic cytotoxicity of MS-275 and 5-azacytidine in leukemic cells. Leuk Res. 2008;32:771–780. doi: 10.1016/j.leukres.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Manero G. Gore SD. Future directions for the use of hypomethylating agents. Semin Hematol. 2005;42:S50–S59. doi: 10.1053/j.seminhematol.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Goetz ME. Luch A. Reactive species: a cell damaging route assisting to chemical carcinogens. Cancer Lett. 2008;266:73–83. doi: 10.1016/j.canlet.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg AL. Functions of the proteasome: from protein degradation and immune surveillance to cancer therapy. Biochem Soc Trans. 2007;35:12–17. doi: 10.1042/BST0350012. [DOI] [PubMed] [Google Scholar]
- 35.Groll M. Clausen T. Molecular shredders: how proteasomes fulfill their role. Curr Opin Struct Biol. 2003;13:665–673. doi: 10.1016/j.sbi.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Grune T. Blasig IE. Sitte N. Roloff B. Haseloff R. Davies KJ. Peroxynitrite increases the degradation of aconitase and other cellular proteins by proteasome. J Biol Chem. 1998;273:10857–10862. doi: 10.1074/jbc.273.18.10857. [DOI] [PubMed] [Google Scholar]
- 37.Hanna J. Finley D. A proteasome for all occasions. FEBS Lett. 2007;581:2854–2861. doi: 10.1016/j.febslet.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heider U. von Metzler I. Kaiser M. Rosche M. Sterz J. Rotzer S. Rademacher J. Jakob C. Fleissner C. Kuckelkorn U. Kloetzel PM. Sezer O. Synergistic interaction of the histone deacetylase inhibitor SAHA with the proteasome inhibitor bortezomib in mantle cell lymphoma. Eur J Haematol. 2008;80:133–142. doi: 10.1111/j.1600-0609.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- 39.Hershko A. The ubiquitin system for protein degradation and some of its roles in the control of the cell division cycle. Cell Death Differ. 2005;12:1191–1197. doi: 10.1038/sj.cdd.4401702. [DOI] [PubMed] [Google Scholar]
- 40.Hose C. Kaur G. Sausville EA. Monks A. Transcriptional profiling identifies altered intracellular labile iron homeostasis as a contributing factor to the toxicity of adaphostin: decreased vascular endothelial growth factor secretion is independent of hypoxia-inducible factor-1 regulation. Clin Cancer Res. 2005;11:6370–6381. doi: 10.1158/1078-0432.CCR-05-0291. [DOI] [PubMed] [Google Scholar]
- 41.Hoye AT. Davoren JE. Wipf P. Fink MP. Kagan VE. Targeting mitochondria. Acc Chem Res. 2008;41:87–97. doi: 10.1021/ar700135m. [DOI] [PubMed] [Google Scholar]
- 42.Ishii N. Role of oxidative stress from mitochondria on aging and cancer. Cornea. 2007;26:S3–S9. doi: 10.1097/ICO.0b013e31812f6745. [DOI] [PubMed] [Google Scholar]
- 43.Kagan VE. Tyurina YY. Bayir H. Chu CT. Kapralov AA. Vlasova II. Belikova NA. Tyurin VA. Amoscato A. Epperly M. Greenberger J. Dekosky S. Shvedova AA. Jiang J. The “pro-apoptotic genies” get out of mitochondria: oxidative lipidomics and redox activity of cytochrome c/cardiolipin complexes. Chem Biol Interact. 2006;163:15–28. doi: 10.1016/j.cbi.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 44.Kamata H. Honda S. Maeda S. Chang L. Hirata H. Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 45.Katayama T. Imaizumi K. Manabe T. Hitomi J. Kudo T. Tohyama M. Induction of neuronal death by ER stress in Alzheimer's disease. J Chem Neuroanat. 2004;28:67–78. doi: 10.1016/j.jchemneu.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Kaur G. Gazit A. Levitzki A. Stowe E. Cooney DA. Sausville EA. Tyrphostin induced growth inhibition: correlation with effect on p210bcr-abl autokinase activity in K562 chronic myelogenous leukemia. Anticancer Drugs. 1994;5:213–222. [PubMed] [Google Scholar]
