Abstract
Honokiol is a small-molecule polyphenol isolated from the genus Magnolia. It is accompanied by other related polyphenols, including magnolol, with which it shares certain biologic properties. Recently, honokiol has been found to have antiangiogenic, antiinflammatory, and antitumor properties in preclinical models, without appreciable toxicity. These findings have increased interest in bringing honokiol to the clinic as a novel chemotherapeutic agent. In addition, mechanistic studies have tried to find the mechanism(s) of action of honokiol, for two major reasons. First, knowledge of the mechanisms of action may assist development of novel synthetic analogues. Second, mechanistic actions of honokiol may lead to rational combinations with conventional chemotherapy or radiation for enhanced response to systemic cancers. In this review, we describe the findings that honokiol has two major mechanisms of action. First, it blocks signaling in tumors with defective p53 function and activated ras by directly blocking the activation of phospholipase D by activated ras. Second, honokiol induces cyclophilin D, thus potentiating the mitochondrial permeability transition pore, and causing death in cells with wild-type p53. Knowledge of the dual activities of honokiol can assist with the development of honokiol derivatives and the design of clinical trials that will maximize the potential benefit of honokiol in the patient setting. Antioxid. Redox Signal. 11, 1139–1148.
Introduction: History of Honokiol
Honokiol was initially described as a component of Magnolia obovata, which is a component of Asian herbal teas, including houpo and saiboku-tu (17). After isolation, several neuronal activities were attributed to honokiol, including suppression of seizure activity induced by intraventricular injection of penicillin G and central muscle relaxation (57). Honokiol was found to inhibit thrombosis by inhibiting thromboxane formation and intracellular calcium mobilization in platelets (21, 56). In the 1990s, honokiol was found to have activity as a scavenger for hydroxyl radicals, lipid peroxidation, and of acetyl-CoA:1-alkyl-sn-glycero-3-phosphocholine acetyltransferase activity, a major intermediate in the production of platelet-activating factor (PAF) (61).
Honokiol was found to potentiate the differentiation of HL60 promyelocytic leukemia cells and cause apoptosis of lung and colon cancer cells in vitro. Magnolol and a methanolic extract of Magnolia were shown to exert a chemopreventive effect in a murine model of skin carcinogenesis (22, 29, 36). Honokiol also was found to be a potent scavenger of hydroxyl radicals, which is likely due to the allyl groups (32, 42). The ortho allyl group could potentially form a six-member ring after absorption of the hydroxyl group. This may account for the superior antioxidant activity to magnolol, which has two para-allyl groups to the hydroxyls, and thus cannot form the six-member ring.
In Vivo and In Vitro Antitumor Activity of Honokiol
Our group was the first to demonstrate the efficacy of honokiol against established tumors in mice (9). Honokiol was selected over magnolol based on the observation that honokiol was more effective on inducing apoptosis in SVR angiosarcoma cells. Treatment of SVR cells with honokiol caused decreased phosphorylation of MAP kinase, akt, and c-src. This implicates an upstream target for honokiol. In addition, honokiol potentiated TRAIL-mediated apoptosis, and honokiol cytotoxicity was partially abrogated by neutralizing antibodies to TRAIL. Honokiol also has direct antiangiogenic activity, in that honokiol blocks the phosphorylation and rac activation due to VEGF-VEGFR2 interactions (1).
Honokiol has been shown to have both direct antiangiogenic and antitumor properties. In a study of patients with chronic lymphocytic leukemia (CLL), honokiol had preferential activity against patient-derived CLL cells versus normal lymphocytes (10). Honokiol causes apoptosis in CLL cells through activation of caspase 8, followed by caspase 9 and 3 activation. Bax is upregulated by honokiol, with no effect on bcl2. Mcl-1 is initially upregulated, followed by rapid cleavage. Honokiol prevented interleukin-4–mediated survival of CLL cells, and potentiated the cytotoxicity of chlorambucil, fludarabine, and cladribine (10).
A concurrent study of honokiol in multiple myeloma demonstrated that honokiol killed myeloma cells from relapsed patients at doses that did not kill PBMCs. Caspase 3, 7, 8, and 9 were induced by honokiol treatment, as well as PARP cleavage. Honokiol caused cleavage of Mcl-1 and downregulation of XIAP, whereas Bad was markedly upregulated; and Bid, p-Bad, Bak, Bax, Bcl-2, and Bcl-xL were unchanged (26). Honokiol also induced release of mitochondrial proapoptotic protein AIF to the cytosol. Honokiol caused apoptosis even in the presence of IGF-1, interleukin-6, bone marrow stroma, and prevented phosphorylation of Akt, Stat-3, and Erk2, again implying an upstream target of action (18, 26).
Subsequent reports have found that honokiol has activity against a variety of tumors. Honokiol has been found to induce apoptosis in the colon cancer cell lines RKO and to inhibit the growth of RKO cells in murine xenografts (54). We have shown that honokiol prevents the growth of MDA-MD-231 breast cancer cells in murine xenografts (59). Of interest, MDA-MD-231 cells demonstrate mutant p53 and mutant K-ras, which is preferentially observed in the triple-negative breast cancer phenotype (24, 62). In the same study, honokiol had less activity on the MCF7 breast cancer cell line, which exhibits wild-type p53 and loss of p16ink4a. Given that SVR cells have defects in p53 signaling because of expression of SV40 large T, and that MDA-MB-231 cells also express mutant p53, tumors that have defects in p53 signaling may be targets of honokiol. Similarly, honokiol caused apoptosis in other solid-tumor cell lines that contain mutant p53 and ras activation, including lung and bladder cell lines (19).
Honokiol has been shown to reverse the multidrug resistance gene, and perhaps the ABCC transporter gene, the two major mechanisms for drug efflux (60). This may be due to decreased activation of NF-κB by honokiol (1). The efficacy of honokiol in combination with docetaxol was assessed in a nude mouse xenograft of lytic bone disease by using the metastatic C4-2 model. Honokiol had efficacy as monotherapy in reducing microvessel count and tumor burden in mice, but in combination with docetaxol, showed a dramatic reduction in tumor volume, lytic disease of bone. Thus, the major role of honokiol may be facilitating the efficacy of conventional chemotherapy, by inhibiting NF-κB (46).
Honokiol has also been shown to potentiate the activity of cisplatin in murine models of ovarian cancer, likely through a similar mechanism (33).
