Abstract
Mutations in human CGI-58/ABHD5 cause Chanarin-Dorfman syndrome (CDS), characterized by excessive storage of triacylglycerol in tissues. CGI-58 is an α/β-hydrolase fold enzyme expressed in all vertebrates. The carboxyl terminus includes a highly conserved consensus sequence (HXXXXD) for acyltransferase activity. Mouse CGI-58 was expressed in Escherichia coli as a fusion protein with two amino terminal 6-histidine tags. Recombinant CGI-58 displayed acyl-CoA-dependent acyltransferase activity to lysophosphatidic acid, but not to other lysophospholipid or neutral glycerolipid acceptors. Production of phosphatidic acid increased with time and increasing concentrations of recombinant CGI-58 and was optimal between pH 7.0 and 8.5. The enzyme showed saturation kinetics with respect to 1-oleoyl-lysophosphatidic acid and oleoyl-CoA and preference for arachidonoyl-CoA and oleoyl-CoA. The enzyme showed slight preference for 1-oleoyl lysophosphatidic acid over 1-palmitoyl, 1-stearoyl, or 1-arachidonoyl lysophosphatidic acid. Recombinant CGI-58 showed intrinsic fluorescence for tryptophan that was quenched by the addition of 1-oleoyl-lysophosphatidic acid, oleoyl-CoA, arachidonoyl-CoA, and palmitoyl-CoA, but not by lysophosphatidyl choline. Expression of CGI-58 in fibroblasts from humans with CDS increased the incorporation of radiolabeled fatty acids released from the lipolysis of stored triacylglycerols into phospholipids. CGI-58 is a CoA-dependent lysophosphatidic acid acyltransferase that channels fatty acids released from the hydrolysis of stored triacylglycerols into phospholipids.
Keywords: Chanarin-Dorfman Syndrome, neutral lipid storage disorder, α/β-hydrolase fold enzymes
Most cells of vertebrates have the capacity to store triacylglycerols in cytoplasmic lipid droplets. The hydrolysis of these lipids provides a source of substrates for the synthesis of phospholipids used for membrane repair and expansion, and metabolism to fuel ATP production. In adipocytes, the binding of catecholamines to β-adrenergic receptors at the cell surface triggers a signaling cascade that ultimately activates lipolysis through a complex mechanism requiring the protein kinase A-mediated phosphorylation of multiple proteins, including a major cytosolic lipase named hormone-sensitive lipase and the structural lipid droplet protein perilipin A (1). Additional components of the lipolytic pathway include adipose triglyceride lipase (ATGL, also known as desnutrin), monoglyceride lipase, and CGI-58/ABHD5 (comparative genome identification 58/α/β hydrolase domain 5). Details of how these various components work together are still poorly understood and the subject of active investigation. Even less is known about how lipolysis of stored neutral lipids is catalyzed and controlled in other nonadipose cells.
CGI-58 plays an important role in the maintenance of triacylglycerol homeostasis in many types of cells. Mutations in the gene for CGI-58 cause a neutral lipid storage disorder in humans called Chanarin-Dorfman syndrome (CDS) (2). CDS is characterized by ichthyosis, hepatosteatosis, and hepatomegaly, developmental defects, and the excessive accumulation of triacylglycerols in lipid droplets in many types of cells, including basal keratinocytes, hepatocytes, myocytes, and leukocytes (2–5). CGI-58 is a member of the lipase subfamily of α/β-hydrolase fold enzymes (2). Unlike lipases, CGI-58 lacks a complete catalytic triad; an asparagine residue replaces the usual nucleophilic serine residue within a conserved consensus sequence of GXSXG (where X represents any amino acid). Consistent with this substitution, recombinant CGI-58 shows no lipase activity (6). Nonetheless, experiments conducted in vitro have shown that the addition of CGI-58 significantly increases triacylglycerol hydrolase activity of cell lysates containing the lipase ATGL (6, 7). Based on these observations, it has been proposed that CGI-58 serves as an activating cofactor for ATGL. Recent studies have shown that CGI-58 interacts directly with ATGL through a protein-protein interaction at the surfaces of lipid droplets (8), although the mechanism by which CGI-58 enhances ATGL activity has not yet been elucidated. Moreover, the relative expression of ATGL and CGI-58 differs in various tissues, and humans with mutations in ATGL (9) show distinctly different phenotypic traits than individuals with mutations in CGI-58 (2–5). Specifically, humans with mutations in ATGL do not have ichthyosis but have cardiomyopathies that are not observed in individuals with mutations in CGI-58. These observations suggest that CGI-58 might serve as a coactivator for several different lipases or have more than one activity or function.
The carboxyl terminus of mouse CGI-58 contains an acyltransferase consensus sequence of HXXXXD that is conserved in homologs of CGI-58 from all vertebrate and some invertebrate species (Fig. 1). Hence, we hypothesized that CGI-58 has acyltransferase activity for glycerolipid acceptors. We tested recombinant mouse CGI-58 for acyltransferase activity using a variety of lipid substrates. While this study was in progress, another research group reported acyltransferase activity of recombinant human CGI-58 (10); however, that report lacked complete characterization of the enzyme properties. We have expanded our studies to provide characterization of Michaelis-Menten kinetics of the acyltransferase reaction catalyzed by recombinant mouse CGI-58. Our report contains further novel identification of substrate specificity of CGI-58, pH, and temperature sensitivity, and inhibition of the acyltransferase reaction by detergents and metal ions. Moreover, we have used measurement of the intrinsic fluorescence of tryptophan residues within CGI-58 to characterize the binding of lipid substrates. Finally, we studied the effects of CGI-58 expression on the metabolism of stored triacylglycerols in skin fibroblasts from an individual with CDS.
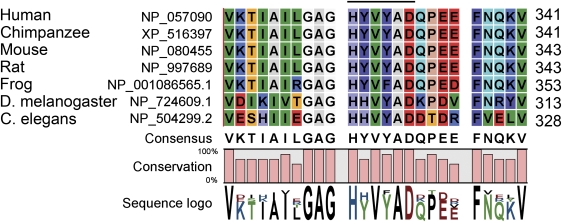
Fig. 1.
Sequence alignment of amino acid sequences of CGI-58 from various species showing the conserved acyltransferase motif. Amino acid sequences for a portion of the carboxyl terminus of CGI-58 are aligned for human (NP_057090), chimpanzee (XP_516397), mouse (NP_080455), rat (NP_997689), frog (Xenopus laevis, NP_001086565.1), Drosophila melanogaster (CG1882, isoform B, NP_724609.1), and Caenorhabditis elegans (hypothetical protein C37H5.2, NP_504299.2) using CLC Genomics Workbench 5.1. The sequences show the conservation of the HXXXXD consensus site for acyltransferase residues, indicated by the over-scoring line. Numbers following each sequence refer to the final residue.
MATERIALS AND METHODS
Materials
MEM and DMEM were obtained from Mediatech, Inc. (Herndon,VA). FBS was purchased from Sigma. Triacsin C was obtained from BIOMOL International (Plymouth Meeting, PA). PfuUltra High-Fidelity DNA polymerase was purchased from Stratagene, Inc. (La Jolla, CA). Unless otherwise noted, all chemicals, scintillation counting supplies, and solvents were purchased from Fisher Scientific or Sigma. [1-14C]Oleoyl-CoA (54 mCi/mmol) and 1-oleoyl [oleoyl-9,10-3H] lysophosphatidic acid (53 Ci/mmol) were purchased from Perkin Elmer Biosciences. [1-14C]oleate (55 mCi/mmol) was obtained from Amersham. Silica gel hard layer fluorescent (HLF) TLC plates were from Analtech (Newark, DE). Oligonucleotide primers were purchased from Operon BioTechnologies, Inc. (Huntsville, AL). Phospholipids and lysophospholipids were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL) and Sigma. The DNA purification kit and nickel-nitrilotriacetic acid (Ni2+-NTA) agarose matrix were purchased from Qiagen. Reagent for protein assays, Coomassie Plus, was purchased from Pierce.