- 47.Kaur G. Narayanan VL. Risbood PA. Hollingshead MG. Stinson SF. Varma RK. Sausville EA. Synthesis, structure-activity relationship, and p210(bcr-abl) protein tyrosine kinase activity of novel AG 957 analogs. Bioorg Med Chem. 2005;13:1749–1761. doi: 10.1016/j.bmc.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Kim JH. Chu SC. Gramlich JL. Pride YB. Babendreier E. Chauhan D. Salgia R. Podar K. Griffin JD. Sattler M. Activation of the PI3K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood. 2005;105:1717–1723. doi: 10.1182/blood-2004-03-0849. [DOI] [PubMed] [Google Scholar]
- 49.Kisselev AF. Akopian TN. Castillo V. Goldberg AL. Proteasome active sites allosterically regulate each other, suggesting a cyclical bite-chew mechanism for protein breakdown. Mol Cell. 1999;4:395–402. doi: 10.1016/s1097-2765(00)80341-x. [DOI] [PubMed] [Google Scholar]
- 50.Kisselev AF. Callard A. Goldberg AL. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J Biol Chem. 2006;281:8582–8590. doi: 10.1074/jbc.M509043200. [DOI] [PubMed] [Google Scholar]
- 51.Kisselev AF. Goldberg AL. Proteasome inhibitors: from research tools to drug candidates. Chem Biol. 2001;8:739–758. doi: 10.1016/s1074-5521(01)00056-4. [DOI] [PubMed] [Google Scholar]
- 52.Kolb JP. Mechanisms involved in the pro- and anti-apoptotic role of NO in human leukemia. Leukemia. 2000;14:1685–1694. doi: 10.1038/sj.leu.2401896. [DOI] [PubMed] [Google Scholar]
- 53.Korsmeyer SJ. Yin XM. Oltvai ZN. Veis-Novack DJ. Linette GP. Reactive oxygen species and the regulation of cell death by the Bcl-2 gene family. Biochim Biophys Acta. 1995;1271:63–66. doi: 10.1016/0925-4439(95)00011-r. [DOI] [PubMed] [Google Scholar]
- 54.Koster AJ. Walz J. Lupas A. Baumeister W. Structural features of archaebacterial and eukaryotic proteasomes. Mol Biol Rep. 1995;21:11–20. doi: 10.1007/BF00990965. [DOI] [PubMed] [Google Scholar]
- 55.Kowaltowski AJ. Fiskum G. Redox mechanisms of cytoprotection by Bcl-2. Antioxid Redox Signal. 2005;7:508–514. doi: 10.1089/ars.2005.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuhn DJ. Chen Q. Voorhees PM. Strader JS. Shenk KD. Sun CM. Demo SD. Bennett MK. van Leeuwen FW. Chanan-Khan AA. Orlowski RZ. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–3290. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Labutti J. Parsons I. Huang R. Miwa G. Gan LS. Daniels JS. Oxidative deboronation of the peptide boronic acid proteasome inhibitor bortezomib: contributions from reactive oxygen species in this novel cytochrome P450 reaction. Chem Res Toxicol. 2006;19:539–546. doi: 10.1021/tx050313d. [DOI] [PubMed] [Google Scholar]
- 58.Le SB. Hailer MK. Buhrow S. Wang Q. Flatten K. Pediaditakis P. Bible KC. Lewis LD. Sausville EA. Pang YP. Ames MM. Lemasters JJ. Holmuhamedov EL. Kaufmann SH. Inhibition of mitochondrial respiration as a source of adaphostin-induced reactive oxygen species and cytotoxicity. J Biol Chem. 2007;282:8860–8872. doi: 10.1074/jbc.M611777200. [DOI] [PubMed] [Google Scholar]