Tumor cells rely on several stimuli for optimal tumor growth, including activation of focal adhesion kinases, downstream of integrins. Retrovirus-based knockdown of shb, an adaptor for integrin-mediated signaling, was accomplished by cre-mediated recombination. Loss of shb causes decreased activation of focal adhesion kinase, but did not affect in vivo or in vitro growth of SVR angiosarcoma cells (7, 18). Although tumor cells lacking shb grow as well in vivo as parental SVR cells, the growth of shb-deficient cells was markedly inhibited by honokiol in comparison with parental cells in vivo. The likely mechanism for this finding is that several redundant inputs contribute to in vivo tumorigenesis, including oncogenic ras, as well as integrin-mediated antiapoptotic signaling. Loss of integrin-mediated signaling causes increased dependence on oncogenic H-ras in the SVR cells. FAK is activated by multiple tyrosine kinases, including VEGFR2 and PDGFRβ (27, 58). This provides a rationale for combination of tyrosine kinase inhibitors plus honokiol (18). It is well known that EGFR inhibitors are ineffective against lung cancers that have mutant ras, and the combination of honokiol and a tyrosine kinase inhibitor may result in improved efficacy (6).
The Mechanisms of Action
Grp94 has been implicated as a target of honokiol. Treatment of a series of gastric cancer cell lines with honokiol reduced expression of grp94, in a calpain-dependent manner. Blockade of calpain by chemical calpain inhibitors reduced honokiol-mediated cytotoxicity (45). SIRNA-mediated downregulation of grp94 results in apoptosis in gastric cancer cell lines. Low-dose honokiol was found to be effective in gastric tumor xenografts in mice. The reason these tumors are so sensitive to honokiol is not currently understood. Of interest, AGS and MKN 45, which have wild-type p53, are less sensitive to honokiol inducing calpain activity compared with N87 gastric cancer cells, which has mutant p53 (37).
An additional mode of cell death was recently described. Honokiol induced expression of the mitochondrial protein cyclophilin D and blockade of cyclophilin D by siRNA or cyclosporin A (31). It induced necrosis through increased outer mitochondrial permeability and, contrary to prior reports, honokiol induced reactive oxygen. However, treatment of cells with antioxidants did not prevent cell death due to honokiol. Honokiol was effective in prolonging life in mice xenografted with HL60 promyelocytic leukemia cells, both intravenously and intraperitoneally injected (31). Given that cyclosporin A is associated with massive increases in cutaneous malignancies with mutant p53 (39), addition of honokiol may potentially block the protumorigenic effects of cyclosporine without compromising the immunosuppressive efficacy. Indeed, honokiol is mildly immunosuppressive as monotherapy, in that it significantly alleviates a murine model of inflammatory arthritis. It is currently believed that cyclosporine exerts immunosuppressive effects through NFAT inhibition, but protumorigenic effects through inhibiting the mitochondrial transition pore.
Honokiol affects NF-κB signaling, but not through a direct effect on NF-κB/DNA binding (1). Honokiol blocked NF-κB and Akt activation as a result of TNF-α stimulation, enhancing TNF-α–mediated cell death. Honokiol inhibits TNF-induced NF-κB activation, IκBα phosphorylation, and IκBα degradation, and RANKL-mediated NF-κB activation. Honokiol inhibits NF-κB–dependent reporter gene expression induced by TNFR1, TRADD, TRAF, NIK, and IKKβ. Consistent with the effect of honokiol on NF-κB is that honokiol decreases levels of NF-κB target genes, including VEGF, matrix metalloproteinase 9 (MMP9), ICAM-1, and cyclooxygenase 2 (COX2) (1). These findings suggest a site of action upstream of NF-κB/IKK, especially because Akt also is downregulated in response to TNF-α. A separate study demonstrated that honokiol blocks NF-κB activation because of a number of stimuli, including TNF-α, phorbol ester, and lipopolysaccharide. Honokiol blocked the production of TNF-α, MCP-1, interleukin-8, and ICAM-1, and was found to act at the level of IKK or upstream of IKK (53).
Honokiol was found to exert apoptotic activity against human prostate cancer cell lines LNCAP and PC3 cells (20). Honokiol treatment of these cells induced phosphorylation of ser 15 of p53, induction of p21, decreased phosphorylation of retinoblastoma (Rb), and inactivation of the transcription factor E2F1. As in the previous study on cyclophilin D, honokiol induced reactive oxygen in prostate cancer cells, but the induction was more pronounced in the LNCAP cells, which have wild-type p53, compared with the PC3 cells, which have mutant p53. Interestingly, oral gavage of 2 mg of honokiol 3 times weekly significantly inhibited PC3 growth in vivo, demonstrating significant oral bioavailability.
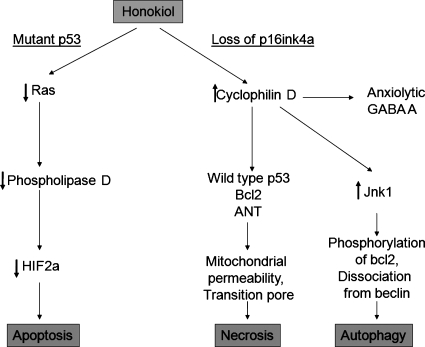
Honokiol has been particularly effective in several tumor xenograft systems with deficits in p53 signaling, including PC3, MDA-MD-231, and SVR cells, above and beyond their effects on cellular proliferation in vivo. Honokiol blocks the growth of bend3 hemangiomas in vitro, but does not affect the proliferation of bend3 hemangiomas in nude mice (unpublished data). Bend3 cells do not have deficits in p53 signaling, unlike SVR endothelial cells. Thus, two modes of cell killing have been observed to be due to honokiol. In cells with p53 deficits, potentiation of extrinsic pathways has been noted (i.e., potentiation of TRAIL), followed by activation of caspase 3, 7, and 9. A second pathway, associated with mitochondrial dysfunction, reactive oxygen generation, and necrosis, may be more important in tumors with wild-type p53 (Fig. 1).
FIG. 1.
Metabolic pathway of honokiol in cells that use a ras signaling schema. Honokiol acts on the ras-Rhoa complex to inhibit PLD expression. The inhibition of PLD expression then causes consequential apoptosis in cells. Honokiol can also act on cyclophilin D, via a loss of the p16ink4a pathway, which can induce necrosis or autophagy via two distinct pathways. The first is the cyclophilin D effect to upregulate Jnk1, causing bcl2 to phosphorylate, causing autophagy. The second is the effect on WT p53 and bcl2, which leads to mitochondrial permeability via the transition pore. The second route leads the breakdown of the cellular system and necrosis.
Recent work by the Huang's laboratory, and that of Singh, suggests that cyclophilin D is most likely a major target of the cytotoxic actions of honokiol. The Huang group found that cells from high-grade esophageal dysplasia are highly sensitive to honokiol, and that honokiol sensitivity could be ablated by cyclophilin D siRNA or cyclosporin A, which inhibits NFAT activity like tacrolimus, but also inhibits cyclophilin D (31). In these cells, honokiol was found to cause cell death in an apoptosis-independent fashion. Intriguingly, mice deficient in cyclophilin D are anxiety prone, and honokiol is a known anxiolytic compound (31).
