Abstract
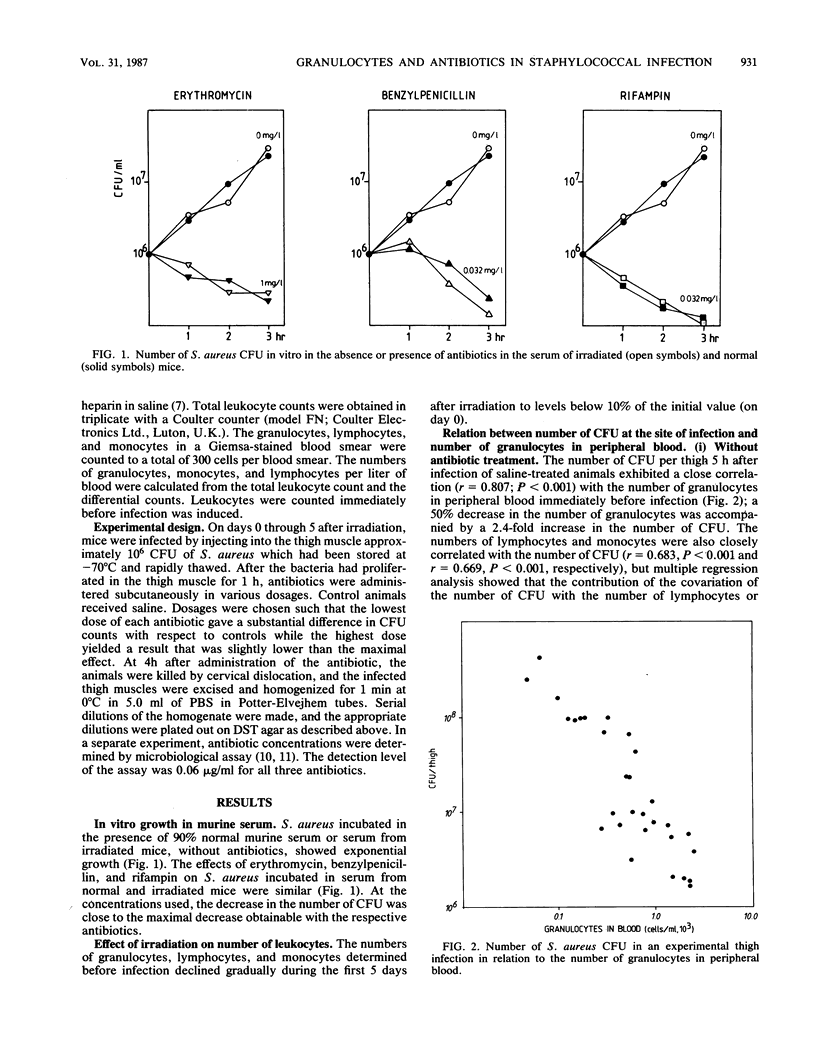
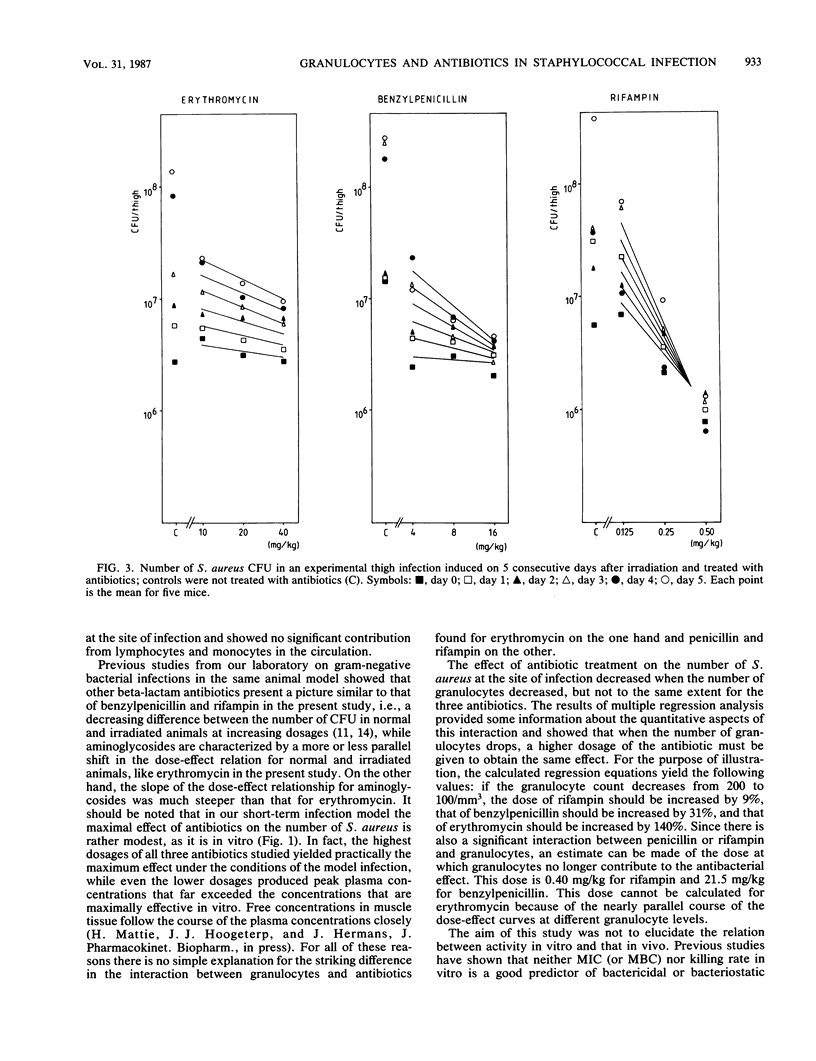
The quantitative relation between granulocytopenia and antibiotic treatment was established for a short-term Staphylococcus aureus infection in the thighs of mice, using rifampin, benzylpenicillin, and erythromycin. Granulocytopenia was induced by total-body irradiation; the number of granulocytes decreased gradually during the first 5 days after irradiation to 10% of the number in normal mice. Experimental infections were established on each of the 5 days after irradiation. In animals not treated with antibiotics, the number of granulocytes in peripheral blood and the number of CFU at the site of infection exhibited a strong negative correlation. The influence of granulocytes on the effect of antibiotics on the number of CFU differed for the three antibiotics. For erythromycin the slope of the dose-effect relation was rather flat, but a decrease in the number of granulocytes caused a significant, nearly parallel, shift in the dose-effect relation, resulting in an increase in the number of CFU. For benzylpenicillin the slope of the dose-effect relation for normal mice was also flat, but as the number of granulocytes decreased the slope became significantly steeper, resulting in a diminishing influence of granulocytes at higher dosages. For rifampin the slope of the dose-effect relation, which was already steep for nonirradiated animals, increased significantly. Here too the effect of granulocytes decreased as the dose increased.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander W. J., Cobbs C. G., Curtiss R., 3rd Modification of bacterial serum susceptibility by rifampin. Infect Immun. 1980 Jun;28(3):923–926. doi: 10.1128/iai.28.3.923-926.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I., MacVittie T. J., Walker R. I. Recovery of aerobic and anaerobic bacteria from irradiated mice. Infect Immun. 1984 Oct;46(1):270–271. doi: 10.1128/iai.46.1.270-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald S. P., Rogers H. J. Bacteriostatic effect of serum: role of antibody to lipopolysaccharide. Infect Immun. 1980 Feb;27(2):302–308. doi: 10.1128/iai.27.2.302-308.