- 59.Lemasters JJ. Modulation of mitochondrial membrane permeability in pathogenesis, autophagy and control of metabolism. J Gastroenterol Hepatol. 2007;22(suppl 1):S31–S37. doi: 10.1111/j.1440-1746.2006.04643.x. [DOI] [PubMed] [Google Scholar]
- 60.Leone M. Zhai D. Sareth S. Kitada S. Reed JC. Pellecchia M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003;63:8118–8121. [PubMed] [Google Scholar]
- 61.Ling YH. Liebes L. Zou Y. Perez-Soler R. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells. J Biol Chem. 2003;278:33714–33723. doi: 10.1074/jbc.M302559200. [DOI] [PubMed] [Google Scholar]
- 62.Llobet D. Eritja N. Encinas M. Sorolla A. Yeramian A. Schoenenberger JA. Llombart-Cussac A. Marti RM. Matias-Guiu X. Dolcet X. Antioxidants block proteasome inhibitor function in endometrial carcinoma cells. Anticancer Drugs. 2008;19:115–124. doi: 10.1097/CAD.0b013e3282f24031. [DOI] [PubMed] [Google Scholar]
- 63.Long J. Manchandia T. Ban K. Gao S. Miller C. Chandra J. Adaphostin cytoxicity in glioblastoma cells is ROS-dependent and is accompanied by upregulation of heme oxygenase-1. Cancer Chemother Pharmacol. 2007;59:527–535. doi: 10.1007/s00280-006-0295-5. [DOI] [PubMed] [Google Scholar]
- 64.Lucas DM. Davis ME. Parthun MR. Mone AP. Kitada S. Cunningham KD. Flax EL. Wickham J. Reed JC. Byrd JC. Grever MR. The histone deacetylase inhibitor MS-275 induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia cells. Leukemia. 2004;18:1207–1214. doi: 10.1038/sj.leu.2403388. [DOI] [PubMed] [Google Scholar]
- 65.MacKenzie SH. Clark AC. Targeting cell death in tumors by activating caspases. Curr Cancer Drug Targets. 2008;8:98–109. doi: 10.2174/156800908783769391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marchion D. Munster P. Development of histone deacetylase inhibitors for cancer treatment. Expert Rev Anticancer Ther. 2007;7:583–598. doi: 10.1586/14737140.7.4.583. [DOI] [PubMed] [Google Scholar]
- 67.Marcucci G. Byrd JC. Dai G. Klisovic MI. Kourlas PJ. Young DC. Cataland SR. Fisher DB. Lucas D. Chan KK. Porcu P. Lin ZP. Farag SF. Frankel SR. Zwiebel JA. Kraut EH. Balcerzak SP. Bloomfield CD. Grever MR. Caligiuri MA. Phase 1 and pharmacodynamic studies of G3139, a Bcl-2 antisense oligonucleotide, in combination with chemotherapy in refractory or relapsed acute leukemia. Blood. 2003;101:425–432. doi: 10.1182/blood-2002-06-1899. [DOI] [PubMed] [Google Scholar]
- 68.Marks PA. Dokmanovic M. Histone deacetylase inhibitors: discovery and development as anticancer agents. Expert Opin Invest Drugs. 2005;14:1497–1511. doi: 10.1517/13543784.14.12.1497. [DOI] [PubMed] [Google Scholar]
- 69.Masdehors P. Merle-Beral H. Magdelenat H. Delic J. Ubiquitin-proteasome system and increased sensitivity of B-CLL lymphocytes to apoptotic death activation. Leuk Lymphoma. 2000;38:499–504. doi: 10.3109/10428190009059268. [DOI] [PubMed] [Google Scholar]