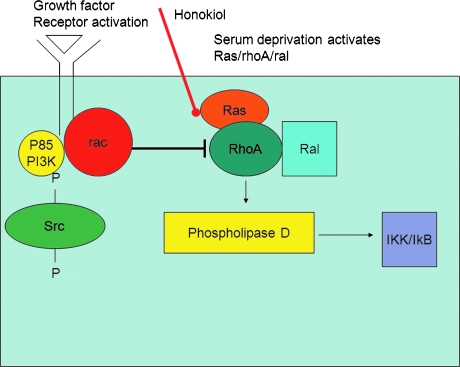
The antagonism of cyclosporin A and honokiol might have beneficial properties. Cyclosporin A is a heavily used immunosuppressant, but has major side effects in terms of hypertension, renal dysfunction, and promotion of skin cancers and lymphoma. Addition of honokiol to cyclosporin A might help alleviate these side effects, which may be due to cyclophilin D inhibition rather than NFAT inhibition, and because honokiol by itself has been shown to have antiinflammatory properties in murine models of arthritis, the addition of honokiol might reduce the dose of cyclosporin A necessary to prevent organ rejection (Fig. 2) (24, 31).
FIG. 2.
A possible chemical mechanism in which honokiol acts as a reactive oxygen (ROS) scavenger. This allows honokiol to act as a significant inhibitor in reactive oxygen species (ROS)-driven tumors. This inhibition is in part a result of the effect that honokiol has on the expression of phospholipase D (PLD) in the ras pathway. The direct effect of honokiol is on the Ras-RhoA-ral complex, which leads to the expression of phospholipase D (PLD). The expression of PLD from this complex is induced by rac, which is involved with PI3K and a receptor activated by growth factors. Ultimately, PLD activated IKK and IκB activity, which leads to tumor growth. The Ras-RhoA-ral is induced by serum deprivation. Studies have been done with the combination of both serum-deficient tumors with treatment of honokiol, varying from 10 to 15% deficient. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Effect of Honokiol on Reactive Oxygen Species
The inhibition by honokiol of reactive oxygen driven tumors is due to its involvement with the NADPH oxidase (NOX) pathway (44). This inhibition was first demonstrated in neutrophils (32), and later, in hepatocytes (41) and human umbilical vein endothelial cells (HUVECs) (44). NF-κB regulates the signaling pathway of NOX-mediated oxidative stress.
A possible chemical mechanism that may take place involves a peroxide intermediate followed by the phenolic hydroxyl group attacking the peroxide carbon chain, yielding a pentose or hexose ring and water (Fig. 4). Both conformations are stable in cells and led to no side reactions. This shows the effectiveness of honokiol on reactive-oxygen scavenging that would be most effective in ROS-driven tumors.
FIG. 4.
Postulated products of peroxide scavenging by honokiol. Such compounds can include endoperoxides, bridged endoperoxides, or nitrophenols. As shown, multiple combinations of oxygenated honokiol can exist with ring enclosures common in scavenging compounds.
A paradox of honokiol is that it may have both pro- and antioxidant activities. We previously showed that honokiol has antioxidant activities, perhaps because of the allyl groups on honokiol, which can react with reactive oxygen species (15), however, the antimitochondrial effects of honokiol may generate reactive oxygen by activating the mitochondrial permeability transition pore (Fig. 1). The ability of honokiol to activate the mitochondrial pore may be dependent on the p53 status, with tumors with wild-type p53 having greater susceptibility to pore formation (31).
Two other polyphenols with similar reactive oxygen species scavenging are magnolol and obovatol. Magnolol is an isomer of honokiol, differing in the relative arrangement of hydroxyl group to allyl group on the phenolic ring. Magnolol has been shown to attenuate oxidized low-density lipoprotein (oxLDL)-induced ROS generation subsequently reducing NF-κB activation (40). The magnolol attenuation of ROS has been proposed as a reason for its inhibitory effect on neutrophil adherence to the extracellular matrix during injury (43). Obovatol, another polyphenolic compound found in Magnolia, inhibits NF-κB activation, leading to apoptotic cell death; its proposed mechanism is through inhibition of nitric oxide (NO) as the reactive oxygen species (13).
Immune Effects of Honokiol
Previous in vitro data showing blockade of TNF-α production, NF-κB activation, and production of additional inflammatory cytokines suggest that honokiol would have antiinflammatory properties at clinically achievable concentrations. To test this, collagen-induced arthritis was induced in the CD40-LMP1 transgenic mouse, in which native CD40 is replaced by a CD40-LMP1 transgene (38). The effect of this is to potentiate CD40 signaling, and the mice have an exaggerated autoimmune response to collagen-induced arthritis. Treatment of these mice with honokiol significantly decreased the clinical scores of collagen-induced arthritis in both normal and transgenic mice. Antibody production, most notably IgG3, was diminished, as were IL-12, IL-6, interferon gamma, and notably IL-17. These findings indicate that honokiol may have benefit against IL-17–mediated inflammatory disorders, including rheumatoid arthritis, psoriasis, and inflammatory bowel disease. Preparations containing honokiol have also been used in preclinical models of asthma, and clinical trials are occurring in Japan (28, 51).
Honokiol is a dimer of allylphenol. The most abundant allylphenols in nature are eugenol and isoeugenol, components of clove oil. Interestingly, eugenol has some modest antitumor activity and GABAA signaling activities (5, 48, 56). The position of the allyl and phenolic hydroxyl groups is critical. We initially tested 2,2' and 4,4'-dihydroxybiphenyl, and found them to be devoid of activity; thus, a requirement for an allyl group was postulated. We made allyl ethers of these compounds and subjected them to the Claisen rearrangement, forming allylated 2,2' and 4,4' dihydroxybiphenyl, but these compounds lacked activity as well, implying that placement of the allylphenols also was critical. Spacing out of the allylphenol groups resulted in activity against SVR cells, with allylated diethylstilbestrol and bisphenol A having potent activity against SVR cells. Unfortunately, the diethylstilbestrol derivative did not have activity in the SVR tumor model, possibly because of rapid metabolism (3).
Analogues and Antiviral Activities
HIV is an attractive target for honokiol and honokiol analogues (Fig. 3). While HIV causes profound immunodeficiencies, paradoxically, IL-17– and TNF-α–mediated autoimmune disorders, such as psoriasis, are often exacerbated (8, 25). The use of both nucleoside and nonnucleoside reverse transcriptase inhibitors are associated with mitochondrial dysfunction, and the protease inhibitors can induce metabolic disturbances, including lipodystrophy and hyperlipidemia. One potential mechanism is through inhibition of ABC transporters, which transport cholesterol, as well as mediate a form of multidrug resistance. Atherosclerosis is inevitable in long-term responders to HAART therapy for HIV, and in general, patients receiving long-term HAART therapy experience premature aging (55).