Expression and purification of recombinant mouse CGI-58
A mouse CGI-58 cDNA (11) was used to amplify the coding sequence by PCR in two steps using the following primers: forward primers (5-CATCACCATCACATGGCGGCGGAGGAGG-3) and (5-GTATTCATATGCACCACCATCACCATCACATGGCG-3) and reverse primer (5-GCCAGCGTCGACTCAGTCTACTGTGTGGCAG-3). Amplification was performed using PfuUltra polymerase for 25 cycles with 30 pmol of each primer. This strategy generates a CGI-58 cDNA with a 6-histidine tag on the 5′ end and NdeI and SalI restriction sites at the 5′ and 3′ ends of the amplified sequence, respectively. This cDNA was ligated into the NdeI and SalI sites of the pET-28a(+) bacterial expression vector (Novagen, EMD Chemicals, Inc., Gibbstown, NJ) in frame with the vector's 6-histidine tag sequence. Thus, the CGI-58 fusion protein encoded by this sequence contains a 12-histidine (His12) tag at the N-terminus; use of a 12-histidine tag was for arbitrary reasons. The CGI-58 cDNA was sequenced to confirm fidelity of amplification.
The His12-CGI-58 fusion protein was expressed in BL21 (DE3) cells induced with 1 mM isopropyl-β-D-1-thiogalactopyranoside for 5 h at 37°C, using a previously described protocol (12). All steps for protein purification were performed at 4°C. Bacteria expressing recombinant CGI-58 were lysed in a solution containing 300 mM sodium chloride, 50 mM Tris-HCL, pH 8.0, 20 mM imidazole, with or without protease inhibitors including 10 mg/l leupeptin, 500 μM benzamidine, and 100 μM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, or complete protease inhibitor cocktail without EDTA (Roche Applied Science, no. 11873580001) (Solution I). Cells were disrupted by two passes through a French press at 20,000 pounds/square inch, using a precooled cell; alternatively, cells were disrupted with glass beads in a Bead-Beater from Biospec Products, Inc. (Bartlesville, OK) using an ice-jacketed chamber with 7 cycles of 30 s disruption followed by 2 min cooling on ice. Probe sonication was found to inactivate enzyme activity, even when accompanied by cooling incubations. The loss of enzyme activity following probe sonication may have resulted from either local heating of bacterial lysates, or from shedding of metal ions from the probe. Cell debris was removed by centrifugation at 11,000 × g for 35 min, followed by collection of the supernatant. A stock solution of 10% polyethyleneimine (Sigma) in water (w/v) was added to the supernatant to a final concentration of 0.2% polyethyleneimine; precipitated material was removed by centrifugation at 11,000 × g for 25 min at 4°C. The resulting supernatant was loaded onto a Ni2+-NTA-agarose column equilibrated with Solution I. The column was washed with 100 ml solution I. His12-CGI-58 was eluted from the column in 1 ml fractions using a total of 5 ml of Solution II (Solution I with 250 mM imidazole and 40% glycerol). Enzyme preparations were stored at −20°C.
Analysis of protein
Determinations of protein concentration employed the method of Bradford (13) with BSA used as a standard. Qualitative measurement of protein overexpression in cultured cells and assessment of purity of recombinant proteins employed 10– 12% SDS-PAGE gels (14) followed by Coomassie brilliant blue staining or immunoblotting. Immunoblotting was performed using nitrocellulose membranes, as described previously (11). The His12-CGI-58 fusion protein was detected with a rabbit polyclonal antiserum raised against mouse CGI-58 (11), followed by peroxidase-conjugated goat anti-rabbit IgG (Sigma) and enhanced chemiluminescence reagents (Amersham Biosciences).
Acyltransferase assay
Acyltransferase activity of recombinant CGI-58 was assessed with a variety of lysophospholipid and neutral lipid acceptors. Unless noted otherwise, the reaction mixture contained 1 µg recombinant His12-CGI-58 in 50 mM Tris-HCl buffer, pH 7.5, with 10 µM [1-14C]oleoyl-CoA (70,000–80,000 dpm/nmol), and 50 µM lipid acceptor (including lysophosphatidic acid, lysophosphatidyl choline, lysophosphatidyl ethanolamine, lysophosphatidyl inositol, lysophosphatidyl serine, 1- or 2-monoacylglycerol, 1,2- or 1,3-diacylglycerol, or glycerol-3-phosphate) in a final volume of 100 µL; the reaction mixture was incubated for 10 min at 30°C. Acyltransferase activity was also assayed using a mixture containing 50 µM [1-oleoyl-9,10-3H]lysophosphatidic acid (70,800 dpm/nmol) and 20 µM acyl-CoA or 5 µM oleate. Reactions were terminated by extracting the lipids with 0.1 N HCl in methanol:chloroform:1 M MgCl2 (1:2:2) to partition [3H] or [14C]labeled lipid products into a chloroform phase (15). Lipids were separated by TLC on silica gel HLF plates using chloroform-methanol-acetone-acetic acid-water (50:10:20:15:5, v/v/v/v/v) as the solvent system (16). TLC plates were subjected to autoradiography using a Storm System Phosphorimager (Molecular Dynamics), and spots coeluting with phosphatidic acid (or other phospholipid) standards were scraped and quantified by scintillation counting. Neutral lipids were resolved on TLC plates eluted with hexane-ethyl ether-acetic acid (80:20:2, v/v/v).
When kinetic parameters were measured as a function of pH, the ternary N-(2-acetamido)-2-aminoethanesulfonic acid-Tris-ethanolamine buffer system was used to minimize variations in ionic strength (17).
Measurement of tryptophan quenching
Fluorescence measurements were made on an SLM-8000C spectrofluorometer (Urbana, IL) using an excitation wavelength of 280 nm (1 mm band-width) with emission wavelength monitored from 300 to 400 nm (4 mm band-width). Data were obtained using 3 µM CGI-58 in 20 mM MOPS buffer, pH 7.3, with addition of various concentrations of oleoyl-CoA, palmitoyl-CoA, arachidonoyl-CoA, 1-oleoyl-lysophosphatidic acid, or 1-oleoyl-lysophosphatidyl choline at room temperature.
Studies with human fibroblasts
Human skin fibroblasts from an individual with CDS (18, 19), lacking functional CGI-58, were generously provided by Dr. Rosalind A. Coleman (University of North Carolina, Chapel Hill, NC) and used to study the effects of overexpression of CGI-58 in triacylglycerol turnover. Cells were cultured as described previously (18, 19).
Adenoviral vectors driving the expression of human CGI-58 and β-galactosidase were prepared by ligating the full length coding sequence of the human CGI-58 cDNA or the cDNA for β-galactosidase into the shuttle vector for the AdEasy XL Adenoviral Vector System (Stratagene); the manufacturer's protocols were used for recombination of the shuttle vector to make adenoviral expression vectors and for assembly of virions in cultured AD293 cells. Preliminary experiments in CDS fibroblasts established the titer of crude viral preparations required to produce consistent expression of CGI-58 and β-galactosidase by immunoblotting without evidence of toxicity, judged by microscopic inspection of cells to assess changes in morphology.
CDS cells were incubated with 100 μM [1-14C]oleic acid complexed to BSA at a 4:1 molar ratio for 12 h prior to transduction with adenoviral preparations for either CGI-58 or β-galactosidase; coincident with addition of virus, supplemental fatty acids were removed. At various times, media and cells were collected; cells were solvent extracted to recover lipids (20). Lipids were resolved by TLC on silica gel HLF plates developed in hexane-ethyl ether-acetic acid (80:20:1, v/v/v). Radioactivity was detected and quantified using a Storm System Phosphorimager. Solvent extraction and TLC of media samples revealed that 97% of the radioactivity comigrated with fatty acid standards. Data were analyzed by ANOVA with Bonferroni's posthoc test.