- 70.McConkey DJ. Zhu K. Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resist Updat. 2008;11:164–179. doi: 10.1016/j.drup.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 71.Miller CP. Ban K. Dujka ME. McConkey DJ. Munsell M. Palladino M. Chandra J. NPI-0052, a novel proteasome inhibitor, induces caspase-8 and ROS-dependent apoptosis alone and in combination with HDAC inhibitors in leukemia cells. Blood. 2007;110:267–277. doi: 10.1182/blood-2006-03-013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Minami T. Adachi M. Kawamura R. Zhang Y. Shinomura Y. Imai K. Sulindac enhances the proteasome inhibitor bortezomib-mediated oxidative stress and anticancer activity. Clin Cancer Res. 2005;11:5248–5256. doi: 10.1158/1078-0432.CCR-05-0085. [DOI] [PubMed] [Google Scholar]
- 73.Mishra MV. Bisht KS. Sun L. Muldoon-Jacobs K. Awwad R. Kaushal A. Nguyen P. Huang L. Pennington JD. Markovina S. Bradbury CM. Gius D. DNMT1 as a molecular target in a multimodality-resistant phenotype in tumor cells. Mol Cancer Res. 2008;6:243–249. doi: 10.1158/1541-7786.MCR-07-0373. [DOI] [PubMed] [Google Scholar]
- 74.Momparler RL. Bouchard J. Samson J. Induction of differentiation and inhibition of DNA methylation in HL-60 myeloid leukemic cells by 5-AZA-2'-deoxycytidine. Leuk Res. 1985;9:1361–1366. doi: 10.1016/0145-2126(85)90123-7. [DOI] [PubMed] [Google Scholar]
- 75.Mukhopadhyay I. Sausville EA. Doroshow JH. Roy KK. Molecular mechanism of adaphostin-mediated G1 arrest in prostate cancer (PC-3) cells: signaling events mediated by hepatocyte growth factor receptor, c-Met, and p38 MAPK pathways. J Biol Chem. 2006;281:37330–37344. doi: 10.1074/jbc.M605569200. [DOI] [PubMed] [Google Scholar]
- 76.Nawrocki ST. Carew JS. Pino MS. Highshaw RA. Andtbacka RH. Dunner K., Jr Pal A. Bornmann WG. Chiao PJ. Huang P. Xiong H. Abbruzzese JL. McConkey DJ. Aggresome disruption: a novel strategy to enhance bortezomib-induced apoptosis in pancreatic cancer cells. Cancer Res. 2006;66:3773–3781. doi: 10.1158/0008-5472.CAN-05-2961. [DOI] [PubMed] [Google Scholar]
- 77.Nerini-Molteni S. Ferrarini M. Cozza S. Caligaris-Cappio F. Sitia R. Redox homeostasis modulates the sensitivity of myeloma cells to bortezomib. Br J Haematol. 2008;141:494–503. doi: 10.1111/j.1365-2141.2008.07066.x. [DOI] [PubMed] [Google Scholar]
- 78.Newton K. Strasser A. The Bcl-2 family and cell death regulation. Curr Opin Genet Dev. 1998;8:68–75. doi: 10.1016/s0959-437x(98)80064-6. [DOI] [PubMed] [Google Scholar]
- 79.Oddo S. The ubiquitin-proteasome system in Alzheimer's disease. J Cell Mol Med. 2008;12:363–373. doi: 10.1111/j.1582-4934.2008.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oltersdorf T. Elmore SW. Shoemaker AR. Armstrong RC. Augeri DJ. Belli BA. Bruncko M. Deckwerth TL. Dinges J. Hajduk PJ. Joseph MK. Kitada S. Korsmeyer SJ. Kunzer AR. Letai A. Li C. Mitten MJ. Nettesheim DG. Ng S. Nimmer PM. O'Connor JM. Oleksijew A. Petros AM. Reed JC. Shen W. Tahir SK. Thompson CB. Tomaselli KJ. Wang B. Wendt MD. Zhang H. Fesik SW. Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 81.Orlowski RZ. Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14:1649–1657. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
- 82.Paoluzzi L. Gonen M. Gardner JR. Mastrella J. Yang D. Holmlund J. Sorensen M. Leopold L. Manova K. Marcucci G. Heaney ML. O'Connor OA. Targeting Bcl-2 family members with the BH3 mimetic AT-101 markedly enhances the therapeutic effects of chemotherapeutic agents in in vitro and in vivo models of B-cell lymphoma. Blood. 2008;111:5350–5358. doi: 10.1182/blood-2007-12-129833. [DOI] [PubMed] [Google Scholar]