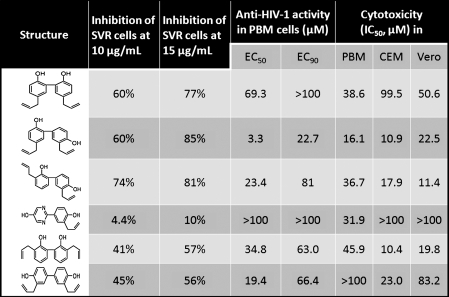
FIG. 3.
A table of analogues, including honokiol and magnolol. This table includes data on compound activity against angiosarcoma (SVR cells) in varying concentrations and its activity against HIV, which demonstrate its antiviral properties. Alongside this information in columns 6, 7, and 8, the data show the cytotoxicity of honokiol and its analogues in three normal human cell lines. With this information, one can gather the benefits of honokiol and its analogues in antitumor and antiviral schemas, with minimal cytotoxic effects (4).
One of the major regulators of HIV-1 virion transcription is NF-κB, which causes transcription of the HIV genome through responsive sites on the HIV long terminal repeat (LTR). Activation of NF-κB likely represents a common mechanism through which inflammatory stimuli, such as concurrent viral infection and TNF-α, production lead to enhanced HIV transcription. Honokiol and honokiol analogues have been demonstrated to inhibit HIV viral replication at doses that do not kill normal peripheral blood mononuclear cells. Further studies are required to determine whether combinations of honokiol analogues with HAART will lead to enhanced antiviral activity with decreased side effects.
Honokiol Protection of Heart and Liver Cells
Along with its antitumor and antiviral properties, honokiol has shown to improve the functions of normal human cells. The first studies were conducted by using honokiol in place of α-tocopherol in treating heart mitochondrial lipid peroxidation caused by ADP and ferrous sulfate (34). Results of oxygen consumption and malondialdehyde (MDA) production showed that the honokiol inhibition was 1,000 times that of α-tocopherol. As honokiol is better than α-tocopherol at inhibiting lipid peroxidation, it was used to protect the myocardium against ischemic injury by suppressing ventricular arrhythmia during ischemia and reperfusion (52).
In light of this, studies were done to show the protective effect of honokiol on hepatocytes from peroxidative injury, oxygen consumption, and malondialdehyde formation. Results showed the mitochondrial control ratio and ADP/O of honokiol-treated hepatocytes to be much higher than control in reperfusion, in a dose-dependant manner (12). The precise mechanism of how honokiol scavenges reactive oxygen and nitrogen species is not known, but could occur through putative intermediates, such as endoperoxides and nitrate esters (Fig. 4).
In recent studies, cyclophilin D has been shown to play a fundamental role in basal brain functions and the homeostasis of body weight (35). Mitochondrial cyclophilin D is the key modulator in mitochondrial permeability transition, which is the regulator of cell death induced by either calcium or oxidative damage. The deletion of cyclophilin D gene in CyPD-KO mice led to anxiety, exploratory avoidance, and learning/memory avoidance. The deletion also led to an observable lack of equilibrium. CyPD-KO mice also had larger amounts of adipose tissue, leading to obesity. The ability of honokiol to upregulate cyclophilin D allows honokiol to have a direct effect on the overall health of the body.
One potential mechanism of the metabolic syndrome/type II diabetes could be endoplasmic reticulum stress, causing depression of cyclophilin D, and honokiol might reverse this by induction of cyclophilin D. Of interest, honokiol induces AMP kinase and mimics adiponectin (unpublished data), suggesting that honokiol may be beneficial in the treatment of metabolic syndrome/type II diabetes.
Neurologic Effects of Honokiol
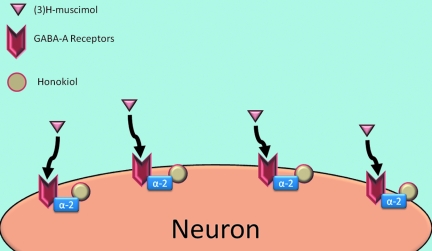
Honokiol was originally isolated as an anxiolytic principle of Magnolia grandiflora. This indicated that, unlike other polyphenols, honokiol can cross the blood–brain barrier, making it an attractive candidate for the treatment of central nervous system primary tumors and metastases. Honokiol also acts on the GABAA receptor subunit α-2 to help to enhance the binding of (3)H-muscimol (2) (Fig. 5). Honokiol exhibited increased potency over the clinically used anesthetic propofol (2,6-di-isopropylphenol) in enhancement of muscimol binding to cerebellar membranes (47). The unsubstituted, 2,2'-dihydroxybiphenyl was completely inactive in this assay, indicating the requirement for substituents for activity. The GABAA agonistic properties of honokiol likely account for its anxiolytic properties (30). Intriguingly, mice deficient in cyclophilin D, a potential target of honokiol, exhibit increased anxiety, as well as obesity mediated by white fat accumulation (35). If honokiol acts through cyclophilin D, cyclophilin D–knockout mice should be resistant to the anxiolytic effects of honokiol. Enhancement of cyclophilin D could potentially modulate the binding of GABA to the GABAA receptor.
FIG. 5.
Honokiol enhances the complexing of the α2 subunit to the GABAA receptor, which allows better binding of (3)H-muscimol to the GABAA receptor. This enhanced binding allows better activation of the neuron and more sustained signal. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Honokiol also has been reported to have neurotropic activities. These include enhancement of MAP kinase–mediated neurite formation, and decreased death due to cerebral ischemia/reperfusion. The mechanism for these findings is unknown and could be due to either reactive oxygen scavenging or involvement of cyclophilin D or ras. These findings on normal tissue are intriguing, especially given the cytotoxic effect of honokiol and MAP kinase inhibition on a variety of tumor cells.
Honokiol Antimicrobial Activity
Honokiol exhibits inhibition of microbial growth, although less than common antibiotics (23), in various assays done. Honokiol has been used to treat such periodontopathic microorganisms, Porphyromonas gingivalis, Prevotella gingivalis, Actinobacillus actinomycetemcomitans, Capnocytophaga gingivalis, and Veillonella disper, and no cytotoxicity against human gingival fibroblasts and epithelial cells; along with other gram-positive bacteria and fungi (11, 14). Mechanisms for the antimicrobial actions of honokiol have yet to be uncovered.
In more recent applications, honokiol has been used to inhibit the growth of vancomycin-resistant enterococci (VRE) and methicillin-resistant Staphylococcus aureus (MRSA), in a dose- and time-dependent manner (49). These results are promising for the honokiol antimicrobial activities, showing the honokiol ability to be effective against more infectious microorganisms where more common antibiotics have failed.