RESULTS
Conservation of a consensus sequence for acyltransferase activity
To deduce potential functions of CGI-58, we aligned the predicted amino acid sequences for vertebrate and invertebrate orthologs of CGI-58 and searched for highly conserved sequences. We observed an invariant consensus sequence for glycerolipid acyltransferase activity of HXXXXD near the carboxyl terminus of all available vertebrate sequences, as well as the sequences of Caenorhabditis elegans and several species of Drosophila (Fig. 1). We then tested recombinant CGI-58 for acyltransferase activity using a variety of lipid acceptors.
Purification of recombinant CGI-58
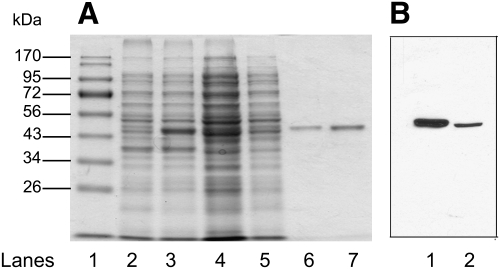
To obtain CGI-58 for in vitro studies of enzyme activity, mouse CGI-58 was expressed in Escherichia coli as a fusion protein with two 6-histidine sequences in tandem. Recombinant His12-CGI-58 was purified over a nickel affinity column. The recovered recombinant His12-CGI-58 was >95% pure by Coomassie staining (Fig. 2A) and migrated in SDS-PAGE gels at an apparent molecular weight of 45 kDa. Immunoblotting of recombinant His12-CGI-58 indicated that the apparent molecular weight of the fusion protein expressed in E. coli was approximately equivalent to the apparent molecular weight of endogenous CGI-58 in cultured mouse 3T3-L1 adipocytes (Fig. 2B); each of these proteins migrated at a higher apparent molecular weight (45 kDa) than that predicted from the amino acid sequence (39.2 kDa for mouse CGI-58). Gel filtration of pure recombinant CGI-58 suggested that the protein elutes as a monomer (data not shown).
Fig. 2.
Purification of recombinant CGI-58. Recombinant His12-CGI-58 was expressed in E. coli and purified over Ni2+-NTA-agarose. A: Coomassie Blue-stained SDS-PAGE gel showing molecular weight markers (lane 1), uninduced E. coli lysate (lane 2), isopropyl-β-D-1-thiogalactopyranoside-induced bacterial lysate (lane 3), supernatant following cell disruption by French press (lane 4), supernatant from the polyethyleneimine precipitation (lane 5), and eluant from Ni2+-NTA-agarose (lanes 6 and 7). B: Immunoblot of 3T3-L1 adipocyte lysate (lane 1) and eluant from Ni2+-NTA- agarose (lane 2) probed with polyclonal antiserum against mouse CGI-58.
CGI-58 displays CoA-dependent acyltransferase activity to lysophosphatidic acid
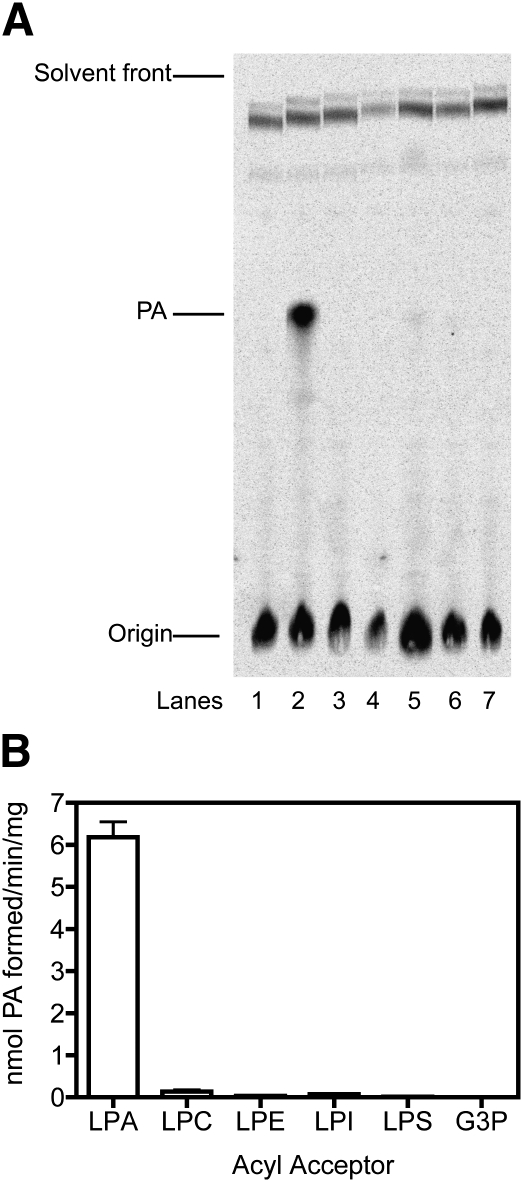
Recombinant His12-CGI-58 was used to examine acyltransferase activity to a variety of glycerolipid acceptors. His12-CGI-58 was added to reactions containing [14C]oleoyl-CoA and unlabeled lipid acceptors, including lysophosphatidic acid, lysophosphatidyl choline, lysophosphatidyl ethanolamine, lysophosphatidyl inositol, lysophosphatidyl serine, and glycerol-3-phosphate. Additional lipid acceptors with amino groups were tested, including sphingosine, sphinganine, phytosphingosine, and phosphatidyl ethanolamine. The reaction mixtures were solvent-extracted and the component lipids were resolved by TLC. Formation of products was observed using phosphorimaging and comparison of the migration of products formed to lipid standards. The only detectable product formed was phosphatidic acid when lysophosphatidic acid was added to the reaction mixture (Fig. 3), indicating that recombinant CGI-58 has lysophosphatidic acid acyltransferase activity but does not use the other lipids as substrates under the conditions of the assay. Additionally, neutral lipid precursors, including 1-monoacylglycerol, 2-monoacylglycerol, 1,2- diacylglycerol, and a mixture of 1,2-diacylglycerol and 1,3-diacylglycerol, were tested as substrates with radiolabeled oleoyl-CoA; no significant radioactivity was recovered in either diacylglycerol or triacylglycerol. Finally, CGI-58 was tested for a reverse reaction, or transacylase activity, by adding recombinant CGI-58 to reaction mixtures containing [32P]labeled phosphatidic acid as an acyl group donor and either lysophosphatidyl choline or 1-monoacylglycerol as acceptor lipids; appearance of [32P]labeled lysophosphatidic acid was used as an endpoint to represent acyl group transfer away from phosphatidic acid. No evidence of acyl group removal was observed.
Fig. 3.
CGI-58 shows lysophosphatidic acid acyltransferase activity. Recombinant His12-CGI-58 (1 μg) was incubated with 10 μM [14C]oleoyl-CoA in the presence of various lipid acceptors (each at 50 μM) and 50 mM Tris-HCl, pH 7.5, for 10 min at 30°C prior to solvent extraction and TLC of extracted lipids. A: Phosphorimager scan of silica gel TLC plate showing radioactive solvent-extracted lipids obtained without addition of recombinant CGI-58 (lane 1) or with addition of CGI-58 to reactions with lysophosphatidic acid (lane 2, LPA), lysophosphatidyl choline (lane 3, LPC), lysophosphatidyl ethanolamine (lane 4, LPE), lysophosphatidyl serine (lane 5, LPS), lysophosphatidyl inositol (lane 6, LPI), or glycerol-3-phosphate (lane 7, G3P) as acceptors. Radioactive phosphatidic acid (PA) formed in lane 2 coeluted with an unlabeled phosphatidic acid standard transiently observed following iodine staining. B: Histogram of enzyme activity of lanes depicted in A. Data represent means ± SD for duplicate samples from one of two experiments.