- 83.Pei XY. Dai Y. Grant S. Synergistic induction of oxidative injury and apoptosis in human multiple myeloma cells by the proteasome inhibitor bortezomib and histone deacetylase inhibitors. Clin Cancer Res. 2004;10:3839–3852. doi: 10.1158/1078-0432.CCR-03-0561. [DOI] [PubMed] [Google Scholar]
- 84.Penta JS. Johnson FM. Wachsman JT. Copeland WC. Mitochondrial DNA in human malignancy. Mutat Res. 2001;488:119–133. doi: 10.1016/s1383-5742(01)00053-9. [DOI] [PubMed] [Google Scholar]
- 85.Perez-Galan P. Roue G. Villamor N. Montserrat E. Campo E. Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–264. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 86.Pritts TA. Hungness ES. Hershko DD. Robb BW. Sun X. Luo GJ. Fischer JE. Wong HR. Hasselgren PO. Proteasome inhibitors induce heat shock response and increase IL-6 expression in human intestinal epithelial cells. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1016–R1026. doi: 10.1152/ajpregu.00492.2001. [DOI] [PubMed] [Google Scholar]
- 87.Qiu J. Levin LR. Buck J. Reidenberg MM. Different pathways of cell killing by gossypol enantiomers. Exp Biol Med (Maywood) 2002;227:398–401. doi: 10.1177/153537020222700605. [DOI] [PubMed] [Google Scholar]
- 88.Reed JC. Bcl-2-family proteins and hematologic malignancies: history and future prospects. Blood. 2008;111:3322–3330. doi: 10.1182/blood-2007-09-078162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reed JC. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006;13:1378–1386. doi: 10.1038/sj.cdd.4401975. [DOI] [PubMed] [Google Scholar]
- 90.Reed JC. Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood. 2005;106:408–418. doi: 10.1182/blood-2004-07-2761. [DOI] [PubMed] [Google Scholar]
- 91.Reed JC. Stein C. Subasinghe C. Haldar S. Croce CM. Yum S. Cohen J. Antisense-mediated inhibition of BCL2 protooncogene expression and leukemic cell growth and survival: comparisons of phosphodiester and phosphorothioate oligodeoxynucleotides. Cancer Res. 1990;50:6565–6570. [PubMed] [Google Scholar]
- 92.Reinheckel T. Sitte N. Ullrich O. Kuckelkorn U. Davies KJ. Grune T. Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem J. 1998;335:637–642. doi: 10.1042/bj3350637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Richardson PG. Hideshima T. Mitsiades C. Anderson KC. The emerging role of novel therapies for the treatment of relapsed myeloma. J Natl Compr Cancer Netw. 2007;5:149–162. doi: 10.6004/jnccn.2007.0015. [DOI] [PubMed] [Google Scholar]
- 94.Riester D. Hildmann C. Schwienhorst A. Histone deacetylase inhibitors: turning epigenic mechanisms of gene regulation into tools of therapeutic intervention in malignant and other diseases. Appl Microbiol Biotechnol. 2007;75:499–514. doi: 10.1007/s00253-007-0912-1. [DOI] [PubMed] [Google Scholar]
- 95.Rivett AJ. Mason GG. Murray RZ. Reidlinger J. Regulation of proteasome structure and function. Mol Biol Rep. 1997;24:99–102. doi: 10.1023/a:1006814306401. [DOI] [PubMed] [Google Scholar]
- 96.Rodenhiser DI. Epigenetic contributions to cancer metastasis. Clin Exp Metastasis. 2009;26:5–18. doi: 10.1007/s10585-008-9166-2. [DOI] [PubMed] [Google Scholar]
- 97.Rosato RR. Almenara JA. Grant S. The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF1 1. Cancer Res. 2003;63:3637–3645. [PubMed] [Google Scholar]