Clinical Uses of Honokiol
Honokiol inhibits multiple facets of signal transduction (Fig. 2). Currently, it is not known whether honokiol has a single major target or several minor targets. However, it has several activities that make it desirable as a therapeutic. First, it is orally bioavailable and crosses the blood–brain barrier. Second, it inhibits NF-κB activity differently from other known inhibitors. This suggests that it can sensitize tumors to apoptosis in the face of conventional chemotherapy and radiation, through downregulation of mdr and mcl-1. It seems to have a particular affinity for bone-seeking tumors, perhaps by affecting wnt/dkk signaling. Honokiol normalizes the Warburg phenomenon in tumor cells, and perhaps may work through a similar fashion to reverse type II diabetes, which can also be caused by mitochondrial dysfunction. Finally, it may be a useful adjunct to antiretroviral drugs, in part by independently inhibiting HIV replication and inhibiting the mitochondrial side effects of current antiretroviral therapy. Further study into the mechanism(s) of honokiol is clearly warranted.
Isolation and Chemical Synthesis of Honokiol and Its Derivatives
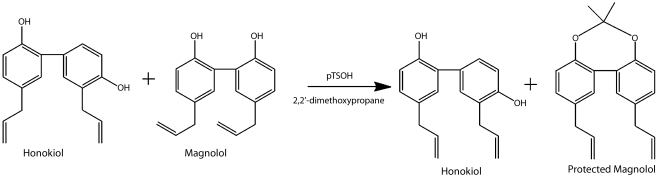
One obstacle to both preclinical and clinical development of honokiol has been isolation of honokiol and separation on a large scale from its close relative, magnolol. Because honokiol exists as a natural product with its structural homologue magnolol, which differs from honokiol only in the position of one hydroxyl group, we devised a large-scale purification method that uses the protection of magnolol to obtain pure honokiol via chromatography (3). The method takes advantage of the close proximity of the phenolic hydroxyl groups of magnolol and forms a protected diol generating the magnolol acetonide, which separates from honokiol (Fig. 6). This purification method leads to 91% yields. The magnolol may be deprotected afterward, by using aqueous HCl.
FIG. 6.
Purification of honokiol from Magnolia. Protection of magnolol with dimethoxypropane to make the magnolol-acetonide enables honokiol to be purified via chromatography. Without the protection, both magnolol and honokiol, which are structural isomers of each other, have the same polarity and cannot be separated. The protected magnolol can be deprotected by using acid.
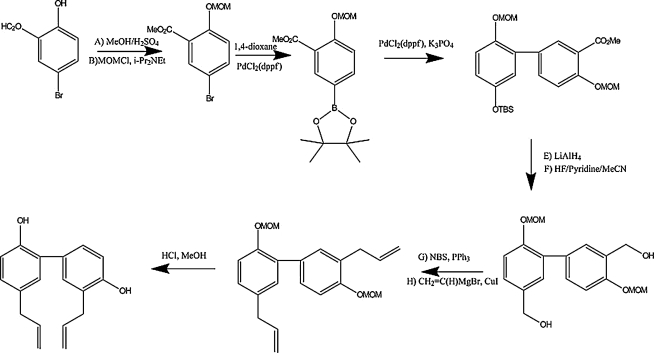
Honokiol can be synthesized chemically via Pd-catalyzed Myaura-Suzuki coupling (16) (Fig. 7). Even older synthetic methods involve quinol-acetates with the Grignard reagent, followed by a subsequent Claisen rearrangement (50). Synthesis leads to only moderate yields, 15–26%, making the purification a more sensible approach.
FIG. 7.
The Muara-Suzuki coupling mechanism. This synthetic process is the palladium-catalyzed cross-coupling between two phenolic rings into a bisphenol compound. The mechanism of the Suzuki reaction is best viewed from the perspective of the palladium catalyst. The first step is the oxidative addition of palladium to the halide to form the organo-palladium species. Reaction with base gives the intermediate, which, via transmetalation with the boronate complex, forms the organopalladium species. Reductive elimination of the desired product restores the original palladium catalyst.
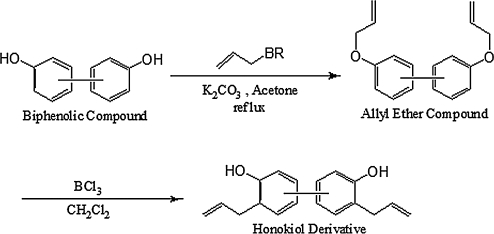
The synthesis of honokiol derivatives is an area of great oncologic interest. Some of these synthetic attempts include the phenolic O-allylation of biphenolic compounds, to make the allyl ether, followed by a Claisen rearrangement (4) (Fig. 8).
FIG. 8.
Synthetic scheme of honokiol and its derivatives. The synthesis of honokiol derivatives by using various bisphenol starting materials via an allylation of the phenolic hydroxyl groups and then the Claisen rearrangement. Various derivatives have different effects on targets and varying toxicity.
Summary
Honokiol is a small molecule with broad antitumor activity. Unlike many other natural products, honokiol exhibits a desirable spectrum of bioavailability. The development of other polyphenolic agents has been hindered by poor absorption and rapid excretion (i.e., curcumin). Honokiol does not have this disability, in that significant systemic levels of honokiol can be obtained in preclinical models and that honokiol can cross the blood–brain barrier. In this review, we demonstrate that honokiol has two distinct mechanisms of action. The first one is through inhibition of ras signaling, and appears to be most active in tumors with defective p53 function. The second one is through induction of cyclophilin D and activation of the mitochondrial transition pore. Honokiol appears to have distinct activities against tumors with mutant p53, through inhibition of ras-phospholipase D activation, and tumors with wild-type p53, through induction of cyclophilin D and potentiation of the mitochondrial transition pore.
Honokiol also exhibits antiviral activity against HIV, perhaps through inhibition of NF-κB signaling. Thus, honokiol may be useful in the setting of immunocompromise. Future clinical trials should be carried out combining honokiol with conventional chemotherapy, and increased efficacy might be observed through alleviation of chemotherapy-induced NF-κB activation. This knowledge is required for the development of future analogues, which may target either of these pathways, and for the development of clinical trials using honokiol and honokiol analogues, in which p53 status may play a key role in efficacy.
Acknowledgments
J. L. Arbiser was supported by a Veterans Administration Merit Award, NIH grant R01 AR02030, R01AR050727, and grants from the Jamie Rabinowitch-Davis Foundation and the Minsk Foundation.
Abbreviations
Bcl2, B-cell lymphoma 2; EGFR, epidermal growth factor receptor; FAK, focal adhesion kinase; GABA, γ-aminobutyric acid; MAP kinase, mitogen-activated protein kinase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; PARP, poly-ADP ribose polymerase; PBMC, peripheral blood mononuclear cell; Src, sarcoma; TNF-α, tumor necrosis factor-α; TRADD, TNFRSF1A-associated via death domain; TRAF, TNF receptor associated factor.