Characterization of the lysophosphatidic acid acyltransferase activity of CGI-58
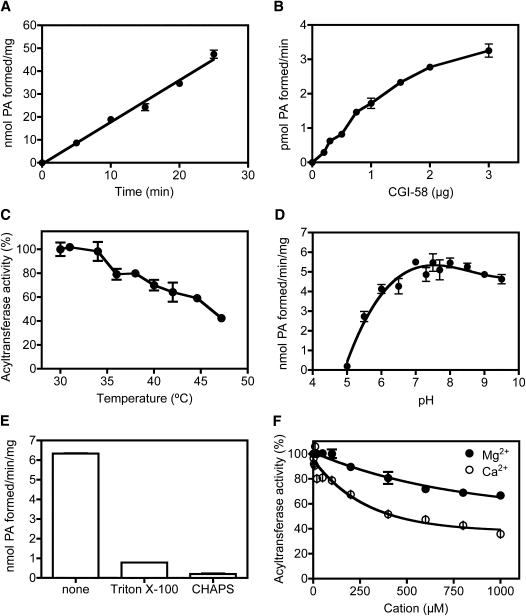
Formation of phosphatidic acid from [14C]oleoyl-CoA and 1-oleoyl lysophosphatidic acid increased with time of the reaction to 25 min (Fig. 4A) and with increasing concentration of recombinant CGI-58 in a dose-dependent manner (Fig. 4B). Although most reactions were carried out at 30°C, incubation of the reaction at 37°C slightly increased formation of phosphatidic acid over 25 min when compared with reactions at 30°C (data not shown). Enzyme activity of recombinant CGI-58 was sensitive to elevated temperature. Preincubation of the enzyme for 10 min at temperatures above 35°C decreased acyltransferase activity subsequently measured at 30°C (Fig. 4C); enzyme activity was reduced by approximately 60% following 10 min preincubation at 47°C. The reaction displayed a broad pH optimum between 7.0 and 8.5 (Fig. 4D). Subsequent reactions were carried out at pH 7.5. The reaction was inhibited by the addition of 0.4 mM Triton X-100 or 6 mM CHAPS (Fig. 4E), indicating that care should be taken when using these detergents to emulsify lipid substrates. For kinetics experiments, lipid substrates were added to the reaction mixtures at concentrations below the critical micellar concentrations of the compounds (21–26); detergents were not used to emulsify lipids. Acyltransferase activity was also inhibited by the presence of magnesium and calcium (Fig. 4F); addition of 1 μM MgCl2 or 1μM CaCl2 inhibited formation of phosphatidic acid by approximately 35 or 60%, respectively. Addition of EDTA to reaction mixtures restored acyltransferase activity that was inhibited by divalent cations (data not shown).
Fig. 4.
Factors affecting lysophosphatidic acid acyltransferase activity of recombinant CGI-58. Recombinant His12-CGI-58 (1 μg) was added to reaction mixtures containing 10 μM [14C]oleoyl-CoA and 50 μM 1-oleoyl-lysophosphatidic acid in 50 mM Tris-HCl, pH 7.5, unless otherwise noted. Reaction mixtures were extracted with solvents and phosphatidic acid was separated from other lipids by TLC. Phosphorimaging and scintillation counting of scrapings from silica gel plates were used to quantify phosphatidic acid formation. A: Time dependence of phosphatidic acid formation. His12-CGI-58 was added to the reaction mixture and samples were incubated at 30°C for the indicated times. Data represent means ± SD for three samples in one representative experiment out of four. Error bars that are not visible are contained within the symbol. B: Enzyme concentration dependence of phosphatidic acid formation: various amounts of His12-CGI-58 were added to reaction mixtures, which were then incubated at 30°C for 10 min. Data represent means ± SD for duplicate samples for one out of two experiments. C: Recombinant His12-CGI-58 (0.5 μg) was incubated at various temperatures for 10 min prior to addition of [14C]oleoyl CoA (10 μM) and lysophosphatidic acid (50 μM) in 50 mM Tris-HCl, pH 7.5 and further incubation at 30°C for 10 min. Data represent mean ± SD for duplicate samples from one of two experiments; error bars that are not visible are contained within the symbol. D: Determination of optimal pH for phosphatidic acid formation. His12-CGI-58 was added to the reaction mixture in solutions buffered with N-(2-acetamido)-2-aminoethanesulfonic acid, Tris, or ethanolamine between pH 5.0 and 9.5. Reactions were incubated at 30°C for 10 min. Data represent means ± SD for triplicate samples for one out of four experiments. E: Detergent inhibition of phosphatidic acid formation. His12-CGI-58 was added to reaction mixtures containing no additions (none), 0.4 mM Triton X-100, or 6 mM CHAPS, and samples were incubated at 30°C for 10 min. Data represent means ± SD for triplicate samples for one out of three experiments. F: Metal ion inhibition of phosphatidic acid formation: 0.5 μg His12-CGI-58 was added to reaction mixtures containing various concentrations of magnesium chloride (•), or calcium chloride (○) up to 1 μM. Acyltransferase activity is expressed as percent of maximal activity in the absence of added metal ions. Data represent means ± SD for duplicate samples from one out of two experiments.
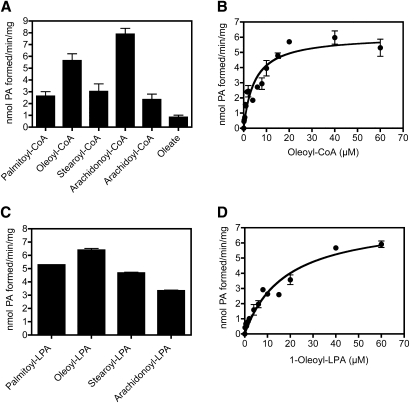
Preference of recombinant CGI-58 for species of substrate lipids was tested. Individual acyl group donors were tested as CoA compounds with 1-oleoyl-lysophosphatidic acid as the acceptor lipid. Arachidonoyl-CoA was the best substrate for the formation of phosphatidic acid followed by oleoyl-CoA (Fig. 5A); palmitoyl-CoA, stearoyl-CoA, and arachidoyl-CoA were relatively poor substrates for the reaction. The concentration of palmitoyl-CoA tested was below the reported critical micellar concentration of 30–40 μM (21, 22, 25, 26); however, it is possible that low reactivity of CGI-58 toward stearoyl-CoA and arachidoyl-CoA was due to low solubility of these lipids in the reaction mixture. Moreover, the combination of the lysophosphatidic acid acceptor and acyl-CoA may have reduced the solubility of saturated species of acyl-CoA. Extremely low acyltransferase activity was observed when oleate was supplied as a substrate; this observation suggests that CGI-58 is a CoA-dependent lysophosphatidic acid acyltransferase. Species of lysophosphatidic acid were tested with oleoyl CoA as the acyl group donor. 1-Oleoyl-lysophosphatidic acid was a slightly better substrate for the acyltransferase reaction than 1-palmitoyl-lysophosphatidic acid, 1-stearoyl-lysophosphatidic acid, or 1-arachidonoyl-lysophosphatidic acid (Fig. 5C).
Fig. 5.