- 98.Ruefli AA. Ausserlechner MJ. Bernhard D. Sutton VR. Tainton KM. Kofler R. Smyth MJ. Johnstone RW. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc Natl Acad Sci U S A. 2001;98:10833–10838. doi: 10.1073/pnas.191208598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sallmyr A. Fan J. Datta K. Kim KT. Grosu D. Shapiro P. Small D. Rassool F. Internal tandem duplication of FLT3 (FLT3/ITD) induces increased ROS production, DNA damage, and misrepair: implications for poor prognosis in AML. Blood. 2008;111:3173–3182. doi: 10.1182/blood-2007-05-092510. [DOI] [PubMed] [Google Scholar]
- 100.Sattler M. Verma S. Shrikhande G. Byrne CH. Pride YB. Winkler T. Greenfield EA. Salgia R. Griffin JD. The BCR/ABL tyrosine kinase induces production of reactive oxygen species in hematopoietic cells. J Biol Chem. 2000;275:24273–24278. doi: 10.1074/jbc.M002094200. [DOI] [PubMed] [Google Scholar]
- 101.Shanafelt TD. Lee YK. Bone ND. Strege AK. Narayanan VL. Sausville EA. Geyer SM. Kaufmann SH. Kay NE. Adaphostin-induced apoptosis in CLL B cells is associated with induction of oxidative stress and exhibits synergy with fludarabine. Blood. 2005;105:2099–2106. doi: 10.1182/blood-2004-06-2205. [DOI] [PubMed] [Google Scholar]
- 102.Shibata T. Yamada T. Kondo M. Tanahashi N. Tanaka K. Nakamura H. Masutani H. Yodoi J. Uchida K. An endogenous electrophile that modulates the regulatory mechanism of protein turnover: inhibitory effects of 15-deoxy-delta 12,14-prostaglandin J2 on proteasome. Biochemistry. 2003;42:13960–13968. doi: 10.1021/bi035215a. [DOI] [PubMed] [Google Scholar]
- 103.Silva G. Cunha A. Gregoire IP. Seldon MP. Soares MP. The antiapoptotic effect of heme oxygenase-1 in endothelial cells involves the degradation of p38 alpha MAPK isoform. J Immunol. 2006;177:1894–1903. doi: 10.4049/jimmunol.177.3.1894. [DOI] [PubMed] [Google Scholar]
- 104.Srimatkandada P. Loomis R. Carbone R. Srimatkandada S. Lacy J. Combined proteasome and Bcl-2 inhibition stimulates apoptosis and inhibits growth in EBV-transformed lymphocytes: a potential therapeutic approach to EBV-associated lymphoproliferative diseases. Eur J Haematol. 2008;80:407–418. doi: 10.1111/j.1600-0609.2008.01044.x. [DOI] [PubMed] [Google Scholar]
- 105.Sterz J. von Metzler I. Hahne JC. Lamottke B. Rademacher J. Heider U. Terpos E. Sezer O. The potential of proteasome inhibitors in cancer therapy. Expert Opin Invest Drugs. 2008;17:879–895. doi: 10.1517/13543784.17.6.879. [DOI] [PubMed] [Google Scholar]
- 106.Stockwin LH. Bumke MA. Yu SX. Webb SP. Collins JR. Hollingshead MG. Newton DL. Proteomic analysis identifies oxidative stress induction by adaphostin. Clin Cancer Res. 2007;13:3667–3681. doi: 10.1158/1078-0432.CCR-07-0025. [DOI] [PubMed] [Google Scholar]
- 107.Suh KS. Goy A. Bortezomib in mantle cell lymphoma. Future Oncol. 2008;4:149–168. doi: 10.2217/14796694.4.2.149. [DOI] [PubMed] [Google Scholar]
- 108.Trachootham D. Zhou Y. Zhang H. Demizu Y. Chen Z. Pelicano H. Chiao PJ. Achanta G. Arlinghaus RB. Liu J. Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 109.Tzung SP. Kim KM. Basanez G. Giedt CD. Simon J. Zimmerberg J. Zhang KY. Hockenbery DM. Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nat Cell Biol. 2001;3:183–191. doi: 10.1038/35055095. [DOI] [PubMed] [Google Scholar]