References
- 1.Ahn KS. Sethi G. Shishodia S. Sung B. Arbiser JL. Aggarwal BB. Honokiol potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through modulation of nuclear factor-kappaB activation pathway. Mol Cancer Res. 2006;4:621–633. doi: 10.1158/1541-7786.MCR-06-0076. [DOI] [PubMed] [Google Scholar]
- 2.Ai J. Wang X. Nielsen M. Honokiol and magnolol selectively interact with GABAA receptor subtypes in vitro. Pharmacology. 2001;63:34–41. doi: 10.1159/000056110. [DOI] [PubMed] [Google Scholar]
- 3.Amblard F. Delinsky D. Arbiser JL. Schinazi RF. Facile purification of honokiol and its antiviral and cytotoxic properties. J Med Chem. 2006;49:3426–3427. doi: 10.1021/jm060268m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amblard F. Govindarajan B. Lefkove B. Rapp KL. Detorio M. Arbiser JL. Schinazi RF. Synthesis, cytotoxicity, and antiviral activities of new neolignans related to honokiol and magnolol. Bioorg Med Chem Lett. 2007;17:4428–4431. doi: 10.1016/j.bmcl.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoshima H. Hamamoto K. Potentiation of GABAA receptors expressed in Xenopus oocytes by perfume and phytoncid. Biosci Biotechnol Biochem. 1999;63:743–748. doi: 10.1271/bbb.63.743. [DOI] [PubMed] [Google Scholar]
- 6.Arbiser JL. Why targeted therapy hasn't worked in advanced cancer. J Clin Invest. 2007;117:2762–2765. doi: 10.1172/JCI33190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arbiser JL. Bingaman A. Durham M. Cowan S. Cohen C. Zarnegar E. Varma V. Larsen CP. SVR angiosarcomas can be rejected by CD4 costimulation dependent and CD8 costimulation independent pathways. Mol Med. 2002;8:551–558. [PMC free article] [PubMed] [Google Scholar]
- 8.Arican O. Aral M. Sasmaz S. Ciragil P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273–279. doi: 10.1155/MI.2005.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai X. Cerimele F. Ushio-Fukai M. Waqas M. Campbell PM. Govindarajan B. Der CJ. Battle T. Frank DA. Ye K. Murad E. Dubiel W. Soff G. Arbiser JL. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278:35501–35507. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- 10.Battle TE. Arbiser J. Frank DA. The natural product honokiol induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia (B-CLL) cells. Blood. 2005;106:690–697. doi: 10.1182/blood-2004-11-4273. [DOI] [PubMed] [Google Scholar]
- 11.Chang B. Lee Y. Ku Y. Bae K. Chung C. Antimicrobial activity of magnolol and honokiol against periodontopathic microorganisms. Planta Med. 1998;64:367–369. doi: 10.1055/s-2006-957453. [DOI] [PubMed] [Google Scholar]
- 12.Chiu JH. Ho CT. Wei YH. Lui WY. Hong CY. In vitro and in vivo protective effect of honokiol on rat liver from peroxidative injury. Life Sci. 1997;61:1961–1971. doi: 10.1016/s0024-3205(97)00836-9. [DOI] [PubMed] [Google Scholar]
- 13.Choi MS. Lee SH. Cho HS. Kim Y. Yun YP. Jung HY. Jung JK. Lee BC. Pyo HB. Hong JT. Inhibitory effect of obovatol on nitric oxide production and activation of NF-kappaB/MAP kinases in lipopolysaccharide-treated RAW 264.7cells. Eur J Pharmacol. 2007;556:181–189. doi: 10.1016/j.ejphar.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 14.Clark AM. El-Feraly FS. Li WS. Antimicrobial activity of phenolic constituents of Magnolia grandiflora L. J Pharm Sci. 1981;70:951–952. doi: 10.1002/jps.2600700833. [DOI] [PubMed] [Google Scholar]
- 15.Dikalov S. Losik T. Arbiser JL. Honokiol is a potent scavenger of superoxide and peroxyl radicals. Biochem Pharmacol. 2008;76:589–596. doi: 10.1016/j.bcp.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esumi T. Makado G. Zhai H. Shimizu Y. Mitsumoto Y. Fukuyama Y. Efficient synthesis and structure-activity relationship of honokiol, a neurotrophic biphenyl-type neolignan. Bioorg Med Chem Lett. 2004;14:2621–2625. doi: 10.1016/j.bmcl.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 17.Fujita M. Itokawa H. Sashida Y. [Studies on the components of Magnolia obovata Thunb, 3: occurrence of magnolol and honokiol in M. obovata and other allied plants] Yakugaku Zasshi. 1973;93:429–434. doi: 10.1248/yakushi1947.93.4_429. [DOI] [PubMed] [Google Scholar]
- 18.Funa NS. Reddy K. Bhandarkar S. Kurenova EV. Yang L. Cance WG. Welsh M. Arbiser JL. Shb gene knockdown increases the susceptibility of SVR endothelial tumor cells to apoptotic stimuli in vitro and in vivo. J Invest Dermatol. 2007 doi: 10.1038/sj.jid.5701057. [DOI] [PubMed] [Google Scholar]
- 19.Garcia A. Zheng Y. Zhao C. Toschi A. Fan J. Shraibman N. Brown HA. Bar-Sagi D. Foster DA. Arbiser JL. Honokiol suppresses survival signals mediated by Ras-dependent phospholipase D activity in human cancer cells. Clin Cancer Res. 2008;14:4267–4274. doi: 10.1158/1078-0432.CCR-08-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hahm ER. Arlotti JA. Marynowski SW. Singh SV. Honokiol, a constituent of oriental medicinal herb Magnolia officinalis, inhibits growth of PC-3 xenografts in vivo in association with apoptosis induction. Clin Cancer Res. 2008;14:1248–1257. doi: 10.1158/1078-0432.CCR-07-1926. [DOI] [PubMed] [Google Scholar]
- 21.Hamasaki Y. Muro E. Miyanji S. Yamamoto S. Kobayashi I. Sato R. Zaitu M. Matsuo M. Ichimaru T. Tasaki H. Miyazaki S. Inhibition of leukotriene synthesis by honokiol in rat basophilic leukemia cells. Int Arch Allergy Immunol. 1996;110:278–281. doi: 10.1159/000237299. [DOI] [PubMed] [Google Scholar]
- 22.Hibasami H. Achiwa Y. Katsuzaki H. Imai K. Yoshioka K. Nakanishi K. Ishii Y. Hasegawa M. Komiya T. Honokiol induces apoptosis in human lymphoid leukemia Molt 4B cells. Int J Mol Med. 1998;2:671–673. doi: 10.3892/ijmm.2.6.671. [DOI] [PubMed] [Google Scholar]