Substrate preference and kinetics of phosphatidic acid formation by recombinant CGI-58. Lysophosphatidic acid acyltransferase activity of recombinant His12-CGI-58 was studied. A: Acyl CoA specificity of phosphatidic acid formation. 0.8 μg His12-CGI-58 was added to reaction mixtures containing 50 μM [3H]1-oleoyl-lysophosphatidic acid and 20 μM palmitoyl-CoA, oleoyl-CoA, stearoyl-CoA, arachidonoyl-CoA, or arachidoyl-CoA, or 5 μM oleate in 50 mM Tris-HCl, pH 7.5. Reactions were incubated at 30°C for 10 min. Recombinant His12-CGI-58 showed dependence upon thioesterified fatty acids with arachidonoyl-CoA>oleoyl-CoA, low reactivity with palmitoyl-CoA, stearoyl-CoA, or arachidoyl-CoA, and very low reactivity with oleate. Data represent means ± SD for averaged values from three experiments, each with triplicate samples. B: Saturation kinetics for phosphatidic acid formation with varying concentrations of oleoyl-CoA. One microgram His12-CGI-58 was added to 75 μM 1-oleoyl-lysophosphatidic acid with varying concentrations of [14C]oleoyl-CoA in 50 mM Tris-HCl, pH 7.5, and the reaction mixture was incubated at 30°C for 10 min. Data represent means ± SD for triplicate samples for one out of two experiments. Calculated values for Km = 4.8 ± 0.9 μM and Vmax = 6.1 ± 0.5 nmol/min/mg. C: Lysophosphatidic acid specificity of phosphatidic acid formation. A total of 0.8 μg His12-CGI-58 was added to reaction mixtures containing 10 μM [14C]oleoyl-CoA, and 50 μM 1-palmitoyl-lysophosphatidic acid, 1-oleoyl-lysophosphatidic acid, 1-stearoyl-lysophosphatidic acid, or 1-arachidonoyl lysophosphatidic acid in 50 mM Tris-HCl, pH 7.5. Reactions were incubated at 30°C for 10 min. Data represent means ± SD of averaged values from two experiments, each with triplicate samples. D: Saturation kinetics for phosphatidic acid formation with varying concentrations of 1-oleoyl-lysophosphatidic acid. A total of 0.8 μg His12-CGI-58 was added to 20 μM-oleoyl CoA with varying concentrations of [3H]1-oleoyl-lysophosphatidic acid and the reaction mixture was incubated at 30°C for 10 min. Data represent means ± SD for triplicate samples for one out of two experiments. Calculated values for Km = 18 ± 3 μM and Vmax = 7.6 ± 0.7 nmol/min/mg.
The lysophosphatidic acid acyltransferase reaction showed saturation kinetics when increasing concentrations of oleoyl-CoA were added to a single concentration of 1-oleoyl-lysophosphatidic acid (75 μM). Analysis of the data using the Michaelis-Menten equation yielded an apparent Km = 4.8 ± 0.9 μM with a Vmax = 6.1 ± 0.5 nmol/min/mg (Fig. 5B). The reaction was also saturable with respect to 1-oleoyl-lysophosphatidic acid at a single concentration of oleoyl-CoA (20 μM); the apparent Km = 18 ± 3 μM and Vmax = 7.6 ± 0.7 nmol/min/mg (Fig. 5D).
CGI-58-mediated formation of phosphatidic acid was not inhibited by the addition of various concentrations of either lysophosphatidy l choline (from 5 to 50 μM) or 2-monoacylglycerol (from 0.5 to 10 μM) to the reaction mixture (data not shown). Neither of these lipids was found to be an alternative substrate for the acyltransferase activity of recombinant mouse CGI-58, nor did they serve as competitive inhibitors for lysophosphatidic acid.
Intrinsic fluorescence of tryptophan residues in CGI-58
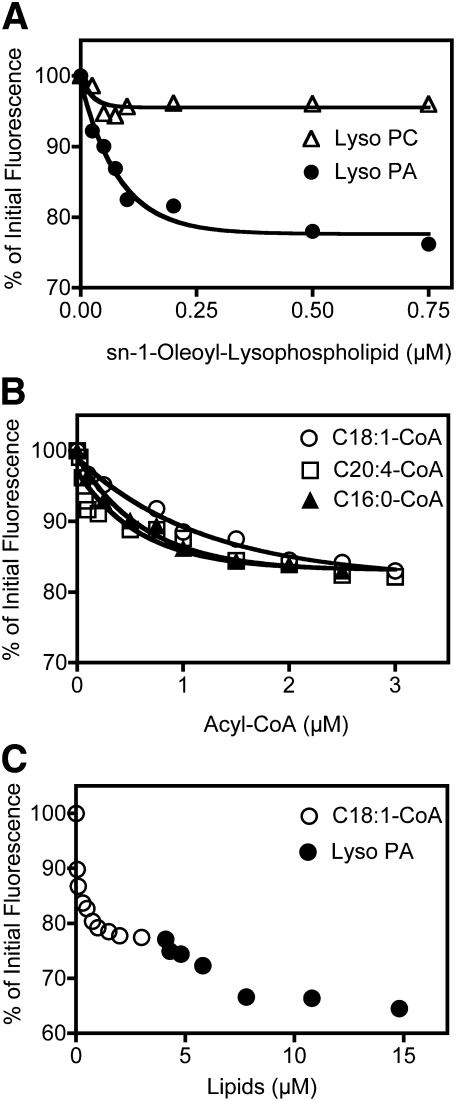
The amino acid sequence of mouse CGI-58 includes nine tryptophan residues. Accordingly, recombinant CGI-58 displays strong intrinsic fluorescence due to tryptophan residues, with maximum fluorescence emission at approximately 332 nm. Various lipids were tested for the ability to bind to CGI-58, and in doing so, alter the fluorescence of tryptophan. Tryptophan fluorescence of CGI-58 was quenched by the addition of substrate lipids; for each of the substrate lipids tested, the wavelength of tryptophan emission was unaltered upon addition of increasing concentrations of the lipid. 1-Oleoyl-lysophosphatidic acid quenched ∼24% of the tryptophan signal, yielding an apparent dissociation constant of Kd = 0.063 ± 0.009 μM (Fig. 6A). In contrast, 1-oleoyl-lysophosphatidyl choline had minimal effects on the tryptophan fluorescence of recombinant CGI-58 (Fig. 6A). Arachidonoyl-CoA, oleoyl-CoA, and palmitoyl-CoA each quenched ∼17% of tryptophan fluorescence with apparent dissociation constants of Kd = 0.16 ± 0.03 μM, Kd = 1.08 ± 0.02 μM, and Kd = 0.62 ± 0.02 μM, respectively (Fig. 6B). When oleoyl-CoA and 1-oleoyl-lysophosphatidic acid were added sequentially to recombinant CGI-58, a pattern of additive quenching of ∼36% of the initial fluorescence was observed (Fig. 6C); these data suggest that acyl-CoA and lysophosphatidic acid bind to separate sites on CGI-58.
Fig. 6.
The intrinsic fluorescence of tryptophan residues in CGI-58 is quenched following addition of substrate lipids. Intrinsic fluorescence of 3 μM His12-CGI-58 in 20 mM MOPS, pH 7.3, was measured using an excitation wavelength of 280 nm and monitoring emission wavelengths from 300 to 400 nm. Quenching of fluorescence was measured at increasing concentrations of (A) 1-oleoyl-lysophosphatidic acid (•), 1-oleoyl-phosphatidyl choline (Δ), (B) oleoyl-CoA (○), arachidonoyl-CoA (□), or palmitoyl-CoA (▴), and (C) oleoyl-CoA (○) followed by 1-oleoyl-lysophosphatidic acid (•). Data shown are representative of two or three experiments.
Expression of CGI-58 in CDS fibroblasts increases partitioning of fatty acids released from triacylglycerol hydrolysis to phospholipids
Fibroblasts from a human with CDS (18, 19) that lack functional CGI-58 and store excessive triacylglycerol under normal culture conditions were used to study effects of the expression of CGI-58 on cellular lipid metabolism. CDS cells were incubated with 100 μM [14C]oleic acid to label cellular lipids; under the conditions of incubation, ∼75% of the radiolabeled oleic acid was incorporated into triacylglycerols, with 23% of the label in phospholipids and 2% in cholesterol esters. Following lipid loading, adenoviruses driving the expression of CGI-58 or a control protein (β-galactosidase) were added to the cells in the absence of supplemental fatty acids.