- 110.Ungerstedt JS. Sowa Y. Xu WS. Shao Y. Dokmanovic M. Perez G. Ngo L. Holmgren A. Jiang X. Marks PA. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2005;102:673–678. doi: 10.1073/pnas.0408732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Usami H. Kusano Y. Kumagai T. Osada S. Itoh K. Kobayashi A. Yamamoto M. Uchida K. Selective induction of the tumor marker glutathione S-transferase P1 by proteasome inhibitors. J Biol Chem. 2005;280:25267–25276. doi: 10.1074/jbc.M501014200. [DOI] [PubMed] [Google Scholar]
- 112.Vaissiere T. Sawan C. Herceg Z. Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutat Res. 2008;659:40–48. doi: 10.1016/j.mrrev.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 113.Voehringer DW. BCL-2 and glutathione: alterations in cellular redox state that regulate apoptosis sensitivity. Free Radic Biol Med. 1999;27:945–950. doi: 10.1016/s0891-5849(99)00174-4. [DOI] [PubMed] [Google Scholar]
- 114.Voehringer DW. Meyn RE. Redox aspects of Bcl-2 function. Antioxid Redox Signal. 2000;2:537–550. doi: 10.1089/15230860050192314. [DOI] [PubMed] [Google Scholar]
- 115.Wang JL. Liu D. Zhang ZJ. Shan S. Han X. Srinivasula SM. Croce CM. Alnemri ES. Huang Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci U S A. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Webster KA. Programmed death as a therapeutic target to reduce myocardial infarction. Trends Pharmacol Sci. 2007;28:492–499. doi: 10.1016/j.tips.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 117.Yamamoto N. Sawada H. Izumi Y. Kume T. Katsuki H. Shimohama S. Akaike A. Proteasome inhibition induces glutathione synthesis and protects cells from oxidative stress: relevance to Parkinson disease. J Biol Chem. 2007;282:4364–4372. doi: 10.1074/jbc.M603712200. [DOI] [PubMed] [Google Scholar]
- 118.Yew EH. Cheung NS. Choy MS. Qi RZ. Lee AY. Peng ZF. Melendez AJ. Manikandan J. Koay ES. Chiu LL. Ng WL. Whiteman M. Kandiah J. Halliwell B. Proteasome inhibition by lactacystin in primary neuronal cells induces both potentially neuroprotective and pro-apoptotic transcriptional responses: a microarray analysis. J Neurochem. 2005;94:943–956. doi: 10.1111/j.1471-4159.2005.03220.x. [DOI] [PubMed] [Google Scholar]
- 119.Yu C. Rahmani M. Almenara J. Sausville EA. Dent P. Grant S. Induction of apoptosis in human leukemia cells by the tyrosine kinase inhibitor adaphostin proceeds through a RAF-1/MEK/ERK- and AKT-dependent process. Oncogene. 2004;23:1364–1376. doi: 10.1038/sj.onc.1207248. [DOI] [PubMed] [Google Scholar]
- 120.Zhang L. Ming L. Yu J. BH3 mimetics to improve cancer therapy: mechanisms and examples. Drug Resist Updat. 2007;10:207–217. doi: 10.1016/j.drup.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zimmermann AK. Loucks FA. Le SS. Butts BD. Florez-McClure ML. Bouchard RJ. Heidenreich KA. Linseman DA. Distinct mechanisms of neuronal apoptosis are triggered by antagonism of Bcl-2/Bcl-x(L) versus induction of the BH3-only protein Bim. J Neurochem. 2005;94:22–36. doi: 10.1111/j.1471-4159.2005.03156.x. [DOI] [PubMed] [Google Scholar]
- 122.Zou W. Yue P. Lin N. He M. Zhou Z. Lonial S. Khuri FR. Wang B. Sun SY. Vitamin C inactivates the proteasome inhibitor PS-341 in human cancer cells. Clin Cancer Res. 2006;12:273–280. doi: 10.1158/1078-0432.CCR-05-0503. [DOI] [PubMed] [Google Scholar]