- 23.Ho KY. Tsai CC. Chen CP. Huang JS. Lin CC. Antimicrobial activity of honokiol and magnolol isolated from Magnolia officinalis. Phytother Res. 2001;15:139–141. doi: 10.1002/ptr.736. [DOI] [PubMed] [Google Scholar]
- 24.Hui L. Zheng Y. Yan Y. Bargonetti J. Foster DA. Mutant p53 in MDA-MB-231 breast cancer cells is stabilized by elevated phospholipase D activity and contributes to survival signals generated by phospholipase D. Oncogene. 2006;25:7305–7310. doi: 10.1038/sj.onc.1209735. Epub June 19, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Ishigame H. Nakajima A. Saijo S. Komiyama Y. Nambu A. Matsuki T. Nakae S. Horai R. Kakuta S. Iwakura Y. The role of TNFalpha and IL-17 in the development of excess IL-1 signaling-induced inflammatory diseases in IL-1 receptor antagonist-deficient mice. Ernst Schering Res Found Workshop. 2006:129–153. doi: 10.1007/3-540-37673-9_8. [DOI] [PubMed] [Google Scholar]
- 26.Ishitsuka K. Hideshima T. Hamasaki M. Raje N. Kumar S. Hideshima H. Shiraishi N. Yasui H. Roccaro AM. Richardson P. Podar K. Le GS. Chauhan D. Tamura K. Arbiser J. Anderson KC. Honokiol overcomes conventional drug resistance in human multiple myeloma by induction of caspase-dependent and -independent apoptosis. Blood. 2005;106:1794–1800. doi: 10.1182/blood-2005-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacamo R. Jiang X. Lunn JA. Rozengurt E. FAK phosphorylation at Ser-843 inhibits Tyr-397 phosphorylation, cell spreading and migration. J Cell Physiol. 2007;210:436–444. doi: 10.1002/jcp.20870. [DOI] [PubMed] [Google Scholar]
- 28.Ko CH. Chen HH. Lin YR. Chan MH. Inhibition of smooth muscle contraction by magnolol and honokiol in porcine trachea. Planta Med. 2003;69:532–536. doi: 10.1055/s-2003-40654. [DOI] [PubMed] [Google Scholar]
- 29.Konoshima T. Kozuka M. Tokuda H. Nishino H. Iwashima A. Haruna M. Ito K. Tanabe M. Studies on inhibitors of skin tumor promotion, IX: neolignans from Magnolia officinalis. J Nat Prod. 1991;54:816–822. doi: 10.1021/np50075a010. [DOI] [PubMed] [Google Scholar]
- 30.Kuribara H. Kishi E. Kimura M. Weintraub ST. Maruyama Y. Comparative assessment of the anxiolytic-like activities of honokiol and derivatives. Pharmacol Biochem Behav. 2000;67:597–601. doi: 10.1016/s0091-3057(00)00401-9. [DOI] [PubMed] [Google Scholar]
- 31.Li L. Han W. Gu Y. Qiu S. Lu Q. Jin J. Luo J. Hu X. Honokiol induces a necrotic cell death through the mitochondrial permeability transition pore. Cancer Res. 2007;67:4894–4903. doi: 10.1158/0008-5472.CAN-06-3818. [DOI] [PubMed] [Google Scholar]
- 32.Liou KT. Shen YC. Chen CF. Tsao CM. Tsai SK. The anti-inflammatory effect of honokiol on neutrophils: mechanisms in the inhibition of reactive oxygen species production. Eur J Pharmacol. 2003;475:19–27. doi: 10.1016/s0014-2999(03)02121-6. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y. Chen L. He X. Fan L. Yang G. Chen X. Lin X. DU L. Li Z. Ye H. Mao Y. Zhao X. Wei Y. Enhancement of therapeutic effectiveness by combining liposomal honokiol with cisplatin in ovarian carcinoma. Int J Gynecol Cancer. 2008;18:652–659. doi: 10.1111/j.1525-1438.2007.01070.x. Epub September 24, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Lo YC. Teng CM. Chen CF. Chen CC. Hong CY. Magnolol and honokiol isolated from Magnolia officinalis protect rat heart mitochondria against lipid peroxidation. Biochem Pharmacol. 1994;47:549–553. doi: 10.1016/0006-2952(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 35.Luvisetto S. Basso E. Petronilli V. Bernardi P. Forte M. Enhancement of anxiety, facilitation of avoidance behavior, and occurrence of adult-onset obesity in mice lacking mitochondrial cyclophilin D. Neuroscience. 2008;155:585–596. doi: 10.1016/j.neuroscience.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maruyama Y. Kuribara H. Morita M. Yuzurihara M. Weintraub ST. Identification of magnolol and honokiol as anxiolytic agents in extracts of saiboku-to, an oriental herbal medicine. J Nat Prod. 1998;61:135–138. doi: 10.1021/np9702446. [DOI] [PubMed] [Google Scholar]
- 37.Mattioli E. Vogiatzi P. Sun A. Abbadessa G. Angeloni G. D'Ugo D. Trani D. Gaughan JP. Vecchio FM. Cevenini G. Persiani R. Giordano A. Claudio PP. Immunohistochemical analysis of pRb2/p130, VEGF, EZH2, p53, p16(INK4A), p27(KIP1), p21(WAF1), Ki-67 expression patterns in gastric cancer. J Cell Physiol. 2007;210:183–191. doi: 10.1002/jcp.20833. [DOI] [PubMed] [Google Scholar]
- 38.Munroe ME. Arbiser JL. Bishop GA. Honokiol, a natural plant product, inhibits inflammatory signals and alleviates inflammatory arthritis. J Immunol. 2007;179:753–763. doi: 10.4049/jimmunol.179.2.753. [DOI] [PubMed] [Google Scholar]
- 39.Otley CC. Pittelkow MR. Skin cancer in liver transplant recipients. Liver Transpl. 2000;6:253–262. doi: 10.1053/lv.2000.6352. [DOI] [PubMed] [Google Scholar]
- 40.Ou HC. Chou FP. Sheu WH. Hsu SL. Lee WJ. Protective effects of magnolol against oxidized LDL-induced apoptosis in endothelial cells. Arch Toxicol. 2007;81:421–432. doi: 10.1007/s00204-006-0172-3. [DOI] [PubMed] [Google Scholar]
- 41.Park EJ. Kim SY. Zhao YZ. Sohn DH. Honokiol reduces oxidative stress, c-jun-NH2-terminal kinase phosphorylation and protects against glycochenodeoxycholic acid-induced apoptosis in primary cultured rat hepatocytes. Planta Med. 2006;72:661–664. doi: 10.1055/s-2006-931571. [DOI] [PubMed] [Google Scholar]
- 42.Park EJ. Zhao YZ. Na M. Bae K. Kim YH. Lee BH. Sohn DH. Protective effects of honokiol and magnolol on tertiary butyl hydroperoxide- or D-galactosamine-induced toxicity in rat primary hepatocytes. Planta Med. 2003;69:33–37. doi: 10.1055/s-2003-37027. [DOI] [PubMed] [Google Scholar]