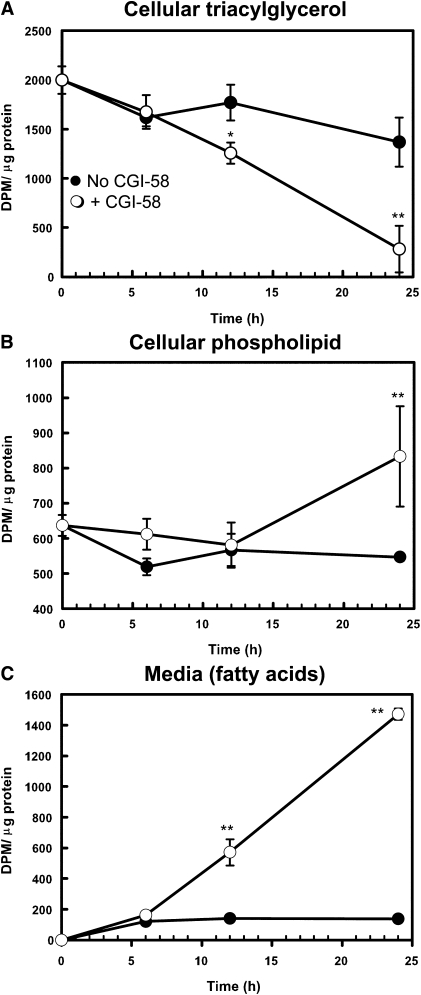
Alterations in the metabolism of radiolabeled triacylglycerol were observed following initiation of expression of CGI-58. The expression of CGI-58 and β-galactosidase was observed by 6 h incubation of cells (data not shown). When CGI-58 was expressed in CDS cells, the majority of radiolabeled triacylglycerol was hydrolyzed within 24 h of incubation (Fig. 7A). In contrast, in cells expressing β-galactosidase, radiolabeled triacylglycerol decreased by only 25% over 24 h. Additionally, within 24 h of expression of ectopic human or mouse CGI-58, the mass of triacylglycerol within CDS fibroblasts was normalized to typically low levels of triacylglycerol observed in normal human fibroblasts (data not shown). In cells expressing CGI-58, a small but reproducible increase in the incorporation of radiolabeled oleate into phospholipids was consistently observed at 24 h that was absent in cells lacking functional CGI-58 (Fig. 7B). The majority of radiolabeled oleate released from the hydrolysis of triacylglycerols was secreted into the culture medium as free fatty acids whether or not CGI-58 was expressed (Fig. 7C), although significantly more fatty acid was secreted when CGI-58 was expressed. The observed increase in fatty acids secreted into the culture medium is consistent with increased hydrolysis of triacylglycerols in cells expressing CGI-58. When triacsin C, an inhibitor of acyl CoA synthetase, was added to the culture medium of cells expressing CGI-58 during the chase period, the incorporation of radiolabeled fatty acids into phospholipid decreased, while radiolabeled fatty acids in the culture medium increased (data not shown). These data suggest that thioesterification of radiolabeled fatty acids by a triacsin-C-sensitive acyl CoA synthetase is required prior to incorporation of fatty acids released during lipolysis into phospholipids.
Fig. 7.
Expression of CGI-58 in CDS fibroblasts increased the rate of triacylglycerol turnover and increased the incorporation of radiolabeled fatty acids released from triacylglycerol hydrolysis into cellular phospholipids. CDS fibroblasts were loaded with 100 μM [14C]oleate to increase radiolabeled triacylglycerol content of cells, followed by withdrawal of supplemental fatty acids and addition of adenoviral vectors to drive the expression of CGI-58 (○) or β-galactosidase (•) at time = 0 h. Data are means ± SD for triplicate samples from one representative experiment out of two; data were analyzed by ANOVA with Bonferroni's posthoc test. A: Radioactivity in cellular triacylglycerols relative to cellular protein over time of the incubation following the addition of adenoviral expression vectors. Cells expressing CGI-58 hydrolyzed significantly more triacylglycerol at 12 h (*, P < 0.01) and 24 h (**, P < 0.001) than control cells expressing β-galactosidase. B: Radioactivity in cellular phospholipids relative to cellular protein over time of the incubation. Cells expressing CGI-58 incorporated significantly more radiolabeled oleate into phospholipids at 24 h (**, P < 0.001) than control cells expressing β-galactosidase. C: Radioactivity released into the culture medium (shown relative to cellular protein content) was primarily fatty acids throughout the incubation. Cells expressing CGI-58 secreted significantly more radiolabeled oleate into the culture medium at 12 and 24 h (**, P < 0.001) than control cells expressing β-galactosidase. Where error bars are not visible, they are contained within the symbol.
DISCUSSION
Herein, we characterize the enzyme properties of recombinant mouse CGI-58, a CoA-dependent lysophosphatidic acid acyltransferase (EC 2.3.1.51). CGI-58 efficiently acylated lyosphosphatidic acid in a time- and enzyme concentration-dependent manner, but failed to show activity toward other lysophospholipid or glycerolipid acceptors. CGI-58 displayed saturation kinetics with respect to both 1-oleoyl-lysophosphatidic acid and oleoyl-CoA and showed preference for unsaturated species of acyl-CoA, arachidonoyl-CoA, and oleoyl-CoA, relative to saturated species of acyl-CoA. The enzyme displayed a slight preference for 1-oleoyl-lysophosphatidic acid over species of lysophosphatidic acid with saturated fatty acids in the sn-1 position. The enzyme was inhibited by detergents, divalent cations, acidic pH, and preincubation at elevated temperatures.
Our study of recombinant mouse CGI-58 is in agreement with prior observations regarding specificity of the reactivity of recombinant human CGI-58 for the acylation of lysophosphatidic acid (10) but fails to confirm inhibition of enzyme activity by lysophosphatidyl choline that was observed in this previous study. Moreover, we failed to detect lysophosphatidyl choline binding to mouse CGI-58 through assessment of changes in the intrinsic tryptophan fluorescence of the enzyme. These differences could reflect species-specific differences in CGI-58 activity. Additionally, we provide new information on the enzyme properties of CGI-58 and the effects of CGI-58 expression on fatty acid channeling between triacylglycerols and phospholipids in cultured human fibroblasts.
CGI-58 is the first lysophospholipid acyltransferase to be described that is a member of the α/β hydrolase fold family of enzymes (pfam00561). The primary amino acid sequence lacks resemblance to previously characterized acyltransferases within the pfam 1553 family of acyltransferases, which includes four isoforms of glycerol-3-phosphate acyltransferases, two isoforms of 1-acylglycerol-3-phosphate-O-acyltransferases possessing the same general activity of CGI-58, and several other lysophospholipid acyltransferases (27–32). CGI-58 also lacks sequence similarity to the pfam03062 family of membrane-bound O-acyltransferases, which includes four lysophospholipid acyltransferases, acyl CoA:diacylglycerol acyltransferase 1, and acyl CoA:cholesterol acyltransferases 1 and 2 (29, 30, 33, 34). Although CGI-58 lacks the majority of sequence motifs that characterize these enzymes with similar functions, it retains an acyltransferase consensus sequence of HXXXXD [this study and (10)], shown to be important for catalysis in some acyltransferases (28, 30, 35). Further experimentation is required to determine whether these amino acids comprise the catalytic diad. The maximal activity of recombinant mouse CGI-58 is comparable to that of other glycerolipid acyltransferases within the various enzyme families (28, 33), although most of these membrane-bound enzymes have not been purified to homogeneity. The enzyme with the highest sequence similarly to CGI-58 is ABHD4, which has recently been demonstrated to have phospholipase B and lysophospholipase activity toward N-acyl phosphatidylethanolamines and N-acyl lysophosphatidylethanolamines to produce precursors for endocannabinoid signaling lipids (36). ABHD4 sequences from multiple species have the conserved carboxyl terminal HXXXXD consensus sequences, but, unlike CGI-58, also have conserved GXSXG consensus sequences harboring the putative nucleophilic serine residue for lipase activity.