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON L. E., RUML D., HAHNE H. J., MILLER C. P. Studies on susceptibility to infection following ionizing radiation. IV. The pathogenesis of the endogenous bacteremias in mice. J Exp Med. 1955 Oct 1;102(4):413–424. doi: 10.1084/jem.102.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst M. W., Mattie H., van Furth R. Antibacterial efficacy of cefazolin and cephradine in neutropenic mice. Infection. 1979;7(1):30–34. doi: 10.1007/BF01640554. [DOI] [PubMed] [Google Scholar]
- MILLER C. P., HAMMOND C. W., TOMPKINS M. The role of infection in radiation injury. J Lab Clin Med. 1951 Sep;38(3):331–343. [PubMed] [Google Scholar]
- Mattie H., Goslings W. R., Noach E. L. Cloxacillin and nafcillin: serum binding and its relationship to antibacterial effect in mice. J Infect Dis. 1973 Aug;128(2):170–177. doi: 10.1093/infdis/128.2.170. [DOI] [PubMed] [Google Scholar]
- Mattie H. Kinetics of antimicrobial action. Rev Infect Dis. 1981 Jan-Feb;3(1):19–27. doi: 10.1093/clinids/3.1.19. [DOI] [PubMed] [Google Scholar]
- Mattie H., van der Voet G. B. The relative potency of amoxycillin and ampicillin in vitro and in vivo. Scand J Infect Dis. 1981;13(4):291–296. doi: 10.3109/inf.1981.13.issue-4.10. [DOI] [PubMed] [Google Scholar]
- Meddens M. J., Thompson J., Mattie H., van Furth R. Role of granulocytes and monocytes in the prevention and therapy of experimental Staphylococcus epidermidis endocarditis in rabbits. J Infect. 1985 Jul;11(1):41–50. doi: 10.1016/s0163-4453(85)90982-x. [DOI] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Voet G. B., Mattie H., van Furth R. Comparison of the antibacterial activity of azlocillin and ticarcillin in vitro and in irradiated neutropenic mice. J Antimicrob Chemother. 1985 Nov;16(5):605–613. doi: 10.1093/jac/16.5.605. [DOI] [PubMed] [Google Scholar]
- van der Voet G. B., Mattie H., van Furth R. Quantitative determination of the effect of granulocytes on the course of experimental infections during antibiotic treatment. Infection. 1984 Jan-Feb;12(1):5–9. doi: 10.1007/BF01641015. [DOI] [PubMed] [Google Scholar]
- van der Voet G. B., Mattie H., van Furth R. The antibacterial activity of combinations of mecillinam and ampicillin in vitro and in normal and granulopenic mice. Scand J Infect Dis. 1983;15(1):91–96. doi: 10.3109/inf.1983.15.issue-1.15. [DOI] [PubMed] [Google Scholar]