- 43.Shen YC. Sung YJ. Chen CF. Magnolol inhibits Mac-1 (CD11b/CD18)-dependent neutrophil adhesion: relationship with its antioxidant effect. Eur J Pharmacol. 1998;343:79–86. doi: 10.1016/s0014-2999(97)01519-7. [DOI] [PubMed] [Google Scholar]
- 44.Sheu ML. Chiang CK. Tsai KS. Ho FM. Weng TI. Wu HY. Liu SH. Inhibition of NADPH oxidase-related oxidative stress-triggered signaling by honokiol suppresses high glucose-induced human endothelial cell apoptosis. Free Radic Biol Med. 2008;44:2043–2050. doi: 10.1016/j.freeradbiomed.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 45.Sheu ML. Liu SH. Lan KH. Honokiol induces calpain-mediated glucose-regulated protein-94 cleavage and apoptosis in human gastric cancer cells and reduces tumor growth. PLoS ONE. 2007;2:e1096. doi: 10.1371/journal.pone.0001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shigemura K. Arbiser JL. Sun SY. Zayzafoon M. Johnstone PA. Fujisawa M. Gotoh A. Weksler B. Zhau HE. Chung LW. Honokiol, a natural plant product, inhibits the bone metastatic growth of human prostate cancer cells. Cancer. 2007;109:1279–1289. doi: 10.1002/cncr.22551. [DOI] [PubMed] [Google Scholar]
- 47.Squires RF. Saederup E. Additivities of compounds that increase the numbers of high affinity [3H]muscimol binding sites by different amounts define more than 9 GABA(A) receptor complexes in rat forebrain: implications for schizophrenia and clozapine research. Neurochem Res. 2000;25:1587–1601. doi: 10.1023/a:1026666419725. [DOI] [PubMed] [Google Scholar]
- 48.Sukumaran K. Unnikrishnan MC. Kuttan R. Inhibition of tumour promotion in mice by eugenol. Indian J Physiol Pharmacol. 1994;38:306–308. [PubMed] [Google Scholar]
- 49.Syu WJ. Shen CC. Lu JJ. Lee GH. Sun CM. Antimicrobial and cytotoxic activities of neolignans from Magnolia officinalis. Chem Biodivers. 2004;1:530–537. doi: 10.1002/cbdv.200490046. [DOI] [PubMed] [Google Scholar]
- 50.Takeya T. Okubo T. Tobinaga S. Synthesis of unsymmetrical biphenyl lignans, honokiol and related compounds, utilizing quinol-acetates as reactive intermediates. Chem Pharm Bull. 1986;34:2066–2075. [Google Scholar]
- 51.Taniguchi C. Homma M. Takano O. Hirano T. Oka K. Aoyagi Y. Niitsuma T. Hayashi T. Pharmacological effects of urinary products obtained after treatment with saiboku-to, a herbal medicine for bronchial asthma, on type IV allergic reaction. Planta Med. 2000;66:607–611. doi: 10.1055/s-2000-8626. [DOI] [PubMed] [Google Scholar]
- 52.Tsai SK. Huang SS. Hong CY. Myocardial protective effect of honokiol: an active component in Magnolia officinalis. Planta Med. 1996;62:503–506. doi: 10.1055/s-2006-957957. [DOI] [PubMed] [Google Scholar]
- 53.Tse AK. Wan CK. Shen XL. Yang M. Fong WF. Honokiol inhibits TNF-alpha-stimulated NF-kappaB activation and NF-kappaB-regulated gene expression through suppression of IKK activation. Biochem Pharmacol. 2005;70:1443–1457. doi: 10.1016/j.bcp.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 54.Wang T. Chen F. Chen Z. Wu YF. Xu XL. Zheng S. Hu X. Honokiol induces apoptosis through p53-independent pathway in human colorectal cell line RKO. World J Gastroenterol. 2004;10:2205–2208. doi: 10.3748/wjg.v10.i15.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X. Mu H. Chai H. Liao D. Yao Q. Chen C. Human immunodeficiency virus protease inhibitor ritonavir inhibits cholesterol efflux from human macrophage-derived foam cells. Am J Pathol. 2007;171:304–314. doi: 10.2353/ajpath.2007.060965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watanabe H. Watanabe K. Hagino K. Chemostructural requirement for centrally acting muscle relaxant effect of magnolol and honokiol, neolignane derivatives. J Pharmacobiodyn. 1983;6:184–190. doi: 10.1248/bpb1978.6.184. [DOI] [PubMed] [Google Scholar]
- 57.Watanabe K. Watanabe HY. Goto Y. Yamamoto N. Yoshizaki M. Studies on the active principles of magnolia bark: centrally acting muscle relaxant activity of magnolol and honokiol. Jpn J Pharmacol. 1975;25:605–607. doi: 10.1254/jjp.25.605. [DOI] [PubMed] [Google Scholar]
- 58.Withers BE. Hanks SK. Fry DW. Correlations between the expression, phosphotyrosine content and enzymatic activity of focal adhesion kinase, pp125FAK, in tumor and nontransformed cells. Cancer Biochem Biophys. 1996;15:127–139. [PubMed] [Google Scholar]
- 59.Wolf I. O'Kelly J. Wakimoto N. Nguyen A. Amblard F. Karlan BY. Arbiser JL. Koeffler HP. Honokiol, a natural biphenyl, inhibits in vitro and in vivo growth of breast cancer through induction of apoptosis and cell cycle arrest. Int J Oncol. 2007;30:1529–1537. [PubMed] [Google Scholar]
- 60.Xu D. Lu Q. Hu X. Down-regulation of P-glycoprotein expression in MDR breast cancer cell MCF-7/ADR by honokiol. Cancer Lett. 2006;18:274–280. doi: 10.1016/j.canlet.2005.11.031. Epub January 10, 2006. [DOI] [PubMed] [Google Scholar]
- 61.Yamazaki R. Sugatani J. Fujii I. Kuroyanagi M. Umehara K. Ueno A. Suzuki Y. Miwa M. Development of a novel method for determination of acetyl-CoA:1-alkyl-sn-glycero-3-phosphocholine acetyltransferase activity and its application to screening for acetyltransferase inhibitors: inhibition by magnolol and honokiol from magnoliae cortex. Biochem Pharmacol. 1994;47:995–1006. doi: 10.1016/0006-2952(94)90410-3. [DOI] [PubMed] [Google Scholar]
- 62.Zhong M. Shen Y. Zheng Y. Joseph T. Jackson D. Foster DA. Phospholipase D prevents apoptosis in v-Src-transformed rat fibroblasts and MDA-MB-231 breast cancer cells. Biochem Biophys Res Commun. 2003;302:615–619. doi: 10.1016/s0006-291x(03)00229-8. [DOI] [PubMed] [Google Scholar]