CGI-58 displayed strong tryptophan fluorescence that was quenched by the addition of substrate lipids; quenching of tryptophan fluorescence was used to calculate apparent dissociation constants for species of acyl-CoA in the micromolar range and ∼60 nmol/L for 1-oleoyl-lysophosphatidic acid. Additive quenching of intrinsic tryptophan fluorescence supported the conclusion that the two substrate lipids bind to separate regions of the active site of the enzyme; however, relatively high concentrations of lysophosphatidic acid were required to observe additive tryptophan quenching in the presence of oleoyl-CoA. Measurements of tryptophan fluorescence were made using substrate lipid concentrations below the critical micellar concentrations for those lipids to avoid the use of detergent micelles of lipids. The stoichiometry of substrate lipid binding to recombinant CGI-58 was ≤1, suggesting that the protein does not have multiple binding sites for these lipids. Coupled with elution of the protein from a gel filtration column as a monomer, these data suggest that CGI-58 is most likely active as a monomer. Solution of the structure of CGI-58 is required to identify the position of tryptophan residues relative to substrate binding sites; at this time, it is unclear whether quenching of tryptophan fluorescence is due to the proximity of substrates to tryptophan residues or conformational changes in the protein upon substrate binding.
Expression of human CGI-58 in cultured human CDS fibroblasts that lack functional CGI-58 and store excessive triacylglycerols ablated excessive triacylglycerol storage and increased the rate of turnover of radiolabeled triacylglycerol. The majority of the released fatty acids were secreted into the culture medium; however, ectopic expression of CGI-58 significantly increased incorporation of fatty acids into cellular phospholipids. The effect of CGI-58 expression on increased turnover of triacylglycerol in CDS fibroblasts was previously described (6); however, the fate of fatty acids released during lipolysis was not reported for these cells. Additionally, slight reductions in cellular triacylglycerol were observed when CGI-58 was overexpressed in COS-1, McA RH7777 hepatoma cells, and HepG2 hepatoma cells (37), which presumably express endogenous CGI-58. In contrast to our results with CDS fibroblasts, when CGI-58-GFP was expressed in CHO-K1 fibroblasts, radiolabeled triacylglycerols were reduced, but there was no increase in radiolabeled fatty acid incorporation into phospholipids (37). Reasons for these differences are unclear; however, CHO-K1 cells express endogenous CGI-58 (38), and effects of CGI-58 overexpression in cultured cells have been modest (37). Because triacsin C inhibited the flux of radiolabeled fatty acids into phospholipids when CGI-58 was expressed, either acyl CoA synthetase 1 or 4 likely thioesterifies fatty acids following hydrolysis of triacylglycerols (39–41). Previous studies in CDS cells have established that there are no major changes in the content or composition of phospholipids in membranes of CDS fibroblasts (18, 19); thus, CGI-58 most likely does not play a major role in cellular phospholipid synthesis or homeostasis. Furthermore, the expression of CGI-58 in most cells and tissues is extremely low (11), yet the phenotypic traits of humans with CDS suggest that CGI-58 nevertheless plays a critical role in triacylglycerol homeostasis in many tissues. We suggest that CGI-58 may act at the lipid droplet (11, 42, 43) to channel fatty acids released from hydrolysis of triacylglycerols into phospholipids, perhaps as a way of reducing end-product inhibition of neutral lipid lipases. Moreover, the apparent selectivity of CGI-58 for arachidonoyl-CoA suggests that CGI-58 may play a role in modulating substrate availability for the production of eicosanoid signaling lipids. Finally, the production of phosphatidic acid or reduction of lysophosphatidic acid through the enzyme activity of CGI-58 may play an important role in signaling functions of these lipids.
CGI-58 plays an important role in synergizing triacylglycerol hydrolysis catalyzed by ATGL (6, 7, 44). It is unclear whether this role of CGI-58 requires lysophosphatidic acid acyltransferase activity or is a separate function mediated by a protein-protein interaction between CGI-58 and the lipase (8). Retention of lysophosphatidic acid acyltransferase activity in recombinant human CGI-58 harboring Q130P and E260Q mutations (10) that cause CDS suggests that CGI-58 may serve two separate functions; however, mouse CGI-58 with mutations at these sites is not recruited to lipid droplets (42), unlike native CGI-58. Thus, mutations in CGI-58 that alter subcellular localization of the protein may eliminate CGI-58 function in maintenance of triacylglycerol homeostasis. Additional experimentation is required to elucidate potential dual functions of CGI-58 in lipid metabolism and homeostasis.
Acknowledgments
The authors thank Dr. Rosalind A. Coleman for the generous donation of the CDS cell line. We thank Deanna Russell and Dr. Jeanelle Morgan for expert technical assistance and helpful discussions.
Footnotes
Abbreviations:
- ATGL
- adipose triglyceride lipase
- CDS
- Chanarin-Dorfman syndrome
- HLF
- hard layer fluorescent
- NTA
- nitrilotriacetic acid
This work is supported by National Institutes of Health Grant DK-054797 and an Established Investigator Award from the American Heart Association (to D. L. B.), postdoctoral fellowships from the American Heart Association (to J. M. C. and Z. X.), National Institutes of Health Grant DK-038389 (to J. S.), and National Institutes of Health Grant GM-28140 (to G. M. C.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Brasaemle D. L.2007. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 48: 2547–2559 [DOI] [PubMed] [Google Scholar]
- 2.Lefevre C., Jobard F., Caux F., Bouadjar B., Karaduman A., Heilig R., Lakhdar H., Wollenberg A., Verret J. L., Weissenbach J., et al. 2001. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am. J. Hum. Genet. 69: 1002–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Igal R. A., Rhoads J. M., Coleman R. A. 1997. Neutral lipid storage disease with fatty liver and cholestasis. J. Pediatr. Gastroenterol. Nutr. 25: 541–547 [DOI] [PubMed] [Google Scholar]
- 4.Srebrnik A., Brenner S., Ilie B., Messer G. 1998. Dorfman-Chanarin syndrome: morphologic studies and presentation of new cases. Am. J. Dermatopathol. 20: 79–85 [DOI] [PubMed] [Google Scholar]
- 5.Wollenberg A., Geiger E., Schaller M., Wolff H. 2000. Dorfman-Chanarin syndrome in a Turkish kindred: conductor diagnosis requires analysis of multiple eosinophils. Acta Derm. Venereol. 80: 39–43 [DOI] [PubMed] [Google Scholar]
- 6.Lass A., Zimmermann R., Haemmerle G., Riederer M., Schoiswohl G., Schweiger M., Kienesberger P., Strauss J. G., Gorkiewicz G., Zechner R. 2006. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 3: 309–319 [DOI] [PubMed] [Google Scholar]
- 7.Schweiger M., Schreiber R., Haemmerle G., Lass A., Fledelius C., Jacobsen P., Tornqvist H., Zechner R., Zimmermann R. 2006. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J. Biol. Chem. 281: 40236–40241 [DOI] [PubMed] [Google Scholar]
- 8.Granneman J. G., Moore H. P., Granneman R. L., Greenberg A. S., Obin M. S., Zhu Z. 2007. Analysis of lipolytic protein trafficking and interactions in adipocytes. J. Biol. Chem. 282: 5726–5735 [DOI] [PubMed] [Google Scholar]
- 9.Fischer J., Lefevre C., Morava E., Mussini J. M., Laforet P., Negre-Salvayre A., Lathrop M., Salvayre R. 2007. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat. Genet. 39: 28–30 [DOI] [PubMed] [Google Scholar]
- 10.Ghosh A. K., Ramakrishnan G., Chandramohan C., Rajasekharan R. 2008. CGI-58, the causative gene for Chanarin-Dorfman syndrome, mediates acylation of lysophosphatidic acid. J. Biol. Chem. 283: 24525–24533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subramanian V., Rothenberg A., Gomez C., Cohen A. W., Garcia A., Bhattacharyya S., Shapiro L., Dolios G., Wang R., Lisanti M. P., et al. 2004. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3–L1 adipocytes. J. Biol. Chem. 279: 42062–42071 [DOI] [PubMed] [Google Scholar]
- 12.Montero-Moran G. M., Li M., Rendon-Huerta E., Jourdan F., Lowe D. J., Stumpff-Kane A. W., Feig M., Scazzocchio C., Hausinger R. P. 2007. Purification and characterization of the FeII- and alpha-ketoglutarate-dependent xanthine hydroxylase from Aspergillus nidulans. Biochemistry. 46: 5293–5304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradford M. M.1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254 [DOI] [PubMed] [Google Scholar]
- 14.Laemmli U. K.1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227: 680–685 [DOI] [PubMed] [Google Scholar]
- 15.Han G. S., Carman G. M. 2004. Assaying lipid phosphate phosphatase activities. Methods Mol. Biol. 284: 209–216 [DOI] [PubMed] [Google Scholar]
- 16.Ghosh A. K., Ramakrishnan G., Rajasekharan R. 2008. YLR099C (ICT1) encodes a soluble Acyl-CoA-dependent lysophosphatidic acid acyltransferase responsible for enhanced phospholipid synthesis on organic solvent stress in Saccharomyces cerevisiae. J. Biol. Chem. 283: 9768–9775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis K. J., Morrison J. F. 1982. Buffers of constant ionic strength for studying pH-dependent processes. Methods Enzymol. 87: 405–426 [DOI] [PubMed] [Google Scholar]
- 18.Igal R. A., Coleman R. A. 1996. Acylglycerol recycling from triacylglycerol to phospholipid, not lipase activity, is defective in neutral lipid storage disease fibroblasts. J. Biol. Chem. 271: 16644–16651 [DOI] [PubMed] [Google Scholar]
- 19.Igal R. A., Coleman R. A. 1998. Neutral lipid storage disease: a genetic disorder with abnormalities in the regulation of phospholipid metabolism. J. Lipid Res. 39: 31–43 [PubMed] [Google Scholar]
- 20.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Med. Sci. 37: 911–917 [DOI] [PubMed] [Google Scholar]
- 21.Constantinides P. P., Steim J. M. 1985. Physical properties of fatty acyl-CoA. Critical micelle concentrations and micellar size and shape. J. Biol. Chem. 260: 7573–7580 [PubMed] [Google Scholar]
- 22.Das A. K., Hajra A. K. 1992. Critical micellar concentrations of palmitoyl dehydroxyacetone phosphate and 1-palmitoyl-rac-glycerol 3-phosphate. J. Biol. Chem. 267: 9731. [PubMed] [Google Scholar]
- 23.Li Z., Mintzer E., Bittman R. 2004. The critical micelle concentrations of lysophosphatidic acid and sphingosylphosphorylcholine. Chem. Phys. Lipids. 130: 197–201 [DOI] [PubMed] [Google Scholar]
- 24.Powell G. L., Grothusen J. R., Zimmerman J. K., Evans C. A., Fish W. W. 1981. A re-examination of some properties of fatty acyl-CoA micelles. J. Biol. Chem. 256: 12740–12747 [PubMed] [Google Scholar]
- 25.Requero M. A., Goni F. M., Alonso A. 1993. The critical micellar concentrations of fatty acyl coenzyme-A and fatty acyl carnitines. J. Colloid Interface Sci. 161: 343–346 [Google Scholar]
- 26.Wei J., Kang H. W., Cohen D. E. 2009. Thioesterase superfamily member 2 (Them2)/acyl-CoA thioesterase 13 (Acot13): a homotetrameric hotdog fold thioesterase with selectivity for long-chain fatty acyl-CoAs. Biochem. J. 421: 311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gimeno R. E., Cao J. 2008. Thematic review series: glycerolipids. Mammalian glycerol-3-phosphate acyltransferases: new genes for an old activity. J. Lipid Res. 49: 2079–2088 [DOI] [PubMed] [Google Scholar]
- 28.Lewin T. M., Wang P., Coleman R. A. 1999. Analysis of amino acid motifs diagnostic for the sn-glycerol-3-phosphate acyltransferase reaction. Biochemistry. 38: 5764–5771 [DOI] [PubMed] [Google Scholar]
- 29.Shindou H., Hishikawa D., Harayama T., Yuki K., Shimizu T. 2009. Recent progress on acyl CoA: lysophospholipid acyltransferase research. J. Lipid Res. 50(Suppl): S46–S51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shindou H., Shimizu T. 2009. Acyl-CoA:lysophospholipid acyltransferases. J. Biol. Chem. 284: 1–5 [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi K., Reue K. 2009. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am. J. Physiol. Endocrinol. Metab. 296: E1195–E1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wendel A. A., Lewin T. M., Coleman R. A. 2009. Glycerol-3-phosphate acyltransferases: Rate limiting enzymes of triacylglycerol biosynthesis. Biochim. Biophys. Acta. 1791: 501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shindou H., Eto M., Morimoto R., Shimizu T. 2009. Identification of membrane O-acyltransferase family motifs. Biochem. Biophys. Res. Commun. 383: 320–325 [DOI] [PubMed] [Google Scholar]
- 34.Yen C. L., Stone S. J., Koliwad S., Harris C., Farese R. V., Jr 2008. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 49: 2283–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heath R. J., Rock C. O. 1998. A conserved histidine is essential for glycerolipid acyltransferase catalysis. J. Bacteriol. 180: 1425–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon G. M., Cravatt B. F. 2006. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J. Biol. Chem. 281: 26465–26472 [DOI] [PubMed] [Google Scholar]
- 37.Brown J. M., Chung S., Das A., Shelness G. S., Rudel L. L., Yu L. 2007. CGI-58 facilitates the mobilization of cytoplasmic triglyceride for lipoprotein secretion in hepatoma cells. J. Lipid Res. 48: 2295–2305 [DOI] [PubMed] [Google Scholar]
- 38.Liu P., Ying Y., Zhao Y., Mundy D. I., Zhu M., Anderson R. G. 2004. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J. Biol. Chem. 279: 3787–3792 [DOI] [PubMed] [Google Scholar]
- 39.Igal R. A., Wang P., Coleman R. A. 1997. Triacsin C blocks de novo synthesis of glycerolipids and cholesterol esters but not recycling of fatty acid into phospholipid: evidence for functionally separate pools of acyl-CoA. Biochem. J. 324: 529–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J. H., Lewin T. M., Coleman R. A. 2001. Expression and characterization of recombinant rat Acyl-CoA synthetases 1, 4, and 5. Selective inhibition by triacsin C and thiazolidinediones. J. Biol. Chem. 276: 24667–24673 [DOI] [PubMed] [Google Scholar]
- 41.Lewin T. M., Kim J. H., Granger D. A., Vance J. E., Coleman R. A. 2001. Acyl-CoA synthetase isoforms 1, 4, and 5 are present in different subcellular membranes in rat liver and can be inhibited independently. J. Biol. Chem. 276: 24674–24679 [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi T., Omatsu N., Matsushita S., Osumi T. 2004. CGI-58 interacts with perilipin and is localized to lipid droplets: possible involvement of CGI-58 mislocalization in Chanarin-Dorfman syndrome. J. Biol. Chem. 279: 30490–30497 [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi T., Omatsu N., Morimoto E., Nakashima H., Ueno K., Tanaka T., Satouchi K., Hirose F., Osumi T. 2007. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J. Lipid Res. 48: 1078–1089 [DOI] [PubMed] [Google Scholar]
- 44.Bezaire V., Mairal A., Ribet C., Lefort C., Girousse A., Jocken J., Laurencikiene J., Anesia R., Rodriguez A. M., Ryden M., et al. 2009. Contribution of adipose triglyceride lipase and hormone-sensitive lipase to lipolysis in hMADS adipocytes. J. Biol. Chem. 284: 18282–18291 [DOI] [PMC free article] [PubMed] [Google Scholar]